Search
- Page Path
- HOME > Search
Review Articles
- Diabetes, obesity and metabolism
- Glucagon-Like Peptide-1 Based Therapies: A New Horizon in Obesity Management
- Jang Won Son, Soo Lim
- Endocrinol Metab. 2024;39(2):206-221. Published online April 16, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1940
- 1,423 View
- 112 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
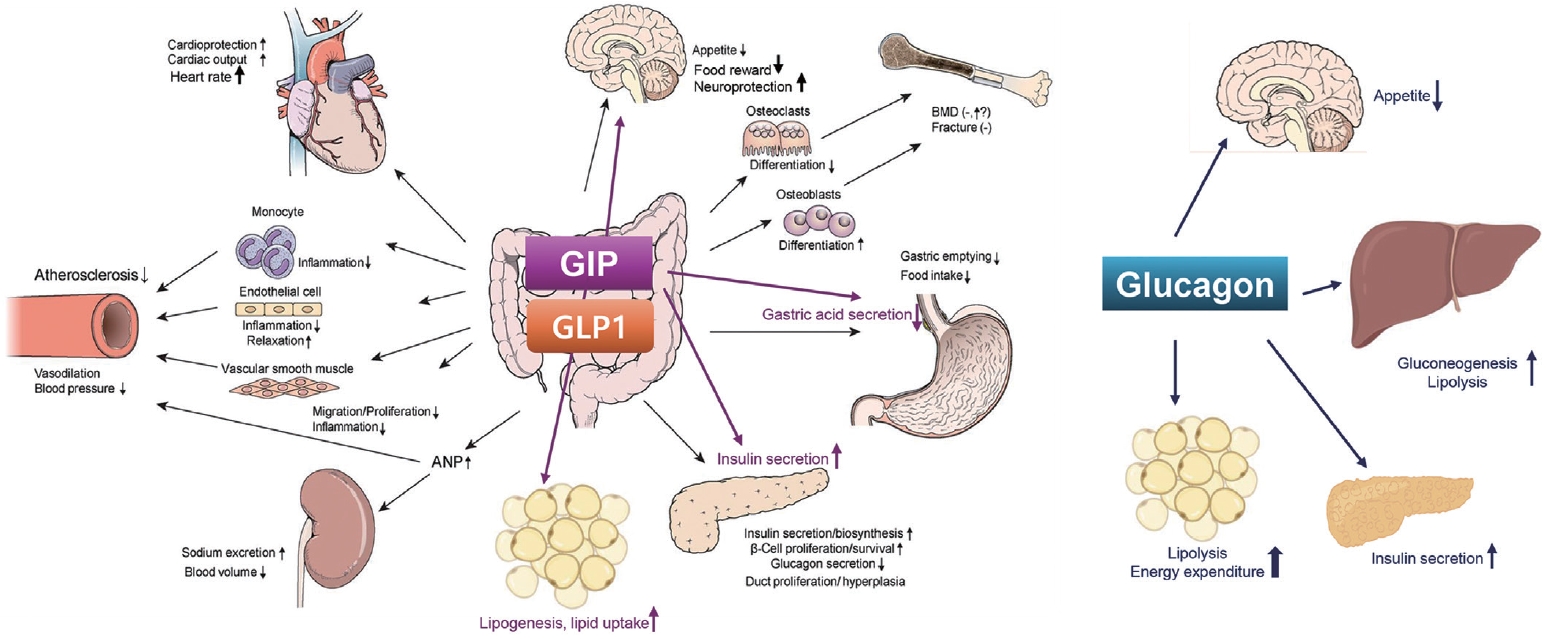
ePub - Obesity is a significant risk factor for health issues like type 2 diabetes and cardiovascular disease. It often proves resistant to traditional lifestyle interventions, prompting a need for more precise therapeutic strategies. This has led to a focus on signaling pathways and neuroendocrine mechanisms to develop targeted obesity treatments. Recent developments in obesity management have been revolutionized by introducing novel glucagon-like peptide-1 (GLP-1) based drugs, such as semaglutide and tirzepatide. These drugs are part of an emerging class of nutrient-stimulated hormone-based therapeutics, acting as incretin mimetics to target G-protein–coupled receptors like GLP-1, glucose-dependent insulinotropic polypeptide (GIP), and glucagon. These receptors are vital in regulating body fat and energy balance. The development of multiagonists, including GLP-1–glucagon and GIP–GLP-1–glucagon receptor agonists, especially with the potential for glucagon receptor activation, marks a significant advancement in the field. This review covers the development and clinical efficacy of various GLP-1-based therapeutics, exploring the challenges and future directions in obesity management.

- Diabetes, obesity and metabolism
- The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor - An Update
- Agnieszka Jakubowska, Carel W. le Roux, Adie Viljoen
- Endocrinol Metab. 2024;39(1):12-22. Published online February 14, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1942
- 2,816 View
- 219 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
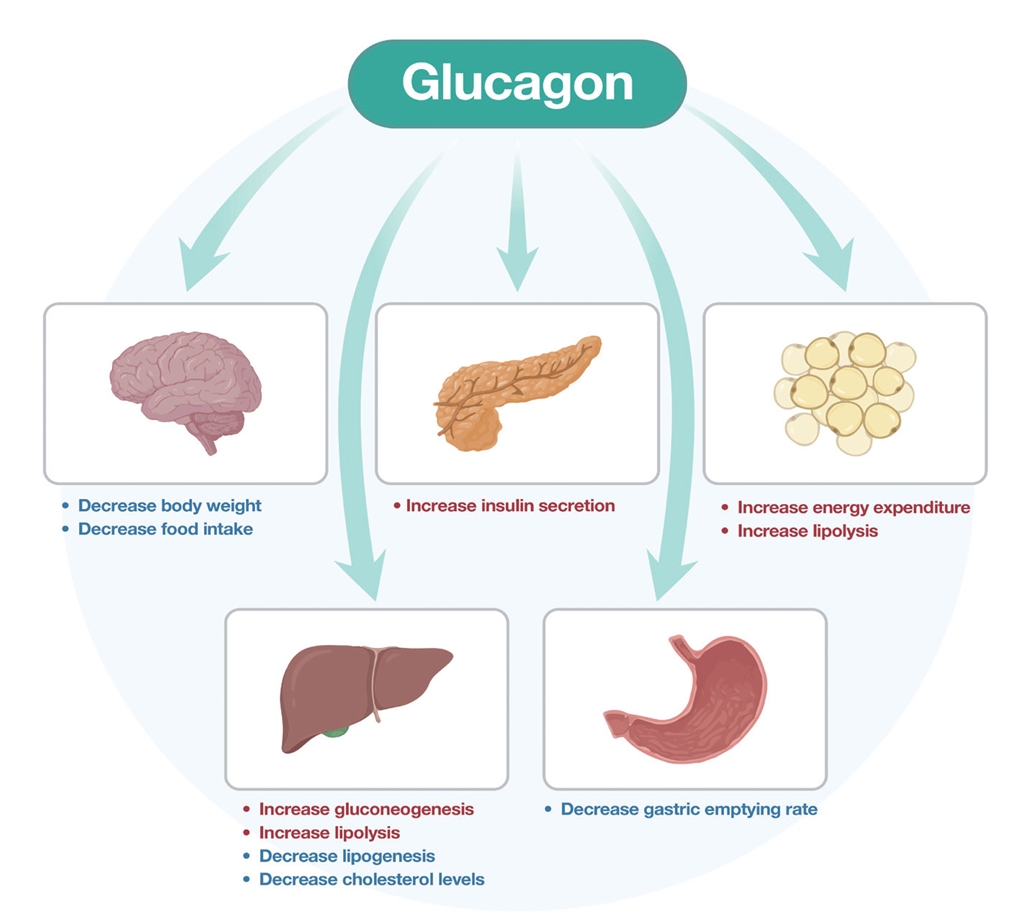
ePub - Obesity is the fifth leading risk factor for global deaths with numbers continuing to increase worldwide. In the last 20 years, the emergence of pharmacological treatments for obesity based on gastrointestinal hormones has transformed the therapeutic landscape. The successful development of glucagon-like peptide-1 (GLP-1) receptor agonists, followed by the synergistic combined effect of glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists achieved remarkable weight loss and glycemic control in those with the diseases of obesity and type 2 diabetes. The multiple cardiometabolic benefits include improving glycemic control, lipid profiles, blood pressure, inflammation, and hepatic steatosis. The 2023 phase 2 double-blind, randomized controlled trial evaluating a GLP-1/GIP/glucagon receptor triagonist (retatrutide) in patients with the disease of obesity reported 24.2% weight loss at 48 weeks with 12 mg retatrutide. This review evaluates the current available evidence for GLP-1 receptor agonists, dual GLP-1/GIP receptor co-agonists with a focus on GLP-1/GIP/glucagon receptor triagonists and discusses the potential future benefits and research directions.
-
Citations
Citations to this article as recorded by- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review
Jorge E. Jalil, Luigi Gabrielli, María Paz Ocaranza, Paul MacNab, Rodrigo Fernández, Bruno Grassi, Paulina Jofré, Hugo Verdejo, Monica Acevedo, Samuel Cordova, Luis Sanhueza, Douglas Greig
International Journal of Molecular Sciences.2024; 25(8): 4407. CrossRef
- New Mechanisms to Prevent Heart Failure with Preserved Ejection Fraction Using Glucagon-like Peptide-1 Receptor Agonism (GLP-1 RA) in Metabolic Syndrome and in Type 2 Diabetes: A Review

- Miscellaneous
- Toward Systems-Level Metabolic Analysis in Endocrine Disorders and Cancer
- Aliya Lakhani, Da Hyun Kang, Yea Eun Kang, Junyoung O. Park
- Endocrinol Metab. 2023;38(6):619-630. Published online November 21, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1814
- 2,553 View
- 111 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
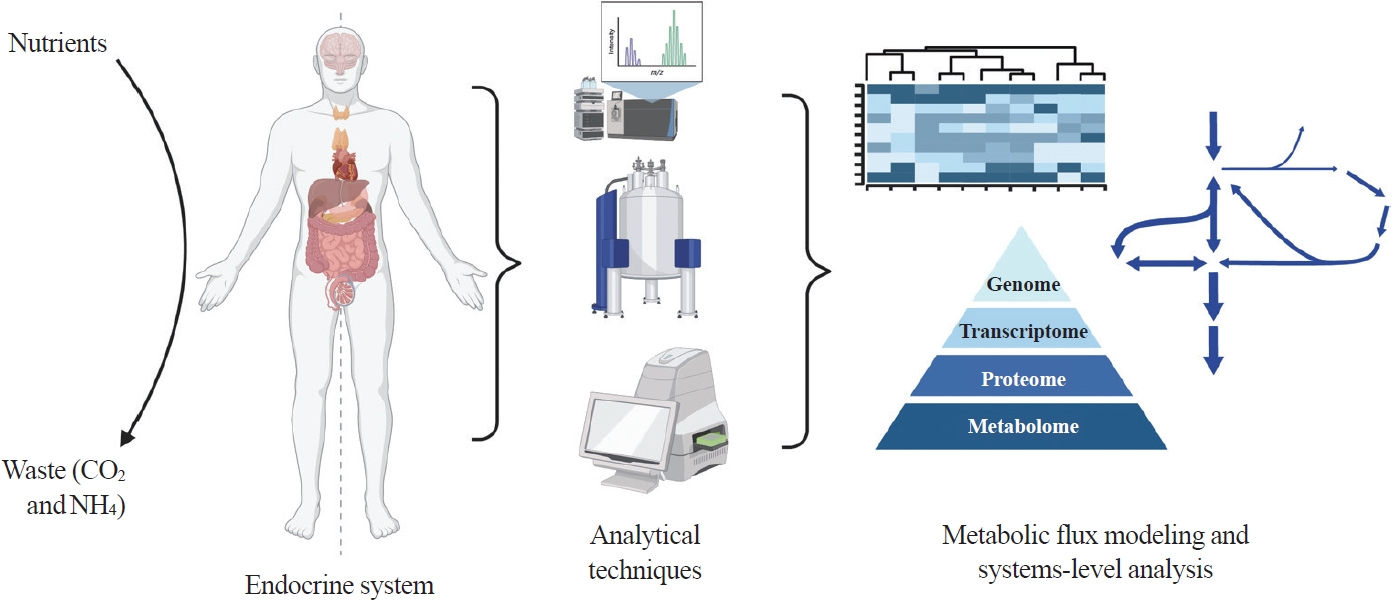
ePub - Metabolism is a dynamic network of biochemical reactions that support systemic homeostasis amidst changing nutritional, environmental, and physical activity factors. The circulatory system facilitates metabolite exchange among organs, while the endocrine system finely tunes metabolism through hormone release. Endocrine disorders like obesity, diabetes, and Cushing’s syndrome disrupt this balance, contributing to systemic inflammation and global health burdens. They accompany metabolic changes on multiple levels from molecular interactions to individual organs to the whole body. Understanding how metabolic fluxes relate to endocrine disorders illuminates the underlying dysregulation. Cancer is increasingly considered a systemic disorder because it not only affects cells in localized tumors but also the whole body, especially in metastasis. In tumorigenesis, cancer-specific mutations and nutrient availability in the tumor microenvironment reprogram cellular metabolism to meet increased energy and biosynthesis needs. Cancer cachexia results in metabolic changes to other organs like muscle, adipose tissue, and liver. This review explores the interplay between the endocrine system and systems-level metabolism in health and disease. We highlight metabolic fluxes in conditions like obesity, diabetes, Cushing’s syndrome, and cancers. Recent advances in metabolomics, fluxomics, and systems biology promise new insights into dynamic metabolism, offering potential biomarkers, therapeutic targets, and personalized medicine.
-
Citations
Citations to this article as recorded by- Editorial: Tumor metabolism and programmed cell death
Dan-Lan Pu, Qi-Nan Wu
Frontiers in Endocrinology.2024;[Epub] CrossRef
- Editorial: Tumor metabolism and programmed cell death

- Diabetes, obesity and metabolism
- The Impact of Taurine on Obesity-Induced Diabetes Mellitus: Mechanisms Underlying Its Effect
- Kainat Ahmed, Ha-Neul Choi, Jung-Eun Yim
- Endocrinol Metab. 2023;38(5):482-492. Published online October 17, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1776
- 2,935 View
- 163 Download
- 2 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
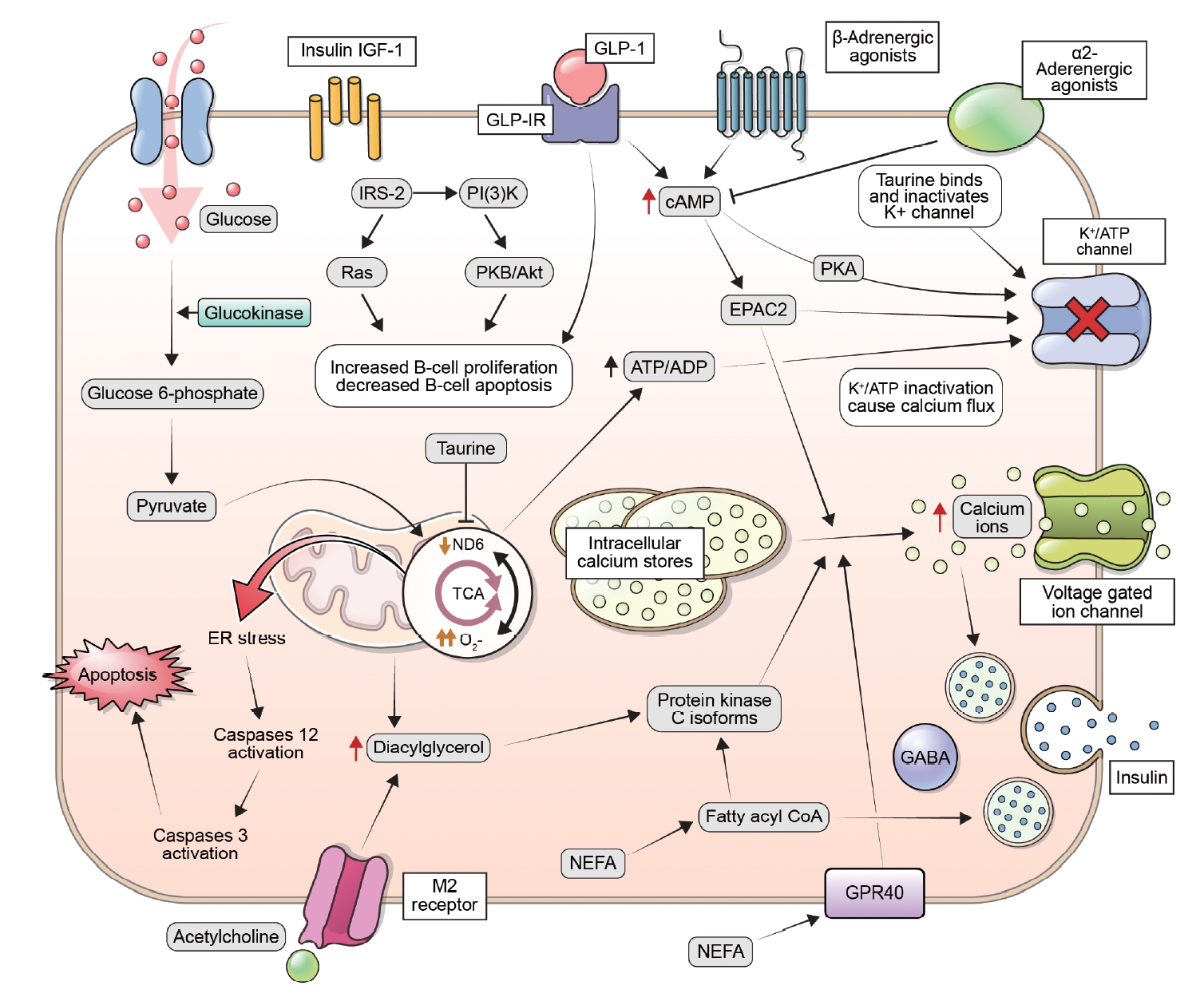
ePub - This review explores the potential benefits of taurine in ameliorating the metabolic disorders of obesity and type 2 diabetes (T2D), highlighting the factors that bridge these associations. Relevant articles and studies were reviewed to conduct a comprehensive analysis of the relationship between obesity and the development of T2D and the effect of taurine on those conditions. The loss of normal β-cell function and development of T2D are associated with obesity-derived insulin resistance. The occurrence of diabetes has been linked to the low bioavailability of taurine, which plays critical roles in normal β-cell function, anti-oxidation, and anti-inflammation. The relationships among obesity, insulin resistance, β-cell dysfunction, and T2D are complex and intertwined. Taurine may play a role in ameliorating these metabolic disorders through different pathways, but further research is needed to fully understand its effects and potential as a therapeutic intervention.
-
Citations
Citations to this article as recorded by- Effect of a Very Low-Calorie Diet on Oxidative Stress, Inflammatory and Metabolomic Profile in Metabolically Healthy and Unhealthy Obese Subjects
Neus Bosch-Sierra, Carmen Grau-del Valle, Christian Salom, Begoña Zaragoza-Villena, Laura Perea-Galera, Rosa Falcón-Tapiador, Susana Rovira-Llopis, Carlos Morillas, Daniel Monleón, Celia Bañuls
Antioxidants.2024; 13(3): 302. CrossRef
- Effect of a Very Low-Calorie Diet on Oxidative Stress, Inflammatory and Metabolomic Profile in Metabolically Healthy and Unhealthy Obese Subjects

Original Article
- Diabetes, obesity and metabolism
- Protective Effects of Melatonin in High-Fat Diet-Induced Hepatic Steatosis via Decreased Intestinal Lipid Absorption and Hepatic Cholesterol Synthesis
- Hyungjune Ku, Yeonji Kim, Alvin Lyle Kim, Garam Lee, Youngsik Choi, Bukyung Kim
- Endocrinol Metab. 2023;38(5):557-567. Published online September 1, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1672
- 2,056 View
- 96 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
The preventative effect of melatonin on the development of obesity and the progression of fatty liver under a high-fat diet (HFD) has been well elucidated through previous studies. We investigated the mechanism behind this effect regarding cholesterol biosynthesis and regulation of cholesterol levels.
Methods
Mice were divided into three groups: normal chow diet (NCD); HFD; and HFD and melatonin administration group (HFD+M). We assessed the serum lipid profile, mRNA expression levels of proteins involved in cholesterol synthesis and reabsorption in the liver and nutrient transporters in the intestines, and cytokine levels. Additionally, an in vitro experiment using HepG2 cells was performed.
Results
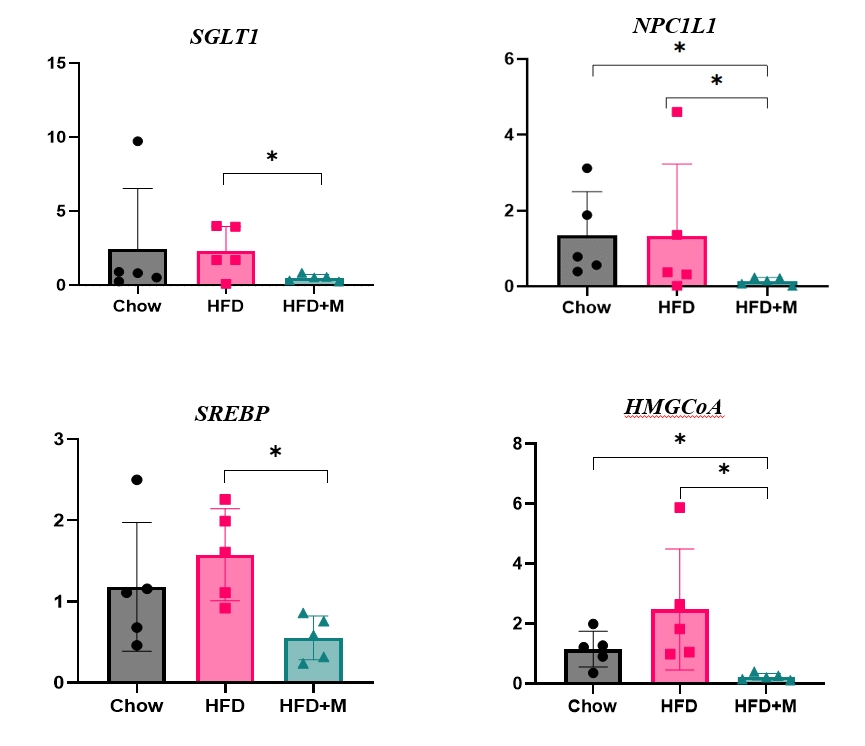
Expression of hepatic sterol regulatory element-binding protein 2 (SREBP-2), 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), and low-density lipoprotein receptor (LDLR) demonstrated that melatonin administration significantly reduces hepatic cholesterol synthesis in mice fed an HFD. Expression of intestinal sodium-glucose transporter 1 (SGLT1), glucose transporter 2 (GLUT2), GLUT5, and Niemann-pick C1-like 1 (NPC1L1) demonstrated that melatonin administration significantly reduces intestinal carbohydrate and lipid absorption in mice fed an HFD. There were no differences in local and circulatory inflammatory cytokine levels among the NCD, HFD, and HFD+M group. HepG2 cells stimulated with palmitate showed reduced levels of SREBP, LDLR, and HMGCR indicating these results are due to the direct mechanistic effect of melatonin on hepatocytes.
Conclusion
Collectively, these data indicate the mechanism behind the protective effects of melatonin from weight gain and liver steatosis under HFD is through a reduction in intestinal caloric absorption and hepatic cholesterol synthesis highlighting its potential in the treatment of obesity and fatty liver disease. -
Citations
Citations to this article as recorded by- Influence of dark deprivation on the ultrastructure and mitochondrial apparatus of rat hepatocytes
D Areshidze
Morphology.2024;[Epub] CrossRef
- Influence of dark deprivation on the ultrastructure and mitochondrial apparatus of rat hepatocytes

Review Article
- Diabetes, Obesity and Metabolism
Big Data Articles (National Health Insurance Service Database) - Big Data Research in the Field of Endocrine Diseases Using the Korean National Health Information Database
- Sun Wook Cho, Jung Hee Kim, Han Seok Choi, Hwa Young Ahn, Mee Kyoung Kim, Eun Jung Rhee
- Endocrinol Metab. 2023;38(1):10-24. Published online February 9, 2023
- DOI: https://doi.org/10.3803/EnM.2023.102
- 3,843 View
- 263 Download
- 16 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - The Korean National Health Information Database (NHID) contains big data combining information obtained from the National Health Insurance Service and health examinations. Data are provided in the form of a cohort, and the NHID can be used to conduct longitudinal studies and research on rare diseases. Moreover, data on the cause and date of death are provided by Statistics Korea. Research and publications based on the NHID have increased explosively in the field of endocrine disorders. However, because the data were not collected for research purposes, studies using the NHID have limitations, particularly the need for the operational definition of diseases. In this review, we describe the characteristics of the Korean NHID, operational definitions of endocrine diseases used for research, and an overview of recent studies in endocrinology using the Korean NHID.
-
Citations
Citations to this article as recorded by- Associations Between Physical Activity and the Risk of Hip Fracture Depending on Glycemic Status: A Nationwide Cohort Study
Kyoung Min Kim, Kyoung Jin Kim, Kyungdo Han, Yumie Rhee
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1194. CrossRef - Weight change in patients with new‐onset type 2 diabetes mellitus and its association with remission: Comprehensive real‐world data
Jinyoung Kim, Bongseong Kim, Mee Kyoung Kim, Ki‐Hyun Baek, Ki‐Ho Song, Kyungdo Han, Hyuk‐Sang Kwon
Diabetes, Obesity and Metabolism.2024; 26(2): 567. CrossRef - Diabetes severity and the risk of depression: A nationwide population-based study
Yunjung Cho, Bongsung Kim, Hyuk-Sang Kwon, Kyungdo Han, Mee Kyoung Kim
Journal of Affective Disorders.2024; 351: 694. CrossRef - Information Bias Might Exaggerate Lung Cancer Risk of Patients With Rheumatoid Arthritis
Nobuyuki Horita, Kaoru Takase-Minegishi
Journal of Thoracic Oncology.2024; 19(2): 348. CrossRef - Diabetes Duration, Cholesterol Levels, and Risk of Cardiovascular Diseases in Individuals With Type 2 Diabetes
Mee Kyoung Kim, Kyu Na Lee, Kyungdo Han, Seung-Hwan Lee
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Risk of fracture in patients with myasthenia gravis: a nationwide cohort study in Korea
Hye-Sun Park, Kyoungsu Kim, Min Heui Yu, Ha Young Shin, Yumie Rhee, Seung Woo Kim, Namki Hong
Journal of Bone and Mineral Research.2024;[Epub] CrossRef - Diabetes severity is strongly associated with the risk of active tuberculosis in people with type 2 diabetes: a nationwide cohort study with a 6-year follow-up
Ji Young Kang, Kyungdo Han, Seung-Hwan Lee, Mee Kyoung Kim
Respiratory Research.2023;[Epub] CrossRef - Research on obesity using the National Health Information Database: recent trends
Eun-Jung Rhee
Cardiovascular Prevention and Pharmacotherapy.2023; 5(2): 35. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef - Risk of Pancreatic Cancer and Use of Dipeptidyl Peptidase 4 Inhibitors in Patients with Type 2 Diabetes: A Propensity Score-Matching Analysis
Mee Kyoung Kim, Kyungdo Han, Hyuk-Sang Kwon, Soon Jib Yoo
Endocrinology and Metabolism.2023; 38(4): 426. CrossRef - Prevalence, Treatment Status, and Comorbidities of Hyperthyroidism in Korea from 2003 to 2018: A Nationwide Population Study
Hwa Young Ahn, Sun Wook Cho, Mi Young Lee, Young Joo Park, Bon Seok Koo, Hang-Seok Chang, Ka Hee Yi
Endocrinology and Metabolism.2023; 38(4): 436. CrossRef - Is Thyroid Dysfunction Associated with Unruptured Intracranial Aneurysms? A Population-Based, Nested Case–Control Study from Korea
Hyeree Park, Sun Wook Cho, Sung Ho Lee, Kangmin Kim, Hyun-Seung Kang, Jeong Eun Kim, Aesun Shin, Won-Sang Cho
Thyroid®.2023; 33(12): 1483. CrossRef - Risk of Cause-Specific Mortality across Glucose Spectrum in Elderly People: A Nationwide Population-Based Cohort Study
Joonyub Lee, Hun-Sung Kim, Kee-Ho Song, Soon Jib Yoo, Kyungdo Han, Seung-Hwan Lee
Endocrinology and Metabolism.2023; 38(5): 525. CrossRef - Risk of depression in patients with acromegaly in Korea (2006-2016): a nationwide population-based study
Shinje Moon, Sangmo Hong, Kyungdo Han, Cheol-Young Park
European Journal of Endocrinology.2023; 189(3): 363. CrossRef - Cumulative effect of impaired fasting glucose on the risk of dementia in middle-aged and elderly people: a nationwide cohort study
Jin Yu, Kyu-Na Lee, Hun-Sung Kim, Kyungdo Han, Seung-Hwan Lee
Scientific Reports.2023;[Epub] CrossRef - Long-Term Cumulative Exposure to High γ-Glutamyl Transferase Levels and the Risk of Cardiovascular Disease: A Nationwide Population-Based Cohort Study
Han-Sang Baek, Bongseong Kim, Seung-Hwan Lee, Dong-Jun Lim, Hyuk-Sang Kwon, Sang-Ah Chang, Kyungdo Han, Jae-Seung Yun
Endocrinology and Metabolism.2023; 38(6): 770. CrossRef - Increased Risk of Hip Fracture in Patients with Acromegaly: A Nationwide Cohort Study in Korea
Jiwon Kim, Namki Hong, Jimi Choi, Ju Hyung Moon, Eui Hyun Kim, Eun Jig Lee, Sin Gon Kim, Cheol Ryong Ku
Endocrinology and Metabolism.2023; 38(6): 690. CrossRef
- Associations Between Physical Activity and the Risk of Hip Fracture Depending on Glycemic Status: A Nationwide Cohort Study

Original Articles
- Diabetes, Obesity and Metabolism
- Sleep Duration and the Risk of Type 2 Diabetes: A Community-Based Cohort Study with a 16-Year Follow-up
- Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Seung Ku Lee, Chol Shin, Nan Hee Kim
- Endocrinol Metab. 2023;38(1):146-155. Published online February 6, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1582
- 2,684 View
- 166 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
We aimed to investigate the moderating effects of obesity, age, and sex on the association between sleep duration and the development of diabetes in Asians.
Methods
We analyzed data from a cohort of the Korean Genome and Epidemiology Study conducted from 2001 to 2020. After excluding shift workers and those with diabetes at baseline, 7,407 participants were stratified into three groups according to sleep duration: ≤5 hours/night, >5 to 7 hours/night (reference), and >7 hours/night. The Cox proportional hazards analyses were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for incident type 2 diabetes mellitus (T2DM). Subgroup analyses were performed according to obesity, age, and sex.
Results
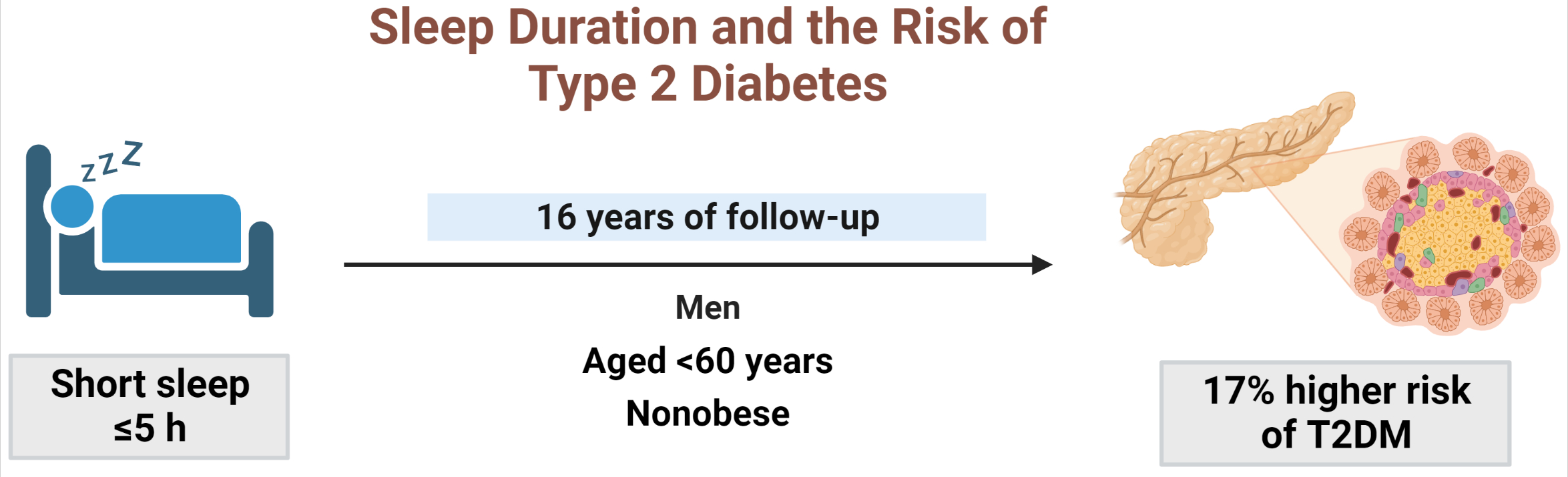
During 16 years of follow-up, 2,024 cases of T2DM were identified. Individuals who slept ≤5 h/night had a higher risk of incident diabetes than the reference group (HR, 1.17; 95% CI, 1.02 to 1.33). The subgroup analysis observed a valid interaction with sleep duration only for obesity. A higher risk of T2DM was observed in the ≤5 hours/night group in non-obese individuals, men, and those aged <60 years, and in the >7 hours/night group in obese individuals (HRs were 1.34 [95% CI, 1.11 to 1.61], 1.22 [95% CI, 1 to 1.49], and 1.18 [95% CI, 1.01 to 1.39], respectively).
Conclusion
This study confirmed the effect of sleep deprivation on the risk of T2DM throughout the 16-year follow-up period. This impact was confined to non-obese or young individuals and men. We observed a significant interaction between sleep duration and obesity. -
Citations
Citations to this article as recorded by- Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
Diabetes & Metabolism Journal.2024; 48(1): 37. CrossRef - Role of Sleep and Sleep Disorders in Cardiometabolic Risk: a Review and Update
Shaden O. Qasrawi, Ahmed S. BaHammam
Current Sleep Medicine Reports.2024; 10(1): 34. CrossRef - Evaluating reliability in wearable devices for sleep staging
Vera Birrer, Mohamed Elgendi, Olivier Lambercy, Carlo Menon
npj Digital Medicine.2024;[Epub] CrossRef - All That Glitters Is Not Gold: The Same Sleep Time, but Different Diabetogenic Outcomes
Bohye Kim, Obin Kwon
Endocrinology and Metabolism.2023; 38(1): 78. CrossRef - The Link Between Sleeping and Type 2 Diabetes: A Systematic Review
Ali Darraj
Cureus.2023;[Epub] CrossRef
- Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes

- Diabetes, Obesity and Metabolism
- Association of Shift Work with Normal-Weight Obesity in Community-Dwelling Adults
- Chul Woo Ahn, Sungjae Shin, Seunghyun Lee, Hye-Sun Park, Namki Hong, Yumie Rhee
- Endocrinol Metab. 2022;37(5):781-790. Published online October 25, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1532
- 3,376 View
- 190 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Shift work is associated with obesity and metabolic syndrome. However, this association in the normal-weight population remains unclear. This study aimed to investigate whether shift work is associated with normal-weight obesity (NWO).
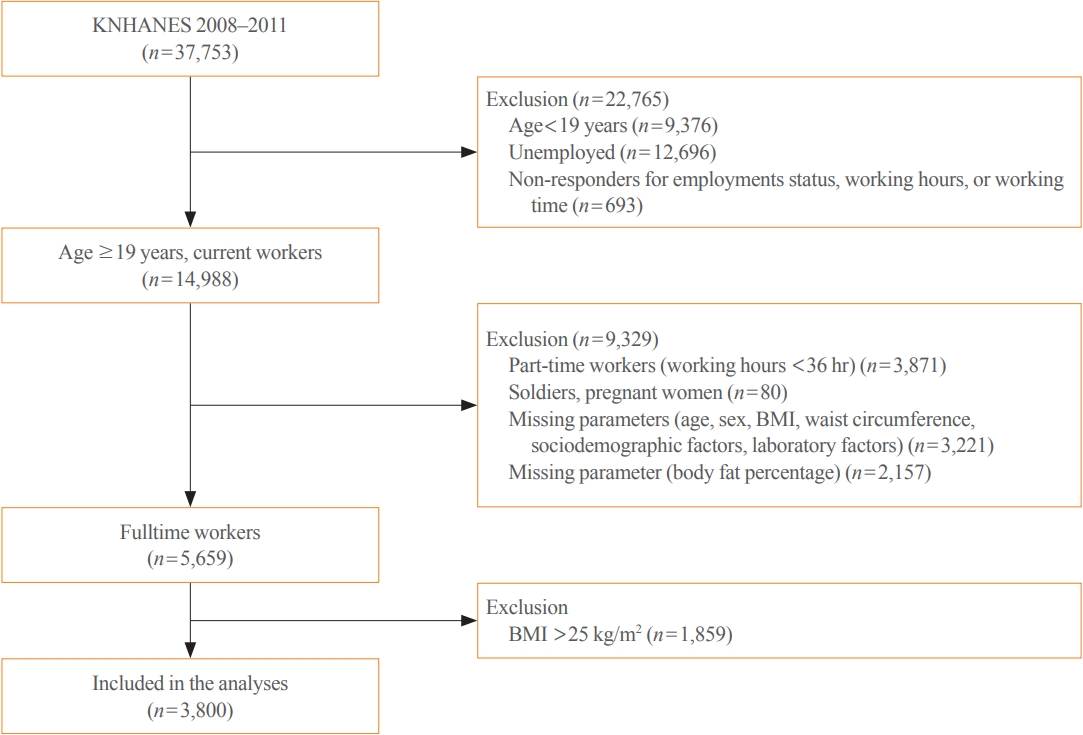
Methods
From the nationally representative Korea National Health and Nutrition Examination Survey (KNHANES) dataset (2008 to 2011), 3,800 full-time workers aged ≥19 years with a body mass index (BMI) ≤25 kg/m2 were analysed. We defined NWO as BMI ≤25 kg/m2 and body fat percentage ≥25% in men and ≥37% in women. Working patterns were classified into “daytime,” “other than daytime,” and “shift.” Multivariable logistic regression analysis was performed to evaluate the relationship between shift work and NWO.
Results
Shift work was associated with higher odds of NWO than daytime work (adjusted odds ratio [aOR], 1.47; 95% confidence interval [CI], 1.04 to 2.09) and night/evening work (aOR, 1.87; 95% CI, 1.11 to 3.14) after adjustment for type of work, working hours, age, sex, BMI, 25-hydroxyvitamin D levels, homeostatic model assessment for insulin resistance, and other sociodemographic factors. In subgroup analyses, the association between shift work and NWO was more robust in those aged ≥60 years and those working ≥56 hours/week.
Conclusion
Shift work was associated with NWO in community-dwelling Korean adults, independent of age, sex, BMI, and other covariates. -
Citations
Citations to this article as recorded by- Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work
Sorina Hohor, Cristina Mandanach, Andreea Maftei, Corina Aurelia Zugravu, Marina Ruxandra Oțelea
Antioxidants.2023; 12(4): 959. CrossRef - Normal-Weight Obesity and Metabolic Syndrome in Korean Adults: A Population-Based Cross-Sectional Study
Jeonghyeon Kim, Seamon Kang, Hyunsik Kang
Healthcare.2023; 11(16): 2303. CrossRef - You Can’t Avoid Shift Work? Then Focus on Body Fat Rather than Weight
Eun Kyung Lee
Endocrinology and Metabolism.2022; 37(5): 756. CrossRef
- Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work

- Diabetes, Obesity and Metabolism
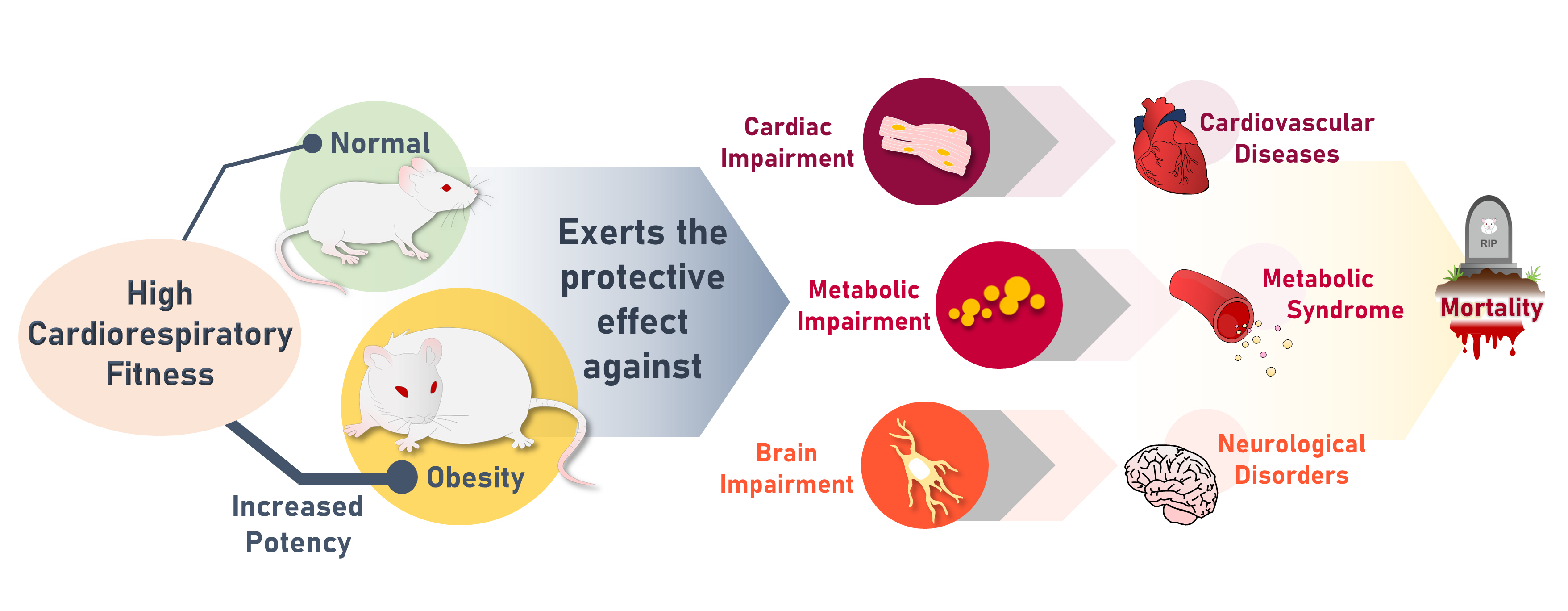
- High Cardiorespiratory Fitness Protects against Molecular Impairments of Metabolism, Heart, and Brain with Higher Efficacy in Obesity-Induced Premature Aging
- Patcharapong Pantiya, Chanisa Thonusin, Natticha Sumneang, Benjamin Ongnok, Titikorn Chunchai, Sasiwan Kerdphoo, Thidarat Jaiwongkam, Busarin Arunsak, Natthaphat Siri-Angkul, Sirawit Sriwichaiin, Nipon Chattipakorn, Siriporn C. Chattipakorn
- Endocrinol Metab. 2022;37(4):630-640. Published online August 5, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1430
- 4,161 View
- 123 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
High cardiorespiratory fitness (CRF) protects against age-related diseases. However, the mechanisms mediating the protective effect of high intrinsic CRF against metabolic, cardiac, and brain impairments in non-obese versus obese conditions remain incompletely understood. We aimed to identify the mechanisms through which high intrinsic CRF protects against metabolic, cardiac, and brain impairments in non-obese versus obese untrained rats.
Methods
Seven-week-old male Wistar rats were divided into two groups (n=8 per group) to receive either a normal diet or a highfat diet (HFD). At weeks 12 and 28, CRF, carbohydrate and fatty acid oxidation, cardiac function, and metabolic parameters were evaluated. At week 28, behavior tests were performed. At the end of week 28, rats were euthanized to collect heart and brain samples for molecular studies.
Results
The obese rats exhibited higher values for aging-related parameters than the non-obese rats, indicating that they experienced obesity-induced premature aging. High baseline CRF levels were positively correlated with several favorable metabolic, cardiac, and brain parameters at follow-up. Specifically, the protective effects of high CRF against metabolic, cardiac, and brain impairments were mediated by the modulation of body weight and composition, the lipid profile, substrate oxidation, mitochondrial function, insulin signaling, autophagy, apoptosis, inflammation, oxidative stress, cardiac function, neurogenesis, blood-brain barrier, synaptic function, accumulation of Alzheimer’s disease-related proteins, and cognition. Interestingly, this effect was more obvious in HFD-fed rats.
Conclusion
The protective effect of high CRF is mediated by the modulation of several mechanisms. These effects exhibit greater efficacy under conditions of obesity-induced premature aging. -
Citations
Citations to this article as recorded by- Associations that Cardiorespiratory Fitness and Body Mass Index Loss Have with Deficit Accumulation Frailty
KAYLONI OLSON, DENISE K. HOUSTON, JOHNATHAN ROSS, RENA R. WING, FELICIA R. SIMPSON, AMBARISH PANDEY, MICHAEL P. WALKUP, MIA YANG, MARK A. ESPELAND
Medicine & Science in Sports & Exercise.2024; 56(4): 717. CrossRef - Interplay between obesity and aging on myocardial geometry and function: Role of leptin-STAT3-stress signaling
Wei Jin, Fei Tu, Feng Dong, Qinqin Deng, Miyesaier Abudureyimu, Wei Yu, Guo-jun Cai, Jian-ming Pei, Zhaohui Pei, Jun Ren
Biochimica et Biophysica Acta (BBA) - General Subjects.2023; 1867(2): 130281. CrossRef - Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings
Jaime A. Gallo-Villegas, Juan C. Calderón
European Journal of Applied Physiology.2023; 123(5): 945. CrossRef
- Associations that Cardiorespiratory Fitness and Body Mass Index Loss Have with Deficit Accumulation Frailty

- Diabetes, Obesity and Metabolism
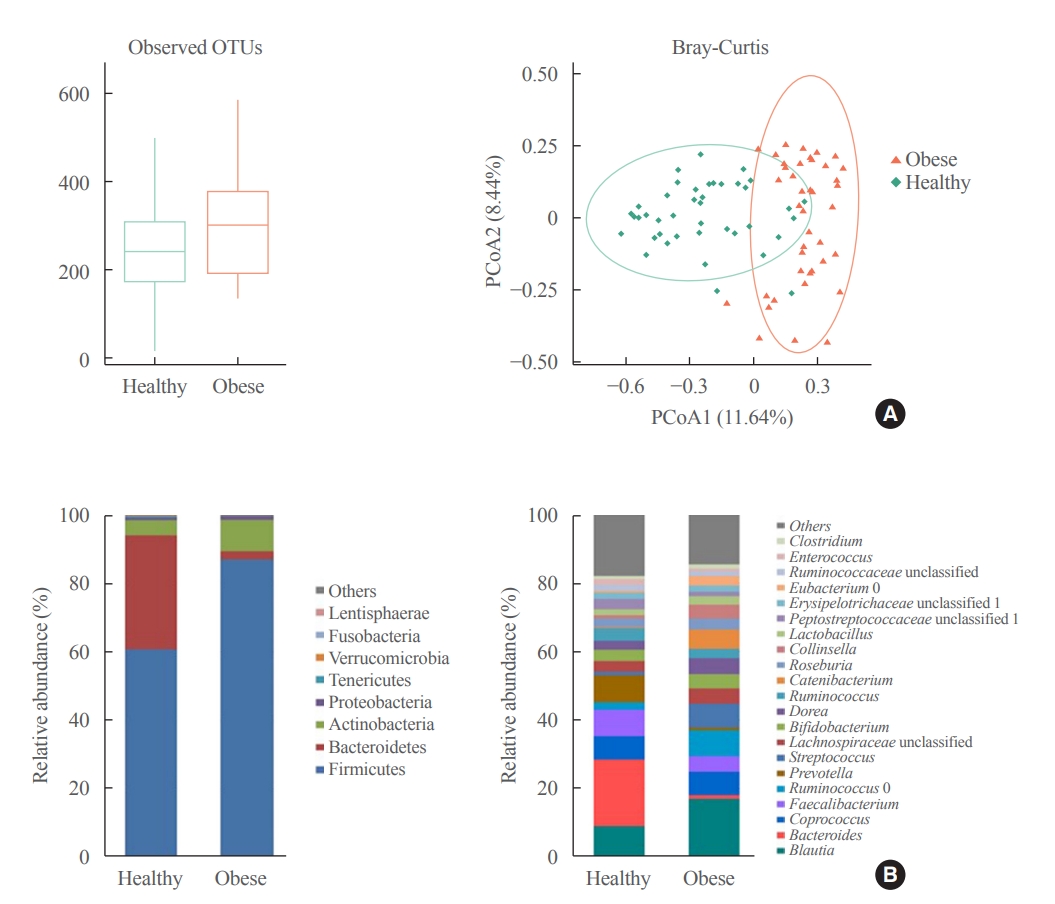
- Association between the Blautia/Bacteroides Ratio and Altered Body Mass Index after Bariatric Surgery
- Yoonhong Kim, Dooheon Son, Bu Kyung Kim, Ki Hyun Kim, Kyung Won Seo, Kyoungwon Jung, Seun Ja Park, Sanghyun Lim, Jae Hyun Kim
- Endocrinol Metab. 2022;37(3):475-486. Published online June 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1481
- Correction in: Endocrinol Metab 2022;37(4):701
- 3,194 View
- 125 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Current evidence support that the gut microbiota plays a potential role in obesity. Bariatric surgery can reduce excess weight and decrease the risk of life-threatening weight-related health problems and may also influence gut microbiota. In this study, we aimed to investigate the changes in gut microbiota before and after bariatric surgery and evaluate the association of the gut microbial shift and altered body mass index (BMI) after bariatric surgery.
Methods
Between January 2019 and July 2020, stools from 58 patients scheduled for bariatric surgery were collected. Six months after bariatric surgery, stools from 22 of these patients were re-collected, and the changes in gut microbiota before and after bariatric surgery were evaluated. In addition, the differences in gut microbiota between patients with severe obesity (BMI >35 kg/m2, n=42) and healthy volunteers with normal BMI (18.8 to 22.8 kg/m2, n=41) were investigated.
Results
The gut microbiota of patients who underwent bariatric surgery showed increased α-diversity and differed β-diversity compared with those before surgery. Interestingly, Blautia was decreased and Bacteriodes was increased at the genus level after bariatric surgery. Further, the Blautia/Bacteroides ratio showed a positive correlation with BMI. To validate these results, we compared the gut microbiota from severely obese patients with high BMI with those from healthy volunteers and demonstrated that the Blautia/Bacteroides ratio correlated positively with BMI.
Conclusion
In the gut microbial analysis of patients who underwent bariatric surgery, we presented that the Blautia/Bacteroides ratio had changed after bariatric surgery and showed a positive correlation with BMI. -
Citations
Citations to this article as recorded by- Modulation of the gut microbiome and Firmicutes phylum reduction by a nutraceutical blend in the obesity mouse model and overweight humans: A double‐blind clinical trial
Victor Nehmi‐Filho, Jessica Alves de Freitas, Lucas Augusto Franco, Roberta Cristina Martins, José Antônio Orellana Turri, Aline Boveto Santamarina, Joyce Vanessa da Silva Fonseca, Ester Cerdeira Sabino, Bruna Carvalho Moraes, Erica Souza, Gilson Masahiro
Food Science & Nutrition.2024; 12(4): 2436. CrossRef - Natural emulsifiers lecithins preserve gut microbiota diversity in relation with specific faecal lipids in high fat-fed mice
Chloé Robert, Armelle Penhoat, Leslie Couëdelo, Magali Monnoye, Dominique Rainteau, Emmanuelle Meugnier, Sofia Bary, Hélène Abrous, Emmanuelle Loizon, Pranvera Krasniqi, Stéphanie Chanon, Aurélie Vieille-Marchiset, François Caillet, Sabine Danthine, Huber
Journal of Functional Foods.2023; 105: 105540. CrossRef - Effects and action mechanisms of lotus leaf (Nelumbo nucifera) ethanol extract on gut microbes and obesity in high-fat diet-fed rats
Zhang Yanan, Ma Lu, Zhang Lu, Huo Jinhai, Wang Weiming
Frontiers in Nutrition.2023;[Epub] CrossRef - First characterization of the intestinal microbiota in healthy Tunisian adults using 16S rRNA gene sequencing
Ahlem Mahjoub Khachroub, Magali Monnoye, Nour Elhouda Bouhlel, Sana Azaiez, Maha Ben Fredj, Wejdene Mansour, Philippe Gérard
FEMS Microbiology Letters.2023;[Epub] CrossRef - Gut microbiota and nonalcoholic fatty liver disease
Boyeon Kim, Bukyung Kim
Kosin Medical Journal.2023; 38(3): 169. CrossRef - Obésité et risque cardiovasculaire : le rôle de la chirurgie bariatrique dans la modulation du microbiote intestinal
Davide Masi, Mickael Massicard, Karine Clément
Nutrition Clinique et Métabolisme.2023; 37(2): 2S8. CrossRef - The Related Metabolic Diseases and Treatments of Obesity
Ming Yang, Shuai Liu, Chunye Zhang
Healthcare.2022; 10(9): 1616. CrossRef
- Modulation of the gut microbiome and Firmicutes phylum reduction by a nutraceutical blend in the obesity mouse model and overweight humans: A double‐blind clinical trial

- Diabetes, Obesity and Metabolism
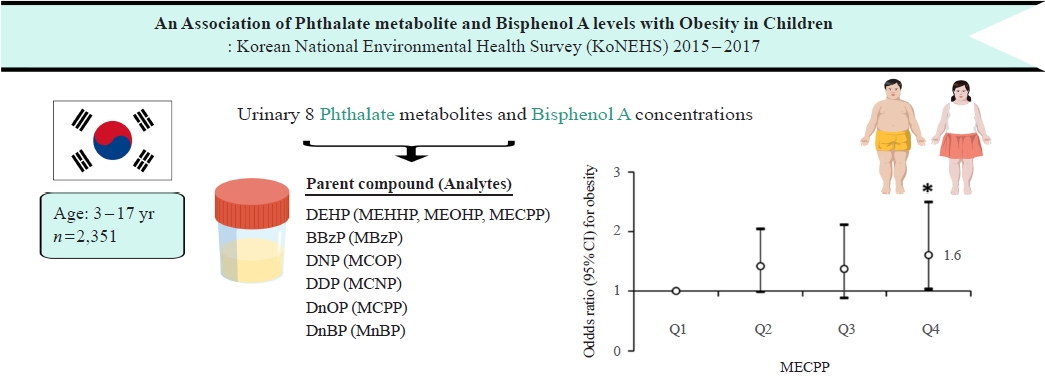
- Associations of Phthalate Metabolites and Bisphenol A Levels with Obesity in Children: The Korean National Environmental Health Survey (KoNEHS) 2015 to 2017
- Moon Young Seo, Shinje Moon, Shin-Hye Kim, Mi Jung Park
- Endocrinol Metab. 2022;37(2):249-260. Published online April 7, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1235
- 5,798 View
- 154 Download
- 7 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Phthalates and bisphenol A (BPA) are synthetic chemicals widely used in daily life. This study investigated urinary phthalate and BPA levels in Korean children and their associations with obesity. Methods: A total of 2,351 children aged 3 to 17 years who participated in the Korean National Environmental Health Survey 2015 to 2017 were included. Urinary dilution was corrected using covariate-adjusted standardization (CAS). We examined the geometric mean (GM) concentrations of urinary phthalate metabolites, including di (2-ethylhexyl) phthalate (DEHP) metabolites (mono [2-ethyl-5-hydroxyhexyl] phthalate, mono [2-ethyl-5-oxohexyl] phthalate, and mono [2-ethyl-5-carboxypentyl] phthalate [MECPP]), mono-benzyl-phthalate (MBzP), mono (carboxyoctyl) phthalate (MCOP), mono (carboxy-isononyl) phthalate (MCNP), mono (3-carboxypropyl) phthalate, and mono-n-butyl-phthalate (MnBP), and BPA. We also analyzed the odds ratio (OR) for obesity according to the quartiles of each analyte. Results: The urinary GM levels of DEHP metabolites and MnBP were notably higher among Korean children than among American, Canadian, and German children. The CAS-applied GM concentrations of most analytes, except for MBzP, MCOP, and MCNP, were higher in children aged 3 to 5 years than in those aged 6 to 17 years. The OR for obesity in the highest quartile of MECPP was significantly higher than in the lowest quartile after adjusting for covariates. However, the other phthalate metabolites and BPA were not significantly associated with obesity. Conclusion: The concentrations of urinary DEHP metabolites and MnBP were higher in Korean children than in children in Western countries. Urinary MECPP exposure, but not other phthalates or BPA, showed a positive association with obesity in Korean children. Further studies are required to elucidate the causal relationships. -
Citations
Citations to this article as recorded by- Diethyl phthalate, a plasticizer, induces adipocyte inflammation and apoptosis in mice after long‐term dietary administration
Shirsha Mondal, Soumyadeep Basu, Songita Ghosh, Suktara Guria, Sutapa Mukherjee
Journal of Biochemical and Molecular Toxicology.2024;[Epub] CrossRef - Nontargeted metabolomic evidence for antagonism between tetracycline and its resistance bacteria underlying their obesogenic effects on Caenorhabditis elegans
Zhuo Li, Di Wu, Zhenyang Yu, Changzheng Cui, Daqiang Yin
Science of The Total Environment.2023; 859: 160223. CrossRef - Prospective association between phthalate exposure in childhood and liver function in adolescence: the Ewha Birth and Growth Cohort Study
Seonhwa Lee, Hye Ah Lee, Bohyun Park, Hyejin Han, Young Sun Hong, Eun Hee Ha, Hyesook Park
Environmental Health.2023;[Epub] CrossRef - Bisphenol A substitutes and childhood obesity at 7 years: a cross-sectional study in Shandong, China
Minyan Chen, Cheng Lv, Shanyu Zhang, Lap Ah Tse, Xinyu Hong, Xi Liu, Yu Ding, Ping Xiao, Ying Tian, Yu Gao
Environmental Science and Pollution Research.2023; 30(29): 73174. CrossRef - Association between Di-2-ethylhexyl phthalate and nonalcoholic fatty liver disease among US adults: Mediation analysis of body mass index and waist circumference in the NHANES
Youming He, Jun Zou, Ting Hong, Dan Feng
Food and Chemical Toxicology.2023; 179: 113968. CrossRef - Association between phthalate exposure and obesity risk: A meta-analysis of observational studies
Qian Wu, Gang Li, Chen-Yang Zhao, Xiao-Lin Na, Yun-Bo Zhang
Environmental Toxicology and Pharmacology.2023; 102: 104240. CrossRef - Levels of Bisphenol A and its analogs in nails, saliva, and urine of children: a case control study
Yolanda Gálvez-Ontiveros, Inmaculada Moscoso-Ruiz, Vega Almazán Fernández de Bobadilla, Celia Monteagudo, Rafael Giménez-Martínez, Lourdes Rodrigo, Alberto Zafra-Gómez, Ana Rivas
Frontiers in Nutrition.2023;[Epub] CrossRef - Nontargeted Metabolomic Evidence for Antagonism between Tetracycline and its Resistance Bacteria Underlying Their Obesogenic Effects on Caenorhabditis Elegans
Zhuo Li, Zhenyang Yu, Changzheng Cui, Daqiang Yin
SSRN Electronic Journal .2022;[Epub] CrossRef
- Diethyl phthalate, a plasticizer, induces adipocyte inflammation and apoptosis in mice after long‐term dietary administration

- Diabetes, Obesity and Metabolism
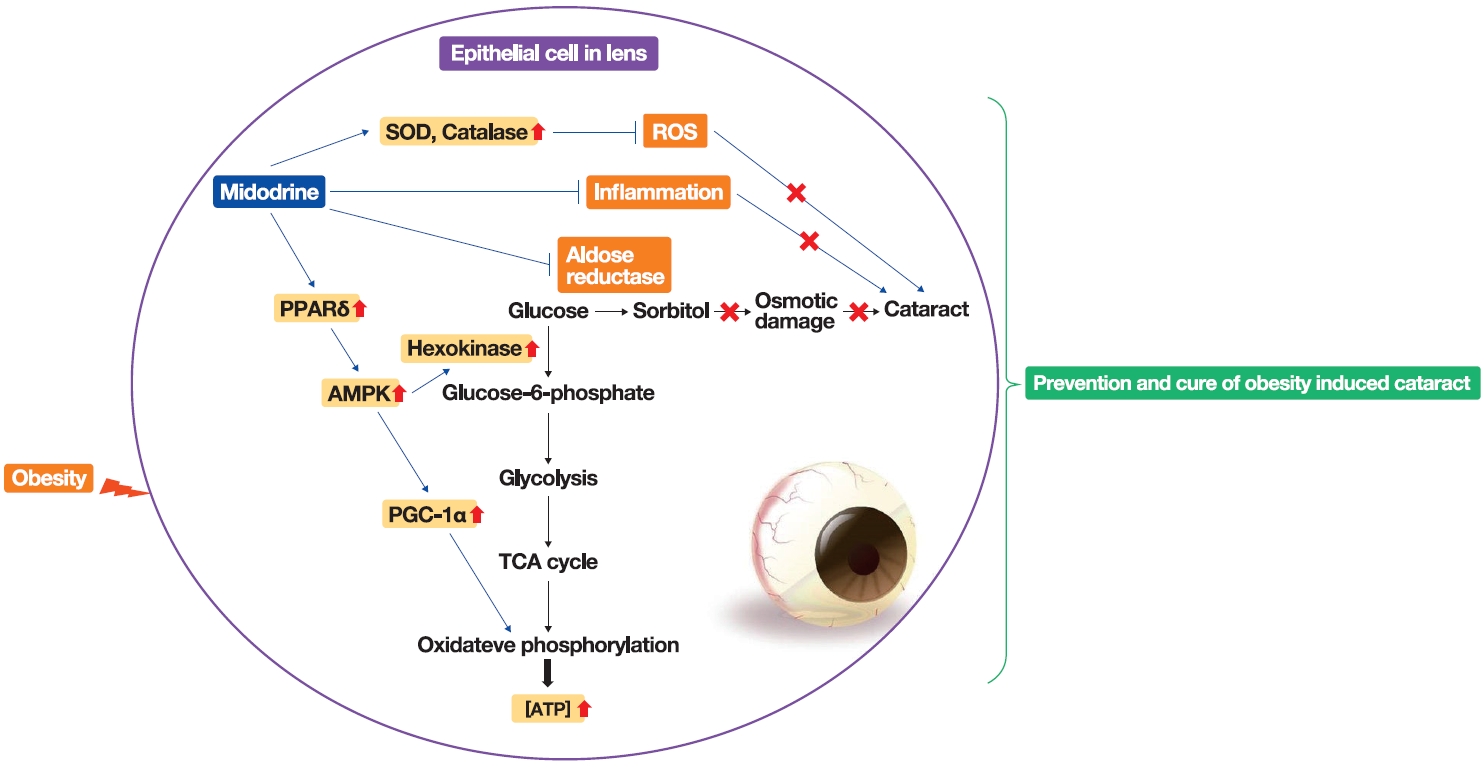
- Stimulation of Alpha-1-Adrenergic Receptor Ameliorates Obesity-Induced Cataracts by Activating Glycolysis and Inhibiting Cataract-Inducing Factors
- Yong-Jik Lee, Yoo-Na Jang, Hyun-Min Kim, Yoon-Mi Han, Hong Seog Seo, Youngsub Eom, Jong-suk Song, Ji Hoon Jeong, Tae Woo Jung
- Endocrinol Metab. 2022;37(2):221-232. Published online March 23, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1237
- 3,673 View
- 138 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Obesity, the prevalence of which is increasing due to the lack of exercise and increased consumption of Westernized diets, induces various complications, including ophthalmic diseases. For example, obesity is involved in the onset of cataracts.
Methods
To clarify the effects and mechanisms of midodrine, an α1-adrenergic receptor agonist, in cataracts induced by obesity, we conducted various analytic experiments in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a rat model of obesity.
Results
Midodrine prevented cataract occurrence and improved lens clearance in OLETF rats. In the lenses of OLETF rats treated with midodrine, we observed lower levels of aldose reductase, tumor necrosis factor-α, and sorbitol, but higher levels of hexokinase, 5’-adenosine monophosphate-activated protein kinase-alpha, adenosine 5´-triphosphate, peroxisome proliferator-activated receptordelta, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, superoxide dismutase, and catalase.
Conclusion
The ameliorating effects of midodrine on cataracts in the OLETF obesity rat model are exerted via the following three mechanisms: direct inhibition of the biosynthesis of sorbitol, which causes cataracts; reduction of reactive oxygen species and inflammation; and (3) stimulation of normal aerobic glycolysis. -
Citations
Citations to this article as recorded by- α1-Adrenergic Receptors: Insights into Potential Therapeutic Opportunities for COVID-19, Heart Failure, and Alzheimer’s Disease
Dianne M. Perez
International Journal of Molecular Sciences.2023; 24(4): 4188. CrossRef - A new use for old drugs: identifying compounds with an anti-obesity effect using a high through-put semi-automated Caenorhabditis elegans screening platform
Freek Haerkens, Charlotte Kikken, Laurens Kirkels, Monique van Amstel, Willemijn Wouters, Els van Doornmalen, Christof Francke, Samantha Hughes
Heliyon.2022; 8(8): e10108. CrossRef
- α1-Adrenergic Receptors: Insights into Potential Therapeutic Opportunities for COVID-19, Heart Failure, and Alzheimer’s Disease

Namgok Lecture 2021
- Diabetes, Obesity and Metabolism
- The Influence of Obesity and Metabolic Health on Vascular Health
- Eun-Jung Rhee
- Endocrinol Metab. 2022;37(1):1-8. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2022.101
- 7,258 View
- 305 Download
- 16 Web of Science
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
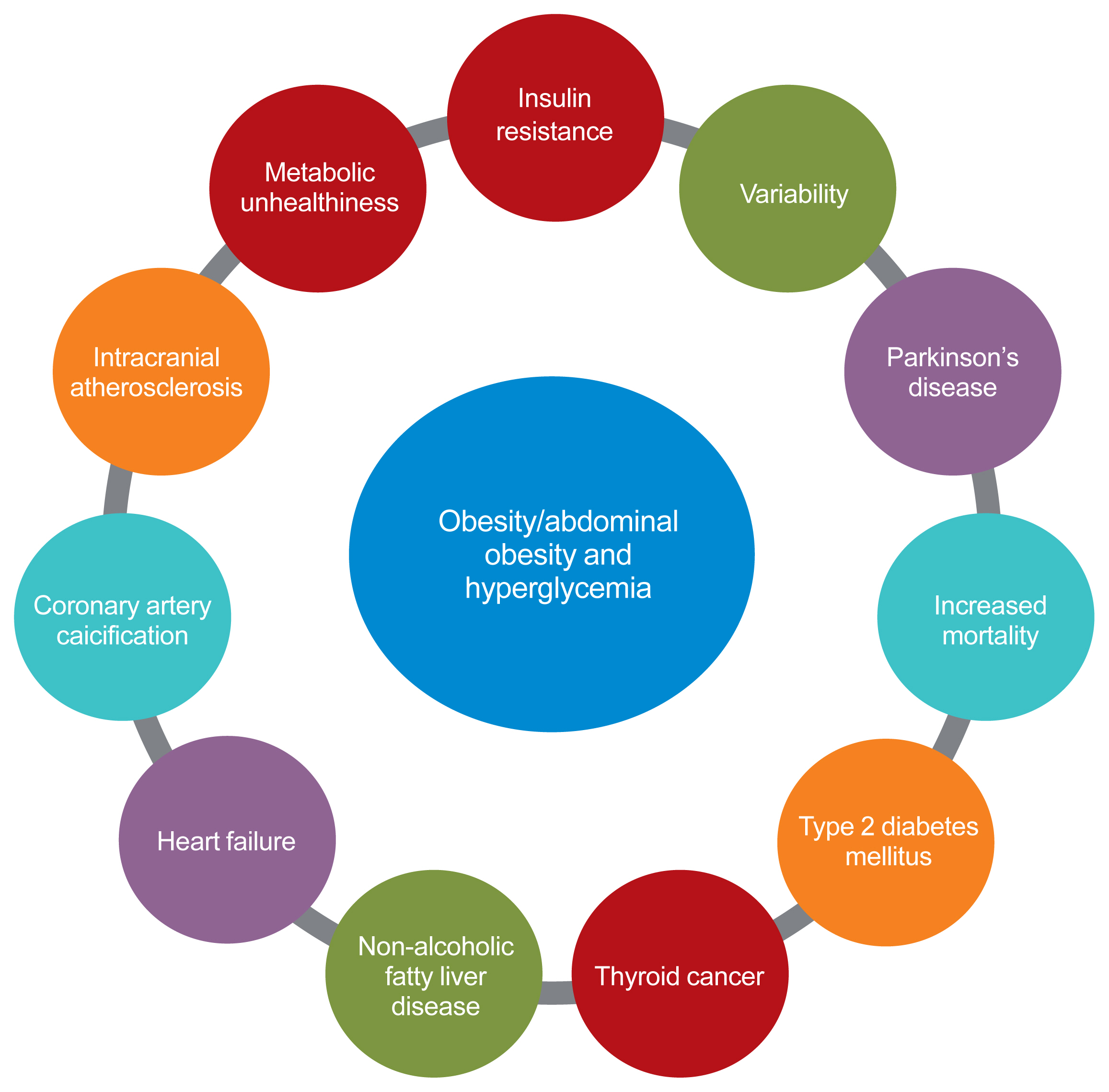
ePub - The prevalence of obesity is rapidly increasing worldwide. Obesity should not be understood only as the accumulation of fat in the body, but instead as a phenomenon that exerts different effects on our health according to the place of fat deposition and its stability. Obesity is the starting point of most metabolic diseases, such as diabetes, hypertension, metabolic syndrome, sleep apnea, and eventually cardiovascular disease. There are different kinds of obesity, ranging from simple obesity to sarcopenic obesity. The main purpose of intervening to address obesity is to decrease the ultimate consequence of obesity—namely, cardiovascular disease. The main mechanism through which obesity, especially abdominal obesity, increases cardiovascular risk is the obesity-induced derangement of metabolic health, leading to the development of metabolic diseases such as diabetes, non-alcoholic fatty liver disease, and metabolic syndrome, which are the main initiators of vascular damage. In this review, I discuss the influence of various types of obesity on the risk of metabolic diseases, and how these diseases increase cardiovascular disease risk.
-
Citations
Citations to this article as recorded by- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ji Yoon Kim, Jimi Choi, Sin Gon Kim
European Heart Journal - Cardiovascular Pharmacotherapy.2024; 10(2): 118. CrossRef - Severity of abdominal obesity and cardiometabolic diseases in US adults
S. Wang, S. Shi, Y. Huang, H. Huang, V.W. Zhong
Public Health.2024; 227: 154. CrossRef - Anti-obesity effects of fucoidan from
Sargassum thunbergii in adipocytes and high fat diet induced obese mice through inhibiting adipogenic specific transcription factor
Hyo-Geun Lee, H.H.A.C.K. Jayawardhana, Fengqi Yang, D.P. Nagahawaththa, N.M. Liyanage, Kyung-Mo Song, Yun-Sang Choi, Seung-Hong Lee, You-Jin Jeon, Min-Cheol Kang
Food Science and Human Wellness.2024; 13(3): 1608. CrossRef - Association of a High Healthy Eating Index Diet with Long-Term Visceral Fat Loss in a Large Longitudinal Study
Sunmin Park
Nutrients.2024; 16(4): 534. CrossRef - Ancistrocladus tectorius Extract Inhibits Obesity by Promoting Thermogenesis and Mitochondrial Dynamics in High-Fat Diet-Fed Mice
Minju Kim, Jin Hyub Paik, Hwa Lee, Min Ji Kim, Sang Mi Eum, Soo Yong Kim, Sangho Choi, Ho-Yong Park, Hye Gwang Jeong, Tae-Sook Jeong
International Journal of Molecular Sciences.2024; 25(7): 3743. CrossRef - Biological Activity Evaluation of Olive, Grape, and Fig at Various Mixing Ratios
Chan-Hwi Lee, So-Young Lee, Ae-Jung Kim
Asian Journal of Beauty and Cosmetology.2024; 22(1): 91. CrossRef - Investigation and Comparison of Maternal Pre-Pregnancy Body Mass Index Coupled with Gestational Weight Gain on Maternal–Fetal Complications Based on US and Chinese Guidelines: A Retrospective Study
Wan-Ju Kung, Hsin-Yi Kuo, Ching-Feng Chang, Yeong-Hwa Zen, Ching-Chiang Lin
Reproductive Sciences.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Mechanistic insights into dietary (poly)phenols and vascular dysfunction-related diseases using multi-omics and integrative approaches: Machine learning as a next challenge in nutrition research
Dragan Milenkovic, Tatjana Ruskovska
Molecular Aspects of Medicine.2023; 89: 101101. CrossRef - Pharmacological Support for the Treatment of Obesity—Present and Future
Marcin Kosmalski, Kacper Deska, Bartłomiej Bąk, Monika Różycka-Kosmalska, Tadeusz Pietras
Healthcare.2023; 11(3): 433. CrossRef - Prioritizing obesity treatment: expanding the role of cardiologists to improve cardiovascular health and outcomes
Donna H. Ryan, John E. Deanfield, Stephan Jacob
Cardiovascular Endocrinology & Metabolism.2023; 12(1): e0279. CrossRef - Adipopenia is associated with osteoporosis in community-dwelling non-underweight adults independent of sarcopenia
Seunghyun Lee, Kyoungmyoung Ko, Sungjae Shin, Hye Sun Park, Namki Hong, Yumie Rhee
Archives of Osteoporosis.2023;[Epub] CrossRef - Design, synthesis and evaluation of 2-pyrimidinylindole derivatives as anti-obesity agents by regulating lipid metabolism
Shi-Yao Guo, Li-Yuan Wei, Bing-Bing Song, Yu-Tao Hu, Zhi Jiang, Dan-Dan Zhao, Yao-Hao Xu, Yu-Wei Lin, Shu-Min Xu, Shuo-Bin Chen, Zhi-Shu Huang
European Journal of Medicinal Chemistry.2023; 260: 115729. CrossRef - Short-Term L-Citrulline Supplementation Does Not Affect Blood Pressure, Pulse Wave Reflection, or Arterial Stiffness at Rest and during Isometric Exercise in Older Males
Andrea Tryfonos, Filippos Christodoulou, George M. Pamboris, Stephanos Christodoulides, Anastasios A. Theodorou
Sports.2023; 11(9): 177. CrossRef - Skinfold Thickness as a Cardiometabolic Risk Predictor in Sedentary and Active Adult Populations
Sughey González-Torres, Luis Miguel Anaya-Esparza, Gabriel Fermín Trigueros del Valle, Edgar Alfonso Rivera-León, Zuamí Villagrán, Sergio Sánchez-Enríquez
Journal of Personalized Medicine.2023; 13(9): 1326. CrossRef - Impact of COVID-19 Lockdown on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in Adults: A before and after Pandemic Lockdown Longitudinal Study
Ángel Arturo López-González, Bárbara Altisench Jané, Luis Masmiquel Comas, Sebastiana Arroyo Bote, Hilda María González San Miguel, José Ignacio Ramírez Manent
Nutrients.2022; 14(14): 2795. CrossRef - Fenofibrate enhances lipid deposition via modulating PPARγ, SREBP-1c, and gut microbiota in ob/ob mice fed a high-fat diet
Ying Zhang, Xiu-Bin Jia, Yun-Chao Liu, Wen-Qian Yu, Yan-Hong Si, Shou-Dong Guo
Frontiers in Nutrition.2022;[Epub] CrossRef - Predictive Roles of Basal Metabolic Rate and Body Water Distribution in Sarcopenia and Sarcopenic Obesity: The link to Carbohydrates
Lizheng Guan, Tiantian Li, Xuan Wang, Kang Yu, Rong Xiao, Yuandi Xi
Nutrients.2022; 14(19): 3911. CrossRef - Metabolic risk factors in patients with comorbidity in Ufa primary health care
O.V. Molchanova, A.V. Mamaeva, A.R. Dunayeva, Z.A. Lust, E.M. Faskhetdinova, R.N. Shepel, D.O. Orlov, L.M. Zhamalov, G.F. Andreeva, O.M. Drapkina
Profilakticheskaya meditsina.2022; 25(9): 39. CrossRef - Assessment of Vitamin D Levels in Relation to Statin Therapy in Elderly Hypertensive Patients with Comorbidities
Kinga-Ilona Nyulas, Zsuzsánna Simon-Szabó, Zoltán Preg, Sándor Pál, Arundhati Sharma, Tünde Pál, Márta Germán-Salló, Enikő Nemes-Nagy
Journal of Interdisciplinary Medicine.2022; 7(4): 88. CrossRef
- Associations of omega-3 fatty acids vs. fenofibrate with adverse cardiovascular outcomes in people with metabolic syndrome: propensity matched cohort study

Original Articles
- Diabetes, Obesity and Metabolism
- The Leg Fat to Total Fat Ratio Is Associated with Lower Risks of Non-Alcoholic Fatty Liver Disease and Less Severe Hepatic Fibrosis: Results from Nationwide Surveys (KNHANES 2008–2011)
- Hyun Min Kim, Yong-ho Lee
- Endocrinol Metab. 2021;36(6):1232-1242. Published online November 23, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1087

- 3,872 View
- 131 Download
- 3 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The prevalence of non-alcoholic fatty liver disease (NAFLD) has rapidly increased worldwide. The aim of this study was to investigate whether there is an independent relationship between regional fat distribution, especially leg fat mass, and the presence of NAFLD using nationally representative data in Korea.
Methods
This cross-sectional study analyzed data from 14,502 participants in the Korea National Health and Nutrition Examination Survey 2008 to 2011. Total fat mass, leg fat mass, and appendicular skeletal muscle mass were measured by dual-energy X-ray absorptiometry. Validated NAFLD prediction models and scoring systems for hepatic fibrosis were used.
Results
The leg fat to total fat (LF/TF) ratio showed a negative relationship with many factors, including body mass index, waist circumference, blood pressure, fasting blood glucose, and liver enzyme levels. When the LF/TF ratio and indices of hepatic steatosis were stratified by quartiles, the LF/TF ratio showed a negative correlation with the scoring systems that were used. The LF/TF ratio showed better accuracy in predicting NAFLD than total fat mass or leg fat mass alone. After adjusting for various traditional and lifestyle factors, a low LF/TF ratio remained a risk factor for NAFLD. Among NAFLD subjects, the LF/TF ratio showed a negative relationship with hepatic fibrosis.
Conclusion
A lower LF/TF ratio was markedly associated with a higher risk of hepatic steatosis and advanced hepatic fibrosis using various predictive models in a Korean population. Therefore, the LF/TF ratio could be a useful anthropometric parameter to predict NAFLD or advanced hepatic fibrosis. -
Citations
Citations to this article as recorded by- A greater ratio of thigh subcutaneous fat to abdominal fat is associated with protection against non-alcoholic fatty liver disease
Yebei Liang, Peizhu Chen, Siyu Chen, Dan Liu, Fusong Jiang, Zhijun Zhu, Keqing Dong, Li Wei, Xuhong Hou
JHEP Reports.2023; 5(7): 100730. CrossRef - Association between Alcohol Consumption and Metabolic Dysfunction-Associated Steatotic Liver Disease Based on Alcohol Flushing Response in Men: The Korea National Health and Nutrition Examination Survey 2019–2021
Dae Eon Kang, Si Nae Oh
Nutrients.2023; 15(18): 3901. CrossRef
- A greater ratio of thigh subcutaneous fat to abdominal fat is associated with protection against non-alcoholic fatty liver disease

- Diabetes, Obesity and Metabolism
Big Data Articles (National Health Insurance Service Database) - Cardiovascular Outcomes of Obesity According to Menopausal Status: A Nationwide Population-Based Study
- Bo Kyung Koo, Sang-Hyun Park, Kyungdo Han, Min Kyong Moon
- Endocrinol Metab. 2021;36(5):1029-1041. Published online October 21, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1197
- 3,732 View
- 117 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
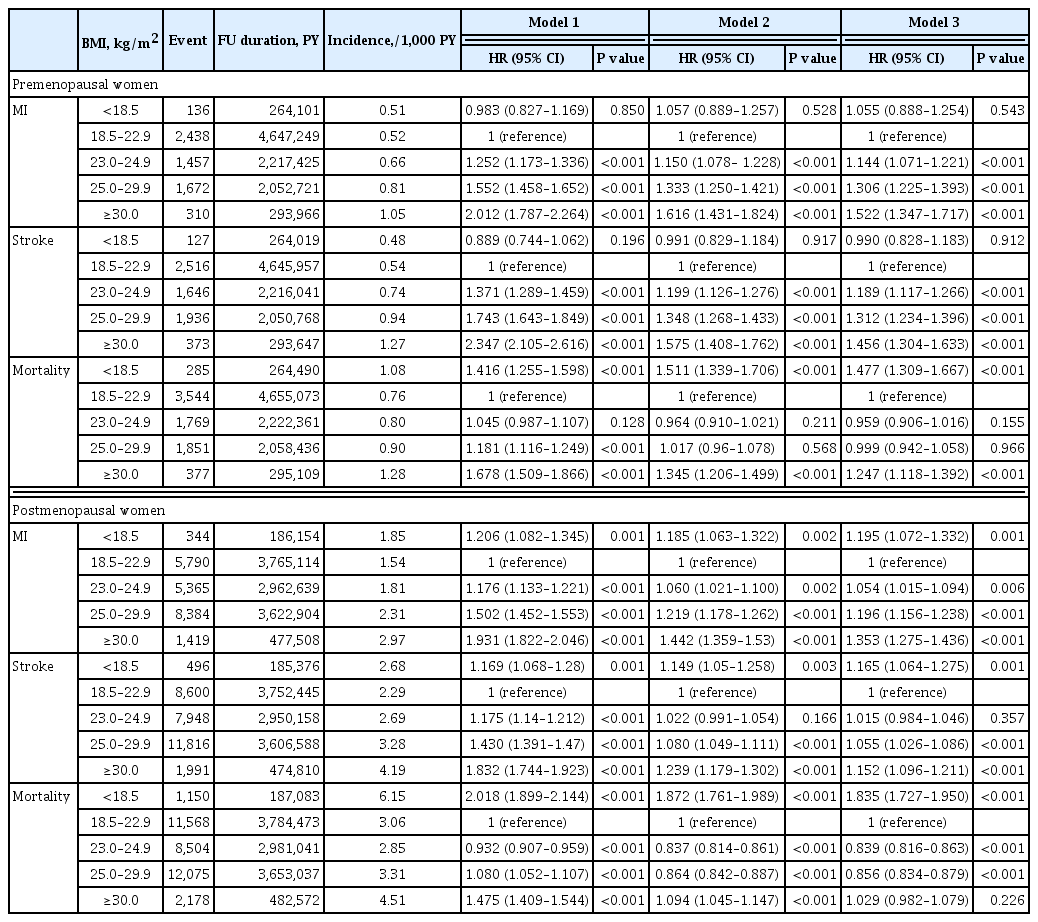
We estimated the effect of obesity on the incidence of cardiovascular disease (CVD) and mortality in women according to menopausal status.
Methods
Women aged 40 to 69 years under routine health check-ups provided by the National Health Insurance Service in 2009 were followed up till 2018 (n=2,208,559).
Results
In premenopausal women, a significant increment of mortality rate was found in underweight and obesity class II (hazard ratio [HR], 1.48; 95% confidence interval [CI], 1.31 to 1.67; and HR, 1.25; 95% CI, 1.12 to 1.39) compared to normal body mass index (BMI); overweight and obesity class I did not affect mortality rate. In postmenopausal women, obesity as well as overweight status reduced the risk of mortality compared to normal BMI (HR, 0.86; 95% CI, 0.83 to 0.88; and HR, 0.84; 95% CI, 0.82 to 0.86). By contrast, there was a linear association between CVD and BMI above the normal range irrespective of menopausal status, which was attenuated in diabetic women.
Conclusion
The current study replicated the J-shaped relationship between BMI and mortality, being more prominent in the postmenopausal group. The risk of CVD was linearly increased as BMI was increased above the normal range irrespective of menopausal status. -
Citations
Citations to this article as recorded by- Biosocial predictors and blood pressure goal attainment among postmenopausal women with hypertension
Geetha Kandasamy, Thangamani Subramani, Gigi Sam, Mona Almanasef, Tahani Almeleebia, Eman Shorog, Asma M. Alshahrani, Amjad Hmlan, Atheer Y. Al Suhaym, Kousalya Prabahar, Vinoth Prabhu Veeramani, Palanisamy Amirthalingam
Frontiers in Cardiovascular Medicine.2024;[Epub] CrossRef - A nationwide cohort study on diabetes severity and risk of Parkinson disease
Kyungdo Han, Bongsung Kim, Seung Hwan Lee, Mee Kyoung Kim
npj Parkinson's Disease.2023;[Epub] CrossRef - Cardiovascular Outcomes according to Comorbidities and Low-Density Lipoprotein Cholesterol in Korean People with Type 2 Diabetes Mellitus
Min Kyong Moon, Junghyun Noh, Eun-Jung Rhee, Sang Hyun Park, Hyeon Chang Kim, Byung Jin Kim, Hae Jin Kim, Seonghoon Choi, Jin Oh Na, Young Youl Hyun, Bum Joon Kim, Kyung-Do Han, In-Kyung Jeong
Diabetes & Metabolism Journal.2023; 47(1): 45. CrossRef - The effect of menopause on cardiovascular risk factors according to body mass index in middle-aged Korean women
Do Kyeong Song, Young Sun Hong, Yeon-Ah Sung, Hyejin Lee, Aysha Almas
PLOS ONE.2023; 18(3): e0283393. CrossRef - Low‐quality muscle mass rather than normal‐quality muscle mass determines fibrosis progression in biopsy‐proven NAFLD
Yun Kyu Lee, Bo Kyung Koo, Sae Kyung Joo, Dong Hyeon Lee, Heejoon Jang, Jee Won Chai, Myoung Seok Lee, Si Won Jang, Young Ho So, Jeong Hwan Park, Mee Soo Chang, Won Kim
Alimentary Pharmacology & Therapeutics.2023; 58(3): 322. CrossRef - Diabetes severity is strongly associated with the risk of active tuberculosis in people with type 2 diabetes: a nationwide cohort study with a 6-year follow-up
Ji Young Kang, Kyungdo Han, Seung-Hwan Lee, Mee Kyoung Kim
Respiratory Research.2023;[Epub] CrossRef - Effects of exercise initiation and smoking cessation after new-onset type 2 diabetes mellitus on risk of mortality and cardiovascular outcomes
Mee Kyoung Kim, Kyungdo Han, Bongsung Kim, Jinyoung Kim, Hyuk-Sang Kwon
Scientific Reports.2022;[Epub] CrossRef - Non-pharmacologic treatment for obesity
Bo Kyung Koo
Journal of the Korean Medical Association.2022; 65(7): 400. CrossRef
- Biosocial predictors and blood pressure goal attainment among postmenopausal women with hypertension


 KES
KES

 First
First Prev
Prev



