Metformin-Associated Lactic Acidosis: Predisposing Factors and Outcome
Article information
Abstract
Background
Metformin is considered the first choice oral treatment for type 2 diabetes patients in the absence of contraindications. Rarely, life-threatening complications associated with metformin treatment are seen in some patients with underlying diseases. The aim of this study was to further investigate the clinical profiles and risk factors for metformin-associated lactic acidosis (MALA) and the treatment modalities according to survival.
Methods
To identify MALA, we performed a retrospective study in seven diabetic patients who were taking metformin and had been diagnosed with lactic acidosis at Inha University Hospital between 1995 and 2012. For each patient, we recorded the age, sex, daily metformin dosage, laboratory test results, admission diagnosis, and risk factors. Also, concurrent conditions, treatment modalities, and outcomes were evaluated.
Results
Six patients had risk factors for lactic acidosis before admission. All patients had renal impairment on admission as a precipitating risk factor. Five patients survived and two patients died despite early renal replacement therapy. Older patients tended to have a poorer prognosis.
Conclusion
Renal function must be monitored in elderly type 2 diabetes mellitus patients with underlying diseases and conditions causing renal impairment who begin metformin treatment. Accurate recognition of MALA and initiation of renal replacement are essential for treatment.
INTRODUCTION
Metformin is an oral antidiabetic drug in the biguanide class that is widely used, alone or in combination with a sulfonylurea or other drugs, in patients with type 2 diabetes. The drug's glucose-lowering effect results mainly from decreased hepatic glucose output with increased glucose utilization in peripheral tissues [1]. Metformin is absorbed mainly from the small intestine, has 40 to 60% oral bioavailability, and has an estimated plasma half-life of 1.5 to 4.9 hours [2]. Unlike sulfonylureas, metformin is not metabolized and does not bind to plasma proteins. Metformin is eliminated by renal tubular secretion and glomerular filtration. Thus, the serum metformin concentration and toxicity are related to renal function.
The most common side effects of metformin are gastrointestinal disturbances, including anorexia, nausea, abdominal discomfort, and diarrhea. These mild symptoms are usually reversed after discontinuation or dose reduction. Rarely, a serious condition called metformin-associated lactic acidosis (MALA) can occur in patients with predisposing factors such as renal insufficiency, hepatic disease, congestive heart failure, or sepsis [3].
The incidence of lactic acidosis in patients on metformin therapy appears to be very low [4,5,6], but it can be fatal when it occurs. In a review of 11,800 patients treated with metformin for a mean duration of approximately 2 years, only two patients developed lactic acidosis (an incidence of nine cases per 100,000 person-years of exposure). In clinical practice, metformin is used for diabetics despite its contraindications in 24.5% to 94% of patients with type 2 diabetes [7,8].
The aim of this study was to further investigate the clinical profiles and risk factors for MALA and to analyze treatment modalities according to survival.
METHODS
We conducted a retrospective analysis of patients admitted into a single academic hospital due to MALA and analyzed the precipitating causes and prognoses. In this study, patients were defined as having MALA if they had metabolic acidosis (arterial pH <7.35, plasma lactate level >5.0 mmol/L [0.50 to 1.50]) and had been treated with metformin before admission.
We searched for cases with diabetes and acidosis including lactic acidosis and ketoacidosis between January 1995 and December 2012 and reviewed the medication histories and causes of acidosis. Among 5,558 patients who were admitted due to diabetes, we identified 198 patients with acidosis, including lactic acidosis or ketoacidosis. Patients with diabetic ketoacidosis and an unknown medication history or low lactic acid level (<5 mmol/L) were excluded. For the selected MALA cases, we recorded the age, sex, daily metformin dosage, laboratory tests, admission diagnosis, and risk factors for lactic acidosis. In addition, accompanying diseases, treatments and outcomes were evaluated. Renal dysfunction was defined as a plasma creatinine level >1.49 mg/dL in men and >1.40 mg/dL in women. Glomerular filtration rate was calculated before initiation of continuous renal replacement therapy using the MDRD eGFR equation (estimated glomerular filtration rate mL/min/1.73 m2=175×Scr-1.154×age-0.203×0.742 if female). Impaired liver function was defined as a serum alanine transaminase concentration >3-fold the upper limit of normal. Values are expressed as means±SD. The relationship between clinical parameters and outcome was examined using the Mann-Whitney test for quantitative variables and the Fisher exact test for qualitative variables in patients who both survived and died. A P≤0.05 was considered to be statistically significant.
RESULTS
Of the 198 diabetic patients reviewed for acidosis, seven patients (five females and two males) were diagnosed with MALA during the study period. Two patients had been misdiagnosed with diabetic ketoacidosis. All patients had been treated with metformin by their primary physicians and had visited the emergency room. Table 1 summarizes the demographics and laboratory data of the seven MALA cases. The patients were 74.5±9.8 years of age (range, 57 to 86), six of whom were older than 70 years except one alcoholic. The mean arterial pH was 6.9±0.2, mean serum lactate was 15.7±8.3 mmol/L, and the mean daily dose of metformin was 1,528±658 mg. Case 1 is described singly here, and the other six cases are summarized in Tables 1, 2.
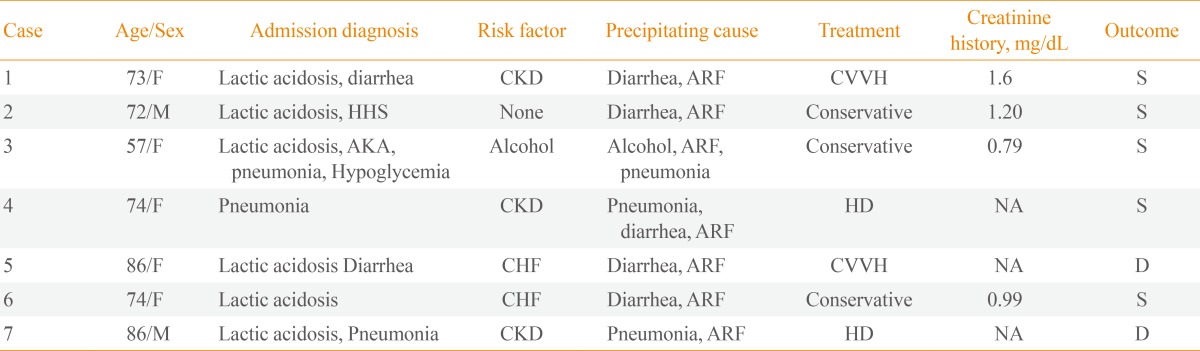
Risk Factors, Diagnosis on Admission, Treatment and Outcome of Patients with Metformin-Associated Lactic Acidosis
Case 1
A 73-year-old woman with a history of hypertension and type 2 diabetes was admitted to the emergency department after 2 days of general weakness, anorexia, vomiting and diarrhea. She was hypotensive, and her mentality was deeply drowsy. She was intubated, ventilated and administered massive fluid resuscitation immediately. Her pharmacologic regimen included metformin 2,000 mg, glimepiride 8 mg, telmisartan/hydrochlorothiazide 80/12.5 mg, amlodipine 10 mg, nifedipine 30 mg, alprazolam 0.5 mg, pantoprazole 20 mg, trimetazidine dihydrochloride 40 mg, and bisoprolol 10 mg. Her physical examination revealed no abnormalities other than severe dehydration. Laboratory results showed leukocytosis (19,660/mm3, 81.4%) with left shift and anemia and levels of hemoglobin of 6.8 g/dL, blood glucose of 419 mg/dL, sodium of 130 mEq/L, potassium of 8 mEq/L, and chloride of 96 mEq/L. Acute renal failure was noted, with a blood urea nitrogen level of 70.6 mg/dL and serum creatinine level of 5.8 mg/dL, and the creatinine clearance was calculated as 7 mL/min/1.73 m2 using the Modification of Diet in Renal Disease (MDRD) formula. The last creatinine level measured before admission was 1.6 mg/dL. Analysis of arterial blood gases indicated a high anion gap (31.5 mEq/L), metabolic acidosis (pH 6.9), and bicarbonate 2.5 mEq/L. Liver function tests were within normal ranges. The serum lactate level was elevated at 9.2 mmol/L. Despite forced diuresis after massive rehydration, urine output was absent and acidosis was aggravated. Continuous venovenous hemodialysis was started using bicarbonate buffered solution. Twenty-four hours of renal replacement therapy led to progressive improvement of metabolic acidosis with pH values of 7.16, 7.36, and 7.41 and bicarbonate values of 6, 12, and 24 mEq/L on each subsequent day. The urinary output also increased progressively. Two days later, the patient returned to a normal mental state with stable vital signs without inotropics or ventilator support. Renal function was improved as indicated by a serum creatinine level of 1.63 mg/dL. After 15 days, the patient was discharged.
Risk factors
All patients who presented with MALA had impaired renal function on admission. Three patients had chronic kidney disease (CKD) before admission. The admission diagnoses, risk factors, treatments and outcomes (60 days after admission) are summarized in Table 2. Six patients (86%) had risk factors for lactic acidosis in their medical histories. Five patients were dehydrated due to vomiting and diarrhea, indicating that acute renal failure resulted from dehydration secondary to diarrhea and poor oral intake and likely was the cause of MALA. Three patients were diagnosed with pneumonia during the treatment. Two patients had been diagnosed previously with heart failure.
Treatment
Four patients (58%) received renal replacement therapy, and the remaining three patients (42%) were treated conservatively with sodium bicarbonate and fluid resuscitation. The bicarbonate-buffered renal replacement therapy was maintained for 24 to 48 hours until the patient's lactate level and urine output were normalized. Three cases underwent continuous venovenous hemodialysis and one underwent intermittent hemodialysis.
Mortality
Two patients (29%) died despite renal replacement therapy on hospitalization days 14 and 60, respectively (Table 3). In case 5, while continuous venovenous hemodialysis improved the acidosis, mental state failed to recover, and the patient was transferred to another hospital. Pneumonia and pulmonary congestion were aggravated in case 7. Both patients who died had acute renal failure with more severe metabolic acidosis (P=0.053) and were older than the patients who survived (P=0.049). Furthermore, their lactate levels were as high as 17 and 32 mmol/L, respectively (P=0.121).
DISCUSSION
Known predisposing factors for MALA include impaired renal function, concurrent liver disease, alcoholism, and decreased tissue perfusion due to infection, heart failure, or other causes [8,9]. In our study, most MALA cases occurred in patients with renal impairment with or without other precipitating conditions. Metformin therapy was contraindicated relatively or absolutely in six patients. Three patients had concurrent pneumonia, suggesting decreased tissue perfusion, since infection precipitates MALA. In MALA, metformin's mechanism of action is thought to inhibit hepatic gluconeogenesis from lactate, pyruvate, and alanine, resulting in additional lactate and substrates for lactate production by decreased pyruvate carboxylase activity, a rate limiting enzyme needed for the formation of glucose from lactate [1]. Another mechanism includes a shift in the intracellular redox potential from aerobic to anaerobic metabolism, with conversion of glucose to lactate in the splanchnic bed of the small intestine.
The main finding in our patients with severe lactic acidosis who were taking metformin was an improvement in shock following a short duration of renal replacement therapy or conservative treatment. Although some of the patients had other diseases such as pneumonia, which could have contributed to the lactic acidosis, this condition alone was not sufficient to cause severe acidosis. The patients recovered from shock rapidly following continuous renal replacement therapy, after which their renal function was normalized.
Scale and Harvey [10] reported that diabetes, rather than metformin therapy, is the major risk factor for the development of lactic acidosis. It remains unclear as to whether this is a causative link or is associated with other causes. Since lactic acidosis continues to develop in patients taking metformin, it is important to clarify the clinical findings and to identify and treat these cases appropriately. We also reviewed five cases of metformin-independent lactic acidosis in our hospital. The mortality rate was 80%, which is higher than that for MALA. In addition, the patients had multiple comorbidities (data not shown).
Symptoms of lactic acidosis are nonspecific and may include anorexia, nausea, vomiting, abdominal pain, lethargy, hyperventilation, and hypotension [11]. Five of our patients (71%) complained of diarrhea, which can be an adverse effect of metformin and can aggravate hypovolemia. Metformin toxicity has been reported previously to mimic the clinical picture of abdominal sepsis [12].
Previously, MALA cases were rare; however, relatively high incidences, such as 47 to 57 cases per 100,000 patient-years, are now being reported [13]. Additionally, the incidence of MALA can be underestimated, because it is not always recorded as acidosis, due to other concomitant diseases, or it can be confused with ketoacidosis. Two of our cases had mildly elevated ketones, and their initial diagnosis at the emergency room was diabetic ketoacidosis. Data from a large clinical trial identified no cases of lactic acidosis [4]. The risk factors of lactic acidosis differ greatly between stable trial patients and those who are acutely unwell or have insufficient renal function.
Dosage guidelines for CKD patients have been published recently [14]. The following maximum daily doses have been recommended for specific creatinine clearance rates: 3 g (120 mL/min), 2 g (60 mL/min), 1 g (15 mL/min), and 500 mg (below 15 mL/min).
Most studies on MALA have focused on its incidence and predisposing factors. However, we were interested in the clinical factors that can guide treatment and predict outcome. In our study, age was the most important factor while the metformin dose and pH had no prognostic value. Also, the mortality was lower than that of other studies evaluating patients with similar cases of acidosis. After correction of acidosis, treatment of the underlying disease affects the outcome. Case 7 recovered from acidosis after hemodialysis treatment, but the pneumonia was aggravated. Recently, it was shown that neither the serum lactate concentration nor metformin dosage is related to the prognosis of MALA [13].
Renal replacement therapy was initiated in four of seven patients. Renal replacement therapy corrects acidosis by filtering out anions and efficiently removes metformin from the plasma. Bicarbonate hemodialysis, continuous venovenous high-flow hemofiltration dialysis, or combined modalities have been reported in the treatment of severe MALA cases [15]. Such severe cases should be treated immediately with renal replacement therapy using bicarbonate-buffered fluids and correction of any associated conditions. A recent study demonstrated that prompt recognition of lactic acidosis and early treatment using bicarbonate dialysis can result in a favorable clinical outcome [16]. Another study reported that continuous venovenous high-flow hemofiltration dialysis can be effective in patients with hemodynamic instability or in those who require urgent removal of metformin and lactic acid [17]. Yet, there is no conclusive data available to indicate which dialysis technique is superior. In our cases, patients with pH <7.0 required renal replacement therapy.
Our report has several limitations. First, serum metformin was not measured in our cases, so its prognostic value could not be assessed. In addition, our sample size was relatively small due to the rarity of the condition and the retrospective nature of our study. We were also unable to compare the findings in our sample patients with those in a control group of metformin users who did not develop lactic acidosis. Also, lactic acidosis may often be misdiagnosed in diabetic patients, although in our study all cases of acidosis were reviewed.
The majority of elderly type 2 diabetes mellitus patients have renal insufficiency and other various comorbidities that increase the risk of lactic acidosis. To prevent MALA in patients at risk of lactic acidosis who are taking metformin, risk factors for MALA should be carefully assessed and renal function monitored. Early recognition of this condition and initiation of renal replacement therapy can improve the outcome.
ACKNOWLEDGMENT
This study was supported by Inha University.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.

