Search
- Page Path
- HOME > Search
- Impact of Antidiabetic Drugs on Clinical Outcomes of COVID-19: A Nationwide Population-Based Study
- Han Na Jang, Sun Joon Moon, Jin Hyung Jung, Kyung-Do Han, Eun-Jung Rhee, Won-Young Lee
- Received October 16, 2023 Accepted January 3, 2024 Published online January 29, 2024
- DOI: https://doi.org/10.3803/EnM.2024.1857 [Epub ahead of print]
- 1,007 View
- 41 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Inconsistent results have been reported regarding the association between the use of antidiabetic drugs and the clinical outcomes of coronavirus disease 2019 (COVID-19). This study aimed to investigate the effect of antidiabetic drugs on COVID-19 outcomes in patients with diabetes using data from the National Health Insurance Service (NHIS) in South Korea.
Methods
We analyzed the NHIS data of patients aged ≥20 years who tested positive for COVID-19 and were taking antidiabetic drugs between December 2019 and June 2020. Multiple logistic regression analysis was performed to analyze the clinical outcomes of COVID-19 based on the use of antidiabetic drugs.
Results
A total of 556 patients taking antidiabetic drugs tested positive for COVID-19, including 271 male (48.7%), most of whom were in their sixties. Of all patients, 433 (77.9%) were hospitalized, 119 (21.4%) received oxygen treatment, 87 (15.6%) were admitted to the intensive care unit, 31 (5.6%) required mechanical ventilation, and 61 (11.0%) died. Metformin was significantly associated with the lower risks of mechanical ventilation (odds ratio [OR], 0.281; 95% confidence interval [CI], 0.109 to 0.720; P=0.008), and death (OR, 0.395; 95% CI, 0.182 to 0.854; P=0.018). Dipeptidylpeptidase-4 inhibitor (DPP-4i) were significantly associated with the lower risks of oxygen treatment (OR, 0.565; 95% CI, 0.356 to 0.895; P=0.015) and death (OR, 0.454; 95% CI, 0.217 to 0.949; P=0.036). Sulfonylurea was significantly associated with the higher risk of mechanical ventilation (OR, 2.579; 95% CI, 1.004 to 6.626; P=0.049).
Conclusion
In patients with diabetes and COVID-19, metformin exhibited reduced risks of mechanical ventilation and death, DPP- 4i was linked with lower risks of oxygen treatment and death, while sulfonylurea was related to the increased risk of mechanical ventilation.

- Diabetes, obesity and metabolism
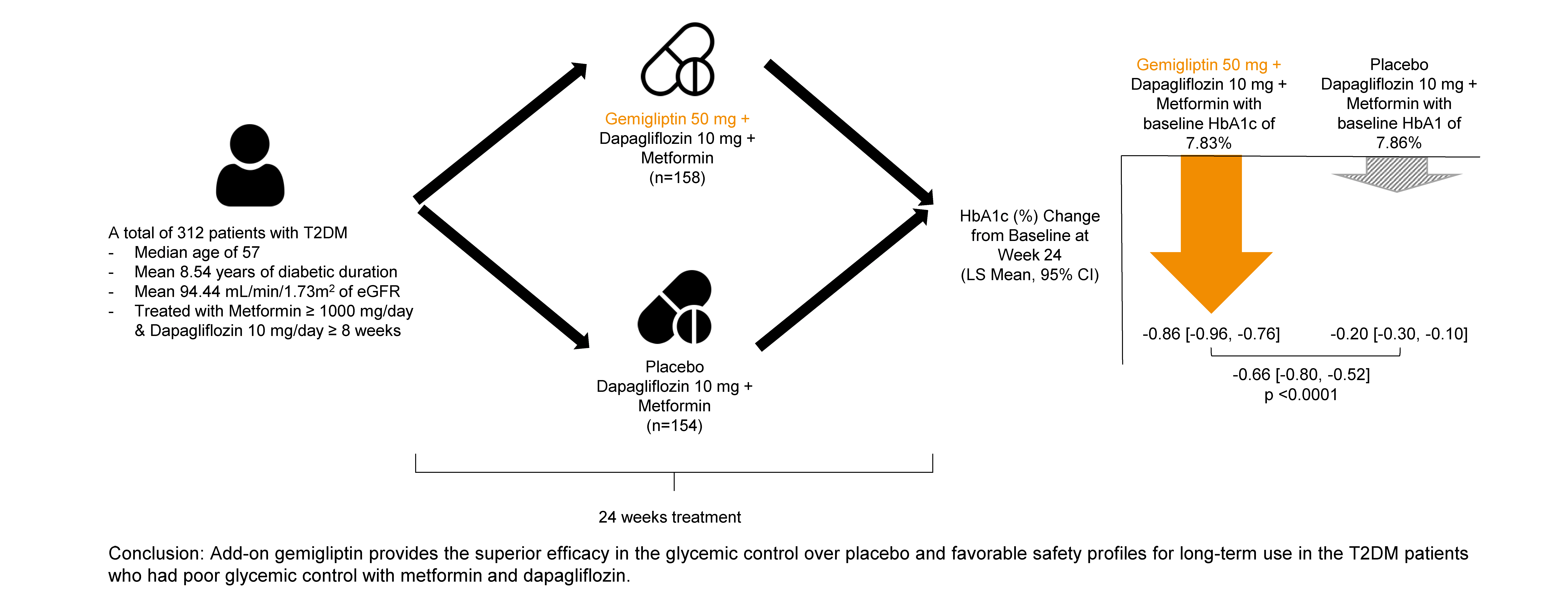
- Efficacy of Gemigliptin Add-on to Dapagliflozin and Metformin in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Study (SOLUTION)
- Byung Wan Lee, KyungWan Min, Eun-Gyoung Hong, Bon Jeong Ku, Jun Goo Kang, Suk Chon, Won-Young Lee, Mi Kyoung Park, Jae Hyeon Kim, Sang Yong Kim, Keeho Song, Soon Jib Yoo
- Endocrinol Metab. 2023;38(3):328-337. Published online June 28, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1688
- 2,419 View
- 264 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study evaluated the efficacy and safety of add-on gemigliptin in patients with type 2 diabetes mellitus (T2DM) who had inadequate glycemic control with metformin and dapagliflozin.
Methods
In this randomized, placebo-controlled, parallel-group, double-blind, phase III study, 315 patients were randomized to receive either gemigliptin 50 mg (n=159) or placebo (n=156) with metformin and dapagliflozin for 24 weeks. After the 24-week treatment, patients who received the placebo were switched to gemigliptin, and all patients were treated with gemigliptin for an additional 28 weeks.
Results
The baseline characteristics were similar between the two groups, except for body mass index. At week 24, the least squares mean difference (standard error) in hemoglobin A1c (HbA1c) changes was –0.66% (0.07) with a 95% confidence interval of –0.80% to –0.52%, demonstrating superior HbA1c reduction in the gemigliptin group. After week 24, the HbA1c level significantly decreased in the placebo group as gemigliptin was administered, whereas the efficacy of HbA1c reduction was maintained up to week 52 in the gemigliptin group. The safety profiles were similar: the incidence rates of treatment-emergent adverse events up to week 24 were 27.67% and 29.22% in the gemigliptin and placebo groups, respectively. The safety profiles after week 24 were similar to those up to week 24 in both groups, and no new safety findings, including hypoglycemia, were noted.
Conclusion
Add-on gemigliptin was well tolerated, providing comparable safety profiles and superior efficacy in glycemic control over placebo for long-term use in patients with T2DM who had poor glycemic control with metformin and dapagliflozin.

- Diabetes, Obesity and Metabolism
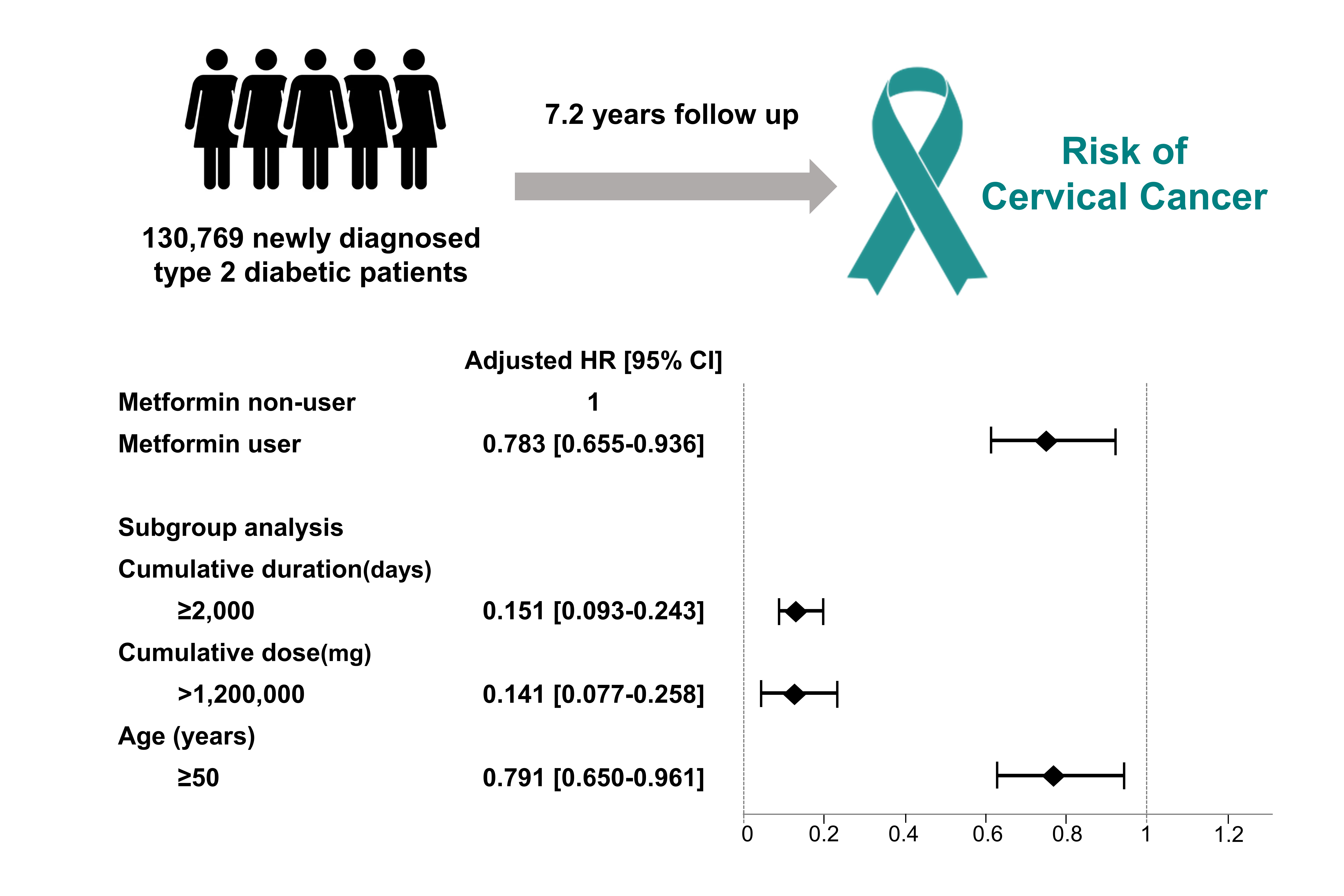
Big Data Articles (National Health Insurance Service Database) - Metformin and Cervical Cancer Risk in Patients with Newly Diagnosed Type 2 Diabetes: A Population-Based Study in Korea
- Hyun Min Kim, Min Jin Kang, Sun Ok Song
- Endocrinol Metab. 2022;37(6):929-937. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1613
- Correction in: Endocrinol Metab 2023;38(1):174
- 2,479 View
- 218 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Cervical cancer is a prevalent malignancy that is a major health problem for women worldwide. The cancer-preventive properties of metformin are well-known, but insufficient data have been reported regarding its relationship to cervical cancer. Therefore, in a nationwide population-based study, we investigated the association between metformin use and cervical cancer incidence in patients with newly diagnosed type 2 diabetes.
Methods
This retrospective cohort study used the Korean National Health Insurance claims database. Individuals newly diagnosed with type 2 diabetes between January 2005 and December 2009 were included. The occurrence of cervical cancer was explored by matching for age, economic status, region of residence, and use of anti-diabetic medication.
Results
In total, 66,013 metformin users and 64,756 non-users were analyzed. Cervical cancer occurred in 219 metformin users (0.33%) and 274 metformin non-users (0.42%) (hazard ratio [HR], 0.783; 95% confidence interval [CI], 0.655 to 0.036; P=0.007). Moreover, cervical cancer risk was considerably reduced in those treated with a high dose (>1,200,000 mg) or for an extended period (≥2,000 days) compared to non-users (HR, 0.151; 95% CI, 0.093 to 0.243; P<0.001; and HR, 0.141; 95% CI, 0.077 to 0.258; P<0.001). The incidence was also significantly lower in metformin users among those over 50 years old (HR, 0.791; 95% CI, 0.650 to 0.961; P<0.001).
Conclusion
Metformin use in patients with newly diagnosed diabetes was associated with a lower risk of cervical cancer in Korea. Furthermore, a significant association was found between the use of metformin and cervical cancer in a dose- and duration-dependent manner and among those over 50 years old. -
Citations
Citations to this article as recorded by- Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp
Mohamad Ali Hijazi, André Gessner, Nahed El-Najjar
Cancers.2023; 15(12): 3199. CrossRef - Network-based drug repurposing for HPV-associated cervical cancer
Faheem Ahmed, Young Jin Yang, Anupama Samantasinghar, Young Woo Kim, Jeong Beom Ko, Kyung Hyun Choi
Computational and Structural Biotechnology Journal.2023; 21: 5186. CrossRef - The Use of Metformin and Postoperative Insulin Pump Were Predictive Factors for Outcomes of Diabetic Colorectal Cancer Patients after Surgery
Xu-Rui Liu, Fei Liu, Zi-Wei Li, Quan Lv, Xin-Peng Shu, Lian-Shuo Li, Yue Tong, Wei Zhang, Dong Peng
Nutrition and Cancer.2023; 75(10): 1926. CrossRef
- Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp

- Obesity and Metabolism
- Myths about Insulin Resistance: Tribute to Gerald Reaven
- Sun H. Kim, Fahim Abbasi
- Endocrinol Metab. 2019;34(1):47-52. Published online March 21, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.1.47
- 6,750 View
- 143 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Gerald Reaven was often called the “father of insulin resistance.” On the 1-year anniversary of his death in 2018, we challenge three myths associated with insulin resistance: metformin improves insulin resistance; measurement of waist circumference predicts insulin resistance better than body mass index; and insulin resistance causes weight gain. In this review, we highlight Reaven's relevant research that helped to dispel these myths associated with insulin resistance.
-
Citations
Citations to this article as recorded by- Assessment of Antidiabetic and Anti-Inflammatory Activities of Carissa carandas Linn Extract: In Vitro and In Vivo Study
Manaschanok Lailerd, Thiri Wai Linn, Narissara Lailerd, Duangporn Amornlerdpison, Arisa Imsumran
Applied Sciences.2023; 13(11): 6454. CrossRef - Association of primary allostatic load mediators and metabolic syndrome (MetS): A systematic review
Francis Osei, Andrea Block, Pia-Maria Wippert
Frontiers in Endocrinology.2022;[Epub] CrossRef - Anthropometric indices and the risk of incident sudden cardiac death among adults with and without diabetes: over 15 years of follow-up in The Tehran Lipid and Glucose Study
Seyyed Saeed Moazzeni, Seyed Saeed Tamehri Zadeh, Samaneh Asgari, Fereidoun Azizi, Farzad Hadaegh
Diabetology & Metabolic Syndrome.2021;[Epub] CrossRef - Metabolic Syndrome or Insulin Resistance: Evolution, Controversies and Association With Cardiovascular Disease Risk
Arun K. Chopra
Indian Journal of Clinical Cardiology.2020; 1(2): 77. CrossRef
- Assessment of Antidiabetic and Anti-Inflammatory Activities of Carissa carandas Linn Extract: In Vitro and In Vivo Study

- Predetermined Anti-Diabetic Drug Regimen Adjustments during Ramadan Fasting: An Observational Study of Safety
- Abdallah M. Beano, Mohammad A. Zmaili, Zaid H. Gheith, Ahmad M. Naser, Munther S. Momani, Al-Motassem F. Yousef, Ayman A. Zayed
- Endocrinol Metab. 2017;32(2):265-273. Published online June 23, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.2.265
- 4,052 View
- 41 Download
- 10 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Many Muslim type 2 diabetes mellitus (T2DM) patients choose to fast the month of Ramadan despite the possible adverse health effects brought about by the change in dietary habits, among other things. Clinical data regarding the safety of multi-drug regimens during fasting are particularly scarce. The aim of the study was to evaluate the safety of a drug protocol devised by the authors to accommodate Ramadan's dietary changes, involving dose adjustments of four anti-diabetic drug regimens in T2DM patients fasting Ramadan.
Methods In this prospective, observational, open-label study, 301 T2DM patients who wished to fast Ramadan were followed during Ramadan and the preceding month. The incidence of hypoglycemia, diabetic ketoacidosis (DKA) and non-ketotic hyperosmolar state (NKHS) was monitored. Patients were classified into four groups: A group (those taking metformin, sulfonylurea and insulin [
n =33]); B group (metformin and sulfonylurea [n =89]); C group (metformin and insulin [n =96]); and D group (premixed 70/30, glargine or regular insulin [n =82]). During Ramadan, drug doses were adjusted as percentages of their pre-Ramadan values: 75% for sulfonylureas, 75% for glargine, 75% for premixed insulin 70/30 in two doses, and 75% for regular insulin. Metformin was adjusted to a twice-daily regimen.Results No cases of DKA or NKHS were reported. Hypoglycemia occurred at a lower rate than pre-Ramadan values in groups C, and D; and a similar rate in groups A, and B.
Conclusion The data suggested that using the above protocol to adjust the doses of anti-diabetic drugs is safe in T2DM patients in regards to hypoglycemia, DKA, and NKHS.
-
Citations
Citations to this article as recorded by- Replicating human characteristics: A promising animal model of central fatigue
Yifei Zhang, Zehan Zhang, Qingqian Yu, Bijuan Lan, Qinghuan Shi, Ruting Li, Ziheng Jiao, Weiyue Zhang, Feng Li
Brain Research Bulletin.2024; 212: 110951. CrossRef - Evaluation of physicians' approaches for the management of patients with diabetes during Ramadan in Iraq
Majid Alabbood, Rafid Alameri, Yasameen Alsaffar
Diabetes Research and Clinical Practice.2023; 195: 110188. CrossRef - The physiological changing and the recommended management plans for the diabetic patient during Ramadan fasting; A review article
Abeer Alhaj, Omar F Shatnawi
JAP Academy Journal.2023;[Epub] CrossRef - Incidence of diabetic ketoacidosis does not differ in Ramadan compared to other months and seasons: results from a 6-year multicenter study
Fateen Ata, Adeel Ahmad Khan, Ibrahim Khamees, Mohammed Bashir
Current Medical Research and Opinion.2023; 39(8): 1061. CrossRef - Evidence-based risk factors for major complications during Ramadan fasting in people with diabetes grouped under IDF-DAR risk categories
Rahmatullah, Muhammad Yakoob Ahmedani, Abdul Basit, Shagufta Zia, Imran Hasan, Qazi Masroor, Abrar Shaikh, Jehangir Khan, Waheed Iqbal
Diabetes Research and Clinical Practice.2022; 185: 109234. CrossRef - A Systematic Review of Insulin Management Recommendations to Improve Glycemic Control and Reduce Hypoglycemic Events During Ramadan Fasting in Patients With Insulin-Requiring Type 2 Diabetes
Alexander Kieu, Ashley Iles, Moien AB Khan, Linda Östlundh, Duston Boyd, MoezAlIslam Ezzat Faris
Frontiers in Nutrition.2022;[Epub] CrossRef - The DAR 2020 Global survey: Ramadan fasting during COVID 19 pandemic and the impact of older age on fasting among adults with Type 2 diabetes
Mohamed Hassanein, Zanariah Hussein, Inass Shaltout, Wan Juani Wan Seman, Chin Voon Tong, Nurain Mohd Noor, Mehmet Akif Buyukbese, Lobna El Tony, Gamal Mohamed Shaker, Reem M. Alamoudi, Khadija Hafidh, M. Fariduddin, Mohammed A. Batais, Shehla Shaikh, Pr
Diabetes Research and Clinical Practice.2021; 173: 108674. CrossRef - Effect of Dosage Reduction of Hypoglycemic Multidrug Regimens on the Incidences of Acute Glycemic Complications in People With Type 2 Diabetes Who Fast During Ramaḍān: A Randomized Controlled Trial
Louay Y. Zaghlol, Amir F. Beirat, Justin Z. Amarin, Amro M. Hassoun Al Najar, Yazan Y. Hasan, Abdallah Qtaishat, Michael E. Tierney, Raja Y. Zaghlol, Ayman A. Zayed
Frontiers in Endocrinology.2021;[Epub] CrossRef - Recommendations for management of diabetes during Ramadan: update 2020, applying the principles of the ADA/EASD consensus
Mahmoud Ibrahim, Melanie J Davies, Ehtasham Ahmad, Firas A Annabi, Robert H Eckel, Ebtesam M Ba-Essa, Nuha Ali El Sayed, Amy Hess Fischl, Pamela Houeiss, Hinde Iraqi, Ines Khochtali, Kamlesh Khunti, Shabeen Naz Masood, Safia Mimouni-Zerguini, Samad Shera,
BMJ Open Diabetes Research & Care.2020; 8(1): e001248. CrossRef - Ramadan and Diabetes: A Narrative Review and Practice Update
Syed H. Ahmed, Tahseen A. Chowdhury, Sufyan Hussain, Ateeq Syed, Ali Karamat, Ahmed Helmy, Salman Waqar, Samina Ali, Ammarah Dabhad, Susan T. Seal, Anna Hodgkinson, Shazli Azmi, Nazim Ghouri
Diabetes Therapy.2020; 11(11): 2477. CrossRef - A systematic review on efficacy and safety of the current hypoglycemic agents in patients with diabetes during Ramadan fasting
Fauzia Rashid, Elamin Abdelgadir
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(2): 1413. CrossRef - Risk of diabetic ketoacidosis during Ramadan fasting: A critical reappraisal
Salem A. Beshyah, Tahseen A. Chowdhury, Nazim Ghouri, Abdulfattah A. Lakhdar
Diabetes Research and Clinical Practice.2019; 151: 290. CrossRef
- Replicating human characteristics: A promising animal model of central fatigue

- Obesity and Metabolism
- Metformin-Associated Lactic Acidosis: Predisposing Factors and Outcome
- Min Ju Kim, Ju Young Han, Jun Young Shin, Shin Il Kim, Jeong Min Lee, Seongbin Hong, So Hun Kim, Moon Suk Nam, Yong Seong Kim
- Endocrinol Metab. 2015;30(1):78-83. Published online March 27, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.1.78
- 4,707 View
- 80 Download
- 28 Web of Science
- 27 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Metformin is considered the first choice oral treatment for type 2 diabetes patients in the absence of contraindications. Rarely, life-threatening complications associated with metformin treatment are seen in some patients with underlying diseases. The aim of this study was to further investigate the clinical profiles and risk factors for metformin-associated lactic acidosis (MALA) and the treatment modalities according to survival.
Methods To identify MALA, we performed a retrospective study in seven diabetic patients who were taking metformin and had been diagnosed with lactic acidosis at Inha University Hospital between 1995 and 2012. For each patient, we recorded the age, sex, daily metformin dosage, laboratory test results, admission diagnosis, and risk factors. Also, concurrent conditions, treatment modalities, and outcomes were evaluated.
Results Six patients had risk factors for lactic acidosis before admission. All patients had renal impairment on admission as a precipitating risk factor. Five patients survived and two patients died despite early renal replacement therapy. Older patients tended to have a poorer prognosis.
Conclusion Renal function must be monitored in elderly type 2 diabetes mellitus patients with underlying diseases and conditions causing renal impairment who begin metformin treatment. Accurate recognition of MALA and initiation of renal replacement are essential for treatment.
-
Citations
Citations to this article as recorded by- An Analysis of Clinical Outcomes of Exploratory Pediatric Metformin Ingestions Reported to the Texas Poison Center Network From 2011 to 2021
Shawn M. Varney, Sarah Watkins, Haylea Stuteville, Mark L. Winter, Han Tony Gao, Thomas G. Martin, Ryan P. Morrissey, Wayne R. Snodgrass, Brett A. Roth
Hospital Pharmacy.2024;[Epub] CrossRef - Metformin Treatment Reduces the Incidence of Rheumatoid Arthritis: A Two-Sample Mendelian Randomized Study
Jialin Liang, Yuanqing Cai, Jianan Zhang, Zhaopu Jing, Leifeng Lv, Guangyang Zhang, Rupeng Zhang, Ruiyu Liu, Kai Nan, Xiaoqian Dang
Journal of Clinical Medicine.2023; 12(7): 2461. CrossRef - PLGA-based microspheres loaded with metformin hydrochloride: Modified double emulsion method preparation, optimization, characterization, and in vitro evaluation
Priyanka Chauhan, Himanshu Paliwal, Chetan Singh Chauhan, Ankit Paliwal
Annales Pharmaceutiques Françaises.2023; 81(6): 997. CrossRef - Metformin-associated lactic acidosis: underlying multiple myeloma
Clara GOMES, Ana FERREIRA, Neuza SOARES, Vanessa CHAVES, Luís LEMOS, Sofia TAVARES, Marta COUTO
Gazzetta Medica Italiana Archivio per le Scienze Mediche.2022;[Epub] CrossRef - Metformin-associated lactic acidosis and factors associated with 30-day mortality
Kanin Thammavaranucupt, Boonchan Phonyangnok, Watanyu Parapiboon, Laddaporn Wongluechai, Watthikorn Pichitporn, Jirut Sumrittivanicha, Somnuek Sungkanuparph, Arkom Nongnuch, Kulapong Jayanama, Donovan Anthony McGrowder
PLOS ONE.2022; 17(8): e0273678. CrossRef - Metformin Associated Lactic Acidosis in Clinical Practice – A Case Series
Philipp Schädle, Otto Tschritter, Monika Kellerer
Experimental and Clinical Endocrinology & Diabetes.2021; 129(11): 842. CrossRef - Effect of continuous use of metformin on kidney function in diabetes patients with acute myocardial infarction undergoing primary percutaneous coronary intervention
Qi Yu, Jia-Jia Zhu, Wen-Xian Liu
BMC Cardiovascular Disorders.2020;[Epub] CrossRef - Metformin: current clinical applications in nondiabetic patients with cancer
Kailin Chen, Yajun Li, Zhen Guo, Yong Zeng, Wei Zhang, Hui Wang
Aging.2020; 12(4): 3993. CrossRef - Specifics of diabetes in old age
Markéta Kubíčková
Interní medicína pro praxi.2019; 21(4): 223. CrossRef - The Association between Metformin Therapy and Lactic Acidosis
Isabelle H. S. Kuan, Ruth L. Savage, Stephen B. Duffull, Robert J. Walker, Daniel F. B. Wright
Drug Safety.2019; 42(12): 1449. CrossRef - Metformin overdose: A serious iatrogenic complication—Western France Poison Control Centre Data Analysis
Alexandre Stevens, Jean‐François Hamel, Ali Toure, Samy Hadjadj, David Boels
Basic & Clinical Pharmacology & Toxicology.2019; 125(5): 466. CrossRef - Risk of Metformin-Associated Lactic Acidosis (MALA) in Patients After Gastric Bypass Surgery
Laura N. Deden, Edo O. Aarts, Stephanie C. W. Aelfers, Marcel M. G. J. van Borren, Ignace M. C. Janssen, Frits J. Berends, Hans de Boer
Obesity Surgery.2018; 28(4): 1080. CrossRef - Metformin-related lactic acidosis: Case report
Jesús Salvador Sánchez-Díaz, Enrique Monares-Zepeda, Enrique Antonio Martínez-Rodríguez, Jorge Samuel Cortés-Román, Oscar Torres-Aguilar, Karla Gabriela Peniche-Moguel, Susana Patricia Díaz-Gutiérrez, Eusebio Pin-Gutiérrez, Gerardo Rivera-Solís, Rosalba C
Colombian Journal of Anesthesiology.2017; 45(4): 353. CrossRef - Metformin-related lactic acidosis: Case report☆
Jesús Salvador Sánchez-Díaz, Enrique Monares-Zepeda, Enrique Antonio Martínez-Rodríguez, Jorge Samuel Cortés-Román, Oscar Torres-Aguilar, Karla Gabriela Peniche-Moguel, Susana Patricia Díaz-Gutiérrez, Eusebio Pin-Gutiérrez, Gerardo Rivera-Solís, Rosalba C
Colombian Journal of Anesthesiology.2017; 45(4): 353. CrossRef - Association between Metformin Use and Risk of Lactic Acidosis or Elevated Lactate Concentration in Type 2 Diabetes
Eun Young Lee, Sena Hwang, Yong-ho Lee, Seo Hee Lee, Young Mi Lee, Hua Pyong Kang, Eugene Han, Woonhyoung Lee, Byung-Wan Lee, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee
Yonsei Medical Journal.2017; 58(2): 312. CrossRef - Hypoglycemia and severe lactic acidosis in a dog following metformin exposure
Nicole Barrella, Beth Eisenberg, Stephanie Nicole Simpson
Clinical Case Reports.2017; 5(12): 2097. CrossRef - Acidosis láctica por metformina: reporte de caso
Jesús Salvador Sánchez-Díaz, Enrique Monares-Zepeda, Enrique Antonio Martínez-Rodríguez, Jorge Samuel Cortés-Román, Oscar Torres-Aguilar, Karla Gabriela Peniche-Moguel, Susana Patricia Díaz-Gutiérrez, Eusebio Pin-Gutiérrez, Gerardo Rivera-Solís, Rosalba C
Revista Colombiana de Anestesiología.2017; 45(4): 353. CrossRef - Towards natural mimetics of metformin and rapamycin
Alexander Aliper, Leslie Jellen, Franco Cortese, Artem Artemov, Darla Karpinsky-Semper, Alexey Moskalev, Andrew G. Swick, Alex Zhavoronkov
Aging.2017; 9(11): 2245. CrossRef - Metformin-Associated Lactic Acidosis
Martin R. Hevesy
Advanced Emergency Nursing Journal.2017; 39(1): 26. CrossRef - Metformin associated lactic acidosis (MALA): clinical profiling and management
Alessandra Moioli, Barbara Maresca, Andrea Manzione, Antonello Maria Napoletano, Daniela Coclite, Nicola Pirozzi, Giorgio Punzo, Paolo Menè
Journal of Nephrology.2016; 29(6): 783. CrossRef - Risk assessment and management of post-transplant diabetes mellitus
Eugene Han, Myoung Soo Kim, Yu Seun Kim, Eun Seok Kang
Metabolism.2016; 65(10): 1559. CrossRef - Temporal trends in the use of antidiabetic medicines: a nationwide 9-year study in older people living in New Zealand
Prasad S. Nishtala, Mohammed Saji Salahudeen
Therapeutic Advances in Drug Safety.2016; 7(5): 184. CrossRef - Metformin-associated lactic acidosis: Current perspectives on causes and risk
Ralph DeFronzo, G. Alexander Fleming, Kim Chen, Thomas A. Bicsak
Metabolism.2016; 65(2): 20. CrossRef - Lactic acidosis and the relationship with metformin usage
Weiyi Huang, Ronald L. Castelino, Gregory M. Peterson
Medicine.2016; 95(46): e4998. CrossRef - Metformin stimulates IGFBP-2 gene expression through PPARalpha in diabetic states
Hye Suk Kang, Ho-Chan Cho, Jae-Ho Lee, Goo Taeg Oh, Seung-Hoi Koo, Byung-Hyun Park, In-Kyu Lee, Hueng-Sik Choi, Dae-Kyu Song, Seung-Soon Im
Scientific Reports.2016;[Epub] CrossRef - Metformin-associated lactic acidosis: time to let it go?
Chantal Mathieu
Journal of Diabetes and its Complications.2015; 29(8): 974. CrossRef - Metformin: risk-benefit profile with a focus on cancer
Nicoletta Provinciali, Matteo Lazzeroni, Massimiliano Cazzaniga, Franco Gorlero, Barbara K Dunn, Andrea DeCensi
Expert Opinion on Drug Safety.2015; 14(10): 1573. CrossRef
- An Analysis of Clinical Outcomes of Exploratory Pediatric Metformin Ingestions Reported to the Texas Poison Center Network From 2011 to 2021

- Obesity and Metabolism
- Vav3, a GEF for RhoA, Plays a Critical Role under High Glucose Conditions
- Jie Sha, Jungsik Na, Jung Ok Lee, Nami Kim, Soo Kyung Lee, Ji Hae Kim, Ji Wook Moon, Su Jin Kim, Hye Jeong Lee, Jong-Il Choi, Sun Hwa Park, Hyeon Soo Kim
- Endocrinol Metab. 2014;29(3):363-370. Published online September 25, 2014
- DOI: https://doi.org/10.3803/EnM.2014.29.3.363
- 3,742 View
- 39 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The role of small GTPase molecules is poorly understood under high glucose conditions.
Methods We analyzed the expression pattern of Vav3 in skeletal muscle C2C12 cells under high glucose culture condition with reverse transcription-polymerase chain reaction and Western blot analysis. We also measured glucose uptake using isotope-labelled glucose.
Results We showed that expression of Vav3 (a guanine nucleotide exchange factor for RhoA) increased. mRNA and protein levels in skeletal muscle C2C12 cells under high glucose conditions. The AMP-activated protein kinase (AMPK) activator AMPK agonist 5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside (AICAR) suppressed high glucose-induced Vav3 induction. In addition, exposure of cells to high glucose concentration increased the phosphorylation of PAK-1, a molecule downstream of RhoA. The phosphorylation of paxillin, a downstream molecule of PAK-1, was also increased by exposure to high glucose. Phosphorylation of these molecules was not observed in the presence of AICAR, indicating that AMPK is involved in the RhoA signal pathway under high glucose conditions. Knock down of Vav3 enhances metformin-mediated glucose uptake. Inhibition of AMPK blocked the increases of Vav3 knock down-induced glucose uptake. Metformin-mediated Glut4 translocation was also increased by Vav3 knock-down, suggesting that Vav3 is involved in metformin-mediated glucose uptake.
Conclusion These results demonstrate that Vav3 is involved in the process of metformin-mediated glucose regulation.
-
Citations
Citations to this article as recorded by- Peripheral origin exosomal microRNAs aggravate glymphatic system dysfunction in diabetic cognitive impairment
Lin Zhang, Dongna Li, Pengrong Yi, Jiangwei Shi, Mengqing Guo, Qingsheng Yin, Dingbin Liu, Pengwei Zhuang, Yanjun Zhang
Acta Pharmaceutica Sinica B.2023; 13(7): 2817. CrossRef - A current overview of RhoA, RhoB, and RhoC functions in vascular biology and pathology
Robert Eckenstaler, Michael Hauke, Ralf A. Benndorf
Biochemical Pharmacology.2022; 206: 115321. CrossRef - Rho Family GTPases and Rho GEFs in Glucose Homeostasis
Polly A. Machin, Elpida Tsonou, David C. Hornigold, Heidi C. E. Welch
Cells.2021; 10(4): 915. CrossRef - Pharmacological Modulators of Small GTPases of Rho Family in Neurodegenerative Diseases
William Guiler, Addison Koehler, Christi Boykin, Qun Lu
Frontiers in Cellular Neuroscience.2021;[Epub] CrossRef - Association of VAV2 and VAV3 polymorphisms with cardiovascular risk factors
Nuria Perretta-Tejedor, Javier Fernández-Mateos, Luis García-Ortiz, Manuel A. Gómez-Marcos, José I. Recio-Rodríguez, Cristina Agudo-Conde, Emiliano Rodriguez-Sánchez, Ana I. Morales, Francisco J. López-Hernández, José M. López-Novoa, Rogelio González-Sarm
Scientific Reports.2017;[Epub] CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef
- Peripheral origin exosomal microRNAs aggravate glymphatic system dysfunction in diabetic cognitive impairment


 KES
KES

 First
First Prev
Prev



