Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(6); 2022 > Article
-
Original ArticleDiabetes, Obesity and Metabolism Metformin and Cervical Cancer Risk in Patients with Newly Diagnosed Type 2 Diabetes: A Population-Based Study in Korea
 Keypoint
Keypoint
The cancer-preventive properties of metformin are well-known, but insufficient data have been reported regarding its relationship to cervical cancer. Therefore, the authors investigated the association between metformin use and cervical cancer incidence in patients with newly diagnosed type 2 diabetes mellitus (T2DM). This retrospective cohort study compared 66,013 metformin users and 64,756 non-users. Metformin use in patients with newly diagnosed T2DM was associated with a lower risk of cervical cancer. Furthermore, a significant association was found between the use of metformin and cervical cancer in a dose- and duration-dependent manner and among those over 50 years old. -
Hyun Min Kim1
 , Min Jin Kang2, Sun Ok Song3
, Min Jin Kang2, Sun Ok Song3
-
Endocrinology and Metabolism 2022;37(6):929-937.
DOI: https://doi.org/10.3803/EnM.2022.1613
Published online: December 26, 2022
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
2Research Institute of National Health Insurance Service Ilsan Hospital, Goyang, Korea
3Division of Endocrinology and Metabolism, Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- Corresponding author: Sun Ok Song. Division of Endocrinology and Metabolism, Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, 100 Ilsan-ro, Ilsandong-gu, Goyang 10444, Korea Tel: +82-31-900-3470, Fax: +82-31-900-0519, E-mail: songsun7777@gmail.com
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Cervical cancer is a prevalent malignancy that is a major health problem for women worldwide. The cancer-preventive properties of metformin are well-known, but insufficient data have been reported regarding its relationship to cervical cancer. Therefore, in a nationwide population-based study, we investigated the association between metformin use and cervical cancer incidence in patients with newly diagnosed type 2 diabetes.
-
Methods
- This retrospective cohort study used the Korean National Health Insurance claims database. Individuals newly diagnosed with type 2 diabetes between January 2005 and December 2009 were included. The occurrence of cervical cancer was explored by matching for age, economic status, region of residence, and use of anti-diabetic medication.
-
Results
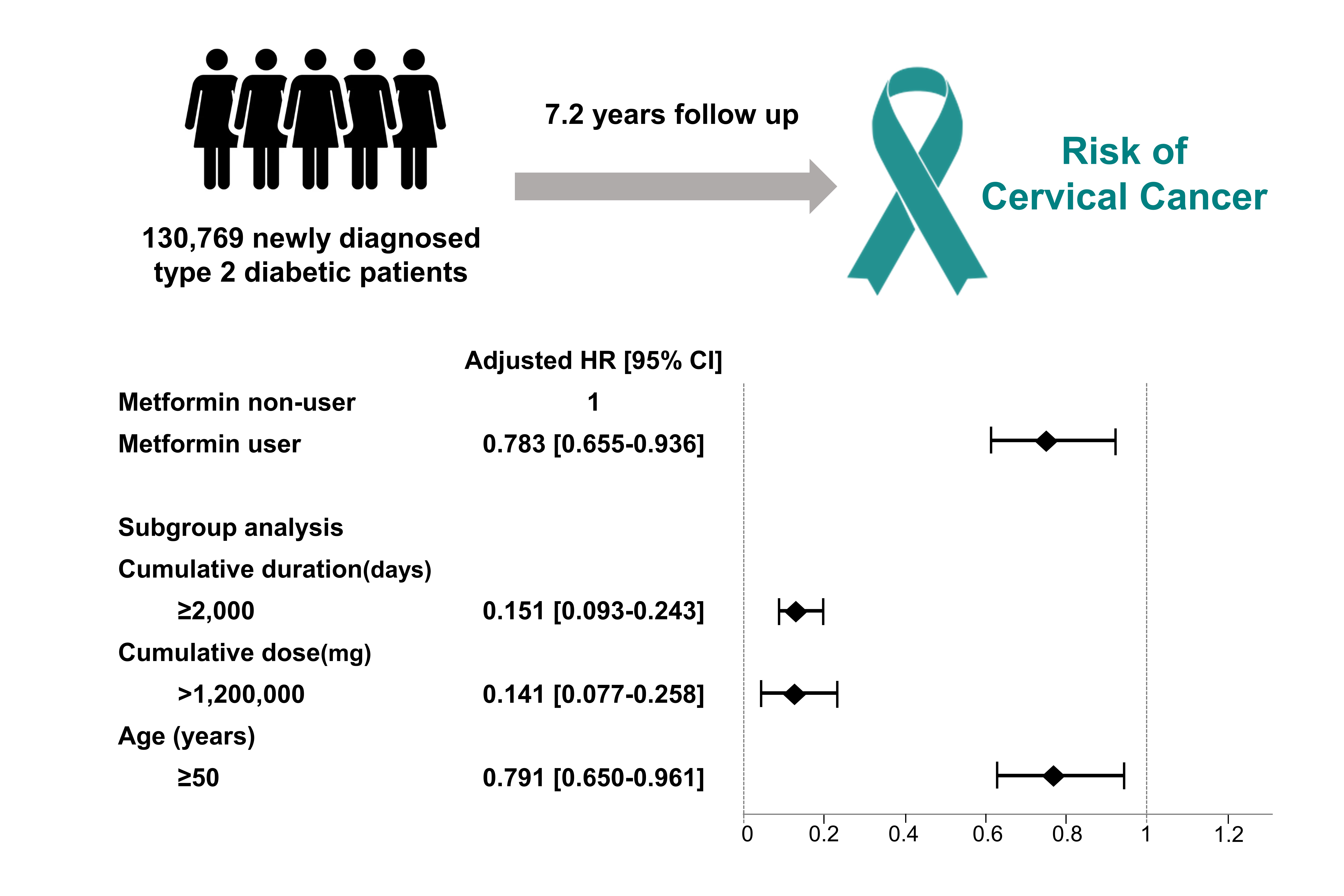
- In total, 66,013 metformin users and 64,756 non-users were analyzed. Cervical cancer occurred in 219 metformin users (0.33%) and 274 metformin non-users (0.42%) (hazard ratio [HR], 0.783; 95% confidence interval [CI], 0.655 to 0.036; P=0.007). Moreover, cervical cancer risk was considerably reduced in those treated with a high dose (>1,200,000 mg) or for an extended period (≥2,000 days) compared to non-users (HR, 0.151; 95% CI, 0.093 to 0.243; P<0.001; and HR, 0.141; 95% CI, 0.077 to 0.258; P<0.001). The incidence was also significantly lower in metformin users among those over 50 years old (HR, 0.791; 95% CI, 0.650 to 0.961; P<0.001).
-
Conclusion
- Metformin use in patients with newly diagnosed diabetes was associated with a lower risk of cervical cancer in Korea. Furthermore, a significant association was found between the use of metformin and cervical cancer in a dose- and duration-dependent manner and among those over 50 years old.
- Diabetes mellitus and cancer are the two most significant health issues worldwide. Multiple epidemiological studies have proven that type 2 diabetes increases both the risk of cancer and the mortality rate from cancer [1]. Cervical cancer is the most prevalent gynecological cancer and the fourth most common cancer in women, with an incidence of 500,000 cases each year. It is also the fourth leading cause of cancer-related mortality [2]. Although incidence is on the decline due to efforts such as human papillomavirus (HPV) vaccination and cytological screening, a substantial number of patients are still newly diagnosed in Korea [3].
- Metformin is the most commonly used anti-diabetic drug, and it has been reported to help prevent or treat various cancers [4]. In addition to improving insulin resistance, metformin has been found to exert anti-cancer effects through multiple mechanisms such as cell cycle arrest via two major signaling pathways, the AMP-activated protein kinase (AMPK) pathway and the phosphatidyl inositol 3-kinase (PI3K) pathway [5]; the modulation of mitochondrial energy metabolism in cancer cells [6]; anti-inflammatory reactions [7]; epigenetic modification [8]; immune modulation [9]; and anti-angiogenic effects [10]. Some of those anti-cancer effects have been studied in cervical cancer cells. However, unlike many preclinical results, epidemiological studies are limited on whether the use of metformin prevents cervical cancer.
- This study aimed to investigate the association between metformin use and the risk of cervical cancer in patients with newly diagnosed type 2 diabetes using claims data from the National Health Insurance Service (NHIS) database in Korea. In addition, we evaluated the impact of metformin use on the development of cervical cancer by cumulative metformin dose, cumulative treatment duration, and age.
INTRODUCTION
- Data source
- This retrospective study evaluated claims data between January 1, 2005, and December 31, 2014 (database no. NHIS-2021-1-473) in the NHIS database. The NHIS is a compulsory national insurance system covering the entire Korean population. The NHIS database has been widely utilized in various epidemiological and health policy studies. The background and data configuration of NHIS have been documented elsewhere in detail [11]. The authors alone are responsible for the content and writing of the paper. The Institutional Review Board of National Health Insurance Service Ilsan Hospital approved this study (Institutional Review Board No: NHIMC-2021-03-053). The need for written informed permission from each subject was waived.
- Study population
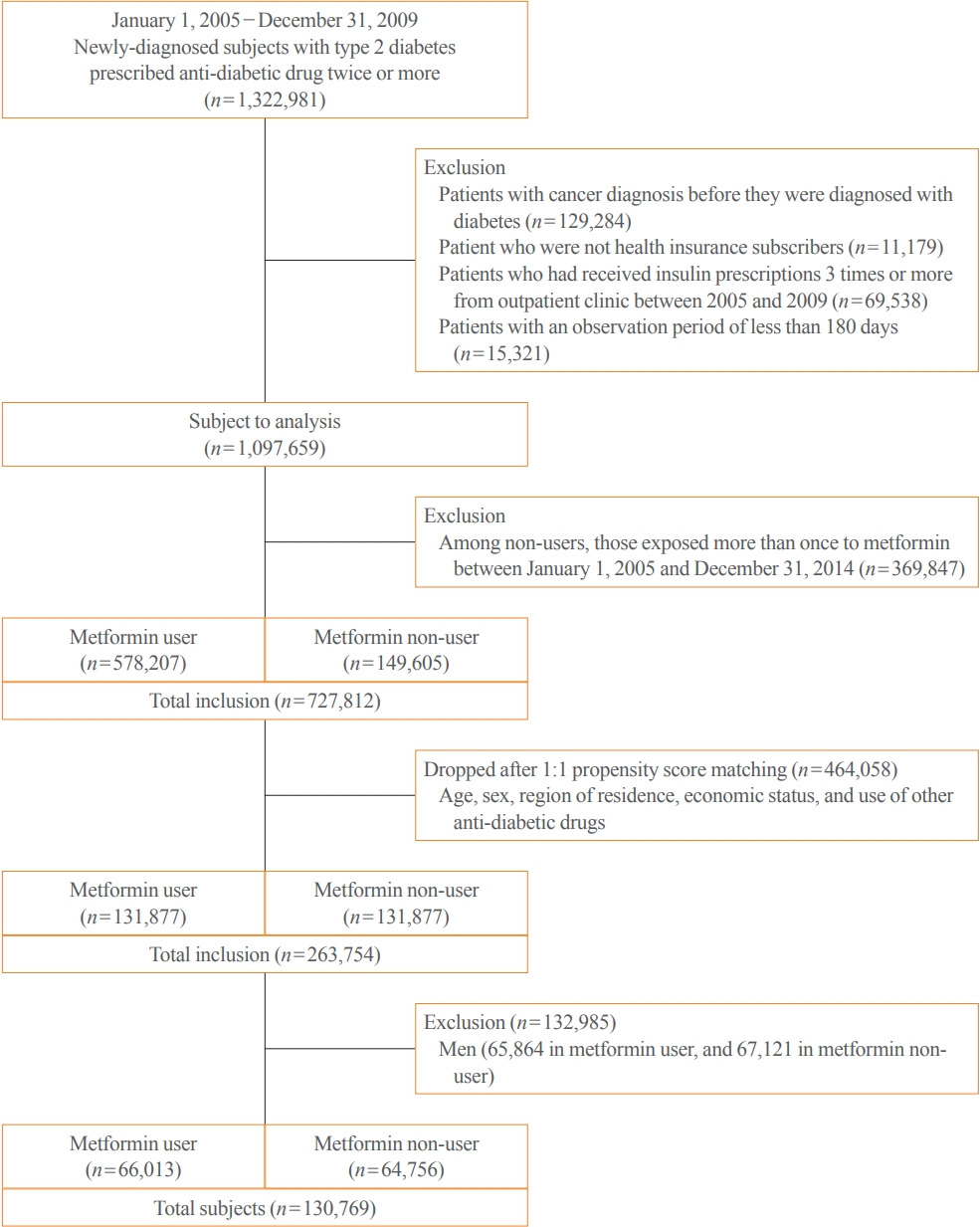
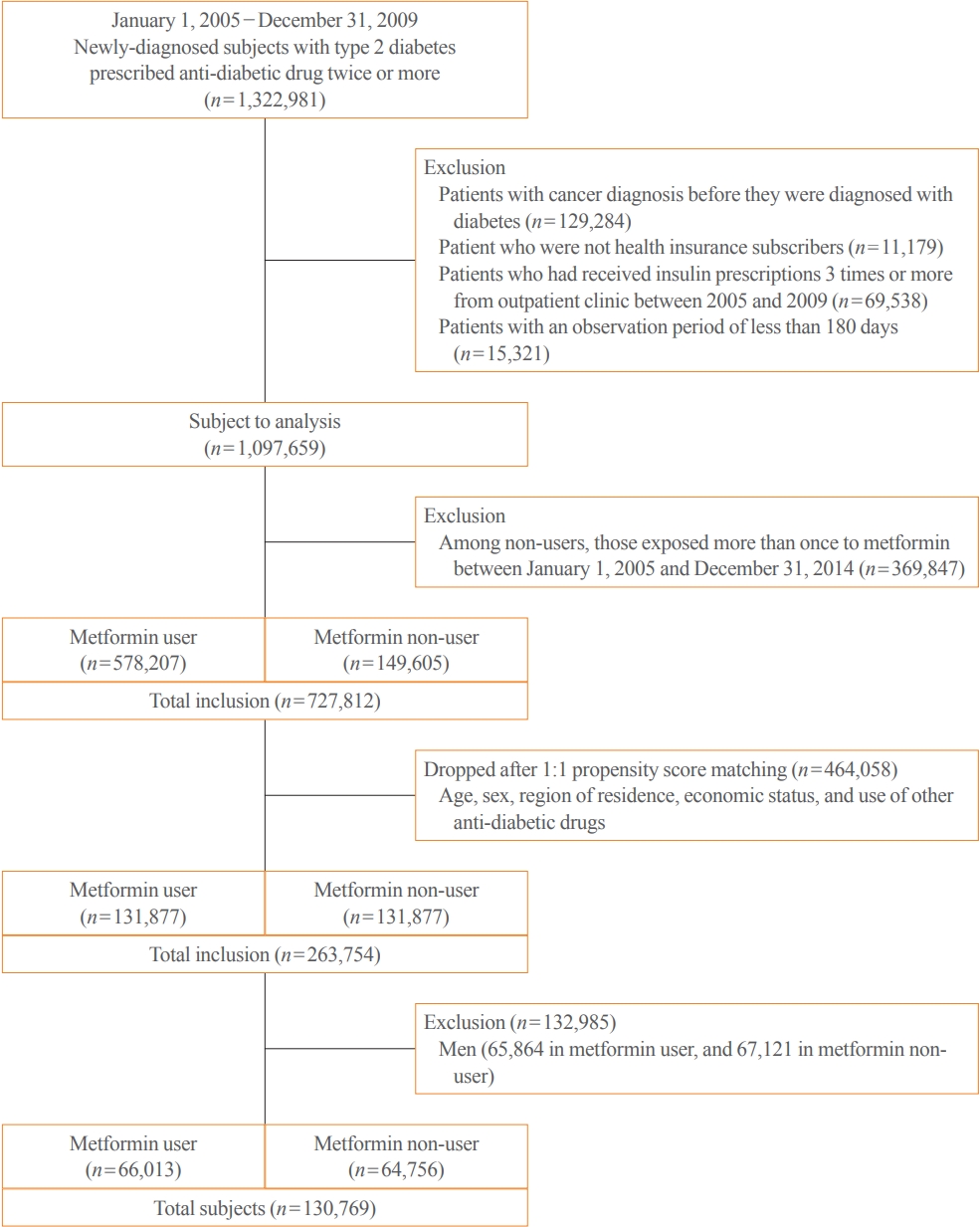
- This study evaluated individuals with newly diagnosed type 2 diabetes between January 2005 and December 2009. We recruited patients diagnosed with diabetes using the International Classification of Diseases, 10th Revision (ICD-10) Clinical Modification codes E11–E14, and who had also been prescribed anti-diabetic medication at least once. Using the same approach as in previous studies [12,13], we identified participants with newly diagnosed diabetes by excluding those who submitted claims for anti-diabetic drugs or diabetes-related claim codes in the 3 years preceding January 2005. We determined 1,322,981 participants to be eligible based on these criteria. After that, we excluded patients diagnosed with cancer before the diagnosis of diabetes and those who were not followed for at least 180 days after the diagnosis of diabetes. We also excluded patients prescribed insulin more than twice during the study period. The reason for excluding those patients was that they are highly likely to have had poor glycemic control, potentially making the clinical characteristics of the subjects more heterogeneous; we also wanted to rule out the possible effect of insulin on the development or progression of cervical cancer.
- Definition of metformin exposure
- Metformin users were defined as individuals who had been prescribed metformin for more than 180 days throughout the 365-day study period; non-users were those who had never used metformin or had received a prescription for less than 180 days in the entire study period. Then, 149,605 non-users and 578,207 metformin users were eligible. They were matched by age, sex, region of residence, economic status, and other anti-diabetic drugs. We enrolled only women to track cervical cancer incidence among 131,877 metformin users and 131,877 non-users in the matched populations (Fig. 1).
- Definitions and covariates
- Cervical cancer was diagnosed with the ICD-10 code C53. We calculated the follow-up duration from inclusion date to cervical cancer diagnosis, death, or the end of the study period. The age of the subjects was divided into 5-year interval for analysis, and the economic status was classified into quartiles. Residential areas were classified into three groups: Seoul (the Korean capital), metropolitan cities (Busan, Daegu, Daejeon, Gwangju, Incheon, and Ulsan), and rural areas. Non-metformin anti-diabetic medications included sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, alpha-glucosidase inhibitors, and meglitinides. Their utilization was collected from claims data and examined as categorical variables.
- Statistical analyses
- The incidence risk of cervical cancer between metformin users and non-users was compared using the Cox proportional hazard regression model adjusted for age, economic status, residential area, and anti-diabetic medications other than metformin. The results of the analysis were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). P values below 0.05 were considered to indicate statistical significance. All analyses were performed using SAS version 9.4 statistical software (SAS Institute, Cary, NC, USA).
METHODS
- Baseline characteristics
- The final cohort included 66,013 metformin users and 64,756 metformin non-users. Table 1 summarizes the demographic information of the study participants. Metformin users were older, had a higher proportion of patients enrolled later in the research, had slightly fewer residents in the capital city, and were more frequently prescribed sulfonylureas or alpha-glucosidase inhibitors than metformin non-users. The economic status of the two groups did not differ significantly; nor did the prescription of thiazolidinediones, dipeptidyl peptidase-4 inhibitors, or meglitinides. The mean daily dose of metformin was 519±148 mg, the mean cumulative dose was 865,999±547,170 mg, and the mean cumulative treatment duration was 1,666±912 days in metformin users.
- Cervical cancer incidence according to metformin use
- When followed up for an average of 7.2 years, cervical cancer occurred in 219 metformin users (0.33%) and 274 metformin non-users (0.42%). Table 2 displays the incidence rate and HRs of cervical cancer according to metformin exposure. The incidence rate of cervical cancer per 100,000 person-years was 24.2 in metformin users and 30.5 in metformin non-users. According to the multiple-adjusted HR, the risk of cervical cancer in metformin users was significantly lower than in metformin non-users (HR, 0.783; 95% CI, 0.655 to 0.936; P=0.007) (Table 2). We also evaluated the risk of cervical cancer according to the cumulative dose and duration of metformin treatment. A dose-and duration-dependent protective effect on cervical cancer development was observed. The risk of cervical cancer was clearly low among those who received the highest category of cumulative dose (>1,200,000 mg), and similar results were found among those treated for the longest period analyzed (≥2,000 days) compared to metformin non-users (HR, 0.151; 95% CI, 0.093 to 0.243; P<0.00; and HR, 0.141; 95% CI, 0.077 to 0.258; P<0.001, respectively). In an analysis according to age, the incidence of cervical cancer was significantly lower in metformin users over 50 years old (HR, 0.791; 95% CI, 0.650 to 0.961; P<0.001), but a non-statistically significant tendency for risk reduction was noted among those under 50 years old (HR, 0.763; 95% CI, 0.481 to 1.252; P=0.235) (Table 3).
RESULTS
- The present study showed a protective effect of metformin against cervical cancer development in women with newly diagnosed type 2 diabetes using a nationwide population-based database in Korea. A significant association was found between the use of metformin and cervical cancer risk in a dose- and duration-dependent manner. The effect was much stronger if patients were treated with a high cumulative dose or if metformin was taken for longer than 5 years. In contrast, no significant effect was observed during the early period of metformin use or with a lower cumulative dose.
- The protective effects and the underlying mechanisms of metformin on various malignancies, including lung, hepatic carcinoma, breast, and colorectal cancers, have been well studied [13]. Research on the mechanisms of metformin’s action in cervical cancer has found that metformin inhibited the transforming growth factor-β1-induced epithelial-to-mesenchymal transition signaling pathway in cervical carcinoma cells [14], suppressed the migration of cancer cells, and induced apoptosis through increased expression of the p-AMPK-activated protein kinase and the suppressor p53 protein [15]. In addition, inactivation mutations of liver kinase B1 (LKB1), a tumor suppressor, can contribute to the development and progression of various solid cancers, including cervical cancer. Xiao et al. [16] investigated the effect of metformin and LKB1 activity in cervical cancer cell lines. They found that metformin could induce both apoptosis and autophagy in cervical cancer cells with intact LKB1 expression via the activation of LKB1-AMPK signaling [16]. Another study revealed that metformin treatment induced apoptosis, cell cycle arrest, and an enhancement of natural killer cell cytotoxicity in cervical cancer cells [17]. These data suggest that metformin may function as a multi-target inhibitor in various processes of cervical cancer.
- However, despite the mechanistic evidence that has been reported to date, there have been few epidemiological studies on metformin and the risk of cervical cancer. According to our knowledge, only one population-based cohort study from Taiwan has investigated the association between metformin use and the incidence of cervical cancer in women with type 2 diabetes [18]. Metformin use was associated with a significantly lower risk of cervical cancer (HR, 0.558; 95% CI, 0.401 to 0.778). In particular, similar to our findings, a longer cumulative duration was associated with a stronger preventative benefit. No significant difference was found in cervical cancer incidence between metformin users and non-users when the drug was administered for less than 23 months. However, when metformin was administered for 23.0 to 47.9 months, the HR was 0.523, and when treated for 47.9 months or more, the HR was 0.109, indicating a substantial reduction compared to metformin non-users. In addition, an observational analysis validated the incidence rates of several malignancies, including cervical cancer, according to drug exposure in two randomized controlled trials [19]. The extracted data for malignancies from these two trials—A Diabetes Outcome Progression Trial (ADOPT) and Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes (RECORD)—did not show a reduction in the overall cancer risk. In ADOPT, however, the number of cervical/uterine cancers was low (four in nonmetformin users and one in metformin users), and in the RECORD trial, the incidence data were presented as uterine cancers, limiting the ability of the data to shed light on the impact of metformin on cervical cancer specifically. Two meta-analyses have examined the association between metformin use and cervical cancer risk [20,21]. A 2019 meta-analysis confirmed that the use of metformin was associated with a reduced risk of gynecological cancer incidence [20]. A subgroup analysis revealed a lower risk of cervical cancer in the Asian population, but no significant difference in the Caucasian population. Only the two studies listed above were included in the cervical cancer analysis [18,19]. The latest meta-analysis examining the relationship between metformin use and the incidence and prognosis of gynecological cancer found no evidence that metformin use reduced the overall risk of gynecological cancer. No detailed analysis regarding the type of cancer was performed [21].
- A strength of this study is that it examined in depth only cases of cervical cancer in a database containing the entire population of newly diagnosed type 2 diabetes cases. In addition to cervical cancer, gynecological cancers include endometrial, ovarian, and other cancers. Although these diseases occur in sites closely located to each other, each of these cancers has a unique mechanism of pathogenesis and distinct risk factors. Studies on the effectiveness of metformin have also shown a wide variety of results [22]. Therefore, combining these cancers makes it difficult to interpret the results and may lead to confusion. Our study also included a sufficient number of young people, the age group most prone to developing cervical cancer. In a subgroup analysis by age, significant risk reduction was only observed in subjects under 50 years. One possible explanation relates to the impact of the length of metformin administration and the cumulative dose. It is also possible that age-related differences in cervical cancer incidence contributed to this result. The incidence of cervical cancer in Korea in younger patients has recently increased, but the economic burden is higher in older patients due to more comorbidities and higher disease severity [23]. Therefore, it is expected that the effect of metformin on cervical cancer may substantially reduce medical expenses.
- This study demonstrated that the protective effect of metformin against cervical cancer was dose- and duration-dependent. Compared to metformin non-users, the risk of cervical cancer was high when metformin was administered at an early stage or at a relatively low cumulative dose. Obesity has been suggested as a weak risk factor for cervical cancer [24]. Although the claims data did not contain information on subjects’ body mass index, we might carefully consider the possibility that physicians preferred to prescribe metformin for obese patients with a slightly higher risk of cervical cancer. This might have resulted in contradictory findings during the early phase of metformin use. In addition, because there were slight differences in the region of residence of the metformin users, there may have been differences in the opportunity for cervical cancer detection through the frequent usage of healthcare services or gynecological screening examinations, and the adjustment performed in this study may not have accounted for this phenomenon. The effect of metformin on reducing cancer incidence was evident after substantial doses were given for a sufficient length of time in previous observational studies [25,26].
- This analysis did not present data about metformin-related cervical cancer outcomes, because it was beyond the scope of this study. However, several observational studies on metformin use and the outcomes of cervical cancer have been published. A retrospective cohort study using the Ontario health database showed that metformin use after cervical cancer diagnosis among older women (>66 years) with diabetes might be associated with a significant decrease in mortality [27]. For every additional 365 g of metformin, there was a 21.4% decrease in cervical cancer–specific mortality. A study from Thailand also showed benefits in disease-free survival in metformin users, but not in overall survival [28]. However, another study in the United States showed no survival benefit at all [29]. A meta-analysis including all three of the above studies showed that metformin use did not affect the overall survival of cervical cancer [21]. In addition to studies that have elucidated the mechanisms of metformin’s anti-cancer effects against cervical cancer cells [15-17], it has recently been observed that adding metformin to conventional therapy can enhance the therapeutic efficacy [30]. Based on the promising results of preclinical studies, a clinical trial evaluating the efficacy of adding metformin to standard chemoradiation treatment for women without diabetes has been completed (NCT02394652), and another is now ongoing (NCT04275713). These findings will clarify whether metformin has a therapeutic effect on cervical cancer.
- This study has some limitations. First, important risk factors for cervical cancer, such as HPV infection or vaccination, sexual behavior, and smoking, as well as information regarding cervical cancer screening that could contribute to a detection bias, could not be considered because they were not included in the claims. Second, the claims data lack laboratory test findings. Thus we were unable to evaluate the level of glycemic control. We believed, however, that the effect of an extreme gap in glycemic control was attenuated since we matched individuals newly diagnosed with diabetes and maintained comparable prescriptions of medications, with the exclusion of those who received insulin therapy. Third, cervical cancer has various stages and prognoses, ranging from carcinoma in situ to invasive cancer. However, it was not possible to analyze these details. Fourth, the cumulative doses and durations of anti-diabetic drugs other than metformin were not considered. Lastly, information on drug adherence was not available.
- In conclusion, this population-based data suggest that metformin use could be associated with reduced cervical cancer risk in Korea. A significant association was found between the use of metformin and cervical cancer in a dose- and duration-dependent manner. In individuals with diabetes who had a longer treatment duration and higher cumulative dose, the risk reduction for cervical cancer was very strong; however, this association was not found in the early phase of treatment, in subjects treated with an insufficient cumulative doses, and in the younger age group. Future large-scale epidemiological research, including sufficient information on the major cervical cancer risk factors in a variety of racial and ethnic groups, may contribute to validating the cervical cancer-preventative effect of metformin.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: M.J.K., S.O.S. Acquisition, analysis, or interpretation of data: H.M.K., M.J.K., S.O.S. Drafting the work or revising: H.M.K. Final approval of the manuscript: H.M.K., S.O.S.
Article information
-
Acknowledgements
- This work was supported by a grant from National Health Insurance Ilsan Hospital (NHIMC-2017-20-031).
| Characteristic | Metformin non-users (n=64,756) | Metformin users (n=66,013) |
|---|---|---|
| Age, yra | 63.9±16.4 | 64.2±13.7 |
| 0–14 | 269 (0.4) | 212 (0.3) |
| 15–20 | 163 (0.3) | 226 (0.3) |
| 20–24 | 333 (0.5) | 295 (0.4) |
| 25–29 | 1,145 (1.8) | 422 (0.6) |
| 30–34 | 1,711 (2.6) | 571 (0.9) |
| 35–39 | 1,593 (2.5) | 1,205 (1.8) |
| 40–44 | 2,274 (3.5) | 2,050 (3.1) |
| 45–49 | 3,543 (5.5) | 3,633 (5.5) |
| 50–54 | 4,890 (7.6) | 5,203 (7.9) |
| 55–59 | 5,792 (8.9) | 5,534 (8.4) |
| 60–64 | 7,540 (11.6) | 9,967 (15.1) |
| 65–69 | 9,960 (15.4) | 11,399 (17.3) |
| 70–74 | 8,784 (13.6) | 11,979 (18.1) |
| 75–79 | 7,962 (12.3) | 8,007 (12.1) |
| ≥80 | 8,797 (13.6) | 5,310 (8.0) |
| Year of study enrollmenta | ||
| 2005 | 15,370 (23.7) | 16,125 (24.4) |
| 2006 | 14,158 (21.9) | 11,613 (17.6) |
| 2007 | 12,823 (19.8) | 12,209 (18.5) |
| 2008 | 11,863 (18.3) | 12,704 (19.2) |
| 2009 | 10,542 (16.3) | 13,362 (20.2) |
| Economic status | ||
| 1–5 (lowest) | 21,860 (33.8) | 22,175 (33.6) |
| 6–10 | 11,162 (17.2) | 11,452 (17.3) |
| 11–15 | 14,349 (22.2) | 14,346 (21.7) |
| 16–20 (highest) | 17,385 (26.8) | 18,040 (27.3) |
| Region of residenceb | ||
| Seoul (capital city) | 13,558 (20.9) | 13,510 (20.5) |
| Metropolitan cities | 15,690 (24.2) | 16,344 (24.8) |
| Rural areas | 35,508 (54.8) | 36,159 (54.8) |
| Anti-diabetic drugs | ||
| Metformin | ||
| Yes | 0 | 66,013 (100.0) |
| No | 64,756 (100.0) | 0 |
| Sulfonylureasb | ||
| Yes | 30,135 (46.5) | 30,155 (45.7) |
| No | 34,621 (53.5) | 35,858 (54.3) |
| TZD | ||
| Yes | 2,190 (3.4) | 2,204 (3.3) |
| No | 62,566 (96.6) | 63,809 (96.7) |
| DPP4i | ||
| Yes | 1,140 (1.8) | 1,138 (1.7) |
| No | 63,616 (98.2) | 64,875 (98.3) |
| AGIa | ||
| Yes | 5,812 (9.0) | 5,534 (8.4) |
| No | 58,944 (91.0) | 60,479 (91.6) |
| Meglitinide | ||
| Yes | 1,968 (3.0) | 1,939 (2.9) |
| No | 62,788 (97.0) | 64,074 (97.1) |
| Daily metformin dose, mean (range), mg | 519 (148) | |
| Cumulative metformin dose, mean (range), mg | 865,999 (547,170) | |
| None | 64,756 (100.0) | 0 |
| 0–239,999 | 0 | 9,611 (14.6) |
| 240,000–1,200,000 | 0 | 39,900 (60.4) |
| >1,200,000 | 0 | 16,502 (25.0) |
| Cumulative duration of therapy, mean (range), day | 1,666 (912) | |
| None | 64,756 (100.0) | 0 |
| 0–469 | 0 | 9,161 (13.9) |
| 470–1,999 | 0 | 30,429 (46.1) |
| ≥2,000 | 0 | 26,423 (40.0) |
| Cervical cancer cases | 274 | 219 |
|
Cancer cases (%) |
Incidence of cancer, /100,000 person-years |
Hazard ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Metformin non-user | Metformin user | Metformin non-user | Metformin user | Univariate | Multivariatea |
| 274 (0.42) | 219 (0.33) | 30.5 | 24.2 | 0.805 (0.674–0.961)b | 0.783 (0.655–0.936)b |
CI, confidence interval.
a The comparisons were made using metformin non-users as the reference group. The hazard ratio was adjusted for age, economic status, region of residence, and anti-diabetic medications (sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, alpha-glucosidase inhibitors, and meglitinide);
b P<0.05.
| Variable | Hazard ratio (95% CI)a | Hazard ratio (95% CI)b |
|---|---|---|
| Cumulative duration, day | ||
| Non-users | 1.000 | - |
| 0–469 | 1.603 (1.205–2.131)d | 1.000 |
| 470–1,999 | 1.140 (0.931–1.396)c | 0.723 (0.530–0.095)d |
| ≥2,000 | 0.151 (0.093–0.243)c | 0.094 (0.055–0.161)c |
| Cumulative dose, mg | ||
| Non-users | 1.000 | - |
| 0–239,999 | 1.798 (1.376–2.349)c | 1.000 |
| 240,000–1,200,000 | 0.849 (0.692–1.042)c | 0.549 (0.356–0.642)c |
| >1,200,000 | 0.141 (0.077–0.258)c | 0.079 (0.042–0.151)c |
| Age, yr | ||
| Non-users | 1.000 | - |
| <50 | 0.763 (0.481–1.252)d | - |
| ≥50 | 0.791 (0.650–0.961)d | - |
CI, confidence interval.
a The comparisons were made using metformin non-users as the reference group. The hazard ratios were adjusted for age, economic status, region of residence, and anti-diabetic medications (sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, alpha-glucosidase inhibitors, and meglitinide);
b The comparisons were made using a cumulative duration of 0–469 days or a cumulative dose of 0–239,999 mg as the reference groups. The hazard ratios were adjusted for age, economic status, region of residence, and anti-diabetic medications (sulfonylureas, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, alpha-glucosidase inhibitors, and meglitinide);
c P<0.001;
d P<0.05.
- 1. Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG, et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 2012;55:1607–18.ArticlePubMedPDF
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49.ArticlePubMedPDF
- 3. Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat 2021;53:301–15.ArticlePubMedPMCPDF
- 4. Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab 2013;24:469–80.ArticlePubMed
- 5. Zhao B, Luo J, Yu T, Zhou L, Lv H, Shang P. Anticancer mechanisms of metformin: a review of the current evidence. Life Sci 2020;254:117717.ArticlePubMed
- 6. Gao ZY, Liu Z, Bi MH, Zhang JJ, Han ZQ, Han X, et al. Metformin induces apoptosis via a mitochondria-mediated pathway in human breast cancer cells in vitro. Exp Ther Med 2016;11:1700–6.ArticlePubMedPMC
- 7. Chu NJ, Armstrong TD, Jaffee EM. Nonviral oncogenic antigens and the inflammatory signals driving early cancer development as targets for cancer immunoprevention. Clin Cancer Res 2015;21:1549–57.ArticlePubMedPMCPDF
- 8. Li K, Zhang TT, Wang F, Cui B, Zhao CX, Yu JJ, et al. Metformin suppresses melanoma progression by inhibiting KAT5-mediated SMAD3 acetylation, transcriptional activity and TRIB3 expression. Oncogene 2018;37:2967–81.ArticlePubMedPDF
- 9. Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res 2018;78:1779–91.ArticlePubMedPMCPDF
- 10. Wang J, Li G, Wang Y, Tang S, Sun X, Feng X, et al. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2015;6:44579–92.ArticlePubMedPMC
- 11. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the Korean National Health Insurance and Health Insurance Review & Assessment database. Diabetes Metab J 2020;44:671–8.ArticlePubMedPMCPDF
- 12. Cho YY, Kang MJ, Kim SK, Jung JH, Hahm JR, Kim TH, et al. Protective effect of metformin against thyroid cancer development: a population-based study in Korea. Thyroid 2018;28:864–70.ArticlePubMed
- 13. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–5.ArticlePubMedPMC
- 14. Cheng K, Hao M. Metformin inhibits TGF-β1-induced epithelial-to-mesenchymal transition via PKM2 relativemTOR/p70s6k signaling pathway in cervical carcinoma cells. Int J Mol Sci 2016;17:2000.ArticlePubMedPMC
- 15. Chen YH, Yang SF, Yang CK, Tsai HD, Chen TH, Chou MC, et al. Metformin induces apoptosis and inhibits migration by activating the AMPK/p53 axis and suppressing PI3K/AKT signaling in human cervical cancer cells. Mol Med Rep 2021;23:88.ArticlePubMedPMC
- 16. Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, et al. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol 2012;127:249–55.ArticlePubMedPMC
- 17. Xia C, Liu C, He Z, Cai Y, Chen J. Metformin inhibits cervical cancer cell proliferation by modulating PI3K/Akt-induced major histocompatibility complex class I-related chain A gene expression. J Exp Clin Cancer Res 2020;39:127.ArticlePubMedPMCPDF
- 18. Tseng CH. Metformin use and cervical cancer risk in female patients with type 2 diabetes. Oncotarget 2016;7:59548–55.ArticlePubMedPMC
- 19. Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti G, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53:1838–45.ArticlePubMedPMC
- 20. Wen Q, Zhao Z, Wen J, Zhou J, Wu J, Lei S, et al. The association between metformin therapy and risk of gynecological cancer in patients: two meta-analyses. Eur J Obstet Gynecol Reprod Biol 2019;237:33–41.ArticlePubMed
- 21. Yao K, Zheng H, Li T. Association between metformin use and the risk, prognosis of gynecologic cancer. Front Oncol 2022;12:942380.ArticlePubMedPMC
- 22. Imai A, Ichigo S, Matsunami K, Takagi H, Yasuda K. Clinical benefits of metformin in gynecologic oncology. Oncol Lett 2015;10:577–82.ArticlePubMedPMC
- 23. Kim J, Lee D, Son KB, Bae S. The burden of cervical cancer in Korea: a population-based study. Int J Environ Res Public Health 2020;17:6308.ArticlePubMedPMC
- 24. Poorolajal J, Jenabi E. The association between BMI and cervical cancer risk: a meta-analysis. Eur J Cancer Prev 2016;25:232–8.PubMed
- 25. Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–5.PubMedPMC
- 26. Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61.ArticlePubMedPDF
- 27. Han K, Pintilie M, Lipscombe LL, Lega IC, Milosevic MF, Fyles AW. Association between metformin use and mortality after cervical cancer in older women with diabetes. Cancer Epidemiol Biomarkers Prev 2016;25:507–12.ArticlePubMedPDF
- 28. Hanprasertpong J, Jiamset I, Geater A, Peerawong T, Hemman W, Kornsilp S. The effect of metformin on oncological outcomes in patients with cervical cancer with type 2 diabetes mellitus. Int J Gynecol Cancer 2017;27:131–7.ArticlePubMed
- 29. Takiuchi T, Machida H, Hom MS, Mostofizadeh S, Frimer M, Brunette LL, et al. Association of metformin use and survival outcome in women with cervical cancer. Int J Gynecol Cancer 2017;27:1455–63.ArticlePubMedPMC
- 30. Tang ZY, Sheng MJ, Qi YX, Wang LY, He DY. Metformin enhances inhibitive effects of carboplatin on HeLa cell proliferation and increases sensitivity to carboplatin by activating mitochondrial associated apoptosis signaling pathway. Eur Rev Med Pharmacol Sci 2018;22:8104–12.PubMed
References
Figure & Data
References
Citations

- Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp
Mohamad Ali Hijazi, André Gessner, Nahed El-Najjar
Cancers.2023; 15(12): 3199. CrossRef - Network-based drug repurposing for HPV-associated cervical cancer
Faheem Ahmed, Young Jin Yang, Anupama Samantasinghar, Young Woo Kim, Jeong Beom Ko, Kyung Hyun Choi
Computational and Structural Biotechnology Journal.2023; 21: 5186. CrossRef - The Use of Metformin and Postoperative Insulin Pump Were Predictive Factors for Outcomes of Diabetic Colorectal Cancer Patients after Surgery
Xu-Rui Liu, Fei Liu, Zi-Wei Li, Quan Lv, Xin-Peng Shu, Lian-Shuo Li, Yue Tong, Wei Zhang, Dong Peng
Nutrition and Cancer.2023; 75(10): 1926. CrossRef
- Figure
- Related articles
-
- Association between Smoking Status and the Risk of Hip Fracture in Patients with Type 2 Diabetes: A Nationwide Population-Based Study
- Prediction of Cardiovascular Complication in Patients with Newly Diagnosed Type 2 Diabetes Using an XGBoost/GRU-ODE-Bayes-Based Machine-Learning Algorithm
- Increased Risk of Hip Fracture in Patients with Acromegaly: A Nationwide Cohort Study in Korea
- Incretin and Pancreatic β-Cell Function in Patients with Type 2 Diabetes
- Sleep Duration and the Risk of Type 2 Diabetes: A Community-Based Cohort Study with a 16-Year Follow-up

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite