Search
- Page Path
- HOME > Search
- Calcium & bone metabolism
- Protein Signatures of Parathyroid Adenoma according to Tumor Volume and Functionality
- Sung Hye Kong, Jeong Mo Bae, Jung Hee Kim, Sang Wan Kim, Dohyun Han, Chan Soo Shin
- Endocrinol Metab. 2024;39(2):375-386. Published online March 21, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1827
- 1,070 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
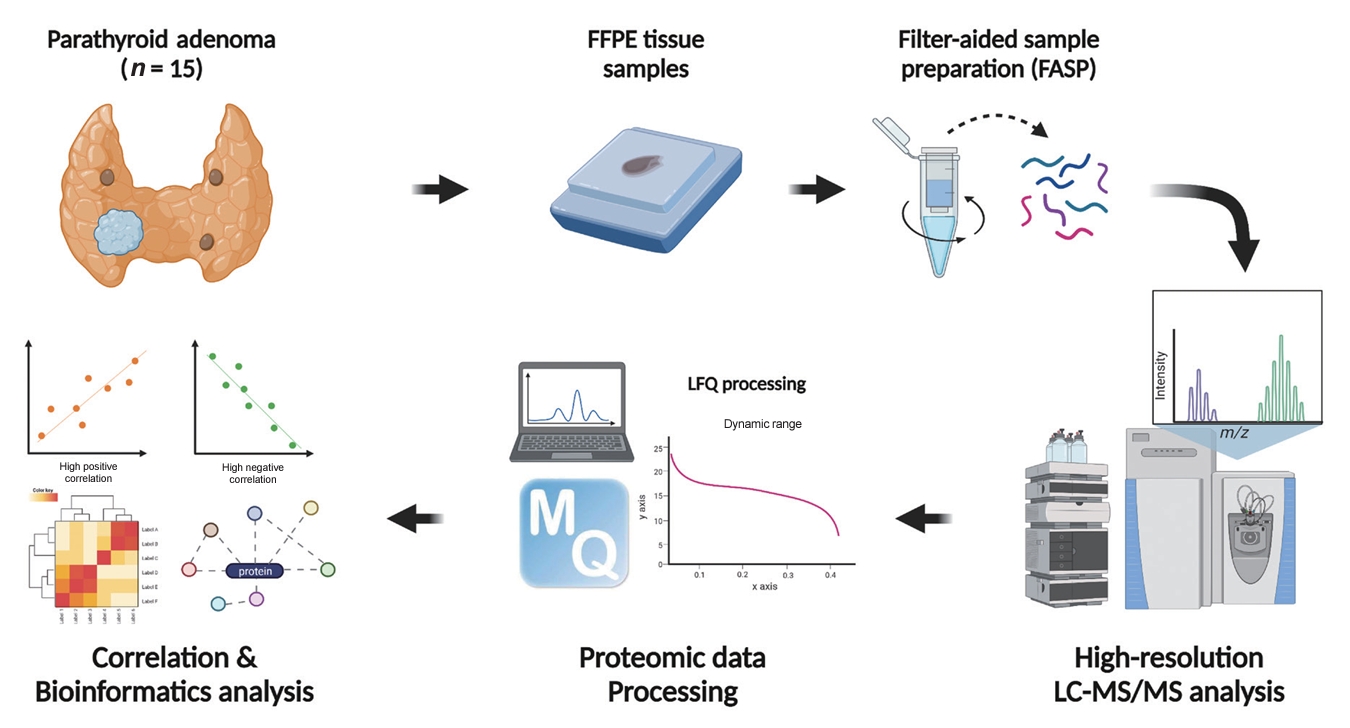
Parathyroid adenoma (PA) is a common endocrine disease linked to multiple complications, but the pathophysiology of the disease remains incompletely understood. The study aimed to identify the key regulator proteins and pathways of PA according to functionality and volume through quantitative proteomic analyses.
Methods
We conducted a retrospective study of 15 formalin-fixed, paraffin-embedded PA samples from tertiary hospitals in South Korea. Proteins were extracted, digested, and the resulting peptides were analyzed using liquid chromatography-tandem mass spectrometry. Pearson correlation analysis was employed to identify proteins significantly correlated with clinical variables. Canonical pathways and transcription factors were analyzed using Ingenuity Pathway Analysis.
Results
The median age of the participants was 52 years, and 60.0% were female. Among the 8,153 protein groups analyzed, 496 showed significant positive correlations with adenoma volume, while 431 proteins were significantly correlated with parathyroid hormone (PTH) levels. The proteins SLC12A9, LGALS3, and CARM1 were positively correlated with adenoma volume, while HSP90AB2P, HLA-DRA, and SCD5 showed negative correlations. DCPS, IRF2BPL, and FAM98A were the main proteins that exhibited positive correlations with PTH levels, and SLITRK4, LAP3, and AP4E1 had negative correlations. Canonical pathway analysis demonstrated that the RAN and sirtuin signaling pathways were positively correlated with both PTH levels and adenoma volume, while epithelial adherence junction pathways had negative correlations.
Conclusion
Our study identified pivotal proteins and pathways associated with PA, offering potential therapeutic targets. These findings accentuate the importance of proteomics in understanding disease pathophysiology and the need for further research.

- Thyroid
- BRAFV600E Mutation Enhances Estrogen-Induced Metastatic Potential of Thyroid Cancer by Regulating the Expression of Estrogen Receptors
- Minjun Kim, Su-jin Kim, Seong Yun Ha, Zhen Xu, Youngjin Han, Hyeon-Gun Jee, Sun Wook Cho, Young Joo Park, Kyu Eun Lee
- Endocrinol Metab. 2022;37(6):879-890. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1563
- 2,908 View
- 217 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
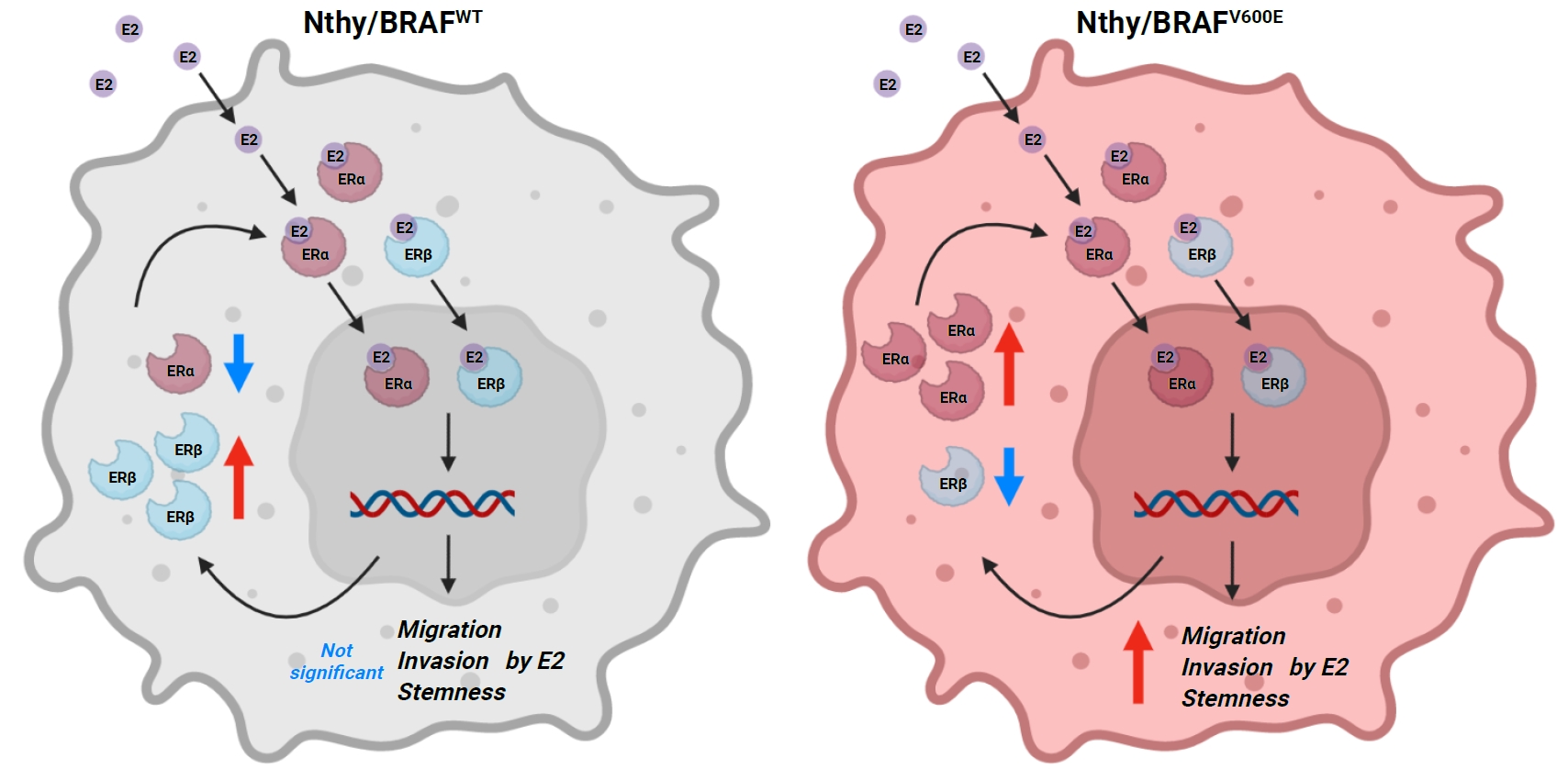
Cross-talk between mitogen-activated protein kinase and estrogen has been reported; however, the role of BRAFV600E in the estrogen responsiveness of thyroid cancer is unknown. We elucidated the effect of BRAFV600E on the estrogen-induced increase in metastatic potential in thyroid cancer.
Methods
Using a pair of cell lines, human thyroid cell lines which harbor wild type BRAF gene (Nthy/WT) and Nthy/BRAFV600E (Nthy/V600E), the expression of estrogen receptors (ERs) and estrogen-induced metastatic phenotypes were evaluated. Susceptibility to ERα- and ERβ-selective agents was evaluated to confirm differential ER expression. ESR expression was analyzed according to BRAFV600E status and age (≤50 years vs. >50 years) using The Cancer Genome Atlas (TCGA) data.
Results
Estradiol increased the ERα/ERβ expression ratio in Nthy/V600E, whereas the decreased ERα/ERβ expression ratio was found in Nthy/WT. BRAFV600E-mutated cell lines showed a higher E2-induced increase in metastatic potential, including migration, invasion, and anchorage-independent growth compared with Nthy/WT. An ERα antagonist significantly inhibited migration in Nthy/V600E cells, whereas an ERβ agonist was more effective in Nthy/WT. In the BRAFV600E group, ESR1/ESR2 ratio was significantly higher in younger age group (≤50 years) compared with older age group (>50 years) by TCGA data analysis.
Conclusion
Our data show that BRAFV600E mutation plays a crucial role in the estrogen responsiveness of thyroid cancer by regulating ER expression. Therefore, BRAFV600E might be used as a biomarker when deciding future hormone therapies based on estrogen signaling in thyroid cancer patients. -
Citations
Citations to this article as recorded by- The importance of protein domain mutations in cancer therapy
Kiran Kumar Chitluri, Isaac Arnold Emerson
Heliyon.2024; 10(6): e27655. CrossRef - Three cases of thyroid cancer in transgender female veterans receiving gender-affirming estrogen treatment
John D. Christensen, Hiba T. Basheer
Endocrine and Metabolic Science.2024; 15: 100177. CrossRef - Thyroid Cancer Prevalence, Risk Exposure, and Clinical Features Among Transgender Female Veterans
John David Christensen, Hiba T Basheer, Jose Joaquin Lado Abeal
Journal of the Endocrine Society.2024;[Epub] CrossRef - Association of DNA Promoter Methylation and BRAF Mutation in Thyroid Cancer
Farzana Jasmine, Briseis Aschebrook-Kilfoy, Mohammad M. Rahman, Garrett Zaagman, Raymon H. Grogan, Mohammed Kamal, Habibul Ahsan, Muhammad G. Kibriya
Current Oncology.2023; 30(3): 2978. CrossRef - Editorial: Recent advances in papillary thyroid carcinoma: Progression, treatment and survival predictors
Erivelto Martinho Volpi, Margarita Carmen Ramirez-Ortega, Jose Federico Carrillo
Frontiers in Endocrinology.2023;[Epub] CrossRef
- The importance of protein domain mutations in cancer therapy

