Articles
- Page Path
- HOME > Endocrinol Metab > Volume 29(1); 2014 > Article
-
Original ArticleCorrelation between Expression of Glucose Transporters in Granulosa Cells and Oocyte Quality in Women with Polycystic Ovary Syndrome
- Eunju Kim1, Hyun Ha Seok2, Su-Yeon Lee1, Dong Ryul Lee1,2, Jisook Moon3, Tae Ki Yoon2, Woo Sik Lee2, Kyung-Ah Lee1,2
-
Endocrinology and Metabolism 2014;29(1):40-47.
DOI: https://doi.org/10.3803/EnM.2014.29.1.40
Published online: March 14, 2014
1Department of Biomedical Science, CHA University, Seoul, Korea.
2Fertility Center, CHA Gangnam Medical Center, CHA University, Seoul, Korea.
3Department of Applied Bioscience, CHA University, Seoul, Korea.
- Corresponding author: Kyung-Ah Lee. Department of Biomedical Science, CHA University, 6-9 Nonhyeon-ro 105-gil, Gangnam-gu, Seoul 135-907, Korea. Tel: +82-2-557-3937, Fax: +82-2-563-2038, leeka@ovary.co.kr
Copyright © 2014 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The glucose transporters (GLUTs) exhibit different tissue-specific expression. This study aimed to investigate the types of GLUTs expressed in human granulosa cells (GCs) obtained from women with polycystic ovary syndrome (PCOS) and their relationship with insulin resistance (IR) and the outcomes of in vitro maturation (IVM) of immature oocytes.
-
Methods
- Expression of GLUTs was evaluated in GCs from women with PCOS with or without IR. Thirty-six women with PCOS undergoing an IVM program were included. Differential gene expression between the insulin sensitive (IS) and IR group was measured by reverse transcription polymerase chain reaction.
-
Results
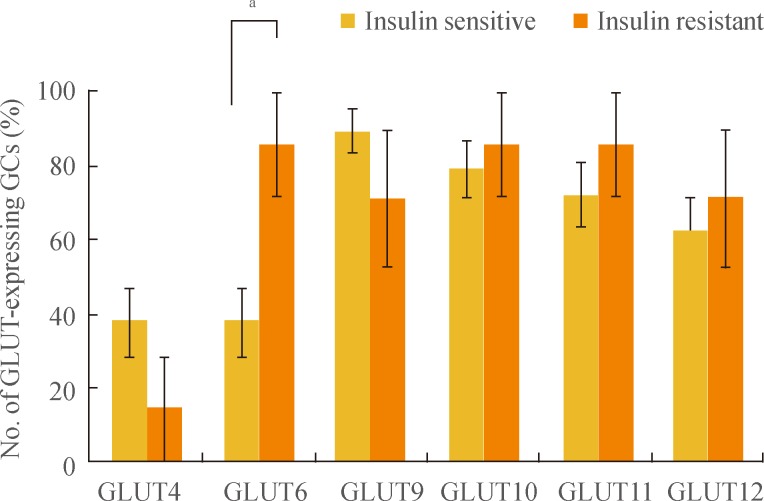
- Expression of GLUTs 1, 3, 5, 8, and 13 was constitutive, whereas expression of GLUTs 2 and 7 was not observed in human GCs. The remaining GLUTs, 4, 6, 9, 10, 11, and 12, were differentially expressed among patients according to metabolic status, such as insulin sensitivity. A higher number of GCs from patients with IR (92%) expressed GLUT6 than GCs from IS PCOS patients (46.3%). Logistic regression showed that expression of GLUTs 9, 11, and 12 correlates with rates of IVM at 48 hours, fertilization, and implantation, respectively.
-
Conclusion
- This is the first report describing the expression pattern of all 13 members of the GLUT family in human GCs. Results of the present study suggest that patients' insulin sensitivity regulates GLUT expression in GCs in PCOS patients, and this may control oocyte quality for IVM and subsequent processes such as fertilization and implantation in patients taking part in an in vitro fertilization program.
- Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women [1,2], affecting 5% to 10% of woman of reproductive age [3]. According to the Rotterdam criteria for diagnosis, PCOS involves at least two out of the following three criteria: clinical or biochemical evidence of hyperandrogenism, oligoovulation and/or anovulation, and polycystic ovarian morphology with an accumulation of small antral follicles [4]. PCOS is a heterogeneous disorder that presents variable features, including obesity, infertility, hyperinsulinemia, and insulin resistance (IR). The etiology of PCOS remains unclear, but IR is believed to play an important role in its pathophysiology by stimulating ovarian androgen production [5]. Moreover, insulin sensitizing drugs such as metformin have been shown to confer therapeutic benefits [6]. In PCOS, IR mainly occurs in classic target tissues, such as skeletal muscle, fat, liver, or fibroblasts [7,8,9], but also in the ovary [10].
- Glucose is a key fuel and an important metabolic substrate that regulates gene transcription, enzyme activity, hormone secretion, and glucoregulatory neuron activity in mammals [11]. The facilitative transport of glucose across the plasma membrane of mammalian cells is catalyzed by a family of glucose transporter proteins (GLUTs). Since the 1990s, 14 members of the human GLUT family have been identified [12,13,14,15]. The various transporters exhibit different tissue-specific expression, substrate specificity, and kinetic properties, and certain cells express two or more isoforms [16].
- Expression of GLUTs is altered in various tissues of PCOS patients. For example, GLUT4 expression is decreased in adipocytes [17] and the endometrium [18], but is not altered in skeletal muscle, where GLUT1 expression is increased [19]. Moreover, insulin receptor signaling is also decreased in PCOS patients [20,21]. However, the expression patterns and functions of recently discovered GLUTs are relatively less well studied in the ovary.
- Oocytes, cumulus cells, and granulosa cells (GCs) communicate by endocrine, paracrine, and autocrine signals during whole process of folliculogenesis [22]. The bidirectional interactions among these cells are essential for the development and function of follicles as well as oocytes [23]. Oocytes lack the ability to carry out glycolysis and cholesterol biosynthesis, and to transport certain amino acids; thus, surrounding somatic cells must compensate for these metabolic insufficiencies [22]. Therefore, the expression of GLUTs in surrounding GCs may be strongly related with oocyte quality. In this study, we evaluated the expression profiles of all members of the GLUT family in GCs and the relationships among GLUT expression in GCs and IR and oocyte quality in PCOS patients participating in an in vitro maturation (IVM) program.
INTRODUCTION
- Patient selection and blood collection
- This study protocol was approved by the Institutional Ethical Review Board of the Fertility Center of the CHA Gangnam Medical Center, CHA University, and all patients provided written informed consent. Diagnosis of PCOS was established according to the revised Rotterdam European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine criteria [4]. Patients with hyperprolactinemia, thyroid disease, congenital adrenal hyperplasia, Cushing syndrome, or androgen-secreting tumors were excluded.
- All participants were undergoing in vitro fertilization (IVF) at the Fertility Center of CHA Hospital, Seoul, Korea. Thirty-four PCOS patients were enrolled to undergo IVF cycles without hormone stimulation. Twenty-eight women had normal glucose metabolism, and were grouped as insulin sensitive (IS) in this study, while six women presented with IR.
- IR was diagnosed using the 75-g oral glucose tolerance test (OGTT) and the homeostasis model assessment (HOMA) index. The HOMA index was calculated as HOMA=(fasting insulin, µIU/mL×fasting glucose, mmol/L)/22.5, and IR was determined when HOMA was >3 in the present study [24]. OGTT and HOMA are well-known validated markers for IR [25].
- Patients' clinical profiles and the blood samples for measuring baseline hormonal status were collected on day 3 of each cycle. Blood pressures, waist circumference, and body mass index were measured as described by Ryu and colleagues [24]. The level of serum gonadotropins and other hormones were measured as described previously [26].
- Preparation of granulosa cells
- Follicular fluid was aspirated transvaginally under ultrasound guidance during follicle puncture for IVF. After isolation of cumulus-oocyte complexes, follicular fluid was pooled and centrifuged at 1,000 g for 10 minutes. The pellet was retained, and red blood cells were removed by a 60% Percoll gradient and subsequent treatment with red blood cell lysis buffer (Roche, Basel, Switzerland). The remaining cells were purified by centrifugation, suspended in phosphate buffered saline (PBS), and filtered through a mesh (Ted Pella Inc., Redding, CA, USA). The single-cell suspension was layered over 100% fetal bovine serum (Gibco, Grand Island, NY, USA) and centrifuged at 250 g for 15 minutes to remove platelets [27]. The final pellet was dissolved in Dynal buffer 1 (2 mM ethylenediaminetetraacetic acid, 0.1% bovine serum albumin in PBS) and subjected to immunobead leukocyte depletion.
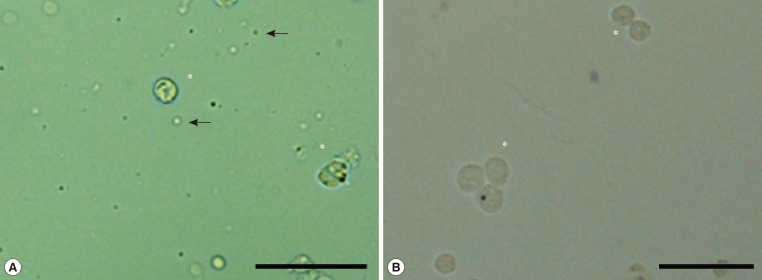
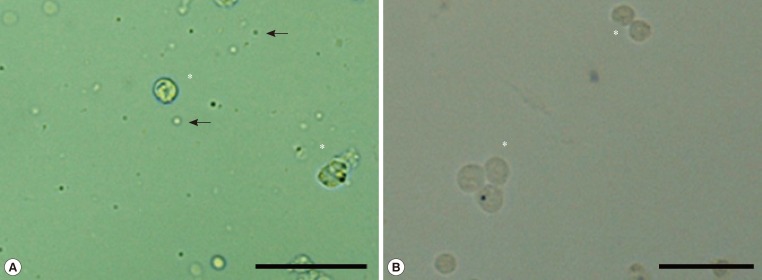
- GCs dispersed in Dynal buffer 1 (2×106 cells/mL) were mixed with 4×107 prewashed paramagnetic beads conjugated with anti-CD45 Ig (Dynal Biotech, Oslo, Norway) at 4℃ for 1 hour. Following capture of the beads with a magnetic separator, the supernatant containing unbound GCs was collected, and the presence of isolated single cells confirmed by microscopic observation (Fig. 1). Bead-bound white blood cells were washed with Dynal buffer 1 four times and lysed for RNA isolation.
- Total RNA isolation and reverse transcription polymerase chain reaction
- Total RNA was extracted using TRIzol agent (Invitrogen, Carlsbad, CA, USA). Cells were added to 1 mL TRIzol, vortexed, and incubated for 5 minutes at room temperature. After addition of 0.2 mL chloroform and incubation for 10 minutes at room temperature, lysates were centrifuged at 10,000 g for 20 minutes at 4℃. The supernatant was transferred to a new tube containing 0.5 mL isopropanol and precipitated. The pellet was washed with 75% ethanol, dried, dissolved in diethylpyrocarbonate-treated water, and stored for reverse transcription (RT) reaction.
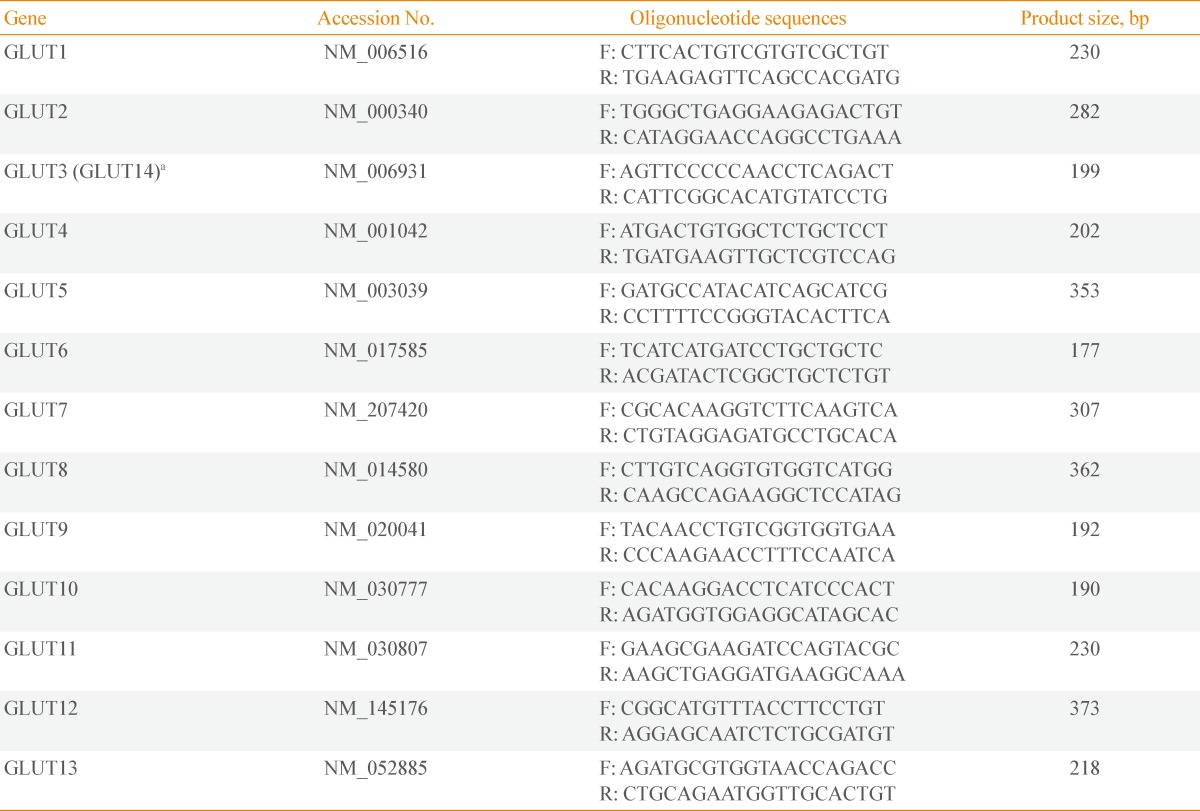
- Complementary DNA (cDNA) was synthesized from 1 µg total RNA using 0.5 µg oligo (dT) primer according to the manufacturer's protocol for M-MLVRT (Promega, Madison, WI, USA). Briefly, 1 µg RNA was treated with DNase I for 15 minutes, followed by enzyme inactivation at 65℃ for 15 minutes. Then oligo (dT) primer was added and incubated at 70℃ for 10 minutes. RT was conducted at 42℃ for 60 minutes, and the reaction was terminated by incubation at 94℃ for 2 minutes. cDNA was used as a template for semiquantitative polymerase chain reaction (PCR) with each set of GLUT primers. To definitively confirm the existence or absence of transcript, the template was amplified by 40 cycles of denaturation at 95℃ for 30 seconds, annealing at 60℃ for 30 seconds, and extension at 72℃ for 30 seconds. Each PCR product was subjected to 2% agarose gel electrophoresis. The primer sequences and product sizes are listed in Table 1. Because GLUT14 shares remarkable identity with GLUT3 and likely resulted from a duplication of GLUT3 [28], we did not design primers specific for GLUT14.
- Statistical analysis
- Statistical analyses were conducted with SAS 9.1 (SAS Institute, Cary, NC, USA) on a Cornell University (Ithaca, NY, USA) mainframe computer. Differences in GLUT expression among groups were analyzed with Fisher exact test. Analyses of relationships between GLUT expression and oocyte maturation rate, fertilization rate, and implantation rate were performed by logistic regression analysis. Values of P<0.05 were considered statistically significant.
METHODS
- Differences in clinical characteristics between insulin sensitive and insulin resistance polycystic ovary syndrome patients
- Clinical profiles of PCOS patients in the two groups, IS and IR, are summarized in Table 2. As predicted, since we grouped patients according to their HOMA-IR values, parameters such as body mass index, blood pressure, waist hip ratio, sex hormone binding globulin, and fasting insulin were significantly different between the IS and IR groups (P<0.05).
- GLUT expression patterns in human granulosa cells in relation to insulin sensitivity
- Expression of GLUTs 1, 3, 5, 8, and 13 was constitutive, whereas expression of GLUTs 2 and 7 was not observed in GCs (Fig. 2A). To confirm that this lack of expression of GLUTs 2 and 7 was not due to PCR conditions, their expression was confirmed with the same primers using cDNA from other cells, such as leukocytes that had been separated from the GCs (Fig. 2B). The demonstration of expression in separated leukocytes but not in GCs verified that we performed clean and absolute separation of the cell types, as well as the absence of the expression of GLUTs 2 and 7 in GCs. Among the 13 GLUT members, expression of the remaining family members, GLUTs 4, 6, 9, 10, 11, and 12, was diverse among GCs from different patients.
- For more detailed analysis of the differential expression pattern of GLUTs, we divided samples according to the existence of IR and compared GLUT expression between these two groups (Fig. 3). There was a trend of GCs from IS samples having increased expression of GLUTs 4, 9, 10, 11, and 12, but these results were not statistically significant. Interestingly, however, GLUT6 expression was detected in a significantly higher percentage of IR GCs than in IS GCs (P<0.05; 85.7% of IR GCs vs. 37.9% of IS GCs). We concluded that GLUT6 expression in GCs is especially sensitive to the patient's insulin sensitivity.
- Association between GLUT expression in granulosa cells and oocyte maturation
- We started this research with the proposition that GLUT expression in GCs could affect oocyte metabolism and consequently influence oocyte quality. We measured the in vitro oocyte maturation rate, since there is no other way to directly measure human oocyte maturity due to ethical limitations. We then analyzed the fertilization rate of oocytes and implantation rate of embryos as an indirect confirmation of the oocytes' quality.
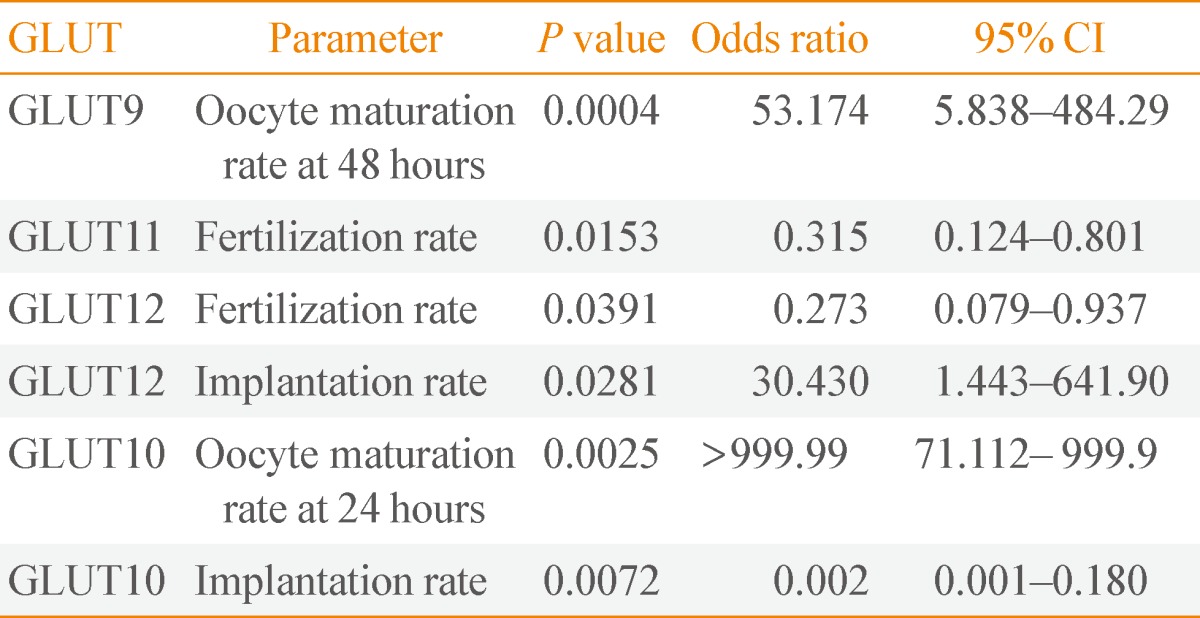
- We used logistic regression to determine whether relationships exist between GLUT expression and variables related to oocytes and embryos, such as oocyte maturation rate at 24 and 48 hours, implantation rate, and fertilization rate. None of these variables were significantly correlated to expression of either GLUTs 4 or 6. In contrast, GLUTs 9, 10, 11, and 12 showed noteworthy relationships with some of these variables (Table 3). An increased oocyte maturation rate at 48 hours was associated with increased GLUT9 expression (odds ratio [OR], 53.174; P=0.0004). Although GLUT9 expression showed a positive correlation with the IVM rate of immature oocytes at 48 hours, there was no relationship between the expression of other GLUTs and oocyte maturation rate at 24 hours. Therefore, we suggest that GLUT9 expression may affect oocyte quality at later stage of in vitro culture. The extreme OR for GLUT10 in terms of the oocyte maturation rate at 24 hours implies that this may not be a meaningful result.
- Association between GLUT expression in granulosa cells and other in vitro fertilization outcomes
- An increased fertilization rate was associated with decreased expression of GLUTs 11 and 12 (OR, 0.315; P=0.0153; and OR, 0.273; P=0.0391, respectively). Moreover, an increased implantation rate was associated with increased GLUT12 expression (OR, 30.43; P=0.0281). These data indicate that increased GLUT9 expression and decreased GLUTs 11 and 12 expressions may be related to improved oocyte maturation after 48 hours of in vitro culture, improved fertilization rate, and improved implantation rate, respectively. Expression of GLUTs 11 and 12 showed a negative correlation with oocyte fertilization rate, whereas expression of GLUT12 showed a positive association with the implantation rate of cultured oocytes.
RESULTS
- Glucose is a major source of metabolic energy for mammalian cells and can regulate gene transcription, enzyme activity, and hormone secretion [11]. Because the plasma membrane is impermeable to polar molecules such as glucose, glucose uptake requires membrane-associated carrier proteins [29]. GLUTs use existing gradients in the concentration of glucose and other hexoses across the membrane to facilitate their translocation, ensuring a continuous supply of glucose to most tissues [30].
- Relatively little is known about the specific functions of the more recently discovered GLUT proteins. GLUT6 cDNA was originally cloned from leukocytes, but at its discovery it was named GLUT9 [31]. In rat adipose cells, GLUT6 appears to recycle between internal membranes and the plasma membrane in a dynamin-dependent manner, but it is unresponsive to stimuli that induce GLUT4 translocation [31]. In our study, we evaluated all 13 GLUT family members' expression in human GCs obtained from IVF patients and found valuable results as follows: 1) GLUTs 2 and 7 are not expressed in human GCs at all, whereas GLUTs 1, 3, 5, 8, and 13 are expressed in all GCs constitutively, and 2) only GLUT6 expression showed differential expression according to the patients' insulin sensitivity.
- Generally, it is known that GLUT4, as a high-affinity GLUT that is predominantly expressed in muscle cells and adiposities, mediates insulin-stimulated glucose transport [32]. Compared to other GLUTs, many aspects of the relationship between GLUT4 and insulin regulation have been revealed. For example, the lack of GLUT4 presence at the plasma membrane in response to insulin is an early step in the development of IR and type 2 diabetes mellitus [30]. Therefore, it is interesting that, in human GCs, the most sensitive GLUT to the patient's insulin sensitivity was GLUT6 rather than GLUT4. Thus, further study will be needed to understand the function and regulatory mechanism of GLUT6 in GCs.
- Functional studies have shown that GLUT9 transports both glucose and fructose, and also uric acid [33]. When plasma uric acid increases, oocyte maturation, fertilization, and embryo cleavage rates decrease [34]. Therefore, according to our results, we suggest that GLUT9 expression by GCs leads to excretion of urea from follicles to create a more favorable environment for oocyte growth and maturation, and it appears to be positively associated with oocyte quality with respect to IVM of immature oocytes over 48 hours. The mechanisms involved these processes should be studied further.
- GLUT11 exhibits detectable glucose and fructose transport activity, but like GLUT6, its principal substrate and function remains unknown [35]. GLUT12 exhibits a distinct expression pattern restricted primarily to IS tissues [36]. In mouse, it has been reported that GLUT12 expression is apparent in ovulated oocytes and 2-cell embryos, declines in morula, then increases again in blastocysts [37], and GLUT12 expression in Xenopus oocytes resulted in increased glucose uptake [38]. We also found that the GLUT12 expression in GCs is positively correlated with implantation rate. Therefore, we conclude that GLUT12 expression is intimately involved in early embryo development.
- Many studies have investigated the factors that regulate GLUT expression. Various physiological conditions, such as fasting, high-fat feeding, obesity, exercise, and cold exposure are related to GLUT expression. Several transcription factors are also known to regulate GLUT expression and many drugs and chemicals, such as antitumor drugs and tamoxifen, also influence GLUT expression [36]. Consequently it can be thought that hormones involved in follicular growth and systemic insulin levels may influence GLUT expression in the follicular cells and ultimately affect oocyte quality. Results of the present study have expanded our understanding of the relationships between metabolism and cell-cell communication in oocyte maturation in relation to GLUT expression, and further studies on the effects of regulatory factors and hormones on specific GLUTs are required in the near future.
DISCUSSION
-
Acknowledgements
- The authors are grateful to the medical doctors and all embryologists of the IVF laboratory involved in sample collection and analysis of clinical and laboratory data. This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084923).
ACKNOWLEDGMENTS
- 1. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800. ArticlePubMed
- 2. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 1998;83:3078–3082. ArticlePubMed
- 3. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745–2749. ArticlePubMed
- 4. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25.Article
- 5. Nestler JE, Usiskin KS, Barlascini CO, Welty DF, Clore JN, Blackard WG. Suppression of serum dehydroepiandrosterone sulfate levels by insulin: an evaluation of possible mechanisms. J Clin Endocrinol Metab 1989;69:1040–1046. ArticlePubMedPDF
- 6. Dunaif A. Drug insight: insulin-sensitizing drugs in the treatment of polycystic ovary syndrome: a reappraisal. Nat Clin Pract Endocrinol Metab 2008;4:272–283. ArticlePubMedPDF
- 7. Book CB, Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 1999;84:3110–3116. ArticlePubMed
- 8. Corbould A, Zhao H, Mirzoeva S, Aird F, Dunaif A. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes 2006;55:751–759. ArticlePubMed
- 9. Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 1992;41:1257–1266. ArticlePubMed
- 10. Wu XK, Zhou SY, Liu JX, Pollanen P, Sallinen K, Makinen M, Erkkola R. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil Steril 2003;80:954–965. ArticlePubMed
- 11. Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab 2010;298:E141–E145. ArticlePubMed
- 12. Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol 2001;18:247–256. ArticlePubMed
- 13. Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr 2004;28:364–371. ArticlePubMed
- 14. Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch 2004;447:480–489. ArticlePubMedPDF
- 15. Wood IS, Hunter L, Trayhurn P. Expression of Class III facilitative glucose transporter genes (GLUT-10 and GLUT-12) in mouse and human adipose tissues. Biochem Biophys Res Commun 2003;308:43–49. ArticlePubMed
- 16. Schurmann A. Insight into the "odd" hexose transporters GLUT3, GLUT5, and GLUT7. Am J Physiol Endocrinol Metab 2008;295:E225–E226. ArticlePubMed
- 17. Rosenbaum D, Haber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol 1993;264(2 Pt 1):E197–E202. ArticlePubMed
- 18. Mozzanega B, Mioni R, Granzotto M, Chiarelli S, Xamin N, Zuliani L, Sicolo N, Marchesoni D, Vettor R. Obesity reduces the expression of GLUT4 in the endometrium of normoinsulinemic women affected by the polycystic ovary syndrome. Ann N Y Acad Sci 2004;1034:364–374. ArticlePubMed
- 19. Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab 2005;288:E1047–E1054. ArticlePubMed
- 20. Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab 2001;281:E392–E399. ArticlePubMed
- 21. Rajkhowa M, Brett S, Cuthbertson DJ, Lipina C, Ruiz-Alcaraz AJ, Thomas GE, Logie L, Petrie JR, Sutherland C. Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem J 2009;418:665–671. ArticlePubMedPDF
- 22. Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 2009;27:32–42. ArticlePubMedPMCPDF
- 23. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001;122:829–838. ArticlePubMed
- 24. Ryu S, Sung KC, Chang Y, Lee WY, Rhee EJ. Spectrum of insulin sensitivity in the Korean population. Metabolism 2005;54:1644–1651. ArticlePubMed
- 25. Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab 2003;88:1019–1023. ArticlePubMed
- 26. Chang EM, Han JE, Seok HH, Lee DR, Yoon TK, Lee WS. Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin Endocrinol (Oxf) 2013;79:93–99. ArticlePubMed
- 27. Fedorcsak P, Raki M, Storeng R. Characterization and depletion of leukocytes from cells isolated from the pre-ovulatory ovarian follicle. Hum Reprod 2007;22:989–994. ArticlePubMedPDF
- 28. Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics 2002;80:553–557. ArticlePubMed
- 29. Suganuma N, Segade F, Matsuzu K, Bowden DW. Differential expression of facilitative glucose transporters in normal and tumour kidney tissues. BJU Int 2007;99:1143–1149. ArticlePubMed
- 30. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806. ArticlePubMedPDF
- 31. Lisinski I, Schurmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J 2001;358(Pt 2):517–522. ArticlePubMedPMCPDF
- 32. Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest 2000;106:165–169. ArticlePubMedPMC
- 33. Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437–442. ArticlePubMedPDF
- 34. Santos P, Marques A, Antunes G, Chaveiro A, Andrade M, Borba A, da Silva FM. Effects of plasma urea nitrogen levels on the bovine oocyte ability to develop after in vitro fertilization. Reprod Domest Anim 2009;44:783–787. ArticlePubMed
- 35. Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schurmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J 2001;359(Pt 2):443–449. ArticlePubMedPMCPDF
- 36. Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab 2002;282:E733–E738. ArticlePubMed
- 37. Zhou Y, Kaye PL, Pantaleon M. Identification of the facilitative glucose transporter 12 gene Glut12 in mouse preimplantation embryos. Gene Expr Patterns 2004;4:621–631. ArticlePubMed
- 38. Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. Glucose transporter GLUT12-functional characterization in Xenopus laevis oocytes. Biochem Biophys Res Commun 2003;308:422–426. ArticlePubMed
References

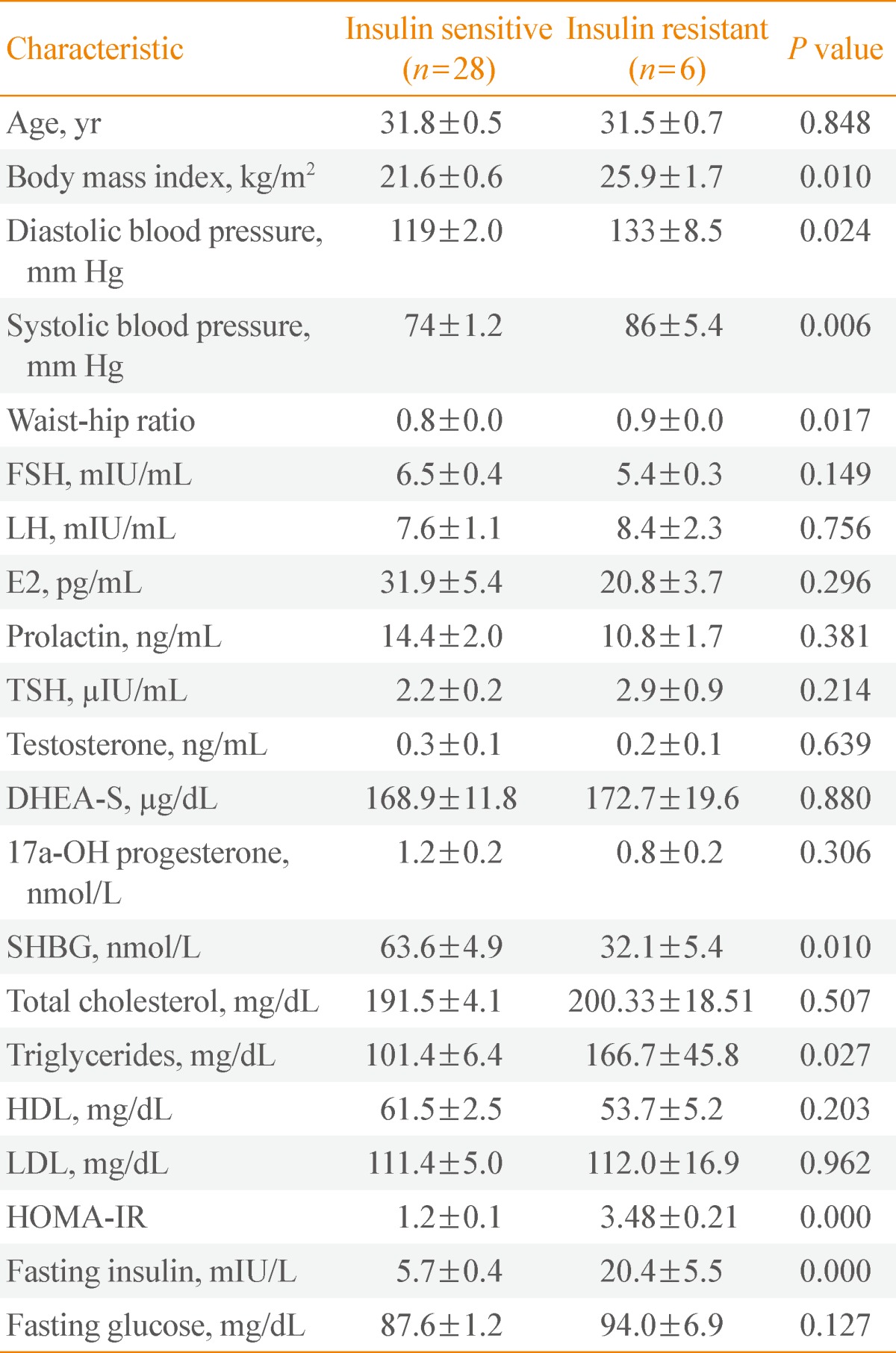
Values are expressed as mean±SD.
FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; TSH, thyroid-stimulating hormone; DHEA-S, dehydroepiandrosterone-sulfate; SHBG, sex hormone binding globulin; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostatic model assessment insulin resistance.
Figure & Data
References
Citations

- Dysregulation of ferroptosis-related genes in granulosa cells associates with impaired oocyte quality in polycystic ovary syndrome
Jialyu Huang, Hancheng Fan, Chenxi Li, Kangping Yang, Chaoyi Xiong, Siyi Xiong, Shenghui Feng, Shen Chen, Bangqi Wang, Yufang Su, Boyun Xu, Haiyan Yang, Ni Wang, Jing Zhu
Frontiers in Endocrinology.2024;[Epub] CrossRef - Chinese herbal medicine alleviates the pathogenesis of polycystic ovary syndrome by improving oxidative stress and glucose metabolism via mitochondrial Sirtuin 3 signaling
Qing Zhang, Jun Ren, Fangfang Wang, Mingqian Li, Manman Pan, Hui Zhang, Fan Qu
Phytomedicine.2023; 109: 154556. CrossRef - Essential Role of Granulosa Cell Glucose and Lipid Metabolism on Oocytes and the Potential Metabolic Imbalance in Polycystic Ovary Syndrome
Chen-Hua Zhang, Xiang-Yi Liu, Jing Wang
International Journal of Molecular Sciences.2023; 24(22): 16247. CrossRef - Effect of Electro-acupuncture on Expression of IRS-1/PI3K/GLUT4 Pathway in Ovarian Granulosa Cells of Infertile Patients with Polycystic Ovary Syndrome-Insulin Resistance of Phlegm-Dampness Syndrome
Shan Xiang, Ming-feng Xia, Jing-yan Song, Dan-qi Liu, Fang Lian
Chinese Journal of Integrative Medicine.2021; 27(5): 330. CrossRef - Comparison of streptozotocin-induced diabetes at different moments of the life of female rats for translational studies
Yuri K Sinzato, Eduardo Klöppel, Carolina A Miranda, Verônyca G Paula, Larissa F Alves, Lívia LS Nascimento, Ana Paula Campos, Barshana Karki, Václav Hampl, Gustavo T Volpato, Débora C Damasceno
Laboratory Animals.2021; 55(4): 329. CrossRef - Molecular Insulin Actions Are Sexually Dimorphic in Lipid Metabolism
Rosa Isela Ortiz-Huidobro, Myrian Velasco, Carlos Larqué, Rene Escalona, Marcia Hiriart
Frontiers in Endocrinology.2021;[Epub] CrossRef - Potential role of tea extract in oocyte development
Lei Zhao, Qing-Yuan Sun, Zhao-Jia Ge
Food & Function.2021; 12(21): 10311. CrossRef - Thioredoxin-interacting protein regulates glucose metabolism and improves the intracellular redox state in bovine oocytes during in vitro maturation
XiaoLong Jiang, YunWei Pang, ShanJiang Zhao, HaiSheng Hao, XueMing Zhao, WeiHua Du, YaChun Wang, HuaBin Zhu
American Journal of Physiology-Endocrinology and Metabolism.2020; 318(3): E405. CrossRef - The Impact of Controlled Ovarian Stimulation Hormones on the Metabolic State and Endocannabinoid System of Human Cumulus Cells
Valentina Notarstefano, Giorgia Gioacchini, Elisabetta Giorgini, Nina Montik, Andrea Ciavattini, Anna Rita Polidori, Fulvia Antonia Candela, Lisa Vaccari, Maurizio Cignitti, Oliana Carnevali
International Journal of Molecular Sciences.2020; 21(19): 7124. CrossRef - Polycystic Ovary Syndrome and Infertility: From Molecular Perspective
Masoumeh Ghafarzadeh
Current Womens Health Reviews.2020; 16(3): 182. CrossRef - Effects of gonadotrophins and insulin on glucose uptake in the porcine cumulus–oocyte complex during IVM
Gabriel Martín Alvarez, María Josefina Barrios Expósito, Evelin Elia, Dante Paz, Sergio Morado, Pablo Daniel Cetica
Reproduction, Fertility and Development.2019; 31(8): 1353. CrossRef - Does the molecular and metabolic profile of human granulosa cells correlate with oocyte fate? New insights by Fourier transform infrared microspectroscopy analysis
Giorgia Gioacchini, Valentina Notarstefano, Elena Sereni, Carlotta Zacà, Giovanni Coticchio, Elisabetta Giorgini, Lisa Vaccari, Oliana Carnevali, Andrea Borini
MHR: Basic science of reproductive medicine.2018;[Epub] CrossRef - Association among genetic variants in the vitamin D pathway and circulating 25-hydroxyvitamin D levels in Korean adults: results from the Korea National Health and Nutrition Examination Survey 2011–2012
So-Young Kwak, Clara Yongjoo Park, Garam Jo, Oh Yoen Kim, Min-Jeong Shin
Endocrine Journal.2018; 65(9): 881. CrossRef - Abnormal expression levels of BMP15/Smad1 are associated with granulosa cell apoptosis in patients with polycystic ovary syndrome
Xiangrong Cui, Xuan Jing, Xueqing Wu, Xingyu Bi, Junfen Liu, Zhijing Long, Xiuping Zhang, Dongdong Zhang, Hongxiang Jia, Dan Su, Kai Huo
Molecular Medicine Reports.2017; 16(6): 8231. CrossRef - Role of the PI3K-Akt Signaling Pathway in the Pathogenesis of Polycystic Ovary Syndrome
Tiantian Li, Hui Mo, Wenfeng Chen, Li Li, Yao Xiao, Jing Zhang, Xiaofang Li, Ying Lu
Reproductive Sciences.2017; 24(5): 646. CrossRef - Nitric Oxide–Mediated Regulation of GLUT by T3 and Follicle-Stimulating Hormone in Rat Granulosa Cells
Ye Tian, Yu Ding, Juan Liu, Dai Heng, Kaili Xu, Wenbo Liu, Cheng Zhang
Endocrinology.2017; 158(6): 1898. CrossRef - Is the canine corpus luteum an insulin-sensitive tissue?
Liza Margareth Medeiros de Carvalho Sousa, Renata dos Santos Silva, Vanessa Uemura da Fonseca, Rafael Magdanelo Leandro, Thiago Senna Di Vincenzo, Ana Bárbara Alves-Wagner, Ubiratan Fabres Machado, Paula de Carvalho Papa
Journal of Endocrinology.2016; 231(3): 223. CrossRef - Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1
Meghan Ruebel, Kartik Shankar, Dana Gaddy, Forrest Lindsey, Thomas Badger, Aline Andres
American Journal of Physiology-Endocrinology and Metabolism.2016; 311(1): E269. CrossRef - Insulin signalling and glucose transport in the ovary and ovarian function during the ovarian cycle
Joëlle Dupont, Rex J. Scaramuzzi
Biochemical Journal.2016; 473(11): 1483. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Chronic ethanol treatment of human hepatocytes inhibits the activation of the insulin signaling pathway by increasing cytosolic free calcium levels
YI-MIN CHEN, JIN-FANG ZHAO, YONG-LIN LIU, JIE CHEN, RONG-LIN JIANG
International Journal of Molecular Medicine.2015; 36(3): 739. CrossRef - Dysregulated hepatic expression of glucose transporters in chronic disease: contribution of semicarbazide-sensitive amine oxidase to hepatic glucose uptake
Sumera Karim, Evaggelia Liaskou, Janine Fear, Abhilok Garg, Gary Reynolds, Lee Claridge, David H. Adams, Philip N. Newsome, Patricia F. Lalor
American Journal of Physiology-Gastrointestinal and Liver Physiology.2014; 307(12): G1180. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite