Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(4); 2022 > Article
-
Review ArticleDiabetes, Obesity and Metabolism Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
 Keypoint
Keypoint
A consensus has been reached that low-density lipoprotein cholesterol, or LDL-C, is an indisputable primary target for lipid-lowering treatment. However, the optimization of triglyceride-rich lipoprotein for reducing the remnant risk of cardiovascular diseases is urged. In this article, the authors focus on the biology of lipoprotein lipase (LPL) and its modulators and review recent clinical applications, including genetic studies and clinical trials of novel therapeutics. In conclusion, the optimization of LPL activity to lower TG levels could eventually reduce incident atherosclerotic cardiovascular disease in conjunction with successful LDL-C reduction. -
Joon Ho Moon1*
 , Kyuho Kim2*
, Kyuho Kim2* , Sung Hee Choi1,3
, Sung Hee Choi1,3
-
Endocrinology and Metabolism 2022;37(4):575-586.
DOI: https://doi.org/10.3803/EnM.2022.402
Published online: August 29, 2022
1Divison of Endocrinology & Metabolism, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
2Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
3Divison of Endocrinology & Metabolism, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- Corresponding author: Sung Hee Choi. Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, 82 Gumi-ro 173beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-7033, Fax: +82-31-787-4070, E-mail: shchoimd@gmail.com
- *These authors contributed equally to this work.
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- High levels of triglycerides (TG) and triglyceride-rich lipoproteins (TGRLs) confer a residual risk of cardiovascular disease after optimal low-density lipoprotein cholesterol (LDL-C)–lowering therapy. Consensus has been made that LDL-C is a non-arguable primary target for lipid lowering treatment, but the optimization of TGRL for reducing the remnant risk of cardiovascular diseases is urged. Omega-3 fatty acids and fibrates are used to reduce TG levels, but many patients still have high TG and TGRL levels combined with low high-density lipoprotein concentration that need to be ideally treated. Lipoprotein lipase (LPL) is a key regulator for TGs that hydrolyzes TGs to glycerol and free fatty acids in lipoprotein particles for lipid storage and consumption in peripheral organs. A deeper understanding of human genetics has enabled the identification of proteins regulating the LPL activity, which include the apolipoproteins and angiopoietin-like families. Novel therapeutic approach such as antisense oligonucleotides and monoclonal antibodies that regulate TGs have been developed in recent decades. In this article, we focus on the biology of LPL and its modulators and review recent clinical application, including genetic studies and clinical trials of novel therapeutics. Optimization of LPL activity to lower TG levels could eventually reduce incident atherosclerotic cardiovascular disease in conjunction with successful LDL-C reduction.
- Therapies that lower low-density lipoprotein cholesterol (LDL-C) levels, including statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, have successfully reduced the risk of atherosclerotic cardiovascular disease (ASCVD) and are currently incorporated in guidelines for the treatment of dyslipidemia [1,2]. Despite this success, recent studies have pointed out that high levels of triglycerides (TGs) and triglyceride-rich lipoproteins (TGRLs) confer substantial residual risk after optimal LDL-C-lowering therapy [3]. Fibrates and omega-3 fatty acids are firstly used or added to treat hypertriglyceridemia in patients whose TG level exceeds 500 mg/dL, which showed high correlation with pancreatitis and lipemia retinalis [4]. Despite the effectiveness of these drugs in lowering TG levels, there have been no other options for patients who do not reach target TG levels than those two, which remains a huge clinical unmet need. Furthermore, inconsistent findings have been reported regarding whether fibrates, omega-3 fatty acids, or TG reduction only can reduce incident ASCVD, with conflicting results from major trials, but still showed benefits in patients with high TG and low high-density lipoprotein cholesterol (HDL-C) [5-8]. However, recent studies, including Asians, have supported the possibility that TG reduction itself may contribute to reducing incident ASCVD [9-11].
- In the era of human genomics, it has become possible to discover genetic variants and elucidate their contributions to various phenotypes and diseases. By studying people with hypolipidemia and severe forms of dyslipidemia, such as familial lipid disorders, potential targets for novel lipid lowering therapeutics have been identified, including the LDL receptor, PCSK9, lipoprotein lipase (LPL), the angiopoietin-like (ANGPTL) family, and apolipoproteins (APOs). In fact, evolocumab and alirocumab were developed based on the findings that people with PCSK9 mutations had low LDL-C levels and were protected against ASCVD [12]. With the expansion of new drug development technologies such as small interfering RNA (siRNA), antisense oligonucleotides, and monoclonal antibodies, attempts have been made to test novel therapeutics for TG reduction as well.
- In this review, we describe the key modulators and biology underlying the regulation of TGs. LPL is a key regulator of lipolysis that hydrolyzes TGs to glycerol and free fatty acids (FFAs), and ANGPTLs and APOs regulate LPL activity. We describe recent attempts to develop novel therapeutics that alter the function of these TG modulators and clinical trials using those new candidate-drugs.
INTRODUCTION
- Serum TG level is mostly determined by the % of TG core in TGRLs such as chylomicron (CM), very low-density lipoprotein (VLDL) and their remnants lipoprotein particles through lipoprotein catabolism. Thus, the elevation of serum TG in humans is dependent on the abnormalities in production and clearance of TGRLs by primary genetic causes or by secondary diseases such as diabetes, metabolic syndrome, Cushing syndrome, nephrotic syndrome, hypothyroidism, etc. [13,14].
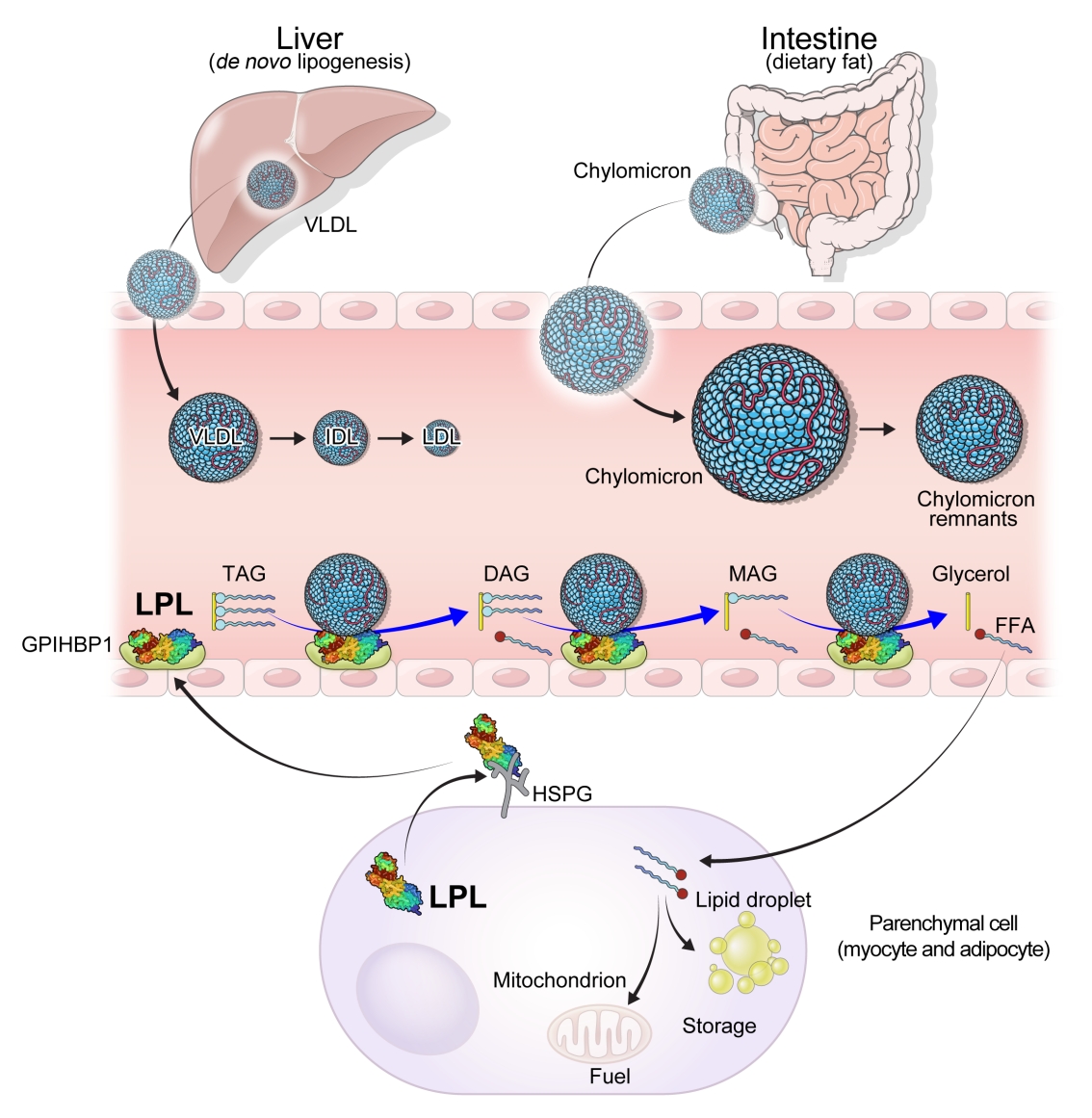
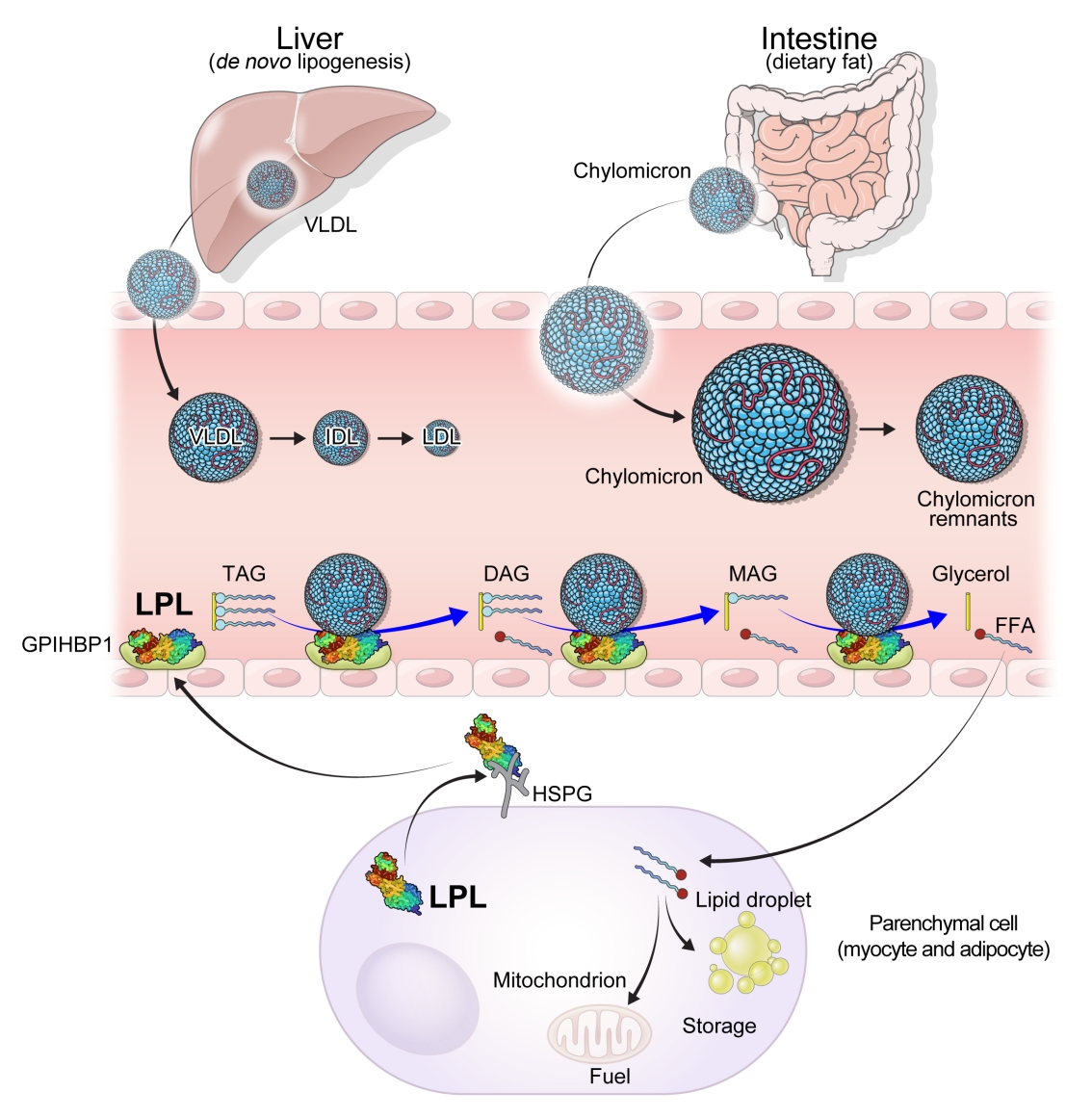
- CMs are large sized lipoprotein particles mainly produced from dietary fats which are absorbed through intestinal enterocytes and CM is conjugated with apolipoprotein B48 (APOB48). It can make systemic circulation via lymphatic system and contains large amount of TG core (more than 80% to 90%), which represent high serum TG levels from exogenous pathway. The hydrolysis of CM are mainly dependent on the activity of LPL, which was controlled by apolipoprotein C2/C3 (APOC2/C3), APOA5, and ANGPTLs action [13].
- VLDL is a representative lipoprotein which contains large % of TG core, secreted from the liver in the endogenous pathway of lipoproteins metabolism. Sources of TG in VLDL particle come from the FFA spillover from peripheral lipolysis, de novo lipogenesis, CM remnants, etc. [15]. Apolipoprotein B100 (APOB100) is a main APO of VLDL and VLDL remnants. Microsomal TG transfer protein is also important to transfer TG from cytosol to endoplasmic reticulum in hepatocyte or in enterocytes for VLDL or CM assembly with APOB100 or with APOB48 incorporation. Cholesteryl ester TG transfer protein (CETP) is activated when the production of TGRLs is abundant such as in insulin resistance and the enhanced LPL action generates more TG hydrolysis of TGRLs, producing more remnants particles. It is well known that lesser size TGRL particles than CMs can be transported through endothelium and be seated for making atherosclerotic plaque [16]. Thus, the optimizing lowering TGRLs is important treatment not only for the hypertriglyceridemia related complications such as severe pancreatitis and lipemia retinalis but also the progression of ASCVDs [17].
LIPOPROTEIN METABOLISM AND HYPERTRIGLYCERIDEMIA
- Lipases are enzymes that catalyze the hydrolysis of TGs. Lipases exist in different contexts; gastric and pancreatic lipases act in an exocrine manner as a component of digestive juices. Hormone-sensitive lipase and adipose TG lipase are located intracellularly and release FFAs and glycerol into the circulation from neutral TGs deposition in adipose tissue. In contrast, LPL and hepatic lipase are located extracellularly on the endothelial cells of blood vessels (Fig. 1).
- LPL is a key and essential enzyme for the catabolism of TGRLs. LPL is produced by the parenchymal cells of peripheral organs, secreted, and anchored to heparan sulfate proteoglycans on the cell surface. Glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1) transports LPL from the cell surface to the capillary endothelium [18]. LPL transported to the vascular endothelium hydrolyzes TGs from CMs and VLDL in the circulation, producing FFAs and glycerol. Insulin induces LPL expression and activity which lead to the uptake of FFAs into peripheral organs for energy storage and consumption. Therefore, LPL is a key regulator of TG levels that is necessary for peripheral organs to store and consume lipids. Familial chylomicronemia syndrome is a rare genetic disorder estimated to affect 1 to 2 individuals per million and characterized by hypertriglyceridemia, which is caused by mutations in LPL or genes that regulate LPL function which include but are not limited to APOC2, APOA5, GPIHBP1, and lipase maturation factor 1 (LMF1) [19,20].
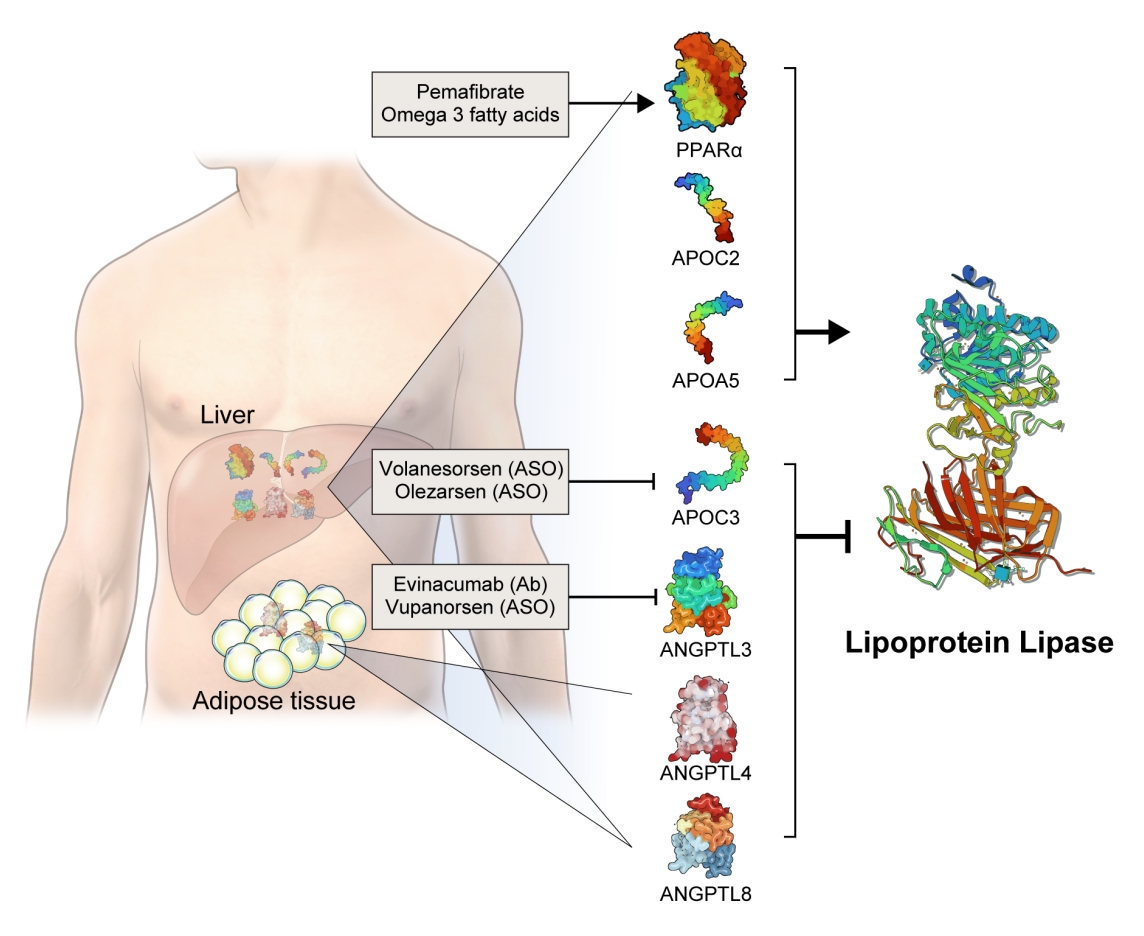
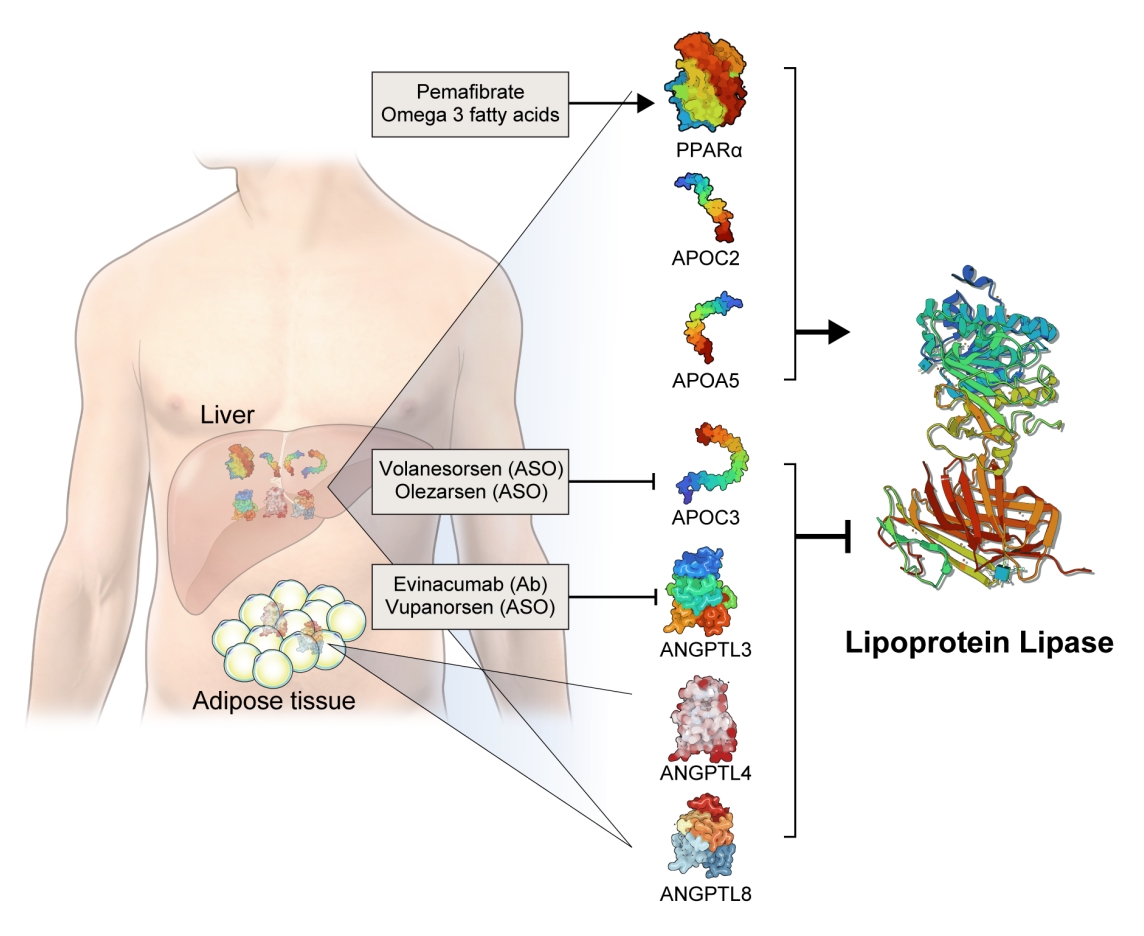
- The activity and/or stability of LPL are regulated by various proteins, including ANGPTLs and APOs (Fig. 2). Loss-of-function mutations in APOC2 and APOA5 inhibit LPL, resulting in severe hypertriglyceridemia [21,22]. In contrast, angiopoietin-like protein 3 (ANGPTL3), ANGPTL4, ANGPTL8, and APOC3 inhibit LPL activity, thereby increasing serum TG levels [23]. The role of each protein in the regulation of LPL activity and circulating TG levels is further discussed below.
- Based on genetic studies exhibiting altered lipid phenotypes in gain- or loss-of-function mutations in the abovementioned proteins regulating LPL activity, pharmaceutical companies have targeted these proteins to develop novel therapeutics to treat hypertriglyceridemia in patients who do not reach the target goal of TG after using the currently available drugs. The clinical evidence regarding modulation of each protein and the corresponding therapeutic developments are discussed in this review (Table 1).
LIPOPROTEIN LIPASE
- APOC3 is a 79-amino-acid-long small glycosylated protein produced by hepatocytes and enterocytes [24]. APOC3 is a component of TGRLs, and it increases TG concentration by directly inhibiting LPL activity and by preventing the clearance of TGRLs. Genetic studies showed that a heterozygous loss-of-function mutation of APOC3 was associated with a 40% reduced level of plasma TGs and a 40% reduction in incident coronary heart disease [25,26]. APOC3 regulates TG levels via both LPL-independent and LPL-dependent pathways, as APOC3 reduction using the antisense oligonucleotide (ISIS 304801) has shown a TG-lowering effect in people defective for LPL [27]. These genetic studies have established the potential of targeting APOC3 as a therapeutic for lowering TG levels.
- Drug development and clinical trials
- An antisense oligonucleotide, volanesorsen (previously called ISIS 304801, ISIS-APOCIIIRx, and IONIS-APOCIIIRx) was developed to decrease the level of APOC3 mRNA. Volanesorsen was well tolerated and efficiently reduced APOC3 and TG levels in preclinical and phase 1 studies [28]. In the A Study of Volanesorsen (Formerly IONIS-APOCIIIRx) in Patients With Familial Chylomicronemia Syndrome (APPROACH) trial (NCT02211209), which was a phase 3 trial including 66 patients with familial chylomicronemia syndrome, volanesorsen reduced APOC3 by 84% and TGs by up to 77% at 3 months [29]. However, thrombocytopenia and injection site reactions were common adverse events observed in 45% and 60% of the participants, respectively.
- The A Study of Volanesorsen (Formally ISIS-APOCIIIRx) in Patients With Hypertriglyceridemia (COMPASS) trial (NCT02-300233) was another phase 3 trial including 113 patients with fasting TG levels ≥500 mg/dL [30]. At 3 months after treatment, TG levels were reduced by 71%, with an absolute reduction of more than 800 mg/dL. Similar to the APPROACH trial, but to a lesser extent, 24% of patients showed injection site reactions, one patient exhibited thrombocytopenia, and one patient exhibited serum sickness. Based on the positive results from the APPROACH and COMPASS trials, the European Union approved volanesorsen for the treatment of familial chylomicronemia syndrome in May 2019 [31].
- To overcome the adverse events observed in volanesorsen trials, an N-acetylgalactosamine-conjugated antisense compound, olezarsen (IONIS-ApoCIII-LRx, AKCEA-APOCIII-LRx or ISIS 678354), was developed to facilitate liver uptake. In a phase 1/2 trial, APOC3 and TG levels decreased by up to 91% and 77%, respectively, at 14 days after a single administration [32]. In a phase 2 trial, TGRLs reduced by 51%, small LDL particles decreased by 39%, and large LDL particles increased by 186% after 6 months of olezarsen treatment suggesting the favorable changes in lipoprotein concentration and particle size remodeling [33]. A phase 3 trial of olezarsen treatment for 53 weeks is underway and is expected to be completed by 2023 (NCT0456-8434).
APOLIPOPROTEIN C3
- Among the eight members of the ANGPTL family, ANGPTL3, ANGPTL4, and ANGPTL8 are responsible for TG regulation by LPL inhibition [34]. The members of the ANGPTL protein family are composed of an N-terminal coiled-coil domain and a fibrinogen-like C-terminal domain, although ANGPTL8 lacks a C-terminal fibrinogen-like domain. The function of ANGPTL3 was first identified in KK/San mice that contained a premature stop codon in Angptl3 and had lower levels of TGs, non-esterified fatty acids, and total cholesterol [35]. Overexpression of Angptl3 or administration of the ANGPTL3 protein restored the features of hypolipidemia.
- Human evidence from a genome-wide association study indicated that TG and LDL-C concentrations are affected by genetic variants in ANGPTL3 [23]. Exome sequencing of two patients with familial combined hypolipidemia who did not have an APOB mutation revealed compound heterozygous nonsense mutations of ANGPTL3; these patients exhibited pan-hypolipidemic phenotypes, including low TG and LDL-C levels, with a clear gene-dose association [36].
- Drug development and clinical trials
- Evinacumab (Evkeeza, Regeneron Pharmaceuticals, Tarrytown, NY, USA) is a human monoclonal antibody inhibiting the action of ANGPTL3 that received approval from the U.S. Food and Drug Administration (FDA) in 2021 as an add-on treatment for patients with homozygous familial hypercholesterolemia who are aged 12 years and older. In a phase 3 trial (the Evinacumab Lipid Studies in Patients with Homozygous Familial Hypercholesterolemia [ELIPSE HoFH]) including 65 patients with homozygous familial hypercholesterolemia whose baseline LDL-C level was 259.5 mg/dL, LDL-C, the primary outcome of this study, was reduced by 47.1% with an absolute change from the baseline of 134.7 mg/dL at week 24 [37]. The mean baseline TG concentration was 97 mg/dL and was reduced by 55.0% at week 24. Adverse events occurred in comparable proportions of patients in the evinacumab and placebo groups (66% and 81%, respectively). An interesting finding was that reductions in LDL-C levels were comparable between those with null-null variants and non-null variants of the LDL receptor. Null-null LDL receptor variants confer higher cardiovascular risk and are less responsive to therapies that depend on LDL receptor activity.
- A phase 2 trial that included subjects with or without heterozygous familial hypercholesterolemia who had relatively milder lipid profiles than homozygous patients (baseline LDL-C of 150 mg/dL and TG of 114.5 mg/dL) consistently showed that evinacumab reduced LDL-C by up to 50% and TG by up to 53% [38]. However, the incidence of adverse events and serious adverse events upon evinacumab treatment was up to 80% and 16%, respectively, suggesting that the safety of long-term treatment should be further validated.
- A mechanistic study using APOB kinetic analyses showed that evinacumab increased the fractional catabolic rate of APOB for intermediate-density-lipoprotein-cholesterol and LDL-C, suggesting that the LDL-C reduction occurred predominantly by increasing APOB-containing lipoprotein clearance from the circulation [39].
- Vupanorsen (AKCEA-ANGPTL3-LRx or IONIS-ANGPTL3-LRx or ISIS 703802) is an N-acetylgalactosamine-conjugated antisense oligonucleotide targeting ANGPTL3. In a preclinical study, vupanorsen reduced levels of hepatic ANGPTL3 and TGs, circulating TG and LDL-C levels, and the progression of atherosclerosis [40]. In a phase 1 study including 44 human participants with TG levels >150 mg/dL, 6-week treatment of vupanorsen reduced TG and LDL up to 63.1% and 32.9%, respectively, without serious adverse events [40]. A phase 2b trial (A Dose-Ranging Study With Vupanorsen [TaRgeting ANGPTL3 with an aNtiSense oLigonucleotide in AdulTs with dyslipidemia, TRANSLATE-TIMI 70]) reached the primary endpoint, achieving a statistically significant reduction in non-HDL-C up to 27.7% at week 24 [41]. Dose-dependent reductions in TG up to 56.8% were observed, while the LDL-C reduction was modest (up to 16.0%). However, adverse events including injection site reactions, elevations in liver enzymes, and a dose-dependent increase in hepatic fat deposition were observed. The magnitude of non-HDL-C and TG reduction was not prominent that the further development of vupanorsen is currently pending. We need to watch for the next movement of this drug.
ANGIOPOIETIN-LIKE PROTEIN 3
- Fibrates are agonists of peroxisome proliferator-activated receptor alpha (PPARα) that reduce TG and increase HDL-C levels [42]. The pharmacological actions of fibrates via PPARα agonism activate LPL transcription in the liver and induce fatty acid uptake and β-oxidation [43,44]. PPARα also inhibits APOC3, which may further enhance LPL activity [45]. Transcription of APOA1, APOA2, and APOA5 is induced via PPARα binding [46]. Fibrates further exert anti-inflammatory and anti-atherogenic activity by reducing vascular cell adhesion molecule (VCAM) and monocyte chemoattractant protein-1 (MCP-1) expression via PPRE-dependent and independent manners [47,48].
- Previous trials of fibrates showed inconsistent results for preventing ASCVDs [10]. After positive results from the Veterans Affairs HDL-C Intervention trial (VA-HIT) trial, showing a 22% reduction in ASCVD with gemfibrozil (1,200 mg/day) in the 1990s [8], major fibrate trials, including the Lower Extremity Arterial Disease Event Reduction (LEADER) [5], Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) [6], and Action to Control Cardiovascular Risk in Diabetes (ACCORD)-Lipid [7] in the 2000s, failed to demonstrate ASCVD prevention with fibrates. Meta-analyses and Cochrane reviews, in contrast, have suggested that fibrates may contribute to primary and secondary prevention of incident ASCVD [9,49,50]. In particular, patients with high TG and low HDL-C levels benefit most from fibrate treatment [7,51].
- Drug development and clinical trials
- Pemafibrate is a selective PPARα agonist with improved potency and selectivity compared to fenofibrate. In a phase 3 Japanese trial, the pemafibrate group showed significantly reduced fasting TG levels by 45%, with significant decreases in non-HDL-C and increases in HDL-C levels [52]. In a phase 3 Japanese comparative trial between pemafibrate versus fibrate in patients with baseline TG levels between 300 and 400 mg/dL, the TG-lowering effect of pemafibrate was comparable to that of fenofibrate, with a reduction of up to 50% [53]. Liver and kidney-related adverse events were lower in the pemafibrate group. The Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study was a phase 3 cardiovascular outcome trial including type 2 diabetes patients with TG levels between 200 and 500 mg/dL, but the Kowa Research Institute decided to stop the trial, concluding that the primary endpoint was unlikely to be met without notable safety concerns [54]. Based on their interim analysis and the absence of safety issues, pemafibrate is now considered for other therapeutic applications, including nonalcoholic fatty liver disease. Although pemafibrate failed to prove a preventive effect for ASCVDs, it can be considered for patients with TG levels >500 mg/dL, and it may be safer than fenofibrate.
- OMEGA-3 FATTY ACIDS
- Omega-3 fatty acids are polyunsaturated fatty acids containing a double bond at the third carbon from the terminal carbon in their chemical structure. Examples of omega-3 fatty acids include α-linolenic acid, which is found in plants, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are found in fish. Mammals are unable to synthesize omega-3 fatty acids; therefore, it is essential to consume them through the diet.
- It has been suggested that omega-3 fatty acids exert TG-lowering effects via different mechanisms: (1) inducing LPL expression and increasing LPL activity; (2) activating PPARα and δ [55]; (3) suppressing hepatic sterol regulatory element-binding protein 1c (SREBP1c); and (4) increasing the β-oxidation of fatty acids [56]. Six-week supplementation of 6 g of omega-3 fatty acids resulted in a 54% increase in LPL mRNA expression in human adipose tissue and up to a 31% increase in LPL activity [57]. By increasing LPL activity, omega-3 fatty acid supplementation accelerated CM TG clearance [58]. Omega-3 fatty acids were more potent ligands or activators for PPARα than saturated fatty acids [59]. The role of PPARα in LPL and TG reduction is described in the previous section.