- Diabetes, Obesity and Metabolism
- Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
- Joon Ho Moon, Kyuho Kim, Sung Hee Choi
- Endocrinol Metab. 2022;37(4):575-586. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.402
- 8,197 View
- 445 Download
- 11 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
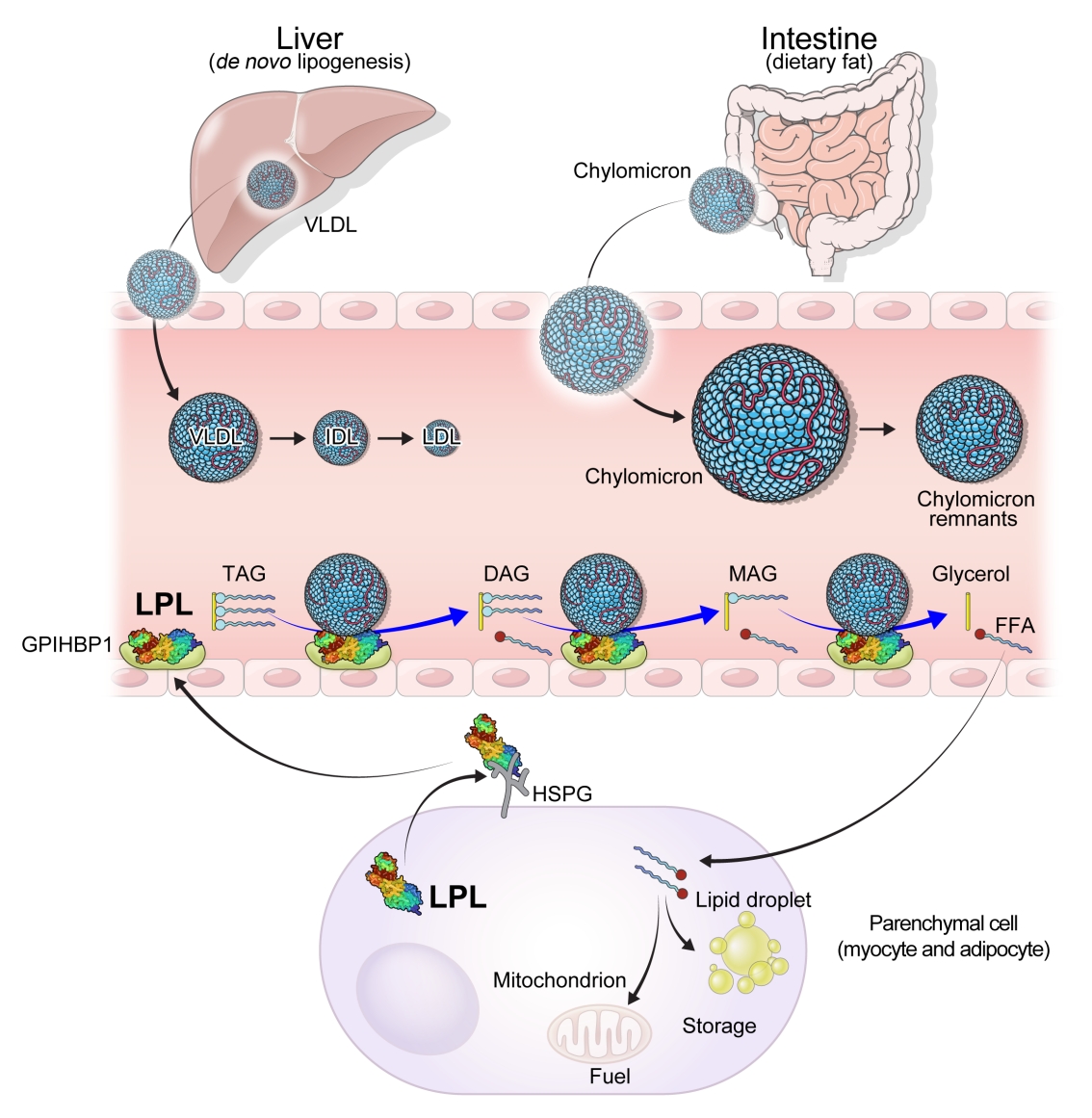
ePub - High levels of triglycerides (TG) and triglyceride-rich lipoproteins (TGRLs) confer a residual risk of cardiovascular disease after optimal low-density lipoprotein cholesterol (LDL-C)–lowering therapy. Consensus has been made that LDL-C is a non-arguable primary target for lipid lowering treatment, but the optimization of TGRL for reducing the remnant risk of cardiovascular diseases is urged. Omega-3 fatty acids and fibrates are used to reduce TG levels, but many patients still have high TG and TGRL levels combined with low high-density lipoprotein concentration that need to be ideally treated. Lipoprotein lipase (LPL) is a key regulator for TGs that hydrolyzes TGs to glycerol and free fatty acids in lipoprotein particles for lipid storage and consumption in peripheral organs. A deeper understanding of human genetics has enabled the identification of proteins regulating the LPL activity, which include the apolipoproteins and angiopoietin-like families. Novel therapeutic approach such as antisense oligonucleotides and monoclonal antibodies that regulate TGs have been developed in recent decades. In this article, we focus on the biology of LPL and its modulators and review recent clinical application, including genetic studies and clinical trials of novel therapeutics. Optimization of LPL activity to lower TG levels could eventually reduce incident atherosclerotic cardiovascular disease in conjunction with successful LDL-C reduction.
-
Citations
Citations to this article as recorded by- The chylomicron saga: time to focus on postprandial metabolism
Alejandro Gugliucci
Frontiers in Endocrinology.2024;[Epub] CrossRef - Sanghuangporus vaninii extract ameliorates hyperlipidemia in rats by mechanisms identified with transcriptome analysis
Ning Gao, Yuanzhen Liu, Guangjie Liu, Bo Liu, Yupeng Cheng
Food Science & Nutrition.2024; 12(5): 3360. CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Obesity Pillars.2024; 10: 100108. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Xanthohumol, a prenylated chalcone, regulates lipid metabolism by modulating the LXRα/RXR-ANGPTL3-LPL axis in hepatic cell lines and high-fat diet-fed zebrafish models
Wan-Yun Gao, Pei-Yi Chen, Hao-Jen Hsu, Je-Wen Liou, Chia-Ling Wu, Ming-Jiuan Wu, Jui-Hung Yen
Biomedicine & Pharmacotherapy.2024; 174: 116598. CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Journal of Clinical Lipidology.2024;[Epub] CrossRef - Factors associated with treatment responses to pioglitazone in patients with steatotic liver disease: A 3‐year prospective cohort study
Ming‐Ling Chang, Jennifer Tai, Jur‐Shan Cheng, Wei‐Ting Chen, Sien‐Sing Yang, Cheng‐Hsun Chiu, Rong‐Nan Chien
Diabetes, Obesity and Metabolism.2024;[Epub] CrossRef - High producer variant of lipoprotein lipase may protect from hepatocellular carcinoma in alcohol-associated cirrhosis
Franziska Schmalz, Janett Fischer, Hamish Innes, Stephan Buch, Christine Möller, Madlen Matz-Soja, Witigo von Schönfels, Benjamin Krämer, Bettina Langhans, Alexandra Klüners, Michael Soyka, Felix Stickel, Jacob Nattermann, Christian P. Strassburg, Thomas
JHEP Reports.2023; 5(4): 100684. CrossRef - Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations
Dieter Lütjohann, Hans-Ulrich Klör, Frans Stellaard
Nutrients.2023; 15(9): 2202. CrossRef - Influence of antipsychotic medications on hyperlipidemia risk in patients with schizophrenia: evidence from a population-based cohort study and in vitro hepatic lipid homeostasis gene expression
Tien-Yuan Wu, Ni Tien, Cheng-Li Lin, Yu-Cun Cheah, Chung Y. Hsu, Fuu-Jen Tsai, Yi-Jen Fang, Yun-Ping Lim
Frontiers in Medicine.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Sugar and Dyslipidemia: A Double-Hit, Perfect Storm
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(17): 5660. CrossRef - Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
Sang Heon Suh, Soo Wan Kim
Diabetes & Metabolism Journal.2023; 47(5): 612. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Developing a model to predict the early risk of hypertriglyceridemia based on inhibiting lipoprotein lipase (LPL): a translational study
Julia Hernandez-Baixauli, Gertruda Chomiciute, Juan María Alcaide-Hidalgo, Anna Crescenti, Laura Baselga-Escudero, Hector Palacios-Jordan, Elisabet Foguet-Romero, Anna Pedret, Rosa M. Valls, Rosa Solà, Miquel Mulero, Josep M. Del Bas
Scientific Reports.2023;[Epub] CrossRef
- The chylomicron saga: time to focus on postprandial metabolism

- Diabetes, Obesity and Metabolism
- The Effects of PPAR Agonists on Atherosclerosis and Nonalcoholic Fatty Liver Disease in ApoE−/−FXR−/− Mice
- Yenna Lee, Bo-Rahm Kim, Geun-Hyung Kang, Gwan Jae Lee, Young Joo Park, Haeryoung Kim, Hak Chul Jang, Sung Hee Choi
- Endocrinol Metab. 2021;36(6):1243-1253. Published online December 28, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1100
- 5,716 View
- 159 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
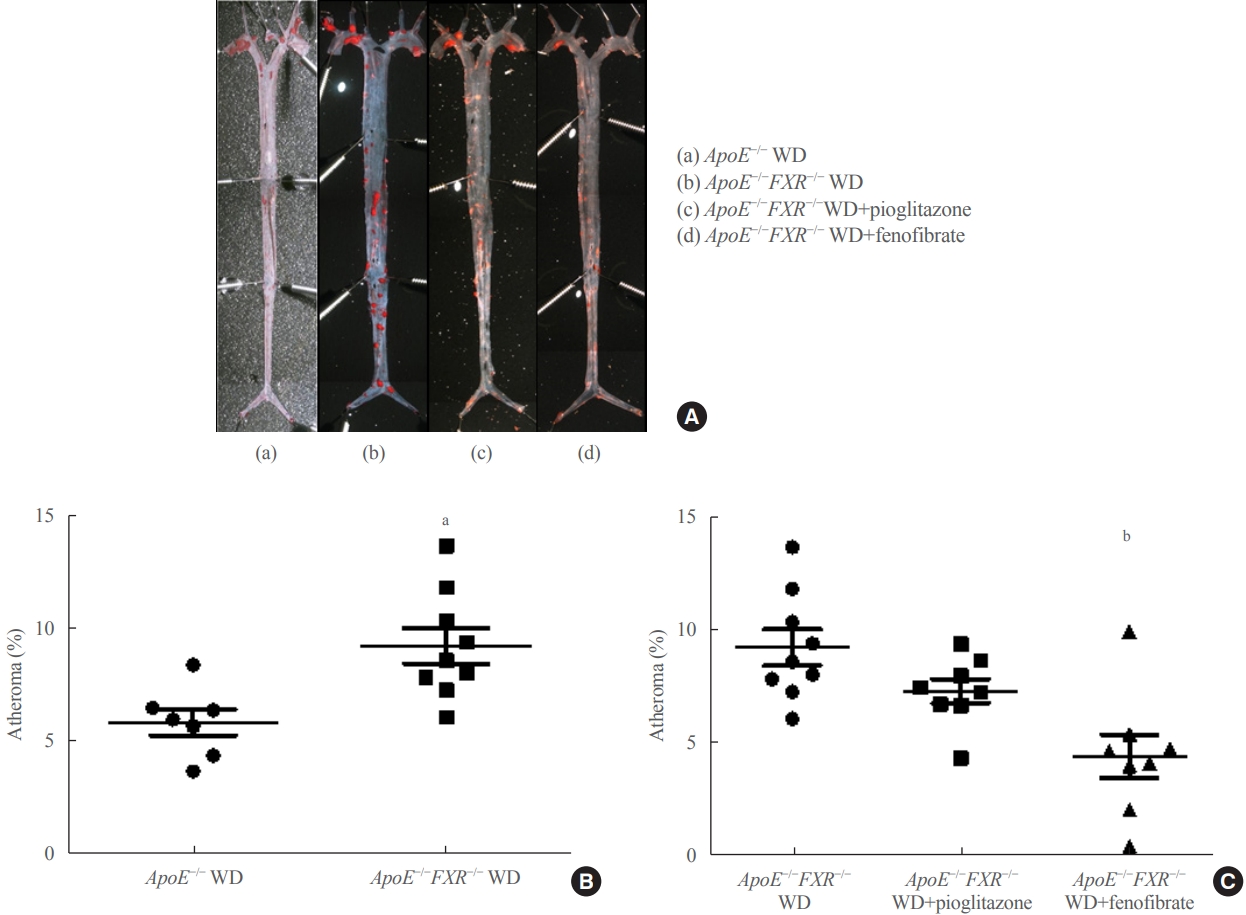
Farnesoid X receptor (FXR), a bile acid–activated nuclear receptor, is a potent regulator of glucose and lipid metabolism as well as of bile acid metabolism. Previous studies have demonstrated that FXR deficiency is associated with metabolic derangements, including atherosclerosis and nonalcoholic fatty liver disease (NAFLD), but its mechanism remains unclear. In this study, we investigated the role of FXR in atherosclerosis and NAFLD and the effect of peroxisome proliferator-activated receptor (PPAR) agonists in mouse models with FXR deficiency.
Methods
En face lipid accumulation analysis, liver histology, serum levels of glucose and lipids, and mRNA expression of genes related to lipid metabolism were compared between apolipoprotein E (ApoE)−/− and ApoE−/−FXR−/− mice. The effects of PPARα and PPARγ agonists were also compared in both groups of mice.
Results
Compared with ApoE−/− mice, ApoE−/−FXR−/− mice showed more severe atherosclerosis, hepatic steatosis, and higher levels of serum cholesterol, low-density lipoprotein cholesterol, and triglycerides, accompanied by increased mRNA expression of FAS, ApoC2, TNFα, IL-6 (liver), ATGL, TGH, HSL, and MGL (adipocytes), and decreased mRNA expressions of CPT2 (liver) and Tfam (skeletal muscle). Treatment with a PPARα agonist, but not with a PPARγ agonist, partly reversed atherosclerosis and hepatic steatosis, and decreased plasma triglyceride levels in the ApoE−/−FXR−/− mice, in association with increased mRNA expression of CD36 and FATP and decreased expression of ApoC2 and ApoC3 (liver).
Conclusion
Loss of FXR is associated with aggravation of atherosclerosis and hepatic steatosis in ApoE-deficient mice, which could be reversed by a PPARα agonist through induction of fatty acid uptake, β-oxidation, and triglyceride hydrolysis. -
Citations
Citations to this article as recorded by- Evaluation of the hepatotoxicity of Psoralea corylifolia L. based on a zebrafish model
Shu-Yan Gao, Jing-Cheng Zhao, Qing Xia, Chen Sun, Maimaiti Aili, Ainiwaer Talifu, Shi-Xia Huo, Yun Zhang, Zhi-Jian Li
Frontiers in Pharmacology.2024;[Epub] CrossRef - Advances in management of metabolic dysfunction-associated steatotic liver disease: from mechanisms to therapeutics
Yuxiao Jiang, Lili Wu, Xiaopeng Zhu, Hua Bian, Xin Gao, Mingfeng Xia
Lipids in Health and Disease.2024;[Epub] CrossRef - Mitochondrial carnitine palmitoyltransferase-II dysfunction: A possible novel mechanism for nonalcoholic fatty liver disease in hepatocarcinogenesis
Min Yao, Ping Zhou, Yan-Yan Qin, Li Wang, Deng-Fu Yao
World Journal of Gastroenterology.2023; 29(12): 1765. CrossRef - Emerging Roles of Gut Microbial Modulation of Bile Acid Composition in the Etiology of Cardiovascular Diseases
Tess Yntema, Debby P. Y. Koonen, Folkert Kuipers
Nutrients.2023; 15(8): 1850. CrossRef - The interplay between nonalcoholic fatty liver disease and atherosclerotic cardiovascular disease
Alexandra C. Finney, Sandeep Das, Dhananjay Kumar, M. Peyton McKinney, Bishuang Cai, Arif Yurdagul, Oren Rom
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - Targeting PPARs for therapy of atherosclerosis: A review
Miao Miao, Xue Wang, Tian Liu, Yan-Jie Li, Wen-Qian Yu, Tong-Mei Yang, Shou-Dong Guo
International Journal of Biological Macromolecules.2023; 242: 125008. CrossRef - Cabernet sauvignon dry red wine ameliorates atherosclerosis in mice by regulating inflammation and endothelial function, activating AMPK phosphorylation, and modulating gut microbiota
Xinlong Cheng, Xue Han, Liangfu Zhou, Yasai Sun, Qian Zhou, Xuan Lin, Zhe Gao, Jie Wang, Wen Zhao
Food Research International.2023; 169: 112942. CrossRef - Impacts of dietary lipids derived from animal or vegetable sources on healthy rats
Mostafa M Dalal, Gamal M Edrees, Hanaa A Hassan, Mamdouh Abdel-Mogib, Mai Alaa El-Dein
Egyptian Journal of Basic and Applied Sciences.2023; 10(1): 618. CrossRef - Whey protein hydrolysate alleviated atherosclerosis and hepatic steatosis by regulating lipid metabolism in apoE-/- mice fed a Western diet
Kai Wang, Zixin Fu, Xiaoyi Li, Hui Hong, Xin Zhan, Xiaohong Guo, Yongkang Luo, Yuqing Tan
Food Research International.2022; 157: 111419. CrossRef - Melatonin alleviates PM2.5‐induced glucose metabolism disorder and lipidome alteration by regulating endoplasmic reticulum stress
Zhou Du, Junjie Hu, Lisen Lin, Qingqing Liang, Mengqi Sun, Zhiwei Sun, Junchao Duan
Journal of Pineal Research.2022;[Epub] CrossRef - Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
Joon Ho Moon, Kyuho Kim, Sung Hee Choi
Endocrinology and Metabolism.2022; 37(4): 575. CrossRef - The role of the gut microbiota in health and cardiovascular diseases
Lu Wang, Shiqi Wang, Qing Zhang, Chengqi He, Chenying Fu, Quan Wei
Molecular Biomedicine.2022;[Epub] CrossRef
- Evaluation of the hepatotoxicity of Psoralea corylifolia L. based on a zebrafish model

- Clinical Study
- Molecular Correlates and Nuclear Features of Encapsulated Follicular-Patterned Thyroid Neoplasms
- Chan Kwon Jung, Andrey Bychkov, Dong Eun Song, Jang-Hee Kim, Yun Zhu, Zhiyan Liu, Somboon Keelawat, Chiung-Ru Lai, Mitsuyoshi Hirokawa, Kaori Kameyama, Kennichi Kakudo
- Endocrinol Metab. 2021;36(1):123-133. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.860

- 5,238 View
- 150 Download
- 11 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Assessing nuclear features is diagnostically challenging in the aspect of thyroid pathology. The aim of this study was to determine whether pathologists could distinguish BRAF-like and RAS-like nuclear features morphologically and identify morphological features to differentiate thyroid tumors with RAS-like mutations from encapsulated papillary thyroid carcinoma (PTC) with predominant follicular growth and BRAFV600E mutation.
Methods
Representative whole slide images of 16 encapsulated thyroid tumors with predominant follicular growth were reviewed by 12 thyroid pathologists using a web browser-based image viewer. Total nuclear score was calculated from semi-quantitatively scored eight nuclear features. The molecular profile of RAS and BRAF genes was determined by Sanger sequencing.
Results
Total nuclear score ranging 0 to 24 could differentiate BRAF-like tumors from RAS-like tumors with a cut-off value of score 14. The interobserver agreement was the highest for the assessment of nuclear pseudoinclusions (NPIs) but the lowest for nuclear elongation and sickle-shaped nuclei. NPIs were found in tumors with BRAFV600E mutation, but not in tumors with RAS-like mutations. Total nuclear scores were significantly higher for tumors with BRAFV600E than for those with RAS-like mutations (P<0.001).
Conclusion
Our results suggest that NPIs and high nuclear scores have diagnostic utility as rule-in markers for differentiating PTC with BRAFV600E mutation from benign or borderline follicular tumors with RAS-like mutations. Relaxation of rigid criteria for nuclear features resulted in an overdiagnosis of PTC. Immunostaining or molecular testing for BRAFV600E mutation is a useful adjunct for cases with high nuclear scores to identify true PTC. -
Citations
Citations to this article as recorded by- Differentiating BRAF V600E- and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study
Chankyung Kim, Shipra Agarwal, Andrey Bychkov, Jen-Fan Hang, Agnes Stephanie Harahap, Mitsuyoshi Hirokawa, Kennichi Kakudo, Somboon Keelawat, Chih-Yi Liu, Zhiyan Liu, Truong Phan-Xuan Nguyen, Chanchal Rana, Huy Gia Vuong, Yun Zhu, Chan Kwon Jung
Virchows Archiv.2024;[Epub] CrossRef - Clinicopathologic Features and Cytologic Correlation of ALK-Rearranged Papillary Thyroid Carcinoma: A Series of Eight Cases
Kun-Ping Shih, Yu-Cheng Lee, Jia-Jiun Tsai, Shu-Hui Lin, Chih-Yi Liu, Wan-Shan Li, Chien-Feng Li, Jen-Fan Hang
Endocrine Pathology.2024;[Epub] CrossRef - The Presence of Typical “BRAFV600E-Like” Atypia in Papillary Thyroid Carcinoma is Highly Specific for the Presence of the BRAFV600E Mutation
John Turchini, Loretta Sioson, Adele Clarkson, Amy Sheen, Leigh Delbridge, Anthony Glover, Mark Sywak, Stan Sidhu, Anthony J. Gill
Endocrine Pathology.2023; 34(1): 112. CrossRef - Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer?
Maria Kościuszko, Angelika Buczyńska, Adam Jacek Krętowski, Anna Popławska-Kita
Cancers.2023; 15(12): 3182. CrossRef - Pitfalls in thyroid pathology and the medicolegal aspects of error
David N Poller
Diagnostic Histopathology.2023; 29(11): 495. CrossRef - Developing Models to Predict BRAFV600E and RAS Mutational Status in Papillary Thyroid Carcinoma Using Clinicopathological Features and pERK1/2 Immunohistochemistry Expression
Agnes Stephanie Harahap, Imam Subekti, Sonar Soni Panigoro, Asmarinah, Lisnawati, Retno Asti Werdhani, Hasrayati Agustina, Dina Khoirunnisa, Mutiah Mutmainnah, Fajar Lamhot Gultom, Abdillah Hasbi Assadyk, Maria Francisca Ham
Biomedicines.2023; 11(10): 2803. CrossRef - The Asian Thyroid Working Group, from 2017 to 2023
Kennichi Kakudo, Chan Kwon Jung, Zhiyan Liu, Mitsuyoshi Hirokawa, Andrey Bychkov, Huy Gia Vuong, Somboon Keelawat, Radhika Srinivasan, Jen-Fan Hang, Chiung-Ru Lai
Journal of Pathology and Translational Medicine.2023; 57(6): 289. CrossRef - Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features (NIFTP): Tumour Entity with a Short History. A Review on Challenges in Our Microscopes, Molecular and Ultrasonographic Profile
Ivana Kholová, Elina Haaga, Jaroslav Ludvik, David Kalfert, Marie Ludvikova
Diagnostics.2022; 12(2): 250. CrossRef - Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
Chan Kwon Jung, Andrey Bychkov, Kennichi Kakudo
Endocrinology and Metabolism.2022; 37(5): 703. CrossRef - Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice
Kennichi Kakudo
Cancers.2022; 14(3): 812. CrossRef - The Incidence of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Meta-Analysis Assessing Worldwide Impact of the Reclassification
Chanchal Rana, Huy Gia Vuong, Thu Quynh Nguyen, Hoang Cong Nguyen, Chan Kwon Jung, Kennichi Kakudo, Andrey Bychkov
Thyroid.2021;[Epub] CrossRef
- Differentiating BRAF V600E- and RAS-like alterations in encapsulated follicular patterned tumors through histologic features: a validation study

- Miscellaneous
- WD40-Repeat Proteins in Ciliopathies and Congenital Disorders of Endocrine System
- Yeonjoo Kim, Soo-Hyun Kim
- Endocrinol Metab. 2020;35(3):494-506. Published online September 8, 2020
- DOI: https://doi.org/10.3803/EnM.2020.302

- 14,969 View
- 200 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - WD40-repeat (WDR)-containing proteins constitute an evolutionarily conserved large protein family with a broad range of biological functions. In human proteome, WDR makes up one of the most abundant protein-protein interaction domains. Members of the WDR protein family play important roles in nearly all major cellular signalling pathways. Mutations of WDR proteins have been associated with various human pathologies including neurological disorders, cancer, obesity, ciliopathies and endocrine disorders. This review provides an updated overview of the biological functions of WDR proteins and their mutations found in congenital disorders. We also highlight the significant role of WDR proteins in ciliopathies and endocrine disorders. The new insights may help develop therapeutic approaches targeting WDR motifs.
-
Citations
Citations to this article as recorded by- Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Alleviate Rheumatoid Arthritis Symptoms via Shuttling Proteins
Lijun Wang, Fei Li, Liting Wang, Bingxing Wu, Min Du, Hua Xing, Shifeng Pan
Journal of Proteome Research.2024; 23(4): 1298. CrossRef - Structural screens identify candidate human homologs of insect chemoreceptors and cryptic Drosophila gustatory receptor-like proteins
Richard Benton, Nathaniel J Himmel
eLife.2023;[Epub] CrossRef - Changes in protein phosphorylation by insulin administration in the central nervous system of the gastropod mollusk Lymnaea stagnalis
Junko Nakai, Kengo Namiki, Yuki Totani, Shigeki Yasumasu, Teruki Yoshimura, Takashi Aoki, Etsuro Ito
Biophysics and Physicobiology.2023; 20(4): n/a. CrossRef - Unveiling Distinct Proteomic Signatures in Complicated Crohn’s Disease That Could Predict the Disease Course
Laura A. Lucaciu, Radu Seicean, Alina Uifălean, Maria Iacobescu, Cristina A. Iuga, Andrada Seicean
International Journal of Molecular Sciences.2023; 24(23): 16966. CrossRef - Aromatic patterns: Tryptophan aromaticity as a catalyst for the emergence of life and rise of consciousness
Amal Alachkar
Physics of Life Reviews.2022; 42: 93. CrossRef - EML2-S constitutes a new class of proteins that recognizes and regulates the dynamics of tyrosinated microtubules
Takashi Hotta, Thomas S. McAlear, Yang Yue, Takumi Higaki, Sarah E. Haynes, Alexey I. Nesvizhskii, David Sept, Kristen J. Verhey, Susanne Bechstedt, Ryoma Ohi
Current Biology.2022; 32(18): 3898. CrossRef - Susceptibility of craniofacial ciliopathies to oral cancer-A proposed research
G Arun Kumar
Journal of Dental Health, Oral Disorders & Therapy.2022; 13(2): 41. CrossRef - A WDR47 homolog facilitates ciliogenesis by modulating intraflagellar transport
Chun-Xue Song, Xian-Ting Zeng, Wan-Xin Zeng, Rong Liu, Xia-Jing Tong, Qian Li
Journal of Cell Science.2022;[Epub] CrossRef - Biallelic loss-of-function variants in WDR11 are associated with microcephaly and intellectual disability
Natja Haag, Ene-Choo Tan, Matthias Begemann, Lars Buschmann, Florian Kraft, Petra Holschbach, Angeline H. M. Lai, Maggie Brett, Ganeshwaran H. Mochida, Stephanie DiTroia, Lynn Pais, Jennifer E. Neil, Muna Al-Saffar, Laila Bastaki, Christopher A. Walsh, In
European Journal of Human Genetics.2021; 29(11): 1663. CrossRef
- Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Alleviate Rheumatoid Arthritis Symptoms via Shuttling Proteins

- Clinical Study
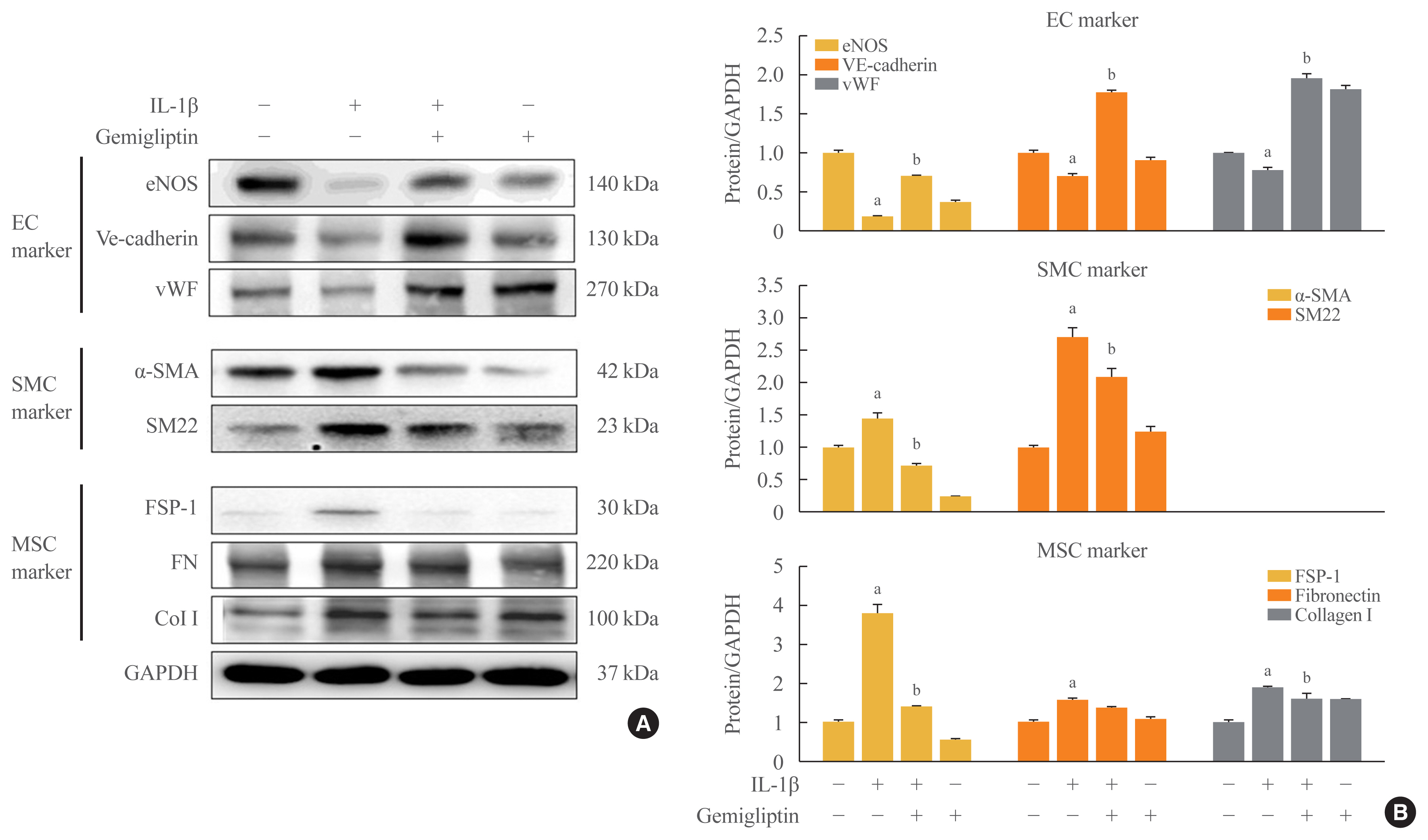
- Gemigliptin Inhibits Interleukin-1β–Induced Endothelial-Mesenchymal Transition via Canonical-Bone Morphogenetic Protein Pathway
- Oak-Kee Hong, Seong-Su Lee, Soon Jib Yoo, Min-Kyung Lee, Mee-Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
- Endocrinol Metab. 2020;35(2):384-395. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.384
- 6,842 View
- 139 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Endothelial-to-mesenchymal transition (EndMT) contributes to inflammatory conditions inducing conversion of endothelial cells (ECs) into activated fibroblasts, promoting fibrotic diseases. Pro-inflammatory cytokine is the most potent inducer of EndMT. We investigated inhibition of interleukin-1β (IL-1β)-induced EndMT by gemigliptin, a dipeptidyl peptidase-IV inhibitor.
Methods
We exposed human umbilical vein endothelial cells (HUVECs) to 10 ng/mL IL-1β/20 μM gemigliptin and analyzed the expression of endothelial, smooth muscle, mesenchymal, and osteoblastic markers, bone morphogenetic protein (BMP), Smad, and non-Smad signaling pathway proteins.
Results
Morphological changes showed gemigliptin blocked IL-1β-induced EndMT, upregulated EC markers, and downregulated smooth muscle and mesenchymal markers. IL-1β activation of HUVECs is initiated by the BMP/Smad and non-smad BMP signaling pathways. Gemigliptin inhibited IL-1β induction of BMP2 and 7, activin receptor type IA, BMP receptor type IA, and BMP receptor type II. Reversal of IL-1β-mediated inhibition of BMP-induced Smad1/5/8, Smad2, and Smad3 phosphorylation by gemigliptin suggests involvement of the Smad pathway in gemigliptin action. In the non-Smad BMP pathway, gemigliptin treatment significantly increased the deactivation of extracellular regulated protein kinase (ERK), p38, and JNK by IL-1β. Gemigliptin treatment suppressed BMP-2-induced expression of key osteoblastic markers including osterix, runt-related transcription factor 2, and hepcidin during IL-1β-induced EndMT.
Conclusion
We demonstrated a novel protective mechanism of gemigliptin against fibrosis by suppressing IL-1β-induced EndMT. -
Citations
Citations to this article as recorded by- Injured Endothelial Cell: A Risk Factor for Pulmonary Fibrosis
Weiming Zhao, Lan Wang, Yaxuan Wang, Hongmei Yuan, Mengxia Zhao, Hui Lian, Shuaichen Ma, Kai Xu, Zhongzheng Li, Guoying Yu
International Journal of Molecular Sciences.2023; 24(10): 8749. CrossRef - Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances
Zuxiang Yu, Chaoyu Xu, Bin Song, Shihao Zhang, Chong Chen, Changlong Li, Shuyu Zhang
Journal of Translational Medicine.2023;[Epub] CrossRef - MiRNAs in Systemic Sclerosis Patients with Pulmonary Arterial Hypertension: Markers and Effectors
Mor Zaaroor Levy, Noa Rabinowicz, Maia Yamila Kohon, Avshalom Shalom, Ariel Berl, Tzipi Hornik-Lurie, Liat Drucker, Shelly Tartakover Matalon, Yair Levy
Biomedicines.2022; 10(3): 629. CrossRef - Recent advance in treatment of atherosclerosis: Key targets and plaque-positioned delivery strategies
Li Li, Sainan Liu, Jianying Tan, Lai Wei, Dimeng Wu, Shuai Gao, Yajun Weng, Junying Chen
Journal of Tissue Engineering.2022; 13: 204173142210885. CrossRef - Vascular Calcification: New Insights Into BMP Type I Receptor A
Zhixing Niu, Guanyue Su, Tiantian Li, Hongchi Yu, Yang Shen, Demao Zhang, Xiaoheng Liu
Frontiers in Pharmacology.2022;[Epub] CrossRef - Yi-Shen-Hua-Shi Granule Alleviates Adriamycin-Induced Glomerular Fibrosis by Suppressing the BMP2/Smad Signaling Pathway
Zhuojing Tan, Yachen Si, Yan Yu, Jiarong Ding, Linxi Huang, Ying Xu, Hongxia Zhang, Yihan Lu, Chao Wang, Bing Yu, Li Yuan
Frontiers in Pharmacology.2022;[Epub] CrossRef - Panax notoginseng Suppresses Bone Morphogenetic Protein-2 Expression in EA.hy926 Endothelial Cells by Inhibiting the Noncanonical NF-κB and Wnt/β-Catenin Signaling Pathways
Tsu-Ni Ping, Shu-Ling Hsieh, Jyh-Jye Wang, Jin-Bor Chen, Chih-Chung Wu
Plants.2022; 11(23): 3265. CrossRef - Vascular calcification: New insights into endothelial cells
Cheng Yuan, Lihua Ni, Changjiang Zhang, Xiaorong Hu, Xiaoyan Wu
Microvascular Research.2021; 134: 104105. CrossRef - Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model
Siwen Zhang, Qiyuan Chang, Pingping Li, Xiaoyu Tong, Yi Feng, Xinyao Hao, Xudong Zhang, Zhengwei Yuan, Jichun Tan
Nanoscale.2021; 13(15): 7334. CrossRef
- Injured Endothelial Cell: A Risk Factor for Pulmonary Fibrosis

- Clinical Study
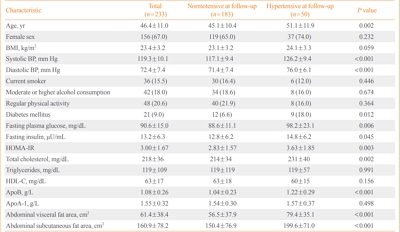
- Apolipoprotein B Levels Predict Future Development of Hypertension Independent of Visceral Adiposity and Insulin Sensitivity
- Seung Jin Han, Wilfred Y. Fujimoto, Steven E. Kahn, Donna L. Leonetti, Edward J. Boyko
- Endocrinol Metab. 2020;35(2):351-358. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.351
- 5,863 View
- 130 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
High plasma apolipoprotein B (apoB) levels have been shown to be associated with hypertension, central obesity, and insulin resistance in cross-sectional research. However, it is unclear whether apoB levels predict future hypertension independent of body composition and insulin sensitivity. Therefore, we prospectively investigated whether plasma apoB concentrations independently predicted the risk of hypertension in a cohort of Japanese Americans.
Methods
A total of 233 normotensive Japanese Americans (77 men, 156 women; mean age, 46.4±11.0 years) were followed over 10 years to monitor them for the development of hypertension. Fasting plasma concentrations of apoB, glucose, and insulin were measured at baseline. Insulin sensitivity was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR). The abdominal visceral and subcutaneous fat areas were measured at baseline using computed tomography. Logistic regression analysis was used to estimate the association between apoB concentrations and the odds of incident hypertension.
Results
The 10-year cumulative incidence of hypertension was 21.5%. The baseline apoB level was found to be positively associated with the odds of incident hypertension over 10 years after adjustment for age, sex, body mass index, systolic blood pressure, abdominal visceral fat area, abdominal subcutaneous fat area, total plasma cholesterol concentration, diabetes status, and HOMA-IR at baseline (odds ratio and 95% confidence interval for a 1-standard deviation increase, 1.89 [1.06 to 3.37]; P=0.030).
Conclusion
Higher apoB concentrations predicted greater risks of future hypertension independent of abdominal visceral fat area and insulin sensitivity in Japanese Americans. -
Citations
Citations to this article as recorded by- Correlation between Central Obesity and Liver Function in Young Adults—A Cross-Sectional Study
John Alvin, Damodara Gowda KM
Journal of Health and Allied Sciences NU.2023; 13(02): 273. CrossRef - Serum amyloid A in children and adolescents: association with overweight and carotid intima-media thickness
Maria Vitória Mareschi Barbosa, João Carlos Pina Faria, Stephanie Ramos Coelho, Fernando Luiz Affonso Fonseca, Andrea Paula Kafejian Haddad, Fabíola Isabel Suano de Souza, Roseli Oselka Saccardo Sarni
einstein (São Paulo).2023;[Epub] CrossRef - The association of the apolipoprotein B/A1 ratio and the metabolic syndrome in children and adolescents: a systematic review and meta-analysis
Kayhan Dinpanah, Toba Kazemi, Sameep Shetty, Saeede Khosravi Bizhaem, Ali Fanoodi, Seyed Mohammad Riahi
Journal of Diabetes & Metabolic Disorders.2023;[Epub] CrossRef - Current Data and New Insights into the Genetic Factors of Atherogenic Dyslipidemia Associated with Metabolic Syndrome
Lăcramioara Ionela Butnariu, Eusebiu Vlad Gorduza, Elena Țarcă, Monica-Cristina Pânzaru, Setalia Popa, Simona Stoleriu, Vasile Valeriu Lupu, Ancuta Lupu, Elena Cojocaru, Laura Mihaela Trandafir, Ștefana Maria Moisă, Andreea Florea, Laura Stătescu, Minerva
Diagnostics.2023; 13(14): 2348. CrossRef - Sex-Based Differences and Risk Factors for Comorbid Nonalcoholic Fatty Liver Disease in Patients with Bipolar Disorder: A Cross-Sectional Retrospective Study
Ying Wang, Yiyi Liu, Xun Zhang, Qing Wu
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 3533. CrossRef - Apolipoprotein B Displays Superior Predictive Value Than Other Lipids for Long-Term Prognosis in Coronary Atherosclerosis Patients and Particular Subpopulations: A Retrospective Study
Chunyan Zhang, Jingwei Ni, Zhenyue Chen
Clinical Therapeutics.2022; 44(8): 1071. CrossRef - Genetics of Cholesterol-Related Genes in Metabolic Syndrome: A Review of Current Evidence
Sok Kuan Wong, Fitri Fareez Ramli, Adli Ali, Nurul ‘Izzah Ibrahim
Biomedicines.2022; 10(12): 3239. CrossRef
- Correlation between Central Obesity and Liver Function in Young Adults—A Cross-Sectional Study

- Thyroid
- Amino Acid Transporters as Potential Therapeutic Targets in Thyroid Cancer
- Keisuke Enomoto, Muneki Hotomi
- Endocrinol Metab. 2020;35(2):227-236. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.227
- 6,846 View
- 173 Download
- 9 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Thyroid cancer cells have a high amino acid demand for proliferation, invasion, and metastasis. Amino acids are taken up by thyroid cancer cells, both thyroid follicular cell and thyroid parafollicular cells (commonly called “C-cells”), via amino acid transporters. Amino acid transporters up-regulate in many cancers, and their expression level associate with clinical aggressiveness and prognosis. This is the review to discuss the therapeutic potential of amino acid transporters and as molecular targets in thyroid cancer.
-
Citations
Citations to this article as recorded by- Applications of spatially resolved omics in the field of endocrine tumors
Yinuo Hou, Yan Gao, Shudi Guo, Zhibin Zhang, Ruibing Chen, Xiangyang Zhang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Taurine and Creatine Transporters as Potential Drug Targets in Cancer Therapy
Dorota Stary, Marek Bajda
International Journal of Molecular Sciences.2023; 24(4): 3788. CrossRef - GLUT1 and ASCT2 Protein Expression in Papillary Thyroid Carcinoma Patients and Relation to Hepatitis C Virus: A Propensity-Score Matched Analysis
Afaf T Ibrahiem, Manal S Fawzy, Jawaher A Abdulhakim, Eman A Toraih
International Journal of General Medicine.2022; Volume 15: 2929. CrossRef - Excellent physicochemical and sensing characteristics of a RexOy based pH sensor at low post-deposition annealing temperature
Munmun Das, Titisha Chakraborty, Kin Fong Lei, Chan Yu Lin, Chyuan Haur Kao
RSC Advances.2022; 12(22): 13774. CrossRef - SLC6A14 Depletion Contributes to Amino Acid Starvation to Suppress EMT-Induced Metastasis in Gastric Cancer by Perturbing the PI3K/AKT/mTORC1 Pathway
Qie Guo, Wen Xu, Xiao Li, Jia-Lin Sun, Xiao-Ce Gu, Fan-Bo Jing, Sercan Ergün
BioMed Research International.2022; 2022: 1. CrossRef - Genetic Engineering in Combination with Semi‐Synthesis Leads to a New Route for Gram‐Scale Production of the Immunosuppressive Natural Product Brasilicardin A
Alma Botas, Michael Eitel, Paul N. Schwarz, Anina Buchmann, Paula Costales, Luz Elena Núñez, Jesús Cortés, Francisco Morís, Michał Krawiec, Marcin Wolański, Bertolt Gust, Mirna Rodriguez, Wolf‐Nicolas Fischer, Bernd Jandeleit, Jolanta Zakrzewska‐Czerwińsk
Angewandte Chemie.2021; 133(24): 13648. CrossRef - Genetic Engineering in Combination with Semi‐Synthesis Leads to a New Route for Gram‐Scale Production of the Immunosuppressive Natural Product Brasilicardin A
Alma Botas, Michael Eitel, Paul N. Schwarz, Anina Buchmann, Paula Costales, Luz Elena Núñez, Jesús Cortés, Francisco Morís, Michał Krawiec, Marcin Wolański, Bertolt Gust, Mirna Rodriguez, Wolf‐Nicolas Fischer, Bernd Jandeleit, Jolanta Zakrzewska‐Czerwińsk
Angewandte Chemie International Edition.2021; 60(24): 13536. CrossRef - Next-Generation Molecular Imaging of Thyroid Cancer
Yuchen Jin, Beibei Liu, Muhsin H. Younis, Gang Huang, Jianjun Liu, Weibo Cai, Weijun Wei
Cancers.2021; 13(13): 3188. CrossRef - Evaluation of 3-l- and 3-d-[18F]Fluorophenylalanines as PET Tracers for Tumor Imaging
Felicia Krämer, Benedikt Gröner, Chris Hoffmann, Austin Craig, Melanie Brugger, Alexander Drzezga, Marco Timmer, Felix Neumaier, Boris D. Zlatopolskiy, Heike Endepols, Bernd Neumaier
Cancers.2021; 13(23): 6030. CrossRef
- Applications of spatially resolved omics in the field of endocrine tumors

- Thyroid
- Mouse Models as a Tool for Understanding Progression in BrafV600E-Driven Thyroid Cancers
- Iñigo Landa, Jeffrey A. Knauf
- Endocrinol Metab. 2019;34(1):11-22. Published online February 15, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.1.11
- 6,256 View
- 94 Download
- 13 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub The development of next generation sequencing (NGS) has led to marked advancement of our understanding of genetic events mediating the initiation and progression of thyroid cancers. The NGS studies have confirmed the previously reported high frequency of mutually-exclusive oncogenic alterations affecting
BRAF andRAS proto-oncogenes in all stages of thyroid cancer. Initially identified by traditional sequencing approaches, the NGS studies also confirmed the acquisition of alterations that inactivate tumor protein p53 (TP53 ) and activate phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA ) in advanced thyroid cancers. Novel alterations, such as those in telomerase reverse transcriptase (TERT ) promoter and mating-type switching/sucrose non-fermenting (SWI/SNF) complex, are also likely to promote progression of the BRAFV600E-driven thyroid cancers. A number of genetically engineered mouse models (GEMM) of BRAFV600E-driven thyroid cancer have been developed to investigate thyroid tumorigenesis mediated by oncogenic BRAF and to explore the role of genetic alterations identified in the genomic analyses of advanced thyroid cancer to promote tumor progression. This review will discuss the various GEMMs that have been developed to investigate oncogenic BRAFV600E-driven thyroid cancers.-
Citations
Citations to this article as recorded by- Strategies to investigate migration and metastases in thyroid cancer
Daniel M. Chopyk, Priya H. Dedhia
Current Opinion in Endocrine and Metabolic Research.2024; 34: 100502. CrossRef - Comparative efficiency of differential diagnostic methods for the identification of BRAF V600E gene mutation in papillary thyroid cancer (Review)
Qian Liu, Xue Jiang, Wenling Tu, Lina Liu, Ying Huang, Yuxiao Xia, Xuliang Xia, Yuhong Shi
Experimental and Therapeutic Medicine.2024;[Epub] CrossRef - Mouse Models to Examine Differentiated Thyroid Cancer Pathogenesis: Recent Updates
Hye Choi, Kwangsoon Kim
International Journal of Molecular Sciences.2023; 24(13): 11138. CrossRef - Mechanistic Insights of Thyroid Cancer Progression
Luis Javier Leandro-García, Iñigo Landa
Endocrinology.2023;[Epub] CrossRef - Anaplastic thyroid cancer: Pathogenesis, prognostic factors and genetic landscape (Review)
Abdul-Mohsen Alhejaily, Omar Alhuzim, Yazeed Alwelaie
Molecular and Clinical Oncology.2023;[Epub] CrossRef - Tissue architecture delineates field cancerization in BRAFV600E-induced tumor development
Elin Schoultz, Ellen Johansson, Carmen Moccia, Iva Jakubikova, Naveen Ravi, Shawn Liang, Therese Carlsson, Mikael Montelius, Konrad Patyra, Jukka Kero, Kajsa Paulsson, Henrik Fagman, Martin O. Bergo, Mikael Nilsson
Disease Models & Mechanisms.2022;[Epub] CrossRef - BRAFV600E Expression in Thyrocytes Causes Recruitment of Immunosuppressive STABILIN-1 Macrophages
Catherine Spourquet, Ophélie Delcorte, Pascale Lemoine, Nicolas Dauguet, Axelle Loriot, Younes Achouri, Maija Hollmén, Sirpa Jalkanen, François Huaux, Sophie Lucas, Pierre Van Meerkeeck, Jeffrey A. Knauf, James A. Fagin, Chantal Dessy, Michel Mourad, Patr
Cancers.2022; 14(19): 4687. CrossRef - Positive BRAFV600E mutation of primary tumor influences radioiodine avidity but not prognosis of papillary thyroid cancer with lung metastases
Shuhui Huang, Mengfang Qi, Tian Tian, Hongyuan Dai, Yuan Tang, Rui Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Preclinical Models of Follicular Cell-Derived Thyroid Cancer: An Overview from Cancer Cell Lines to Mouse Models
Min Ji Jeon, Bryan R. Haugen
Endocrinology and Metabolism.2022; 37(6): 830. CrossRef - An Animal Model Further Uncovers the Role of Mutant Braf during Papillary Thyroid Cancer Development
Bernd Koelsch, Sarah Theurer, Magdalena Staniszewska, Jacqueline Heupel, Amelie Koch, Svenja Mergener, Franziska Walk, Christine Fischer, Andrea Kutritz, Kurt W. Schmid, Andrea Kindler-Röhrborn
The American Journal of Pathology.2020; 190(3): 702. CrossRef - Intratumoral Genetic Heterogeneity in Papillary Thyroid Cancer: Occurrence and Clinical Significance
Laura Fugazzola, Marina Muzza, Gabriele Pogliaghi, Mario Vitale
Cancers.2020; 12(2): 383. CrossRef - The Aryl Hydrocarbon Receptor Is Expressed in Thyroid Carcinoma and Appears to Mediate Epithelial-Mesenchymal-Transition
Sonia Moretti, Nicole Nucci, Elisa Menicali, Silvia Morelli, Vittorio Bini, Renato Colella, Martina Mandarano, Angelo Sidoni, Efisio Puxeddu
Cancers.2020; 12(1): 145. CrossRef
- Strategies to investigate migration and metastases in thyroid cancer

- Epigenetic Modifications: Novel Therapeutic Approach for Thyroid Cancer
- Xuguang Zhu, Sheue-yann Cheng
- Endocrinol Metab. 2017;32(3):326-331. Published online September 18, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.3.326
- 3,954 View
- 40 Download
- 13 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The incidence of thyroid cancer is growing the fastest among all cancers in the United States, especially in women. The number of patients with thyroid neoplasm is part of an even larger number of patients who often need to undergo an operation to exclude a cancer diagnosis. While differentiated thyroid cancer (papillary thyroid cancer and follicular thyroid cancer) accounts for most cases of thyroid cancer and has a relatively good prognosis, effective treatments for patients with de-differentiated and anaplastic thyroid cancer are still gravely needed. Despite progress in the identification of genetic changes in thyroid cancer, the impact of aberrant epigenetic alterations on thyroid cancer remains to be fully elucidated. Understanding of the roles of epigenetic changes in thyroid cancer could open new opportunities for the identification of innovative molecular targets for novel treatment modalities, especially for anaplastic thyroid cancer for which treatment is very limited. This article briefly reviews the studies that exemplify the potential for and promise of using epigenetic regulators in the treatment of thyroid cancer.
-
Citations
Citations to this article as recorded by- Challenges and Coping Strategies of Older Adults in the Aftermath of Kahramanmaraş Earthquake in Türkiye: A Qualitative Research
Nilgun Kuru Alici, Bilge Kalanlar
Journal of Applied Gerontology.2024;[Epub] CrossRef - CircRTN1 stimulates HMGB1 to regulate the malignant progression of papillary thyroid cancer by sponging miR-101-3p
Mei Zheng, Lingli Xu, Cuifeng Wei, Wenzhen Guan
Hormones.2023; 22(2): 281. CrossRef - The expression of HDAC9 and P300 in papillary thyroid carcinoma cell line
Hatice Ozisik, Berrin Ozdil, Aslı Suner, Murat Sipahi, Mehmet Erdogan, Sevki Cetinkalp, Gokhan Ozgen, Fusun Saygili, Gulgun Oktay, Huseyin Aktug
Pathology - Research and Practice.2023; 243: 154385. CrossRef - Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications
Valerie Jacquemin, Mathieu Antoine, Geneviève Dom, Vincent Detours, Carine Maenhaut, Jacques E. Dumont
Cancers.2022; 14(2): 280. CrossRef - Thyroid Carcinoma: A Review for 25 Years of Environmental Risk Factors Studies
Eva Kruger, Eman A. Toraih, Mohammad H. Hussein, Shaimaa A. Shehata, Amani Waheed, Manal S. Fawzy, Emad Kandil
Cancers.2022; 14(24): 6172. CrossRef - Study of Essential and Toxic Metal Imbalances in the Scalp Hair of Thyroid Cancer Patients in Comparison with Healthy Donors
Kalsoom Bibi, Munir H. Shah
Biological Trace Element Research.2021; 199(2): 500. CrossRef - Modern concepts of the molecular pathogenesis of thyroid cancer
A. A. Mikhailova, A. V. Shestakov, K. A. Chubakova, E. V. Kolokolova, V. Yu. Eliseev, M. Ya. Kostyaeva, E. G. Akperov, V. E. Pilipenko, T. V. Saprina, M. R. Mukhamedov, E. L. Choinzonov
Advances in Molecular Oncology.2021; 8(2): 8. CrossRef - Effect of valproic acid on miRNAs affecting histone deacetylase in a model of anaplastic thyroid cancer
Nur Selvi Gunel, Nihal Birden, Cansu Caliskan Kurt, Bakiye Goker Bagca, Behrouz Shademan, Fatma Sogutlu, Neslihan Pinar Ozates, Cigir Biray Avci
Molecular Biology Reports.2021; 48(8): 6085. CrossRef - Histone Deacetylase Inhibitors and Papillary Thyroid Cancer
Eleftherios Spartalis, Konstantinos Kotrotsios, Dimosthenis Chrysikos, Michael Spartalis, Stavroula A. Paschou, Dimitrios Schizas, Konstantinos Tsamakis, Dimitrios Dimitroulis, Theodore Troupis, Nikolaos Nikiteas
Current Pharmaceutical Design.2021; 27(18): 2199. CrossRef - HDAC1 and HDAC2 Double Knockout Triggers Cell Apoptosis in Advanced Thyroid Cancer
Ching-Ling Lin, Ming-Lin Tsai, Chun-Yu Lin, Kai-Wen Hsu, Wen-Shyang Hsieh, Wei-Ming Chi, Li-Chi Huang, Chia-Hwa Lee
International Journal of Molecular Sciences.2019; 20(2): 454. CrossRef - Systems Biology Approaches to Investigate Genetic and Epigenetic Molecular Progression Mechanisms for Identifying Gene Expression Signatures in Papillary Thyroid Cancer
Shan-Ju Yeh, Chien-Yu Lin, Cheng-Wei Li, Bor-Sen Chen
International Journal of Molecular Sciences.2019; 20(10): 2536. CrossRef - Human telomerase reverse transcriptase in papillary thyroid cancer: gene expression, effects of silencing and regulation by BET inhibitors in thyroid cancer cells
Valentina Maggisano, Marilena Celano, Saverio Massimo Lepore, Marialuisa Sponziello, Francesca Rosignolo, Valeria Pecce, Antonella Verrienti, Federica Baldan, Catia Mio, Lorenzo Allegri, Marianna Maranghi, Rosa Falcone, Giuseppe Damante, Diego Russo, Stef
Endocrine.2019; 63(3): 545. CrossRef - Role of Emerging Environmental Risk Factors in Thyroid Cancer: A Brief Review
Maria Fiore, Gea Oliveri Conti, Rosario Caltabiano, Antonino Buffone, Pietro Zuccarello, Livia Cormaci, Matteo Angelo Cannizzaro, Margherita Ferrante
International Journal of Environmental Research and Public Health.2019; 16(7): 1185. CrossRef
- Challenges and Coping Strategies of Older Adults in the Aftermath of Kahramanmaraş Earthquake in Türkiye: A Qualitative Research

- Obesity and Metabolism
- High-Density Lipoprotein, Lecithin: Cholesterol Acyltransferase, and Atherosclerosis
- Alice Ossoli, Chiara Pavanello, Laura Calabresi
- Endocrinol Metab. 2016;31(2):223-229. Published online June 10, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.223
- 5,723 View
- 78 Download
- 37 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Epidemiological data clearly show the existence of a strong inverse correlation between plasma high-density lipoprotein cholesterol (HDL-C) concentrations and the incidence of coronary heart disease. This relation is explained by a number of atheroprotective properties of HDL, first of all the ability to promote macrophage cholesterol transport. HDL are highly heterogeneous and are continuously remodeled in plasma thanks to the action of a number of proteins and enzymes. Among them, lecithin:cholesterol acyltransferase (LCAT) plays a crucial role, being the only enzyme able to esterify cholesterol within lipoproteins. LCAT is synthetized by the liver and it has been thought to play a major role in reverse cholesterol transport and in atheroprotection. However, data from animal studies, as well as human studies, have shown contradictory results. Increased LCAT concentrations are associated with increased HDL-C levels but not necessarily with atheroprotection. On the other side, decreased LCAT concentration and activity are associated with decreased HDL-C levels but not with increased atherosclerosis. These contradictory results confirm that HDL-C levels
per se do not represent the functionality of the HDL system.-
Citations
Citations to this article as recorded by- Association between Mycoplasma pneumoniae infection, high‑density lipoprotein metabolism and cardiovascular health (Review)
Tao Shen, Yanfang Li, Tingting Liu, Yunzhi Lian, Luke Kong
Biomedical Reports.2024;[Epub] CrossRef - Cholesterol transport and beyond: Illuminating the versatile functions of HDL apolipoproteins through structural insights and functional implications
Aishwarya Sudam Bhale, Olivier Meilhac, Christian Lefebvre d'Hellencourt, Mookambeswaran A. Vijayalakshmi, Krishnan Venkataraman
BioFactors.2024;[Epub] CrossRef - The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients
Roxana Nartea, Brindusa Ilinca Mitoiu, Ioana Ghiorghiu
Current Issues in Molecular Biology.2023; 45(4): 3146. CrossRef - Mannose-Coated Reconstituted Lipoprotein Nanoparticles for the Targeting of Tumor-Associated Macrophages: Optimization, Characterization, and In Vitro Evaluation of Effectiveness
Akpedje S. Dossou, Morgan E. Mantsch, Ammar Kapic, William L. Burnett, Nirupama Sabnis, Jeffery L. Coffer, Rance E. Berg, Rafal Fudala, Andras G. Lacko
Pharmaceutics.2023; 15(6): 1685. CrossRef - Abdominal obesity negatively influences key metrics of reverse cholesterol transport
Jennifer Härdfeldt, Marica Cariello, Sara Simonelli, Alice Ossoli, Natasha Scialpi, Marilidia Piglionica, Emanuela Pasculli, Alessia Noia, Elsa Berardi, Patrizia Suppressa, Giuseppina Piazzolla, Carlo Sabbà, Laura Calabresi, Antonio Moschetta
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2022; 1867(2): 159087. CrossRef - Effects of Lactobacillus paracasei N1115 on dyslipidaemia: A randomized controlled study
Hua Jiang, Shengjie Tan, Ke Ning, Hao Li, Wenzhi Zhao, Ai Zhao, Hong Zhu, Shijie Wang, Peiyu Wang, Yumei Zhang
Journal of Functional Foods.2022; 89: 104956. CrossRef - LCAT- targeted therapies: Progress, failures and future
Kaixu Yang, Junmin Wang, Hongjiao Xiang, Peilun Ding, Tao Wu, Guang Ji
Biomedicine & Pharmacotherapy.2022; 147: 112677. CrossRef - Cyclodextrin boostered-high density lipoprotein for antiatherosclerosis by regulating cholesterol efflux and efferocytosis
Yanyan Wang, Hai Gao, Xinya Huang, Zhaoan Chen, Pengyu Kang, Yunyi Zhou, Danhua Qin, Wenli Zhang, Jianping Liu
Carbohydrate Polymers.2022; 292: 119632. CrossRef - Lipidomic Approaches to Study HDL Metabolism in Patients with Central Obesity Diagnosed with Metabolic Syndrome
Gabriele Mocciaro, Simona D’Amore, Benjamin Jenkins, Richard Kay, Antonio Murgia, Luis Vicente Herrera-Marcos, Stefanie Neun, Alice P. Sowton, Zoe Hall, Susana Alejandra Palma-Duran, Giuseppe Palasciano, Frank Reimann, Andrew Murray, Patrizia Suppressa, C
International Journal of Molecular Sciences.2022; 23(12): 6786. CrossRef - rHDL modeling and the anchoring mechanism of LCAT activation
Tommaso Laurenzi, Chiara Parravicini, Luca Palazzolo, Uliano Guerrini, Elisabetta Gianazza, Laura Calabresi, Ivano Eberini
Journal of Lipid Research.2021; 62: 100006. CrossRef - Vasculoprotective properties of plasma lipoproteins from brown bears (Ursus arctos)
Matteo Pedrelli, Paolo Parini, Jonas Kindberg, Jon M. Arnemo, Ingemar Bjorkhem, Ulrika Aasa, Maria Westerståhl, Anna Walentinsson, Chiara Pavanello, Marta Turri, Laura Calabresi, Katariina Öörni, Gérman Camejo, Ole Fröbert, Eva Hurt-Camejo
Journal of Lipid Research.2021; 62: 100065. CrossRef - The Fate of Dietary Cholesterol in the Kissing Bug Rhodnius prolixus
Petter F. Entringer, David Majerowicz, Katia C. Gondim
Frontiers in Physiology.2021;[Epub] CrossRef - Excesive consumption of unsaturated fatty acids leads to oxidative and inflammatory instability in Wistar rats
Jelica D. Grujić-Milanović, Zoran Z. Miloradović, Nevena D. Mihailović-Stanojević, Vojislav V. Banjac, Strahinja Vidosavljević, Milan S. Ivanov, Danijela J. Karanović, Una-Jovana V. Vajić, Djurdjica M. Jovović
Biomedicine & Pharmacotherapy.2021; 139: 111691. CrossRef - Cholesterol Metabolic Reprogramming in Cancer and Its Pharmacological Modulation as Therapeutic Strategy
Isabella Giacomini, Federico Gianfanti, Maria Andrea Desbats, Genny Orso, Massimiliano Berretta, Tommaso Prayer-Galetti, Eugenio Ragazzi, Veronica Cocetta
Frontiers in Oncology.2021;[Epub] CrossRef - Association between Serum Concentrations of Apolipoprotein A-I (ApoA-I) and Alzheimer’s Disease: Systematic Review and Meta-Analysis
Marco Zuin, Carlo Cervellati, Alessandro Trentini, Angelina Passaro, Valentina Rosta, Francesca Zimetti, Giovanni Zuliani
Diagnostics.2021; 11(6): 984. CrossRef - Dapagliflozin reduces thrombin generation and platelet activation: implications for cardiovascular risk reduction in type 2 diabetes mellitus
Christina Kohlmorgen, Stephen Gerfer, Kathrin Feldmann, Sören Twarock, Sonja Hartwig, Stefan Lehr, Meike Klier, Irena Krüger, Carolin Helten, Petra Keul, Sabine Kahl, Amin Polzin, Margitta Elvers, Ulrich Flögel, Malte Kelm, Bodo Levkau, Michael Roden, Jen
Diabetologia.2021; 64(8): 1834. CrossRef - Impact of Dietary Lipids on the Reverse Cholesterol Transport: What We Learned from Animal Studies
Bianca Papotti, Joan Carles Escolà-Gil, Josep Julve, Francesco Potì, Ilaria Zanotti
Nutrients.2021; 13(8): 2643. CrossRef - Supramolecular copolymer modified statin-loaded discoidal rHDLs for atherosclerotic anti-inflammatory therapy by cholesterol efflux and M2 macrophage polarization
Qiqi Zhang, Jianhua He, Fengfei Xu, Xinya Huang, Yanyan Wang, Wenli Zhang, Jianping Liu
Biomaterials Science.2021; 9(18): 6153. CrossRef - Association between Small Dense Low-Density Lipoproteins and High-Density Phospolipid Content in Patients with Coronary Artery Disease with or without Diabetes
Hanene Aoua, Ymène Nkaies, Ali Ben Khalfallah, Mohsen Sakly, Ezzedine Aouani, Nebil Attia
Laboratory Medicine.2020; 51(3): 271. CrossRef - Biomechanical and biochemical investigation of erythrocytes in late stage human leptospirosis
J.A.X. Silva, A.V.P. Albertini, C.S.M. Fonseca, D.C.N. Silva, V.C.O. Carvalho, V.L.M. Lima, A. Fontes, E.V.L. Costa, R.A. Nogueira
Brazilian Journal of Medical and Biological Research.2020;[Epub] CrossRef - Cardioprotective Properties of HDL: Structural and Functional Considerations
Eleni Pappa, Moses S. Elisaf, Christina Kostara, Eleni Bairaktari, Vasilis K. Tsimihodimos
Current Medicinal Chemistry.2020; 27(18): 2964. CrossRef - Relationship between non–high-density lipoprotein cholesterol/apolipoprotein A-I and monocyte/high-density lipoprotein cholesterol ratio and coronary heart disease
Ya Li, Shu Li, Yulin Ma, Jialing Li, Mingying Lin, Jing Wan
Coronary Artery Disease.2020; 31(7): 623. CrossRef - Lipid transfer to high‐density lipoproteins in coronary artery disease patients with and without previous cerebrovascular ischemic events
Carlos J.D.G. Barbosa, Raul C. Maranhão, Renata S. Barreiros, Fatima R. Freitas, André Franci, Célia M.C. Strunz, Flávia B.B. Arantes, Thauany M. Tavoni, José A.F. Ramires, Roberto Kalil Filho, José C. Nicolau
Clinical Cardiology.2019; 42(11): 1100. CrossRef - Antibodies Against the C-Terminus of ApoA-1 Are Inversely Associated with Cholesterol Efflux Capacity and HDL Metabolism in Subjects with and without Type 2 Diabetes Mellitus
Robin Dullaart, Sabrina Pagano, Frank Perton, Nicolas Vuilleumier
International Journal of Molecular Sciences.2019; 20(3): 732. CrossRef - The effect of chronic kidney disease on lipid metabolism
Neris Dincer, Tuncay Dagel, Baris Afsar, Adrian Covic, Alberto Ortiz, Mehmet Kanbay
International Urology and Nephrology.2019; 51(2): 265. CrossRef - Biological Consequences of Dysfunctional HDL
Angela Pirillo, Alberico Luigi Catapano, Giuseppe Danilo Norata
Current Medicinal Chemistry.2019; 26(9): 1644. CrossRef - Plasma lecithin:cholesterol acyltransferase and phospholipid transfer protein activity independently associate with nonalcoholic fatty liver disease
Karlijn J. Nass, Eline H. van den Berg, Eke G. Gruppen, Robin P. F. Dullaart
European Journal of Clinical Investigation.2018;[Epub] CrossRef - An integrated metabolomic strategy for the characterization of the effects of Chinese yam and its three active components on septic cardiomyopathy
Ning Zhou, Meng-Nan Zeng, Kai Li, Yan-Yun Yang, Zhi-Yao Bai, Xiao-Ke Zheng, Wei-Sheng Feng
Food & Function.2018; 9(9): 4989. CrossRef - Effect of Rosuvastatin on Cholesterol Efflux Capacity and Endothelial Function in Type 2 Diabetes Mellitus and Dyslipidemia
Kyong Yeun Jung, Kyoung Min Kim, Sun Kyoung Han, Han Mi Yun, Tae Jung Oh, Sung Hee Choi, Kyong Soo Park, Hak Chul Jang, Soo Lim
Circulation Journal.2018; 82(5): 1387. CrossRef - Association Between Serum LDL-C and ApoB and SYNTAX Score in Patients With Stable Coronary Artery Disease
Taiwu Lin, Luzhao Wang, Jingbin Guo, Peng Liu, Liheng Chen, Mengqiu Wei, Gongxin Li
Angiology.2018; 69(8): 724. CrossRef - Influence of i.v. lipid emulsion on lipoprotein subclass in preterm infants
Hiroki Suganuma, Naho Ikeda, Natsuki Ohkawa, Hiromichi Shoji, Toshiaki Shimizu
Pediatrics International.2018; 60(9): 839. CrossRef - The HDL cholesterol/apolipoprotein A-I ratio: an indicator of cardiovascular disease
Eun-Jung Rhee, Christopher D. Byrne, Ki-Chul Sung
Current Opinion in Endocrinology, Diabetes & Obesity.2017; 24(2): 148. CrossRef - Moringa Leaves Prevent Hepatic Lipid Accumulation and Inflammation in Guinea Pigs by Reducing the Expression of Genes Involved in Lipid Metabolism
Manal Almatrafi, Marcela Vergara-Jimenez, Ana Murillo, Gregory Norris, Christopher Blesso, Maria Fernandez
International Journal of Molecular Sciences.2017; 18(7): 1330. CrossRef
- Association between Mycoplasma pneumoniae infection, high‑density lipoprotein metabolism and cardiovascular health (Review)

- Mechanisms of Vascular Calcification: The Pivotal Role of Pyruvate Dehydrogenase Kinase 4
- Jaechan Leem, In-Kyu Lee
- Endocrinol Metab. 2016;31(1):52-61. Published online March 16, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.1.52
- 4,558 View
- 69 Download
- 30 Web of Science
- 28 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Vascular calcification, abnormal mineralization of the vessel wall, is frequently associated with aging, atherosclerosis, diabetes mellitus, and chronic kidney disease. Vascular calcification is a key risk factor for many adverse clinical outcomes, including ischemic cardiac events and subsequent cardiovascular mortality. Vascular calcification was long considered to be a passive degenerative process, but it is now recognized as an active and highly regulated process similar to bone formation. However, despite numerous studies on the pathogenesis of vascular calcification, the mechanisms driving this process remain poorly understood. Pyruvate dehydrogenase kinases (PDKs) play an important role in the regulation of cellular metabolism and mitochondrial function. Recent studies show that PDK4 is an attractive therapeutic target for the treatment of various metabolic diseases. In this review, we summarize our current knowledge regarding the mechanisms of vascular calcification and describe the role of PDK4 in the osteogenic differentiation of vascular smooth muscle cells and development of vascular calcification. Further studies aimed at understanding the molecular mechanisms of vascular calcification will be critical for the development of novel therapeutic strategies.
-
Citations
Citations to this article as recorded by- Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases
Ankan Sarkar, Sandip V. Pawar, Kanwaljit Chopra, Manish Jain
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2024; 1870(3): 167021. CrossRef - MAPK14 as a key gene for regulating inflammatory response and macrophage M1 polarization induced by ferroptotic keratinocyte in psoriasis
Lin Zhou, Yingdong Zhong, Chaowei Li, Yu Zhou, Xi Liu, Lincai Li, Zhengwei Zou, Zhihui Zhong, Junsong Ye
Inflammation.2024;[Epub] CrossRef - Pyruvate dehydrogenase kinase 4 promotes osteoblastic potential of BMP9 by boosting Wnt/β-catenin signaling in mesenchymal stem cells
Yuan-Yuan Yang, Hong-Hong Luo, Yi-Xuan Deng, Xin-Tong Yao, Jie Zhang, Yu-Xi Su, Bai-Cheng He
The International Journal of Biochemistry & Cell Biology.2023; 154: 106341. CrossRef - lncRNA MEG3 Promotes PDK4/GSK-3β/β-Catenin Axis in MEFs by Targeting miR-532-5p
Yuan-Yuan Yang, Yi-Xuan Deng, Xin-Tong Yao, Hong-Hong Luo, Wen-Ge He, Xuan-Ling Cao, Rong-Chun Chen, Bai-Cheng He, Hai-Tao Jiang, Jing Wang, Sedat Kacar
Oxidative Medicine and Cellular Longevity.2023; 2023: 1. CrossRef - Mitochondrial dynamics in vascular remodeling and target-organ damage
Tong Zhu, Qingxun Hu, Yanggang Yuan, Huijuan Yao, Jian Zhang, Jia Qi
Frontiers in Cardiovascular Medicine.2023;[Epub] CrossRef - PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy
Xuefeng Dou, Qiang Fu, Qilai Long, Shuning Liu, Yejun Zou, Da Fu, Qixia Xu, Zhirui Jiang, Xiaohui Ren, Guilong Zhang, Xiaoling Wei, Qingfeng Li, Judith Campisi, Yuzheng Zhao, Yu Sun
Nature Metabolism.2023; 5(11): 1887. CrossRef - Identification of PDK4 as Hub Gene for Diabetic Nephropathy Using Co-Expression Network Analysis
Yuanyuan Han, Liangzi Jin, Liangzhi Wang, Lan Wei, Chao Tu
Kidney and Blood Pressure Research.2023; 48(1): 522. CrossRef - Flavocoxid Ameliorates Aortic Calcification Induced by Hypervitaminosis D3 and Nicotine in Rats Via Targeting TNF-α, IL-1β, iNOS, and Osteogenic Runx2
Ahmed E. Amer, George S. G. Shehatou, Hassan A. El-Kashef, Manar A. Nader, Ahmed R. El-Sheakh
Cardiovascular Drugs and Therapy.2022; 36(6): 1047. CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - Insights Into the Role of Mitochondria in Vascular Calcification
ZL Zeng, Qing Yuan, Xuyu Zu, Jianghua Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Induced pluripotent stem cell-derived smooth muscle cells to study cardiovascular calcification
Samantha K. Atkins, Abhijeet R. Sonawane, Romi Brouwhuis, Johana Barrientos, Anna Ha, Maximillian Rogers, Takeshi Tanaka, Takehito Okui, Shiori Kuraoka, Sasha A. Singh, Masanori Aikawa, Elena Aikawa
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Phenotypic plasticity of vascular smooth muscle cells in vascular calcification: Role of mitochondria
Yan Zhong Liu, Zong Xiang Li, Lin Lin Zhang, Dan Wang, Yi Ping Liu
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Febuxostat attenuates vascular calcification induced by vitamin D3 plus nicotine in rats
Ahmed E. Amer, Ahmed R. El-Sheakh, Mohamed F. Hamed, Hassan A. El-Kashef, Manar A. Nader, George S.G. Shehatou
European Journal of Pharmaceutical Sciences.2021; 156: 105580. CrossRef - Mitochondria and traffic-related air pollution linked coronary artery calcification: exploring the missing link
Bhavana Sivakumar, Gino A. Kurian
Reviews on Environmental Health.2021; 36(4): 545. CrossRef - Mitochondria Homeostasis and Vascular Medial Calcification
Min li, Yi Zhu, Sandip Kumar Jaiswal, Nai-Feng Liu
Calcified Tissue International.2021; 109(2): 113. CrossRef - POSTN promotes diabetic vascular calcification by interfering with autophagic flux
Xue-Jiao Sun, Wen-Qi Ma, Yi Zhu, Nai-Feng Liu
Cellular Signalling.2021; 83: 109983. CrossRef - The MAMs Structure and Its Role in Cell Death
Nan Wang, Chong Wang, Hongyang Zhao, Yichun He, Beiwu Lan, Liankun Sun, Yufei Gao
Cells.2021; 10(3): 657. CrossRef - Pyruvate dehydrogenase kinases (PDKs): an overview toward clinical applications
Xiuxiu Wang, Xiaoyue Shen, Yuting Yan, Hongmin Li
Bioscience Reports.2021;[Epub] CrossRef - Label-Free Multiphoton Microscopy for the Detection and Monitoring of Calcific Aortic Valve Disease
Ishita Tandon, Kyle P. Quinn, Kartik Balachandran
Frontiers in Cardiovascular Medicine.2021;[Epub] CrossRef - Vascular Calcification—New Insights into Its Mechanism
Sun Joo Lee, In-Kyu Lee, Jae-Han Jeon
International Journal of Molecular Sciences.2020; 21(8): 2685. CrossRef - Osteocalcin Regulates Arterial Calcification Via Altered Wnt Signaling and Glucose Metabolism
Nabil A Rashdan, Alisia M Sim, Lin Cui, Kanchan Phadwal, Fiona L Roberts, Roderick Carter, Derya D Ozdemir, Peter Hohenstein, John Hung, Jakub Kaczynski, David E Newby, Andrew H Baker, Gerard Karsenty, Nicholas M Morton, Vicky E MacRae
Journal of Bone and Mineral Research.2020; 35(2): 357. CrossRef - The role of mitochondria in vascular calcification
Pengbo Wang, Naijin Zhang, Boquan Wu, Shaojun Wu, Ying Zhang, Yingxian Sun
Journal of Translational Internal Medicine.2020; 8(2): 80. CrossRef - Cerebral blood flow in dystonia due to pantothenate kinase-associated neurodegeneration
Peter Stoeter, Pedro Roa-Sanchez, Cesar F Gonzalez, Herwin Speckter, Jairo Oviedo, Pamela Bido
The Neuroradiology Journal.2020; 33(6): 479. CrossRef - PDK4 promotes vascular calcification by interfering with autophagic activity and metabolic reprogramming
Wen-Qi Ma, Xue-Jiao Sun, Yi Zhu, Nai-Feng Liu
Cell Death & Disease.2020;[Epub] CrossRef - Restoring mitochondrial biogenesis with metformin attenuates β-GP-induced phenotypic transformation of VSMCs into an osteogenic phenotype via inhibition of PDK4/oxidative stress-mediated apoptosis
Wen-Qi Ma, Xue-Jiao Sun, Ying Wang, Yi Zhu, Xi-Qiong Han, Nai-Feng Liu
Molecular and Cellular Endocrinology.2019; 479: 39. CrossRef - Salusin-β Promotes Vascular Calcification via Nicotinamide Adenine Dinucleotide Phosphate/Reactive Oxygen Species-Mediated Klotho Downregulation
Haijian Sun, Feng Zhang, Yu Xu, Shuo Sun, Huiping Wang, Qiong Du, Chenxin Gu, Stephen M. Black, Ying Han, Haiyang Tang
Antioxidants & Redox Signaling.2019; 31(18): 1352. CrossRef - Fibroblast Growth Factor 21 (FGF21) Promotes Formation of Aerobic Myofibers via the FGF21‐SIRT1‐AMPK‐PGC1α Pathway
Xinyi Liu, Yongliang Wang, Liming Hou, Yuanzhu Xiong, Shuhong Zhao
Journal of Cellular Physiology.2017; 232(7): 1893. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases

- Thyroid
- Mutation Profile of Well-Differentiated Thyroid Cancer in Asians
- Young Shin Song, Jung Ah Lim, Young Joo Park
- Endocrinol Metab. 2015;30(3):252-262. Published online September 22, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.3.252
- 5,280 View
- 64 Download
- 60 Web of Science
- 52 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Recent advances in molecular diagnostics have led to significant insights into the genetic basis of thyroid tumorigenesis. Among the mutations commonly seen in thyroid cancers, the vast majority are associated with the mitogen-activated protein kinase pathway. B-Raf proto-oncogene (
BRAF ) mutations are the most common mutations observed in papillary thyroid cancers (PTCs), followed byRET/PTC rearrangements andRAS mutations, while follicular thyroid cancers are more likely to harborRAS mutations orPAX8 /peroxisome proliferator-activated receptor γ (PPARγ ) rearrangements. Beyond these more common mutations, alterations in the telomerase reverse transcriptase (TERT ) promoter have recently been associated with clinicopathologic features, disease prognosis, and tumorigenesis in thyroid cancer. While the mutations underlying thyroid tumorigenesis are well known, the frequency of these mutations is strongly associated with geography, with clear differences reported between Asian and Western countries. Of particular interest is the prevalence ofBRAF mutations, with Korean patients exhibiting the highest rate ofBRAF -associated thyroid cancers in the world. Here, we review the prevalence of each of the most common mutations in Asian and Western countries, and identify the characteristics of well-differentiated thyroid cancer in Asians.-
Citations
Citations to this article as recorded by- BRAF V600E Mutation Lacks Association with Poorer Clinical Prognosis in Papillary Thyroid Carcinoma
Hon-Fan Lai, Jen-Fan Hang, Po-Chung Kuo, Chin-Sung Kuo, San-Fan Yao, Jui-Yu Chen, Chen-Hsen Lee
Annals of Surgical Oncology.2024; 31(5): 3495. CrossRef - Clinical value of multi-gene testing in distinguishing benign and malignant thyroid nodules
Murui Zhang, Xiaotong Hu, Lunming Liu, Yihong Wang, Junchang Jiang, Hui Li, Weiqiang Fei, Tingting Zhong, Zhinong Jiang
Medicine.2024; 103(4): e35960. CrossRef - The Association of Socioeconomic Factors and Well-Differentiated Thyroid Cancer
Andrew Bonner, Brendon Herring, Rongzhi Wang, Andrea Gillis, Polina Zmijewski, Brenessa Lindeman, Jessica Fazendin, Herbert Chen
Journal of Surgical Research.2023; 283: 973. CrossRef - BRAFV600E and TERT promoter C228T mutations on ThyroSeq v3 analysis of delayed skin metastasis from papillary thyroid cancer: a case report and literature review
Jee-Hye Choi, Hyeong Won Yu, Ja Kyung Lee, Woochul Kim, June Young Choi, Hee Young Na, So Yeon Park, Chang Ho Ahn, Jae Hoon Moon, Sang Il Choi, Ho-Young Lee, Won Woo Lee, Wonjae Cha, Woo-Jin Jeong
World Journal of Surgical Oncology.2023;[Epub] CrossRef - Less is more meets do more with less: Exploring differences in thyroid FNA molecular testing between Asian and Western practices
Michiya Nishino
Cancer Cytopathology.2023; 131(7): 421. CrossRef - Histological and Genetic Diversity in Ovarian Mucinous Carcinomas: A Pilot Study
Sultana Razia, Kentaro Nakayama, Hitomi Yamashita, Tomoka Ishibashi, Masako Ishikawa, Kosuke Kanno, Seiya Sato, Satoru Kyo
Current Oncology.2023; 30(4): 4052. CrossRef - Effective Use of microRNA, BRAF and Sonographic Risk Assessment in Bethesda III Thyroid Nodules Requires a Different Approach to Nodules with Features of Nuclear Atypia and Other Types of Atypia
Dorota Słowińska-Klencka, Bożena Popowicz, Dominika Kulczycka-Wojdala, Bożena Szymańska, Joanna Duda-Szymańska, Martyna Wojtaszek-Nowicka, Krzysztof Kaczka, Mariusz Klencki
Cancers.2023; 15(17): 4287. CrossRef - Long-Term Changes in the Mortality Rates of Thyroid Cancer in Korea: Analysis of Korean National Data from 1985 to 2020
Yun Mi Choi, Min-Ju Kim, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
Endocrinology and Metabolism.2023; 38(5): 588. CrossRef - 遺伝子から頭頸部がんを診る : 甲状腺癌 (分化癌を中心に)
季吉 森谷
Nippon Jibiinkoka Tokeibugeka Gakkai Kaiho(Tokyo).2023; 126(12): 1277. CrossRef - Toward Systems-Level Metabolic Analysis in Endocrine Disorders and Cancer
Aliya Lakhani, Da Hyun Kang, Yea Eun Kang, Junyoung O. Park
Endocrinology and Metabolism.2023; 38(6): 619. CrossRef - Risk and Prognostic Factors for BRAFV600E Mutations in Papillary Thyroid Carcinoma
Xiaojing Wei, Xiaodong Wang, Jie Xiong, Chen Li, Yixuan Liao, Yongjun Zhu, Jingxin Mao, Gitana Maria Aceto
BioMed Research International.2022; 2022: 1. CrossRef - Disparities in the impact of the AJCC 8th edition staging system on differentiated thyroid cancer outcomes
Juan A. Santamaria‐Barria, Amanda N. Graff‐Baker, Shu‐Ching Chang, Adam Khader, Anthony J. Scholer, Mary Garland‐Kledzik, Melanie Goldfarb
Head & Neck.2022; 44(10): 2129. CrossRef - Relationship Between The BRAF V600E And Tumor Size, Lymph Node, And Distant Metastasis In Papillary Thyroid Carcinoma
Edmond Rukmana Wikanta, Yan Wisnu Prajoko, Benny Issakh, Hermawan Istiadi, Dik Puspasari
Russian Open Medical Journal.2022;[Epub] CrossRef - Lactate Dehydrogenase A as a Potential New Biomarker for Thyroid Cancer
Eun Jeong Ban, Daham Kim, Jin Kyong Kim, Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Kee-Hyun Nam, Woong Youn Chung, Kunhong Kim
Endocrinology and Metabolism.2021; 36(1): 96. CrossRef - Association of Hyperparathyroidism and Papillary Thyroid Cancer: A Multicenter Retrospective Study (Endocrinol Metab 2020;35:925-32, Chaiho Jeong et al.)
Chaiho Jeong, Jeonghoon Ha, Moo Il Kang
Endocrinology and Metabolism.2021; 36(1): 205. CrossRef - Molecular pathogenesis of pediatric thyroid carcinoma
Norisato Mitsutake, Vladimir Saenko
Journal of Radiation Research.2021; 62(Supplement): i71. CrossRef - The Incidence of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Meta-Analysis Assessing Worldwide Impact of the Reclassification
Chanchal Rana, Huy Gia Vuong, Thu Quynh Nguyen, Hoang Cong Nguyen, Chan Kwon Jung, Kennichi Kakudo, Andrey Bychkov
Thyroid.2021;[Epub] CrossRef - The Association Between Radioiodine Refractory in Papillary Thyroid Carcinoma, Sodium/Iodide Symporter Expression, and BRAFV600E Mutation
Tauangtham Anekpuritanang, Maythad Uataya, Apichaya Claimon, Natthawadee Laokulrath, Warut Pongsapich, Paveena Pithuksurachai
OncoTargets and Therapy.2021; Volume 14: 3959. CrossRef - Genomic landscape of metastatic papillary thyroid carcinoma and novel biomarkers for predicting distant metastasis
Xiabin Lan, Hua Bao, Xinyang Ge, Jun Cao, Xiaojun Fan, Qihong Zhang, Kaihua Liu, Xian Zhang, Zhuo Tan, Chuanming Zheng, Ao Wang, Chao Chen, Xin Zhu, Jiafeng Wang, Jiajie Xu, Xuhang Zhu, Xue Wu, Xiaonan Wang, Yang Shao, Minghua Ge
Cancer Science.2020; 111(6): 2163. CrossRef - Did Introducing a New Category of Thyroid Tumors (Non-invasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features) Decrease the Risk of Malignancy for the Diagnostic Categories in the Bethesda System for Reporting Thyroid Cytopathology?
Janusz Kopczyński, Agnieszka Suligowska, Kornelia Niemyska, Iwona Pałyga, Agnieszka Walczyk, Danuta Gąsior-Perczak, Artur Kowalik, Kinga Hińcza, Ryszard Mężyk, Stanisław Góźdź, Aldona Kowalska
Endocrine Pathology.2020; 31(2): 143. CrossRef - The Significance of Transcriptomic Signatures in the Multifocal Papillary Thyroid Carcinoma: Two mRNA Expression Patterns with Distinctive Clinical Behavior from The Cancer Genome Atlas (TCGA) Database
Yea Eun Kang, Boyoung Hwang, Ju Hee Lee, Minho Shong, Hyon-Seung Yi, Bon Seok Koo, Dong Jin Lee
International Journal of Thyroidology.2020; 13(1): 1. CrossRef - High Genetic Diversity and No Evidence of Clonal Relation in Synchronous Thyroid Carcinomas Associated with Hashimoto’s Thyroiditis: A Next-Generation Sequencing Analysis
Csaba Molnár, Emese Sarolta Bádon, Attila Mokánszki, Anikó Mónus, Lívia Beke, Ferenc Győry, Endre Nagy, Gábor Méhes
Diagnostics.2020; 10(1): 48. CrossRef - Low Prevalence of TERT Promoter, BRAF and RAS Mutations in Papillary Thyroid Cancer in the Greek Population
Marilena Argyropoulou, Aristidis S. Veskoukis, Pagona-Maria Karanatsiou, Aikaterini Manolakelli, Ifigenia Kostoglou-Athanassiou, George Vilaras, Andreas Karameris, Kalliopi Liadaki
Pathology & Oncology Research.2020; 26(1): 347. CrossRef - Predominant DICER1 Pathogenic Variants in Pediatric Follicular Thyroid Carcinomas
Young Ah Lee, Sun-Wha Im, Kyeong Cheon Jung, Eun-Jae Chung, Choong Ho Shin, Jong-Il Kim, Young Joo Park
Thyroid.2020; 30(8): 1120. CrossRef - BRAF and KRAS mutations in papillary thyroid carcinoma in the United Arab Emirates
Suhail Al-Salam, Charu Sharma, Bachar Afandi, Khaled Al Dahmani, Ali S. Al-Zahrani, Amal Al Shamsi, Juma Al Kaabi, Paula Soares
PLOS ONE.2020; 15(4): e0231341. CrossRef - VE1 Immunohistochemistry Improves the Limit of Genotyping for Detecting BRAFV600E Mutation in Papillary Thyroid Cancer
Sonam Choden, Somboon Keelawat, Chan Kwon Jung, Andrey Bychkov
Cancers.2020; 12(3): 596. CrossRef - Highly Sensitive and Specific Molecular Test for Mutations in the Diagnosis of Thyroid Nodules: A Prospective Study of BRAF-Prevalent Population
Yoon Young Cho, So Young Park, Jung Hee Shin, Young Lyun Oh, Jun-Ho Choe, Jung-Han Kim, Jee Soo Kim, Hyun Sook Yim, Yoo-Li Kim, Chang-Seok Ki, Tae Hyuk Kim, Jae Hoon Chung, Sun Wook Kim
International Journal of Molecular Sciences.2020; 21(16): 5629. CrossRef - Is there adenoma-carcinoma sequence between benign adenoma and papillary cancer of thyroid: A genomic linkage study
Ramesh Bangaraiahgari, Ramakanth Bhargav Panchangam, Pradeep Puthenveetil, Sabaretnam Mayilvaganan, Rajesh Bangaraiahgari, Rajkiran reddy Banala, Poongkodi Karunakaran, Rafi Md
Annals of Medicine and Surgery.2020; 60: 695. CrossRef - Genomic Characterization of Differentiated Thyroid Carcinoma
Young Shin Song, Young Joo Park
Endocrinology and Metabolism.2019; 34(1): 1. CrossRef - Association between BRAFV600E Mutations and Clinicopathological Features of Papillary Thyroid Microcarcinoma (PTMC)
Sung Min Lee, Cho Rok Lee, Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Kee-Hyun Nam, Woong Youn Chung, Cheong Soo Park
Journal of Endocrine Surgery.2019; 19(3): 76. CrossRef - Combined quantitation of HMGA2 mRNA, microRNAs, and mitochondrial-DNA content enables the identification and typing of thyroid tumors in fine-needle aspiration smears
Sergei E. Titov, Mikhail K. Ivanov, Pavel S. Demenkov, Gevork A. Katanyan, Eugenia S. Kozorezova, Anastasia V. Malek, Yulia A. Veryaskina, Igor F. Zhimulev
BMC Cancer.2019;[Epub] CrossRef - Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer
Young Shin Song, Seong-Keun Yoo, Hwan Hee Kim, Gyeongseo Jung, Ah-Reum Oh, Ji-Young Cha, Su-jin Kim, Sun Wook Cho, Kyu Eun Lee, Jeong-Sun Seo, Young Joo Park
Endocrine-Related Cancer.2019; 26(6): 629. CrossRef - Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice: Perspectives for Surgical Pathology and Cytopathology
Andrey Bychkov, Chan Kwon Jung, Zhiyan Liu, Kennichi Kakudo
Endocrine Pathology.2018; 29(3): 276. CrossRef - Understanding Malignancies of the Thyroid Gland: Institutional Experience
Jaimanti Bakshi, Sourabha Kumar Patro, Navjot Kaur, Naresh Kumar Panda, Grace Budhiraja
Indian Journal of Otolaryngology and Head & Neck Surgery.2018; 70(4): 482. CrossRef - Case–Control Study of Papillary Thyroid Carcinoma on Urinary and Dietary Iodine Status in South Korea
Joon‐Hyop Lee, Ra‐Yeong Song, Jin Wook Yi, Hyeong Won Yu, Hyungju Kwon, Su‐jin Kim, Young Jun Chai, June Young Choi, Jae Hoon Moon, Kyu Eun Lee, Young Joo Park, Sue K. Park
World Journal of Surgery.2018; 42(5): 1424. CrossRef - Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries
Andrey Bychkov, Somboon Keelawat, Shipra Agarwal, Deepali Jain, Chan Kwon Jung, SoonWon Hong, Chiung-Ru Lai, Shinya Satoh, Kennichi Kakudo
Pathology.2018; 50(4): 411. CrossRef - Genetic landscape of papillary thyroid carcinoma in the Chinese population
Jialong Liang, Wanshi Cai, Dongdong Feng, Huajing Teng, Fengbiao Mao, Yi Jiang, Shanshan Hu, Xianfeng Li, Yujie Zhang, Baoguo Liu, Zhong Sheng Sun
The Journal of Pathology.2018; 244(2): 215. CrossRef - Aberrant expression of CD20 in thyroid cancer and its clinicopathologic significance
Andrey Bychkov, Chan Kwon Jung
Human Pathology.2018; 71: 74. CrossRef - Molecular markers in well-differentiated thyroid cancer
Anil K. D’Cruz, Richa Vaish, Abhishek Vaidya, Iain J. Nixon, Michelle D. Williams, Vincent Vander Poorten, Fernando López, Peter Angelos, Ashok R. Shaha, Avi Khafif, Alena Skalova, Alessandra Rinaldo, Jennifer L. Hunt, Alfio Ferlito
European Archives of Oto-Rhino-Laryngology.2018; 275(6): 1375. CrossRef - Meta-Analysis Confirms the Deleterious Effects of CombinedBRAFV600Eand Telomerase Reverse Transcriptase Promoter Mutations on the Course and Mortality of Papillary Thyroid Carcinoma
Charles H. Emerson
Clinical Thyroidology.2017; 29(5): 176. CrossRef - Genetic Alterations and Their Clinical Implications in High-Recurrence Risk Papillary Thyroid Cancer
Min-Young Lee, Bo Mi Ku, Hae Su Kim, Ji Yun Lee, Sung Hee Lim, Jong-Mu Sun, Se-Hoon Lee, Keunchil Park, Young Lyun Oh, Mineui Hong, Han-Sin Jeong, Young-Ik Son, Chung-Hwan Baek, Myung-Ju Ahn
Cancer Research and Treatment.2017; 49(4): 906. CrossRef - Frequent BRAF
V600E
and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan
Naoki Oishi, Tetsuo Kondo, Tadao Nakazawa, Kunio Mochizuki, Tomohiro Inoue, Kazunari Kasai, Ippei Tahara, Tomonori Yabuta, Mitsuyoshi Hirokawa, Akira Miyauchi, Ryohei Katoh
Endocrine Pathology.2017; 28(2): 103. CrossRef - Predicting Factors for Bilaterality in Papillary Thyroid Carcinoma with Tumor Size <4 cm
Seo Ki Kim, Inhye Park, Jung-Woo Woo, Jun Ho Lee, Jun-Ho Choe, Jung-Han Kim, Jee Soo Kim
Thyroid.2017; 27(2): 207. CrossRef - Low Rate of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice
Andrey Bychkov, Mitsuyoshi Hirokawa, Chan Kwon Jung, Zhiyan Liu, Yun Zhu, Soon Won Hong, Shinya Satoh, Chiung-Ru Lai, Lien Huynh, Kennichi Kakudo
Thyroid.2017; 27(7): 983. CrossRef - Changes in the clinicopathological characteristics and genetic alterations of follicular thyroid cancer
Young Shin Song, Jung Ah Lim, Hye Sook Min, Min Joo Kim, Hoon Sung Choi, Sun Wook Cho, Jae Hoon Moon, Ka Hee Yi, Do Joon Park, Bo Youn Cho, Young Joo Park
European Journal of Endocrinology.2017; 177(6): 465. CrossRef - Effects of CoexistentBRAFV600EandTERTPromoter Mutations on Poor Clinical Outcomes in Papillary Thyroid Cancer: A Meta-Analysis
Shinje Moon, Young Shin Song, Ye An Kim, Jung Ah Lim, Sun Wook Cho, Jae Hoon Moon, Seokyung Hahn, Do Joon Park, Young Joo Park
Thyroid.2017; 27(5): 651. CrossRef - TERT Promoter Mutation in an Aggressive Cribriform Morular Variant of Papillary Thyroid Carcinoma
Eun Ji Oh, Sohee Lee, Ja Seong Bae, Yourha Kim, Sora Jeon, Chan Kwon Jung
Endocrine Pathology.2017; 28(1): 49. CrossRef - TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer
Xue Yang, Jiao Li, Xiaoyi Li, Zhiyong Liang, Wen Gao, Jun Liang, Shujun Cheng, Yansong Lin
Journal of Nuclear Medicine.2017; 58(2): 258. CrossRef - Molecular Diagnosis Using Residual Liquid-Based Cytology Materials for Patients with Nondiagnostic or Indeterminate Thyroid Nodules
Hyemi Kwon, Won Gu Kim, Markus Eszlinger, Ralf Paschke, Dong Eun Song, Mijin Kim, Suyeon Park, Min Ji Jeon, Tae Yong Kim, Young Kee Shong, Won Bae Kim
Endocrinology and Metabolism.2016; 31(4): 586. CrossRef - TERT promoter mutations and long-term survival in patients with thyroid cancer
Tae Hyuk Kim, Young-Eun Kim, Soomin Ahn, Ji-Youn Kim, Chang-Seok Ki, Young Lyun Oh, Kyunga Kim, Jae Won Yun, Woong-Yang Park, Jun-Ho Choe, Jung-Han Kim, Jee Soo Kim, Sun Wook Kim, Jae Hoon Chung
Endocrine-Related Cancer.2016; 23(10): 813. CrossRef - Reply:
Y. Che
American Journal of Neuroradiology.2016; 37(1): E9. CrossRef - Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients
Young Shin Song, Jung Ah Lim, Hoonsung Choi, Jae‐Kyung Won, Jae Hoon Moon, Sun Wook Cho, Kyu Eun Lee, Young Joo Park, Ka Hee Yi, Do Joon Park, Jeong‐Sun Seo
Cancer.2016; 122(9): 1370. CrossRef
- BRAF V600E Mutation Lacks Association with Poorer Clinical Prognosis in Papillary Thyroid Carcinoma

- Obesity and Metabolism
- Correlation between Expression of Glucose Transporters in Granulosa Cells and Oocyte Quality in Women with Polycystic Ovary Syndrome
- Eunju Kim, Hyun Ha Seok, Su-Yeon Lee, Dong Ryul Lee, Jisook Moon, Tae Ki Yoon, Woo Sik Lee, Kyung-Ah Lee
- Endocrinol Metab. 2014;29(1):40-47. Published online March 14, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.1.40
- 4,804 View
- 53 Download
- 22 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The glucose transporters (GLUTs) exhibit different tissue-specific expression. This study aimed to investigate the types of GLUTs expressed in human granulosa cells (GCs) obtained from women with polycystic ovary syndrome (PCOS) and their relationship with insulin resistance (IR) and the outcomes of
in vitro maturation (IVM) of immature oocytes.Methods Expression of GLUTs was evaluated in GCs from women with PCOS with or without IR. Thirty-six women with PCOS undergoing an IVM program were included. Differential gene expression between the insulin sensitive (IS) and IR group was measured by reverse transcription polymerase chain reaction.
Results Expression of GLUTs 1, 3, 5, 8, and 13 was constitutive, whereas expression of GLUTs 2 and 7 was not observed in human GCs. The remaining GLUTs, 4, 6, 9, 10, 11, and 12, were differentially expressed among patients according to metabolic status, such as insulin sensitivity. A higher number of GCs from patients with IR (92%) expressed GLUT6 than GCs from IS PCOS patients (46.3%). Logistic regression showed that expression of GLUTs 9, 11, and 12 correlates with rates of IVM at 48 hours, fertilization, and implantation, respectively.
Conclusion This is the first report describing the expression pattern of all 13 members of the GLUT family in human GCs. Results of the present study suggest that patients' insulin sensitivity regulates GLUT expression in GCs in PCOS patients, and this may control oocyte quality for IVM and subsequent processes such as fertilization and implantation in patients taking part in an
in vitro fertilization program.-
Citations
Citations to this article as recorded by- Dysregulation of ferroptosis-related genes in granulosa cells associates with impaired oocyte quality in polycystic ovary syndrome
Jialyu Huang, Hancheng Fan, Chenxi Li, Kangping Yang, Chaoyi Xiong, Siyi Xiong, Shenghui Feng, Shen Chen, Bangqi Wang, Yufang Su, Boyun Xu, Haiyan Yang, Ni Wang, Jing Zhu
Frontiers in Endocrinology.2024;[Epub] CrossRef - Chinese herbal medicine alleviates the pathogenesis of polycystic ovary syndrome by improving oxidative stress and glucose metabolism via mitochondrial Sirtuin 3 signaling
Qing Zhang, Jun Ren, Fangfang Wang, Mingqian Li, Manman Pan, Hui Zhang, Fan Qu
Phytomedicine.2023; 109: 154556. CrossRef - Essential Role of Granulosa Cell Glucose and Lipid Metabolism on Oocytes and the Potential Metabolic Imbalance in Polycystic Ovary Syndrome
Chen-Hua Zhang, Xiang-Yi Liu, Jing Wang
International Journal of Molecular Sciences.2023; 24(22): 16247. CrossRef - Effect of Electro-acupuncture on Expression of IRS-1/PI3K/GLUT4 Pathway in Ovarian Granulosa Cells of Infertile Patients with Polycystic Ovary Syndrome-Insulin Resistance of Phlegm-Dampness Syndrome
Shan Xiang, Ming-feng Xia, Jing-yan Song, Dan-qi Liu, Fang Lian
Chinese Journal of Integrative Medicine.2021; 27(5): 330. CrossRef - Comparison of streptozotocin-induced diabetes at different moments of the life of female rats for translational studies
Yuri K Sinzato, Eduardo Klöppel, Carolina A Miranda, Verônyca G Paula, Larissa F Alves, Lívia LS Nascimento, Ana Paula Campos, Barshana Karki, Václav Hampl, Gustavo T Volpato, Débora C Damasceno
Laboratory Animals.2021; 55(4): 329. CrossRef - Molecular Insulin Actions Are Sexually Dimorphic in Lipid Metabolism
Rosa Isela Ortiz-Huidobro, Myrian Velasco, Carlos Larqué, Rene Escalona, Marcia Hiriart
Frontiers in Endocrinology.2021;[Epub] CrossRef - Potential role of tea extract in oocyte development
Lei Zhao, Qing-Yuan Sun, Zhao-Jia Ge
Food & Function.2021; 12(21): 10311. CrossRef - Thioredoxin-interacting protein regulates glucose metabolism and improves the intracellular redox state in bovine oocytes during in vitro maturation
XiaoLong Jiang, YunWei Pang, ShanJiang Zhao, HaiSheng Hao, XueMing Zhao, WeiHua Du, YaChun Wang, HuaBin Zhu
American Journal of Physiology-Endocrinology and Metabolism.2020; 318(3): E405. CrossRef - The Impact of Controlled Ovarian Stimulation Hormones on the Metabolic State and Endocannabinoid System of Human Cumulus Cells
Valentina Notarstefano, Giorgia Gioacchini, Elisabetta Giorgini, Nina Montik, Andrea Ciavattini, Anna Rita Polidori, Fulvia Antonia Candela, Lisa Vaccari, Maurizio Cignitti, Oliana Carnevali
International Journal of Molecular Sciences.2020; 21(19): 7124. CrossRef - Polycystic Ovary Syndrome and Infertility: From Molecular Perspective
Masoumeh Ghafarzadeh
Current Womens Health Reviews.2020; 16(3): 182. CrossRef - Effects of gonadotrophins and insulin on glucose uptake in the porcine cumulus–oocyte complex during IVM
Gabriel Martín Alvarez, María Josefina Barrios Expósito, Evelin Elia, Dante Paz, Sergio Morado, Pablo Daniel Cetica
Reproduction, Fertility and Development.2019; 31(8): 1353. CrossRef - Does the molecular and metabolic profile of human granulosa cells correlate with oocyte fate? New insights by Fourier transform infrared microspectroscopy analysis
Giorgia Gioacchini, Valentina Notarstefano, Elena Sereni, Carlotta Zacà, Giovanni Coticchio, Elisabetta Giorgini, Lisa Vaccari, Oliana Carnevali, Andrea Borini
MHR: Basic science of reproductive medicine.2018;[Epub] CrossRef - Association among genetic variants in the vitamin D pathway and circulating 25-hydroxyvitamin D levels in Korean adults: results from the Korea National Health and Nutrition Examination Survey 2011–2012
So-Young Kwak, Clara Yongjoo Park, Garam Jo, Oh Yoen Kim, Min-Jeong Shin
Endocrine Journal.2018; 65(9): 881. CrossRef - Abnormal expression levels of BMP15/Smad1 are associated with granulosa cell apoptosis in patients with polycystic ovary syndrome
Xiangrong Cui, Xuan Jing, Xueqing Wu, Xingyu Bi, Junfen Liu, Zhijing Long, Xiuping Zhang, Dongdong Zhang, Hongxiang Jia, Dan Su, Kai Huo
Molecular Medicine Reports.2017; 16(6): 8231. CrossRef - Role of the PI3K-Akt Signaling Pathway in the Pathogenesis of Polycystic Ovary Syndrome
Tiantian Li, Hui Mo, Wenfeng Chen, Li Li, Yao Xiao, Jing Zhang, Xiaofang Li, Ying Lu
Reproductive Sciences.2017; 24(5): 646. CrossRef - Nitric Oxide–Mediated Regulation of GLUT by T3 and Follicle-Stimulating Hormone in Rat Granulosa Cells
Ye Tian, Yu Ding, Juan Liu, Dai Heng, Kaili Xu, Wenbo Liu, Cheng Zhang
Endocrinology.2017; 158(6): 1898. CrossRef - Is the canine corpus luteum an insulin-sensitive tissue?
Liza Margareth Medeiros de Carvalho Sousa, Renata dos Santos Silva, Vanessa Uemura da Fonseca, Rafael Magdanelo Leandro, Thiago Senna Di Vincenzo, Ana Bárbara Alves-Wagner, Ubiratan Fabres Machado, Paula de Carvalho Papa
Journal of Endocrinology.2016; 231(3): 223. CrossRef - Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1
Meghan Ruebel, Kartik Shankar, Dana Gaddy, Forrest Lindsey, Thomas Badger, Aline Andres
American Journal of Physiology-Endocrinology and Metabolism.2016; 311(1): E269. CrossRef - Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle
Joëlle Dupont, Rex J. Scaramuzzi
Biochemical Journal.2016; 473(11): 1483. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Chronic ethanol treatment of human hepatocytes inhibits the activation of the insulin signaling pathway by increasing cytosolic free calcium levels
YI-MIN CHEN, JIN-FANG ZHAO, YONG-LIN LIU, JIE CHEN, RONG-LIN JIANG
International Journal of Molecular Medicine.2015; 36(3): 739. CrossRef - Dysregulated hepatic expression of glucose transporters in chronic disease: contribution of semicarbazide-sensitive amine oxidase to hepatic glucose uptake
Sumera Karim, Evaggelia Liaskou, Janine Fear, Abhilok Garg, Gary Reynolds, Lee Claridge, David H. Adams, Philip N. Newsome, Patricia F. Lalor
American Journal of Physiology-Gastrointestinal and Liver Physiology.2014; 307(12): G1180. CrossRef
- Dysregulation of ferroptosis-related genes in granulosa cells associates with impaired oocyte quality in polycystic ovary syndrome


 KES
KES

 First
First Prev
Prev