- Drug development and clinical trials
- Omega-3 fatty acids, including EPA and DHA, were shown to reduce TG by 25% to 31% with the administration of 2 to 4 g/day in the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial [60]. However, cardiovascular outcome trials with different preparations of omega-3 fatty acids have shown inconsistent results. A Study of Cardiovascular Events in Diabetes (ASCEND) and Vitamin D and Omega-3 Trial (VITAL), which administered EPA and DHA in the setting of primary prevention, did not find a significant reduction in ASCVD events [61,62]. The Outcomes Study to Assess Statin Residual Risk With Epanova in High Cardiovascular Risk Patients With Hypertriglyceridemia (STRENGTH) and Omega-3 Fatty acids in Elderly with Myocardial Infarction (OMEMI) trial, which were designed for secondary prevention, did not show a reduction in ASCVD events when a combination of EPA and DHA was administered [63,64]. In contrast, the A Study of AMR101 to Evaluate Its Ability to Reduce Cardiovascular Events in High-Risk Patients With Hypertriglyceridemia and on Statin (REDUCE-IT) trial administered 2 g of a icosapent ethyl, highly purified EPA ethyl ester, and showed a 25% relative risk reduction (17.2% in the icosapent ethyl group vs. 22.0% in the placebo group) in the primary composite endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, or unstable angina in patients with established ASCVD or with diabetes and other risk factors [65]. This study included subjects who had been receiving statin therapy and achieved LDL-C target levels of LDL-C of 41 to 100 mg/dL. During a median duration of follow-up of 4.9 years, mean fasting TG levels decreased by 21.6% (from 216 to 170 mg/dL), suggesting that high TG levels confer a residual risk of ASCVD after optimal LDL-C lowering therapy, which can be prevented by the administration of EPA.
- Although the REDUCE-IT study clearly demonstrated the cardiovascular benefit of EPA, the conflicting results between REDUCE-IT and STRENGTH are in debate based on their study design: active oil (EPA vs. EPA plus DHA); comparator oil (mineral vs. corn); study population (high vs. moderate cardiovascular risk). A post hoc analysis of the Copenhagen General Population Study which mimicked the two trials showed that the contrasting results between the REDUCE-IT and STRENGTH study can partly be explained by a difference in the comparator oil [66]. The mineral oil arm (a comparator oil in the REDUCE-IT study) showed unfavorable changes in LDL-C and C-reactive protein levels resulting in increased ASCVD risk compared to the entire cohort, suggesting that the protective effect of EPA over EPA plus DHA might have been overestimated. This study supports the protective effects against ASCVD in both EPA and EPA plus DHA.
- In conclusion, omega-3 fatty acids have shown to reduce TG level and incident ASCVD, but the biological function for different composition of omega-3 fatty acids should be further studied. Adverse events including atrial fibrillation and bleeding should be considered when prescribing omega-3 fatty acids [67].
PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR ALPHA
- In this review, we focused on novel targets for lowering TG levels based on the modulation of LPL activity. Evinacumab, a monoclonal antibody inhibiting ANGPTL3, is approved for use in the United States in patients with homozygous familial hypercholesterolemia. Volanesorsen inhibits APOC3 and was approved in Europe for the treatment of familial chylomicronemia syndrome, but has yet to be approved by the U.S. FDA due to safety issues including thrombocytopenia [68]. Other attempts to enhance LPL activity by inhibiting ANGPTL3/8 complex with monoclonal Ab, showed dose dependent reduction in TG, LDL-C, non-HDL-C in phase 1 trial [69]. We hope more clinical result from on-going studies in near future.
- Pemafibrate was found to be effective in lowering TG, with fewer side effects than fenofibrates, but its cardiovascular outcome trial was stopped due to futility issue in ASCVD prevention, but still testing its efficacy in nonalcoholic fatty liver disease. EPA was found to effectively reduce TG levels as well as incident ASCVD, but adverse events, including atrial fibrillation and bleeding, should be kept in mind. Beyond the abovementioned drugs, other therapeutics have been attempted to lower LDL-C with different targets which include inclisiran, bempedoic acid, lomitapide, mipomersen, etc. [70]. But most studies of new lipid lowering treatment were the result on the top of statin therapies and still have very narrow indication for extremely high risk population such as homozygous familial hypercholesterolemia.
- For the past 20 years, there have been few convincing novel drugs for dyslipidemia, but many new candidate-drugs have been developed recently. In the field of hypertriglyceridemia treatment, we also had very narrow option in the clinical practice. Understanding the pathophysiological mechanism of TGRLs metabolism and targeting LPL as a key molecule with recent therapeutic approach in the treatment of hypertriglyceridemia can be a very bright signal to optimize patients with intractable TG levels and with high residual risks for ASCVDs. However, it is necessary to understand the mechanisms of action, precise metabolic effects, and possible side effects of these drugs to use them appropriately in clinical practice.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
-
Acknowledgements
- This work was supported by the National Research Foundation of Korea funded by the Korea Government (grant number NRF-2018R1A5A2024425 and NRF-2021R1C1C1009875). Special thanks to Dong Su Jang for his effort to illustrate figures.
| Name | Target | Characteristics | Clinical outcome | Trial phase and developmental status | Adverse events | Reference | |
|---|---|---|---|---|---|---|---|
| Volanesorsen | APOC3 | Antisense oligonucleotide | APPROACH | Phase 3 | Thrombocytopenia, injection site reactions | [29] | |
| TG: 77% reduction at 3 months (baseline 2,267–590 mg/dL) | |||||||
| COMPASS | Phase 3 | [30] | |||||
| TG: 71% reduction at 3 months (baseline 1,183–294 mg/dL) | |||||||
| Olezarsen | APOC3 | Antisense oligonucleotide | TG: up to 77% reduction at 2 weeks after a single dose (baseline 235–52 mg/dL) TG: up to 73% reduction at 3 months (baseline 189–53 mg/dL) | Phase 1/2b | Generally well tolerated compared to volanesorsen | [32] | |
| TG-rich lipoproteins 51% reduction at 6 months (baseline 200.3 mg/dL) The total LDL particle concentration was not changed, but large LDL particles increased by 186% and small LDL particles decreased by 39% | Phase 2 | [33] | |||||
| Evinacumab | ANGPTL3 | Monoclonal antibody | LDL-C: 47.1% reduction at week 24 (baseline 259.5–124.8 mg/dL) TG: 55.0% reduction at week 24 (baseline 91 mg/dL) | Phase 3 | No significant difference compared with placebo | [37] | |
| LDL-C: up to 56% reduction at 16 weeks (subcutaneous) (baseline 146.3 mg/dL) TG: up to 53% reduction at 16 weeks (subcutaneous) (baseline 109.5 mg/dL) | Phase 2 | [38] | |||||
| Vupanorsen | ANGPTL3 | Antisense oligonucleotide | TG: up to 56.8% reduction at 24 weeks (baseline 228.4–101.9 mg/dL) | Phase 2b Discontinued further development from Pfizer. Developmental rights returned back to Ionis. | Dose-dependent increase in hepatic fat fraction up to 76% | [41] | |
| Pemafibrate | PPARα | Small molecule, PPARα agonist | TG: ~45% reduction at 24 weeks (baseline ~250 to ~30 mg/dL) | Phase 3 | No significant difference from placebo and lower liver and kidney-related adverse events compared to fenofibrate | [52] | |
| PROMINENT | Phase 3, discontinued as the primary endpoint was unlikely to be met. Pemafibrate is considered for other therapeutic applications, including nonalcoholic fatty liver disease. | [54] | |||||
| Cardiovascular outcome trial including type 2 diabetes patients with TG between 200 and 500 mg/dL | |||||||
| Icosapent ethyl | PPARα | Omega-3 fatty acid | REDUCE-IT | Phase 3b, cardiovascular outcome trial | No significant difference compared with placebo | [65] | |
| ASCVD 25% relative risk reduction (17.2% in the icosapent ethyl group vs. 22.0% in the placebo group) during 4.9 years of follow-up TG: 21.6% reduction during 4.9 years of follow-up (baseline 216–170 mg/dL) | |||||||
APOC3, apolipoprotein C3; APPROACH, A Study of Volanesorsen (Formerly IONIS-APOCIIIRx) in Patients With Familial Chylomicronemia Syndrome; TG, triglyceride; COMPASS, A Study of Volanesorsen (Formally ISIS-APOCIIIRx) in Patients With Hypertriglyceridemia; LDL, low-density lipoprotein; ANGPTL3, angiopoietin-like protein 3; LDL-C, low-density lipoprotein cholesterol; PPARα, peroxisome proliferator-activated receptor alpha; PROMINENT, Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes; REDUCE-IT, A Study of AMR101 to Evaluate Its Ability to Reduce Cardiovascular Events in High-Risk Patients With Hypertriglyceridemia and on Statin; ASCVD, atherosclerotic cardiovascular disease.
- 1. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019;73:3168–209.PubMed
- 2. Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 Guidelines for the management of dyslipidemia in Korea. J Lipid Atheroscler 2019;8:78–131.ArticlePubMedPMCPDF
- 3. Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep 2012;14:1–10.ArticlePubMedPMCPDF
- 4. Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998;27:551–67.ArticlePubMed
- 5. Meade T, Zuhrie R, Cook C, Cooper J. Bezafibrate in men with lower extremity arterial disease: randomised controlled trial. BMJ 2002;325:1139.ArticlePubMedPMC
- 6. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849–61.ArticlePubMed
- 7. ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–74.ArticlePubMedPMC
- 8. Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–8.ArticlePubMed
- 9. Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010;375:1875–84.ArticlePubMed
- 10. Kim NH, Kim SG. Fibrates revisited: potential role in cardiovascular risk reduction. Diabetes Metab J 2020;44:213–21.ArticlePubMedPMCPDF
- 11. Ference BA, Kastelein JJ, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321:364–73.ArticlePubMedPMC
- 12. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72.ArticlePubMed
- 13. Packard CJ, Boren J, Taskinen MR. Causes and consequences of hypertriglyceridemia. Front Endocrinol (Lausanne) 2020;11:252.ArticlePubMedPMC
- 14. Santos-Baez LS, Ginsberg HN. Hypertriglyceridemia-causes, significance, and approaches to therapy. Front Endocrinol (Lausanne) 2020;11:616.ArticlePubMedPMC
- 15. Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 2011;22:353–63.ArticlePubMedPMC
- 16. Nakano T, Nakajima K, Niimi M, Fujita MQ, Nakajima Y, Takeichi S, et al. Detection of apolipoproteins B-48 and B-100 carrying particles in lipoprotein fractions extracted from human aortic atherosclerotic plaques in sudden cardiac death cases. Clin Chim Acta 2008;390:38–43.ArticlePubMed
- 17. Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J 2020;41:99–109c.ArticlePubMedPMCPDF
- 18. Wen Y, Chen YQ, Konrad RJ. The regulation of triacylglycerol metabolism and lipoprotein lipase activity. Adv Biol (Weinh) 2022 Jun 8 [Epub]. https://doi.org/10.1002/adbi.202200093.Article
- 19. Ameis D, Kobayashi J, Davis RC, Ben-Zeev O, Malloy MJ, Kane JP, et al. Familial chylomicronemia (type I hyperlipoproteinemia) due to a single missense mutation in the lipoprotein lipase gene. J Clin Invest 1991;87:1165–70.ArticlePubMedPMC
- 20. Falko JM. Familial chylomicronemia syndrome: a clinical guide for endocrinologists. Endocr Pract 2018;24:756–63.ArticlePubMed
- 21. Breckenridge WC, Little JA, Steiner G, Chow A, Poapst M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med 1978;298:1265–73.ArticlePubMed
- 22. Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science 2001;294:169–73.ArticlePubMed
- 23. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013;45:1274–83.ArticlePubMedPMCPDF
- 24. Olkkonen VM, Sinisalo J, Jauhiainen M. New medications targeting triglyceride-rich lipoproteins: can inhibition of ANGPTL3 or apoC-III reduce the residual cardiovascular risk? Atherosclerosis 2018;272:27–32.ArticlePubMed
- 25. TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute, Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31.ArticlePubMedPMC
- 26. Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41.ArticlePubMed
- 27. Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 2014;371:2200–6.ArticlePubMed
- 28. Graham MJ, Lee RG, Bell TA 3rd, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res 2013;112:1479–90.ArticlePubMed
- 29. Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med 2019;381:531–42.ArticlePubMed
- 30. Gouni-Berthold I, Alexander VJ, Yang Q, Hurh E, Steinhagen-Thiessen E, Moriarty PM, et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021;9:264–75.ArticlePubMed
- 31. Paik J, Duggan S. Volanesorsen: first global approval. Drugs 2019;79:1349–54.ArticlePubMedPDF
- 32. Alexander VJ, Xia S, Hurh E, Hughes SG, O’Dea L, Geary RS, et al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J 2019;40:2785–96.ArticlePubMedPMCPDF
- 33. Karwatowska-Prokopczuk E, Tardif JC, Gaudet D, Ballantyne CM, Shapiro MD, Moriarty PM, et al. Effect of olezarsen targeting APOC-III on lipoprotein size and particle number measured by NMR in patients with hypertriglyceridemia. J Clin Lipidol 2022 Jun 23 [Epub]. https://doi.org/10.1016/j.jacl.2022.06.005.Article
- 34. Li J, Li L, Guo D, Li S, Zeng Y, Liu C, et al. Triglyceride metabolism and angiopoietin-like proteins in lipoprotein lipase regulation. Clin Chim Acta 2020;503:19–34.ArticlePubMed
- 35. Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet 2002;30:151–7.ArticlePubMedPDF
- 36. Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med 2010;363:2220–7.ArticlePubMedPMC
- 37. Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJ, Rubba P, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med 2020;383:711–20.ArticlePubMed
- 38. Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017;377:211–21.ArticlePubMedPMC
- 39. Reeskamp LF, Millar JS, Wu L, Jansen H, van Harskamp D, Schierbeek H, et al. ANGPTL3 inhibition with evinacumab results in faster clearance of IDL and LDL apoB in patients with homozygous familial hypercholesterolemia: brief report. Arterioscler Thromb Vasc Biol 2021;41:1753–9.ArticlePubMedPMC
- 40. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–32.ArticlePubMed
- 41. Bergmark BA, Marston NA, Bramson CR, Curto M, Ramos V, Jevne A, et al. Effect of vupanorsen on non-high-density lipoprotein cholesterol levels in statin-treated patients with elevated cholesterol: TRANSLATE-TIMI 70. Circulation 2022;145:1377–86.ArticlePubMedPMC
- 42. Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A 1997;94:4318–23.PubMedPMC
- 43. Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 1996;15:5336–48.ArticlePubMedPMCPDF
- 44. Lee Y, Kim BR, Kang GH, Lee GJ, Park YJ, Kim H, et al. The effects of PPAR agonists on atherosclerosis and nonalcoholic fatty liver disease in ApoE-/-FXR-/- Mice. Endocrinol Metab (Seoul) 2021;36:1243–53.ArticlePubMedPMCPDF
- 45. Hertz R, Bishara-Shieban J, Bar-Tana J. Mode of action of peroxisome proliferators as hypolipidemic drugs: suppression of apolipoprotein C-III. J Biol Chem 1995;270:13470–5.PubMed
- 46. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol 2015;62:720–33.ArticlePubMed
- 47. Staels B, Maes M, Zambon A. Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med 2008;5:542–53.PubMed
- 48. Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 1999;99:3125–31.ArticlePubMedPMC
- 49. Wang D, Liu B, Tao W, Hao Z, Liu M. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev 2015;2015:CD009580.ArticlePubMedPMC
- 50. Jakob T, Nordmann AJ, Schandelmaier S, Ferreira-Gonzalez I, Briel M. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev 2016;11:CD009753.ArticlePubMedPMC
- 51. Kim NH, Han KH, Choi J, Lee J, Kim SG. Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study. BMJ 2019;366:l5125.ArticlePubMedPMC
- 52. Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, et al. Effects of pemafibrate, a novel selective PPARα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2018;41:538–46.ArticlePubMedPDF
- 53. Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S, et al. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb 2018;25:521–38.ArticlePubMedPMC
- 54. Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J 2018;206:80–93.ArticlePubMed
- 55. Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr 2012;142:592S–9S.ArticlePubMed
- 56. Backes J, Anzalone D, Hilleman D, Catini J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis 2016;15:118.ArticlePubMedPMC
- 57. Khan S, Minihane AM, Talmud PJ, Wright JW, Murphy MC, Williams CM, et al. Dietary long-chain n-3 PUFAs increase LPL gene expression in adipose tissue of subjects with an atherogenic lipoprotein phenotype. J Lipid Res 2002;43:979–85.ArticlePubMed
- 58. Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res 2003;44:455–63.ArticlePubMed
- 59. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A 1997;94:4312–7.PubMedPMC
- 60. Kastelein JJ, Maki KC, Susekov A, Ezhov M, Nordestgaard BG, Machielse BN, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol 2014;8:94–106.ArticlePubMed
- 61. ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–50.ArticlePubMed
- 62. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32.ArticlePubMedPMC
- 63. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH Randomized Clinical Trial. JAMA 2020;324:2268–80.ArticlePubMedPMC
- 64. Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation 2021;143:528–39.ArticlePubMed
- 65. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22.ArticlePubMed
- 66. Doi T, Langsted A, Nordestgaard BG. A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: cohort study mimicking trial designs. Eur Heart J 2021;42:4807–17.ArticlePubMedPDF
- 67. Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine 2021;38:100997.ArticlePubMedPMC
- 68. Endocrinologic & Metabolic Drugs Advisory Committee. FDA Briefing Document: EMDAC Meeting for Volanesorsen (Waylivra). Silver Spring: Food and Drug Administration; 2018 [cited 2022 Aug 10]. Available from: https://pink.pharmaintelligence.informa.com/-/media/supporting-documents/pink-sheet/2018/05/waylivrafda_backgrounder.pdf?rev=b873388a6381495c9df19ee1e1895d3c&hash=E57C40AEF44E4C8839C9542AA533EE5B.
- 69. Gaudet D, Gonciarz M, Shen X. Late Breaker Session 1: A first in-human single ascending dose study of a monoclonal antibody against the ANGPTL3/8 complex in subjects with mixed hyperlipidaemia. In: In: 90th European Atherosclerosis Society Congress; 2022 May 22-25; Milan, IT.
- 70. Kim K, Ginsberg HN, Choi SH. New, novel lipid-lowering agents for reducing cardiovascular risk: beyond statins. Diabetes Metab J 2022;46:517–32.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations

- The chylomicron saga: time to focus on postprandial metabolism
Alejandro Gugliucci
Frontiers in Endocrinology.2024;[Epub] CrossRef - Sanghuangporus vaninii extract ameliorates hyperlipidemia in rats by mechanisms identified with transcriptome analysis
Ning Gao, Yuanzhen Liu, Guangjie Liu, Bo Liu, Yupeng Cheng
Food Science & Nutrition.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the obesity medicine association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Obesity Pillars.2024; : 100108. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Xanthohumol, a prenylated chalcone, regulates lipid metabolism by modulating the LXRα/RXR-ANGPTL3-LPL axis in hepatic cell lines and high-fat diet-fed zebrafish models
Wan-Yun Gao, Pei-Yi Chen, Hao-Jen Hsu, Je-Wen Liou, Chia-Ling Wu, Ming-Jiuan Wu, Jui-Hung Yen
Biomedicine & Pharmacotherapy.2024; 174: 116598. CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Journal of Clinical Lipidology.2024;[Epub] CrossRef - High producer variant of lipoprotein lipase may protect from hepatocellular carcinoma in alcohol-associated cirrhosis
Franziska Schmalz, Janett Fischer, Hamish Innes, Stephan Buch, Christine Möller, Madlen Matz-Soja, Witigo von Schönfels, Benjamin Krämer, Bettina Langhans, Alexandra Klüners, Michael Soyka, Felix Stickel, Jacob Nattermann, Christian P. Strassburg, Thomas
JHEP Reports.2023; 5(4): 100684. CrossRef - Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations
Dieter Lütjohann, Hans-Ulrich Klör, Frans Stellaard
Nutrients.2023; 15(9): 2202. CrossRef - Influence of antipsychotic medications on hyperlipidemia risk in patients with schizophrenia: evidence from a population-based cohort study and in vitro hepatic lipid homeostasis gene expression
Tien-Yuan Wu, Ni Tien, Cheng-Li Lin, Yu-Cun Cheah, Chung Y. Hsu, Fuu-Jen Tsai, Yi-Jen Fang, Yun-Ping Lim
Frontiers in Medicine.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Sugar and Dyslipidemia: A Double-Hit, Perfect Storm
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(17): 5660. CrossRef - Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
Sang Heon Suh, Soo Wan Kim
Diabetes & Metabolism Journal.2023; 47(5): 612. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Developing a model to predict the early risk of hypertriglyceridemia based on inhibiting lipoprotein lipase (LPL): a translational study
Julia Hernandez-Baixauli, Gertruda Chomiciute, Juan María Alcaide-Hidalgo, Anna Crescenti, Laura Baselga-Escudero, Hector Palacios-Jordan, Elisabet Foguet-Romero, Anna Pedret, Rosa M. Valls, Rosa Solà, Miquel Mulero, Josep M. Del Bas
Scientific Reports.2023;[Epub] CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite