Articles
- Page Path
- HOME > Endocrinol Metab > Volume 38(6); 2023 > Article
-
Review ArticleHypothalamus and pituitary gland Update on Current Evidence for the Diagnosis and Management of Nonfunctioning Pituitary Neuroendocrine Tumors
 Keypoint
Keypoint
Pituitary neuroendocrine tumors, especially nonfunctioning ones, are noteworthy intracranial tumors often found incidentally. They typically do not produce excess hormones, complicating diagnosis and monitoring. Surgery is the main treatment, but their tricky location often leads to incomplete removal. This highlights the critical need for new biomarkers and treatments for nonfunctional pituitary neuroendocrine tumors. -
Elizabeth Whyte
 , Masahiro Nezu, Constance Chik, Toru Tateno
, Masahiro Nezu, Constance Chik, Toru Tateno
-
Endocrinology and Metabolism 2023;38(6):631-654.
DOI: https://doi.org/10.3803/EnM.2023.1838
Published online: November 15, 2023
Division of Endocrinology and Metabolism, Department of Medicine, University of Alberta, Edmonton, AB, Canada
- Corresponding author: Toru Tateno. Division of Endocrinology and Metabolism, Department of Medicine, University of Alberta, Room 9-112J Clinical Sciences Building, 11350-83 Avenue, Edmonton, AB, Canada Tel: +1-780-492-3626, Fax: +1-780-492-6444, E-mail: tateno@ualberta.ca
Copyright © 2023 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,335 Views
- 148 Download
ABSTRACT
- Pituitary neuroendocrine tumors (PitNETs) are the third most frequently diagnosed intracranial tumors, with nonfunctioning PitNETs (nfPitNETs) accounting for 30% of all pituitary tumors and representing the most common type of macroPitNETs. NfPitNETs are usually benign tumors with no evidence of hormone oversecretion except for hyperprolactinemia secondary to pituitary stalk compression. Due to this, they do not typically present with clinical syndromes like acromegaly, Cushing’s disease or hyperthyroidism and instead are identified incidentally on imaging or from symptoms of mass effects (headache, vision changes, apoplexy). With the lack of effective medical interventions, first-line treatment is transsphenoidal surgical resection, however, nfPitNETs often have supra- or parasellar extension, and total resection of the tumor is often not possible, resulting in residual tumor regrowth or reoccurrence. While functional PitNETs can be easily followed for recurrence using hormonal biomarkers, there is no similar parameter to predict recurrence in nfPitNETs, hence delaying early recognition and timely management. Therefore, there is a need to identify prognostic biomarkers that can be used for patient surveillance and as therapeutic targets. This review focuses on summarizing the current evidence on nfPitNETs, with a special focus on potential new biomarkers and therapeutics.
- Pituitary neuroendocrine tumors (PitNETs) are the third most frequently diagnosed intracranial tumors [1]. In the 2022 World Health Organization (WHO) classification of pituitary tumors, a major nomenclature change was introduced to refer to pituitary adenomas as PitNETs as the term adenoma refers to a benign disease that is not harmful to health or life which is inconsistent with the behaviors of pituitary tumors [2]. PitNETs are classified into functioning or nonfunctioning tumors. Functioning PitNETs (fPitNETs) have hormone oversecretion leading to conditions associated with elevated hormones, including acromegaly, prolactinoma and Cushing’s disease. Nonfunctioning PitNETs (nfPitNETs) account for 30% of all PitNETs and represent the commonest of all macroadenomas [1,3]. NfPitNETs are usually benign tumors with no evidence of hormone oversecretion except for hyperprolactinemia, secondary to pituitary stalk compression [3]. Whereas active surveillance with regular clinical, hormonal and imaging assessment is appropriate for selected patients with nfPitNETs, the first-line treatment for most patients is transsphenoidal surgical resection. However, nfPitNETs often have supra- or parasellar extension, which preclude the total resection of the tumor [4]. This results in residual tumor regrowth in 47% of patients and 24% reoccurrence after complete macroscopic resection [5]. While fPitNETs can be more easily followed for recurrence using hormone levels as biomarkers, there is no similar parameter to predict recurrence in nfPitNETs, hence delaying early recognition and timely management [6]. The delay in diagnosis is approximately 1.96±2.9 years and at the time of diagnosis 67% to 90% have grown into macroadenomas [7,8]. Therefore, there is a need to identify prognostic biomarkers that can not only be used for patient surveillance but also for therapeutic targets [6]. Treatment of residual and recurrent tumors is often necessary, which includes a second surgery, radiation or medical therapy, even though there are currently no established drug options for nfPitNETs [4]. There are some potential new therapeutics being investigated; however, currently there are only minimal in vitro and in vivo studies proving their efficacies. Due to the lack of potential biomarkers and effective treatments for nfPitNETs, more large-scale studies with long-term follow-up need to be performed to provide effective patient care, particularly the subset of aggressive tumors. This review will summarize the current evidence on nfPitNET epidemiology, classification, pathophysiology, biomarkers, clinical presentation, and management, with a special focus on potential new therapeutics.
INTRODUCTION
- NfPitNETs are the most prevalent macroadenomas and the second most common microadenomas, following prolactinomas [3]. A recent review of population studies from UK, Belgium, Switzerland, Northern Finland, Western Sweden, Malta, Iceland, Canada, and Argentina estimated that the prevalence of clinically relevant nfPitNETs is 7–41.3 cases per 100,000 people [3] with a standardized incidence rate of 0.65–2.34/100,000. This is likely an underestimate of the true prevalence, as many nfPitNETs go undiagnosed until they are large and cause mass effects or are incidentally discovered or found at autopsy. Indeed, a recent population-based study from South Korea reported a higher annual incidence of 3.5/100,000 for nfPitNETs [9]. The peak occurrence is from the fourth to the eighth decade of life; however, sex predominance data are conflicting [3]. Two recent studies have examined the role of socioeconomic status (SES) on the presenting characteristics and extent of disease in patients with surgically resected nfPitNETs [10,11]. Both studies found lower SES was associated with more severe disease at the time of diagnosis, including larger tumor size and lower rates of incidental diagnosis [10,11], and in one study, having a primary care provider was the single most crucial factor impacting hospital lengths of stay, readmission rates, follow-up adherence and tumor recurrence [10].
EPIDEMIOLOGY
- Pathological classification
- The 2017 WHO classification recognized the use of immunostaining for transcription factors to define adenohypophyseal tumors, including: pituitary specific transcription factor 1 (PIT1), T-box transcription factor (TPIT), and steroidogenic factor 1 (SF1) [12]. The 2022 WHO classification provided detailed histological subtyping of a PitNET based on the tumor cell lineage, cell type, and related characteristics [2]. The routine use of immunohistochemistry for pituitary transcription factors (PIT1, TPIT, SF1, GATA binding protein 3 [GATA3], and estrogen receptor alpha [ERα]) was endorsed in this classification [2].
- The adenohypophysis is composed of at least six different cell types, including somatotrophs, lactotrophs, mammosomatotrophs, thyrotrophs, corticotrophs, and gonadotrophs. Lactotroph, somatotroph, and thyrotroph are classified as PIT1 lineage, corticotroph as TPIT lineage and gonadotroph as SF1 lineage (Table 1). Immunostaining for hormones can then determine specifically what cell lineage the tumor is derived from, which should include adrenocorticotropic hormone (ACTH), growth hormone (GH), prolactin (PRL), β-thyroid-stimulating hormone (β-TSH), β-follicle-stimulating hormone (β-FSH), and β-luteinizing hormone (β-LH) as well as the α-subunit of glycoprotein hormones [2]. NfPitNETs can be gonadotroph, null cell, plurihormonal PIT1 positive and silent tumors of corticotroph, somatotroph, or lactotroph origin. The inclusion of transcription factors resulted in reclassification of 95% of cases of hormonenegative nfPitNETs based on hormone immunohistochemistry [13].
- Silent gonadotroph tumors comprise approximately 75% of clinically nfPitNETs [13,14]. These tumors showed variable immunostaining for β-FSH, β-LH, α-subunit, SF1, and GATA2. In tumors with only a few cells positive for β-FSH and β-LH, immunostaining for SF1 and/or GATA2 are required to identify the cell lineage of the tumors. ERα seems to be a prognostic factor for reintervention in males and the combination of the absence of ERα expression and young age serve as good predictors of aggressiveness for this tumor subtype [14].
- Silent corticotroph tumors make up approximately 5.5% to 20% of nfPitNETs, although this reporting is heterogenous and might be underreported [13,15-17]. In the 2017 WHO classification, silent corticotroph tumors were characterized as highrisk, as some studies have shown they have increased invasiveness and recurrence [13,16,18] but other studies have found no difference between silent corticotroph tumors and other nfPitNET subtypes [19-21]. A recent meta-analysis concluded that silent corticotroph tumors have a 31% recurrence rate but did not exhibit a significantly higher recurrence rate than other nfPitNET subtypes, questioning their classification as an aggressive subtype [19]. However, a subset of the tumors had increased aggressiveness over time [17]; therefore, it is unclear whether these tumors should be treated with more aggressive interventions. Moreover, these tumors have also been associated with increased incidences of apoplexy and intratumoral hemorrhage compared to other nfPitNETs [21]. Therefore, it seems a subset of silent corticotroph tumors have increased aggressive behavior and perhaps increased recurrence and invasiveness; however, there is no current agreed upon prognostic markers to identify them from the less aggressive silent corticotroph tumors.
- PIT1 positive plurihormonal tumors, previously called silent subtype 3, make up approximately 0.9% to 1.8% of nfPitNETs [17,22]. These tumors may have positive staining for hormones, such as GH, PRL, TSH, and α-subunit. The majority of these tumors remain hormonally silent; however, some of these tumors can also cause hormonal excess, leading to acromegaly, hyperprolactinemia, and hyperthyroidism [23]. In a retrospective study, the resected PIT1 positive plurihormonal tumors were all macroadenomas, aggressive, invasive and had a high rate of recurrence [22]. Recent studies reported a lack or low expression of O6-methylguanine DNA methyltransferase (MGMT) in PIT1 positive plurihormonal tumors, suggesting that temozolomide (TMZ) may be effective in the treatment of PIT1 positive plurihormonal tumors [24,25]. Due to the rarity of this type, further studies are required to better understand the features of PIT1 positive plurihormonal tumors and establish effective treatments.
- Null cell tumors are tumors that do not display any transcriptional factors or hormonal staining and currently have no defined origin. Null cell tumors are decreasing in incidence with the inclusion of transcription factors in tumor classification [26]. Previously, null cell tumors and silent gonadatroph tumors were thought to be the same type of tumor. However, the inclusion of immunostaining for SF1 and ERα in tumor classification revealed that 73% of resected nfPitNETs were silent gonadotroph tumor [13]. This distinction between null cell and silent gonadatroph tumors is important because null cell tumors have been labeled by the WHO 2017 as a high-risk tumor with increased invasiveness and aggressiveness. One study also reported increased residual tumor growth after cavernous sinus invasion, reduced rates of complete tumor resection, increased cavernous sinus invasion, higher proliferation indices and worse clinical outcomes [27,28]. A retrospective study of 516 patients with nfPitNETs, including 23% of tumors originally classified as null cell tumors using classical immunohistochemistry techniques, the inclusion of transcription factor testings resulted in reclassification of 95% of tumors [13]. A recent study confirmed this by finding that less than 5% of nfPitNETs in 1,055 samples were null cell tumors [29].
- Silent somatotroph tumors make up approximately 2% of nfPitNETs while silent lactotroph tumors make up about 0.6% to 1.6% and silent thyrotroph tumors comprise about 0.9% [17,30- 32]. In addition, functional gonadotroph tumors are infrequent [33,34]. These tumors are quite rare and there are limited studies with sufficient sample sizes to make conclusions about their behaviors.
- Radiological and operative classification
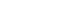
- Cavernous sinus invasion is the most common cause for incomplete resection during tumor removal, leading to increased recurrence rates [35] and additional treatment. Established radiographic and operative classifications to assess PitNET invasion included the initial one by Hardy and Vezina [36] with modification by Wilson [37] that distinguished between the different grades of extrasellar extension. Knosp et al. [38] subsequently described a classification system for tumor parasellar invasion noted on magnetic resonance imaging (MRI) which determined the likelihood of cavernous sinus invasion by the PitNETs (Fig. 1). This is especially important for nfPitNETs as the tumors have high rates of recurrence when they invade into the cavernous sinus; therefore, knowing if the MRI classification can be used in these tumors is critical for therapy decisions [35]. In a recent study in 247 patients with PitNETs including 167 with nfPitNETs, a significant positive association between Knosp classification and Ki-67 expression in nfPitNETs compared with a negative association in patients with fPitNETs [39].
- The grade 3 Knosp classification was further divided into inferior (3A) and superior (3B) cavernous sinus invasion by Micko et al. [40]. In a study in 275 patients with nfPitNETs, Hwang et al. [35] found a significant difference in surgical outcomes between the low (grade 1 and 2) and the high (grade 3A, 3B, and 4) grade groups, but no difference in the gross tumor resection rate between grade 3A and grade 3B. Moreover, the Knosp classification was found to provide a reasonable estimation of surgical cure and the risk of complications, whereas the Hardy-Wilson scale was not useful for these purposes [35]. In another study of 228 PitNET patients, including 140 patients with nfPitNETs, no difference in the diagnostic accuracy of the Knosp and modified Knosp classification for the prediction of surgical cure was found [41]. However, the differentiation between grades 3A and 3B was important as patients with grade 3A had a higher probability of surgical cure, more similar to grade 2 of Knosp, whereas the behavior of Knosp 3B was more similar to grade 4 of the classical Knosp [41].
- A new radiological classification based on shape has been proposed by Berkmann et al. [42] to evaluate the impact of different tumor shapes on gross tumor resection rates and outcomes based on previously defined growth patterns: spherical (shape I), oval (shape II), dumbbell (shape III), mushroom (shape IV), and polylobulated or mushroom (shape V). Based on a retrospective study of 191 patients with nfPitNETs, gross tumor resection was achieved in 53% of patients with decreasing likelihood in higher shape grades (82% in shape I vs. 0% in shape V). Moreover, the higher the “shape grade,” the higher the likelihood for larger tumor remnants and need for further therapies.
- TRANSSPHER (Transsphenoidal Extent of Resection Study) grade is another grading system that has been proposed to address the likelihood of achieving gross tumor resection of nfPiTNETs after transsphenoidal surgery [43]. Based on a multicenter study with 222 patients with nfPitNETs, three MRI characteristics were identified as strong independent predictors of gross tumor resection: tumor diameter >40 mm, nodular tumor extension and Knosp grades 3 to 4 [43]. In the TRANSSPHER grading system, one point was assigned for each of the three MRI characteristics, and the likelihood of achieving gross tumor resection was inversely related to the TRANSSPHER grade.
- Combined classification based on radiology and proliferation index
- Trouillas et al. [44] proposed a classification system to better classify pituitary tumors based on proliferation and invasiveness in 2013, as proliferation characteristics in the 2004 WHO classification were vague and nonspecific, and invasion biomarkers were excluded all together. This classification system defined invasion as histological and/or radiological signs of cavernous or sphenoidal invasion. Proliferation was considered on the presence of at least two of three of the following criteria: Ki-67: >1% (Bouin-Holland fixative) or ≥3% (formalin fixative); mitoses: n >2/10 high power field (HPF); and p53: positive (>10 strongly positive nuclei/10 HPF) [44]. This five-tier classification system was retrospectively validated [44,45] and more recently in nfPitNETs [46]. The study in nfPitNETs reported that this classification system proved to be very useful in predicting the risk of recurrence of nfPitNETs after primary surgery. In particular, grade 2b lesions (proliferative and invasive) showed an overall likelihood of recurrence that was 8.6 times greater than those of grade 1a (noninvasive and nonproliferative tumor) [46]. In another study, a 4.8-fold higher risk of progression/recurrence in grade 2b as compared to grade 1a was found in a cohort of 607 patients with pituitary tumors, including 52% of patients with nfPitNETs followed for a median duration of 38 months [47]. Moreover, patients with proliferative tumors have a higher risk to be retreated after primary surgery [47].
CLASSIFICATION
- Pathogenesis of nfPitNETs comprises many hypotheses that might promote tumor growth and proliferation, including genetic and epigenetic events, microRNA (miRNA) deregulation, immune resistance mechanisms, growth factor overproduction and pituitary stem cells.
- A meta-analysis of the expression of genes in nfPitNETs demonstrated that 67 genes, including paired like homeodomain 2 (PITX2), involved in the regulation of cell growth, proliferation or the cell cycle were deregulated compared to the normal pituitary tissue [48]. PITX2 induced by the Wnt/Dvl/β-catenin pathway activates cyclin D2 expression, resulting in cell-typespecific proliferation during pituitary development [49].
- Several germline mutations result in clinical syndromes associated with PitNET development, including multiple endocrine neoplasia type 1 (MEN1) gene and MEN4 syndrome and aryl hydrocarbon receptor interacting protein (AIP) gene mutations [50-52]. Chromosome 11 deletions have been associated with pituitary tumorigenesis in familial pituitary adenoma syndromes in which MEN1 or AIP, both tumor suppressors, are mutated in germline DNA [53]. This loss of chromosome 11 leads to MEN1 or AIP inactivation, decreasing the cells’ ability to suppress tumor growth. Chromosome 11 deletion has also been associated with the development of some atypical adenomas [53].
- Additionally, the mitogen activated protein kinase (MAPK) signaling pathway has been implicated in cancer, with studies showing that cancerous mutations in MAPK pathways frequently affect Ras, a GTPase, and Raf, a protein kinase [54]. Ras/Raf mutation-activated pathways are important for cell survival and proliferation, whereas stress-activated pathways such as Jun Nterminal kinase (JNK) and p38 oppose malignant transformation. The balance between the two signaling pathways could significantly contribute to tumorigenesis and response to drug therapy. Raf, the main effector of Ras, has been shown to be overexpressed in nfPitNETs. This overexpression suggests overactivity of the Ras-Raf/MAPK pathway to promote pituitary tumorigenesis [55]. Moreover, some somatic mutations of the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene have been shown to have a role in nfPitNET development through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway [56]. Therefore, both the Ras-Raf/MAPK and PI3K/Akt pathways might contribute to nfPitNET development.
- Gene expression may also be modified by epigenetic changes, including tumor suppressor protein p16, a cyclin-dependent kinase (CDK) inhibitor encoded by the CDKN2A gene, which is often downregulated in nfPitNETs, possibly resulting in uncontrolled proliferation [57]. Some p53-dependent genes are also downregulated in nfPitNETs, including DNA damage inducible gene 45g (GADD45g) and the maternally expressed gene 3 (MEG3), both can act as tumor suppressor genes, thus limiting the control of programmed cell death through apoptotic mechanisms [58,59]. Therefore, enhanced cell survival and proliferation might promote nfPitNET growth. Current research seems to point to epigenetic mutations as the more likely cause for the development of nfPitNETs, suggesting genetic predisposition is less important.
- CDK6 might also play a role in tumor proliferation as CDKs bind to cyclins, specifically cyclin D1, to form active cyclinCDK complexes that progress cells from G1 to S phase in the cell cycle [26]. Previous studies have shown that cyclin D1 is overexpressed in nfPitNETs compared to other tumor types and normal pituitary tissue [60]. As well, CDK6 has been shown to be overexpressed in invasive nfPitNETs [61]. CDK6 also has a role in phosphorylating retinoblastoma protein (Rb), which activates E2 transcription factor (E2F) and proliferation [62]. Cyclin-CDK complexes are regulated by p27, which are seen at lower levels in PitNETs compared to normal pituitary tissue, suggesting that p27 loss of function and dysregulation of cell cycle regulation can lead to tumor formation and proliferation [26].
- DNA methylation is one of the crucial epigenetic modifications regulating gene expression. Several studies identified genes methylated differently in nfPitNETs compared to the normal pituitary tissues [63]. Genome-wide DNA methylation and mRNA analyses using 71 nfPitNET samples demonstrated differential gene expression and methylation profiles between a PitNET regrowth group and a nonregrowth group. The DNA methylation and expression levels of two genes, family with sequence similarity 90 member A1 (FAM90A1) gene and inhibitor of growth family member 2 (ING2) gene, are related to nfPitNET regrowth, suggesting the methylation status and expression levels as biomarkers that may predict nfPitNET behaviors [63]. Another study integrating epigenome and transcriptome data found DNA methylation alterations and differential gene expression profiles in nfPitNETs compared to normal pituitary tissues as well as key protein molecules in the protein-protein interaction network, which can be novel therapeutic targets [64].
- miRNAs are small noncoding RNA molecules that by pairing to the complementary mRNA sequence, negatively regulate posttranscriptional gene expression and influencing cell growth [65]. Several studies have found that miRNAs are overexpressed or downregulated in nfPitNETs compared to normal pituitary cells. These downregulated miRNAs, including miR134, are thought to restrain cell cycle progression in G2/M phase. Differential miRNA expression has been predicted to downregulate transforming growth factor beta signaling pathway and Wee1, a mitotic inhibitor that hampers cell cycle progression in nfPitNETs [66,67]. Notch and PRL signaling are also deeply regulated by miRNAs in nfPitNETs [68]. These studies have provided preliminary evidence and additional work is required to understand their functional consequences in nfPitNETs better.
- The proteins that negatively regulate T-cell activation, such as cytotoxic T-lymphocyte-associated protein 4 and programmed cell death 1 (PD-1) have both been implicated in playing a role in nfPitNET growth. Tumor infiltrating lymphocytes express PD-1 and bind to programmed death ligand-1 (PD-L1), which is expressed by tumor cells or antigen-presenting cells [69]. The binding of PD-1 to PD-L1 reduces lymphocytic cancer cell killing [70,71]. In support of this is the observation that nfPitNETs display lower PD-L1 mRNA and protein levels as compared to fPitNETs [72]. Also, nfPitNETs had increased PD-1 expression and a reduced lymphocyte infiltration, as compared to fPitNETs, suggesting that nfPitNETs may evade immune surveillance by triggering this checkpoint. Therefore, immune resistance mechanisms might facilitate nfPitNET growth, however when treated with immune checkpoint inhibitors, hypophysitis can occur [73].
- Another hypothesis for development of nfPitNETs is the role of pituitary stem cells which have been demonstrated in these tumors [74,75]. These stem cells display clonogenic ability in vitro, express stem cell markers, are multipotent and resist to cytotoxic drugs and capable of forming tumor spheres and generating tumors in nude mice [76,77]. One study provided evidence that nfPitNETs contain stem-like cells that express stem cell specific markers, as well as pituitary embryonic transcription factors involved in gonadotroph differentiation [78]. Sphereforming cells displayed long-term proliferation ability and tumorigenic potential in animal models, where they showed invasive behavior and pro-angiogenic activity. This is further supported by studies showing that Notch3 and Jagged1 are overexpressed in human nfPitNETs as compared to normal pituitary tissue [79]. The Notch pathway participates in stem cell signaling and pituitary embryonic development. Therefore, stem cells may also play a role in tumorigenesis and growth in nfPitNETs.
- Angiogenesis is another mechanism by which nfPitNETs might develop as it is one of the most potent triggers for tumor development in other tissues. However, the current research seems to conclude that angiogenic factors might facilitate nfPitNET survival and growth but may not represent the initiating event forming the tumor [65].
PATHOGENESIS
- Clinical presentation of nfPitNETs varies from being asymptomatic to panhypopituitarism, mass effects, and pituitary apoplexy. Asymptomatic tumors are usually discovered incidentally by MRI and computed tomography scan when performed to investigate a nonpituitary disease, known as a pituitary incidentaloma [80]. Since nfPitNETs do not cause hormonal hypersecretion, there is usually a delay in diagnosis of approximately 1.96±2.9 years and at the time of diagnosis 67% to 90% are macroadenomas [7,8]. A recent prospective study of 269 patients with nfPitNETs reported that at presentation 48.7% were incidental and 51.3% were discovered from symptoms [80]. In the incidental group, 27.4% of patients had hypopituitarism compared to 58.7% of patients in the symptomatic group. Of all the patients, 87% presented with macroadenomas, which were larger in the symptomatic group than the incidental group.
- Most patients with symptoms will present with mass effects, such as headaches, visual field defects, ophthalmoplegia, hypopituitarism, pituitary apoplexy, or hyperprolactinemia due to pituitary stalk compression [3]. The most common symptom is headache, which occurs in 19% to 75% of patients regardless of size [81-83]. There are different mechanisms that can produce headaches including suprasellar extension, intrasellar pressure, and stretching of dural membranes containing pain receptors, or activation of trigeminal pain pathways by tumors invading the cavernous sinus [84].
- Due to the pituitary’s close proximity to the optic chiasm, any enlargement can cause compression, leading to visual field defects in about 40% of patients [15]. The degree and location of the vision loss depend on where on the optic chiasm the tumor is compressing but most often results in bitemporal hemianopia [85]. However, it can result in unilateral or altitudinal vision loss in 33% and 16% of cases, respectively [86]. Often the loss of vision is gradual and unnoticed, with the median duration before diagnosis being 6.5 months, and older age being the only factor associated with delayed diagnosis [87].
- Ophthalmoplegia occurs when the tumors invade into the cavernous sinus and compress the cranial nerves. Ophthalmoplegia usually involves the third (oculomotor), fourth (trochlear), or sixth (abducens) cranial nerves, leading to paralysis of the extraocular muscles that control the movements of the eye. Double vision is a characteristic symptom in all three cases [3]. In rare cases, when the tumor invades the cavernous sinus it might cause temporal lobe epilepsy, intracranial hypertension, hydrocephalus, cerebrospinal fluid rhinorrhea or occlusion of the internal carotid artery [88,89].
- Apoplexy is a vascular hemorrhage that can occur in macroadenomas. Apoplexy is seen more commonly in nfPitNETs than other PitNETs, occurring in 7% to 9.5% of all nfPitNETs with nfPitNETs accounting for 45% to 82% of all apoplexy cases [3,90-93]. However, another cohort study which followed patients for 42 weeks had no apoplexy cases even with tumor growth [94]. A systematic review and meta-analysis reported that apoplexy occurs in nfPitNETs that are macroadenomas at an incidence of 1.1% per year [95]. Apoplexy occurs when there is acute expansion of the tumor, presenting with acute onset headache that may or may not be associated with neuroophthalmological signs and symptoms like intracranial hypertension, altered levels of consciousness and hypopituitarism [82]. Radiologic features of pituitary apoplexy on MRI scan varies, as the signal of infarction changes [92]. In the acute phase up to day 7, isointense or slightly hypointense signals are seen on T1 scans and hypointense signals on T2 scans; during the subacute phase from 7 to 21 days and the chronic phase after 21 days, hyperintense signals are observed on T1 and T2 scans and hypointense signals on T1 and T2 scans, respectively.
- Hypopituitarism occurs when there is compression of the adenohypophysis and/or pituitary stalk leading to deficiency of at least one of the adenohypophyseal hormones since hypothalamic factors cannot reach the pituitary to signal the hormones’ release [96]. Hyperprolactinemia can also occur from pituitary stalk compression as it prevents dopamine, an inhibitor of PRL release, from reaching the adenohypophysis. Therefore, hypogonadism can occur due to hyperprolactinemia, usually <95 ng/mL, or from the compression of the anterior pituitary itself [97]. The most common axis affected is the GH axis, followed by the gonadal axis and the adrenal axis [3].
CLINICAL PRESENTATION
- Ki-67 is a common cell proliferation marker, determined by the percentage of cells with immunostaining for mindbomb homolog-1 (MIB-1) antibody. Within nfPitNET subtypes, Ki-67 variability suggests that proliferative indices and higher risk subtypes may act as independent predictors of disease. A recent study reported that Ki-67 labeling index >3% and Knosp grade ≥3 was positively correlated in nfPitNETs but negatively correlated in fPitNETs [39]. The authors attributed this to the delay in diagnosis of nfPitNETs until the tumor has become more extensive and more invasive. While the correlations might be a clinical manifestation rather than evidence that high proliferation is associated with low invasive behavior in fPitNETs [39]. Additionally, it has been reported that nfPitNETs have lower Ki-67 expression than fPitNETs [98]. In nfPitNETs, high Ki-67 expression has been associated with tumor recurrence [99], tumor size >3 cm [99], infiltration into cavernous sinus [39], residual tumor growth [100,101], shorter time to repeat surgery [100], and negatively associated with tumor volume doubling time [102]. However, other studies have found no association between high Ki-67 expression and tumor growth [103], invasiveness [104], recurrence [104], and volume [104]. Additionally, no difference was found between tumors that infiltrated into the cavernous sinus compared to those that did not [32,105].
- MGMT is a DNA repair enzyme, which repairs naturally occurring mutagenic DNA O6-methylguanine back to guanine. In PitNETs, low MGMT expression has been reported in more aggressive tumors, suggesting MGMT expression might play a role in tumor progression [106,107]. Gene expression studies have reported that MGMT’s role in tumorigenesis is through the MAPK and PI3K pathways [108]. Many studies have shown the relationship between low MGMT expression and increased TMZ treatment response in aggressive pituitary tumors, with one review article reporting 76% treatment response rate in low MGMT expressing tumors [109]. Widhalm et al. [106] compared MGMT expression in patients who had progressive, regrowing nfPitNETs with MGMT expression in tumors from patients who remained tumor free after the first operation. In the group with regrowing tumors, low MGMT expression was observed in 50% of the patients, compared with 24% in the tumorfree group. Moreover, the interval to a second surgery in patients who had low MGMT expression was 6.2 years compared to patients with high MGMT expression which was 10.7 years [105]; however, none of these differences reached significance. Therefore, more research is needed on the MGMT expression levels and its relationship to aggressive behavior and TMZ resistance in nfPitNETs.
- ERα is a possible prognostic biomarker for nfPitNETs. In breast cancer, ERα is activated by estrogens leading to enhanced proliferation, which is counteracted by the presence of ERβ, which exerts an antiproliferative effect [110]. Therefore, ERα antagonists and ERβ agonists might be beneficial therapeutic options. However, the expression of these two receptors differ in different types of tumors and cancer stages, likely impacting proliferation [110]. Therefore, detecting the expression of these two receptors in nfPitNETs could prove beneficial in determining who would benefit from estrogen receptor therapeutics. A study by Zhou et al. [111], demonstrated that increased ERα together with decreased E-cadherin and ERβ expression were found in invasive compared to noninvasive nfPitNETs. However, low levels of ERα were associated with earlier and higher rates of reintervention in males with nfPitNETs but not females [14]. Moreover, estrogen, through its receptor, induces expression of pituitary tumor transforming gene (PTTG) [112]. Furthermore, the same authors demonstrated that antiestrogens reduced PTTG expression in human PitNETs, specifically prolactinomas, in vitro and suppressed tumor growth in vivo, indicating a role of antiestrogenic therapy in treatment of PitNETs [113]. Similar studies have not been completed in nfPitNETs.
- PTTG is a proto-oncogene that is involved in a variety of physiological processes, specifically it is one of the key factors in the formation of various tumors. PTTG plays an important role in the formation of PitNETs as it has been demonstrated that most PitNETs express PTTG [99]. As mentioned above, estrogen has been shown to stimulate PTTG expression and PTTG has been shown to promote expression of basic fibroblast growth factor and vascular endothelial growth factor (VEGF) which are closely related to angiogenesis [114,115]. Minematsu et al. [115] suggested that PTTG promotes tumor growth by stimulating angiogenesis instead of through proliferation, as no relationship between PTTG and Ki-67 expression was found. However, Filippella et al. [116] reported conflicting results that PTTG expression was associated with Ki-67 expression, and that they were both correlated to more aggressive behavior and recurrence. Additionally, PTTG expression has been reported to be significantly higher in invasive compared to noninvasive macroadenomas [117]. Another study confirmed the correlation between PTTG expression with the invasiveness of nfPitNETs; however, there was no association with E-cadherin and Ki-67 expression [105]. In addition, association with age and female sex were found though there was no significant relationship with tumor regrowth [118]. However, other studies have found no relationship between PTTG expression and invasiveness in PitNETs [119] or nfPitNETs specifically [99].
- VEGFs are a family of angiogenic and lymphangiogenic growth factors that have been implicated in playing a role in endothelial cell proliferation, angiogenesis, and vascular permeability [120]. High VEGF expression has been reported in nfPitNETs [121-123] and has been associated with tumor invasiveness, specifically cavernous sinus invasion [124,125]. Additionally, VEGF RNA and protein levels have been shown to be higher in nfPitNETs than in normal pituitary tissue; however, no difference was found in VEGF expression, RNA or protein levels between nfPitNETs and fPitNETs [125,126].
- Mismatch repair mechanisms, including MutS homolog 2 (MSH2) and MSH6, correct errors from nucleotide misincorporation by DNA polymerase. Therefore, they are important in repairing DNA damage. MSH2 and MSH6 expression have been positively associated with tumor volume doubling time and inversely associated with cell proliferation and invasiveness in nfPitNETs [127,128].
- E-cadherin has a vital role in cell-cell adhesion, where the loss of these tumor suppression genes can lead to tumor development, progression, and metastases [129]. No association was found in E-cadherin expression between nfPitNETs with cavernous sinus invasion and those without [32]. However, a different study reported that E-cadherin mRNA and protein levels were lower in invasive tumors compared to noninvasive ones [111]. Additionally, Slug, a repressor of E-cadherin, was significantly increased in invasive compared to noninvasive nfPitNETs. Furthermore, Slug was positively correlated with ERα and inversely correlated with ERβ, whereas E-cadherin was positively correlated with ERβ and inversely correlated with ERα. Therefore, ERα and ERβ may act in opposite directions to regulate the Slug-E-cadherin pathway, contributing to the invasiveness of nfPitNETs. The absence of E-cadherin staining served as an independent predictor of reintervention [130].
- Matrix melloproteinase-9 (MMP9) is a zinc-containing protease that has a role in extracellular matrix degradation and angiogenesis. No difference was reported in MMP9 expression between nfPitNETs that extended into the cavernous sinus and those that did not [32,105]. Gong et al. [131] reported that MMP9 activity was significantly higher in invasive nfPitNETs compared to noninvasive nfPitNETs and there was a significant correlation between tumor size and tumor invasion into the cavernous and sphenoid sinus and MMP9 expression levels; however, there was no difference in MMP9 expression between fPitNETs and nfPitNETs [131,132]. Turner et al. [133] also reported that although there was no difference in whether MMP9 was present or not in nfPitNETs that recurred, compared to those that did not, recurrent tumors were more likely to express MMP9. Therefore, MMP9 might be a possible prognostic biomarker for nfPitNETs; however, more research is needed.
- Cathepsin K, a protease that degrades type I collagen and extracellular matrix, thereby contributing to bone resorption and tumor invasion, has recently been shown as a potential marker for sphenoid sinus invasion in 176 patients with nfPitNETs [134]. In the same study, the expression of MMP9 and MMP2 was higher in patients with cavernous sinus invasion. Moreover, higher expression of cathepsin K was an independent risk factor for recurrence.
- Fibroblast growth factor receptor 4 (FGFR4) interacts with fibroblast growth factors, that regulate many processes, including cell proliferation, migration, differentiation and more. In nfPitNETs, no association was found between FGFR4 expression and tumor invasiveness [99]. Additionally, FGFR4 expression was not significantly different between tumors that invaded the cavernous sinus and those that did not [32]. Therefore, FGFR4 might not be a good predictor of tumor invasiveness for nfPitNETs, but more research is needed to see its use in predicting other tumor behaviors.
- Using high-throughput mass spectrometry-based phosphoproteomic analysis, a unique phosphopeptide enrichment pattern was found to correlate with disease recurrence in nfPitNETs in a recent study [135]. A cluster of 22 phosphopeptides was found to be upregulated in recurrent nfPitNETs and a significant phosphorylation of the β-catenin at Ser552 in recurrent and invasive nfPitNETs, compared to noninvasive/nonrecurrent nfPitNET subgroup, suggesting the phosphorylation status of β-catenin at Ser552 could act as a potential biomarker of tumor recurrence in nfPitNETs. This merits additional investigation, given the importance of the Wnt/β-catenin pathway in tumorigenesis and pituitary development.
- The role of cofilin as a biomarker for invasion in nfPitNETs has also been investigated in a couple of studies [136,137]. Actin cytoskeleton rearrangement is regulated by phosphorylated cofilin, inhibiting actin binding and leading to cell migration. Cofilin phosphorylation increased in nfPitNET cells treated with dopamine agonist (DA), with lower phospho-cofilin immunostaining in tumors with cavernous sinus invasion [136]. Moreover, differential expression of phospho-cofilin was associated with GH deficiency and compressive pituitary mass effects in a second study that investigated pituitary tumors with dural invasion [137].
- Whether phosphorylated epidermal growth factor receptor (pEGFR T693) can predict recurrence in nfPitNETs has also been examined in 102 patients followed for a median of 123 months [6]. Patients with tumor recurrence had higher pEGFR T693 positivity and nuclear pEGFR T693 may serve as a predictor for tumor recurrence [6].
BIOMARKERS
- Evaluation
- According to the most recent guidelines, any patient presenting with a macroadenoma or a microadenoma >6 mm should have a laboratory assessment to test for hormonal abnormalities, whether they present with symptoms or not [138,139]. The most common hormonal findings are hypogonadism and GH deficiency, followed by central hypothyroidism and secondary adrenal insufficiency [139]. However, patients with PitNETs rarely present with diabetes insipidus; therefore, if this is a finding, other diagnoses should be considered. Panhypopituitarism occurs in 6% to 29% of patients at diagnosis, and 25% to 65% of patients have hyperprolactinemia due to pituitary stalk compression. However, it is important to determine if the tumor is a nfPitNET or a prolactinoma as the treatment for the two tumors differs considerably, with surgery being the first-line treatment for nfPitNETs and medical therapy for prolactinomas. The level of PRL in the blood can give an indication as to the tumor type. A retrospective study reported that nfPitNETs usually present with PRL levels <100 ng/mL, whereas prolactinomas will have PRL levels >250 ng/mL [140]. Clinical judgement is therefore required when the PRL levels fall within 100 to 250 ng/mL.
- The next step in evaluation is radiological assessment. Usually, this involves MRI with or without contrast [141] unless it is contraindicated. Contrast can help identify the tumor as PitNETs exhibit delayed enhancement, so after contrast, the tumor will appear hypointense compared to the surrounding pituitary gland. Radiological assessment is crucial in the diagnosis of PitNETs as it can give precise measurements on size, degree of invasion into the cavernous sinus and proximity to important structures, including the optic chiasm and carotid arteries [141]. This information is necessary to grade and classify the tumor, as described above, and decide treatment for the individual.
- If, after MRI evaluation, the tumor is distant from the optic chiasm and cavernous sinus and the patient has no visual abnormalities, then an ophthalmologic assessment is not needed [85]. However, if the patient does develop visual symptoms in the future, this would be a strong indication for surgery. Although ophthalmologic assessment is not always required for nfPitNETs, it was recommended by the Congress of Neurological Surgeons systematic review and evidence-based guideline in 2016 [142]. Such evaluation can provide prognostic factors for recovery and, when paired with postoperative evaluation, documents postoperative change. In addition to formal ophthalmologic examinations, tests of value include automatic static perimetry and optical coherence tomography [142]. Older patients and patients with longer duration (>4 months) of vision loss should be counseled regarding the reduced chance of postoperative vision improvement [142].
- Treatment
- Treatment options vary depending on the characteristics of the tumor and the presenting symptoms of the patients. Treatment options include active surveillance, surgery, radiotherapy, and medical therapy.
- Unfortunately, there are few natural history studies on asymptomatic nfPitNETs to determine an optimal treatment strategy [143]. A large retrospective cohort of 371 patients with nfPitNETs found at least one pituitary deficiency in 23.7% of incidental nfPitNETs at diagnosis and identified older age and larger tumor size as risk factors for secondary hormonal insufficiency [144]. Another retrospective study analyzing 347 patients with micro-nfPitNETs demonstrated 2.1 per 100 person-years of growth incidence [145]. Currently, an observational approach has been used in select patients without visual field defects with microadenomas; however, if the patient is younger and has a large lesion, earlier surgical intervention may be advised. If the observational approach is selected for a microadenoma, routine follow-up by MRI imaging is needed to monitor for tumor enlargement every year for 3 years [138]. However, the interval of follow-up MRI could be extended based on a recent study that found a growth incidence of 2.1 per 100 person-years with a mean and median time to growth of 38.1 and 24.5 months, respectively, in a cohort of 347 patients with micro-nfPitNETs followed for a median of 29 months [145]. After 3 years, repeat imaging can be done less frequently if there is no growth. No surveillance is routinely recommended for microadenomas with a diameter <5 mm. If the tumor does grow or compress the optic chiasm, then surgery would be advised. Surgery is not immediately recommended as microadenoma growth only occurs in 3% to 12.5% of patients, with less than 5% growing >1 cm during follow-up [94,95].
- For nfPitNETs larger than 1 cm in size that are asymptomatic when surgery is not performed, visual field testing and MRI imaging should be performed every 6 months to start, and then annually for 3 years following. After those 3 years, imaging can occur less frequently. Overall, approximately 40% to 50% of asymptomatic nfPitNETs will enlarge during long-term follow-up and close to 20% of tumors will become symptomatic, with 9.5% developing apoplexy [90,146]. Surgery is also often required in 21% to 29% of the tumors studied. The median growth of macroadenomas is 0.6 mm/year. Therefore, they grow quite slowly, but monitoring for hypopituitarism every 6 to 12 months should be considered [138,146]. Depending on the distance to the optic chiasm, visual field testing should be considered at an appropriate interval [147]. Unfortunately, there are no current prognostic biomarkers to predict whether the tumor will grow or require surgical intervention. More research is needed on the natural history of these tumors and finding biomarkers to predict their growth behavior, resulting in effective management of these tumors.
- In patients who present with symptoms of visual disturbances or pituitary apoplexy with visual disturbances, surgery is the firstline treatment [138]. However, if the patient presents instead with hypopituitarism, headache, or a tumor close to the optic chiasm, the recommendation for surgery is less clear-cut. A study has reported that surgery can improve hypopituitarism in 30% of patients, but the risk of developing a new hormone deficiency after the surgery is approximately 3% to 15% [148,149]. Therefore, surgery is not usually recommended if hypopituitarism is the only symptom [150]. If the headaches are extreme, surgery can be performed, although there is no guarantee it will resolve the headaches. However, a recent review article reported that 89.7% to 100% of patients who presented with preoperative headaches had relief after surgery, 5.6% reported stable headaches and no patients experienced worse symptoms after longterm follow-up [151].
- Surgery
- The first-line treatment is endoscopy or microscopy-assisted transsphenoidal surgical resection; however, nfPitNETs often have supra- or parasellar extension so total resection of the tumor is often not possible, with total resection occurring in 60% to 73% of patients [4]. This results in residual tumor regrowth in 47% of patients and 24% reoccurrence after complete macroscopic resection [5]. If the tumor is predominantly suprasellar, transcranial surgery might be performed in specific cases [152]. After more than 20 years, there has yet to be a consensus on whether microscopy or endoscopy is the superior visualization technique. Therefore, both are still used around the world. However, recent review articles have reported that the endoscopy approach resulted in significantly higher rates of gross total resection and lower rates of complications, including lower rates of hypopituitarism, in nfPitNETs compared to the microscopic approach [153,154]. Recently, intraoperative MRI has been used for pituitary surgery. The benefit over the usual technique is that it shows the tumor status during the surgery, so the surgeon is able to resect more of the tumor. Therefore, it is thought to improve patient outcomes; however, the results have been mixed with some finding improved resection [155,156] and others finding no difference [157]. Complications from surgery are quite rare, with a mortality rate <1% and <5% of patients experiencing postoperative complications such as cerebrospinal fluid leakage, fistula, meningitis, vascular injury, persistent diabetes insipidus, or new visual field defects [148]. As the experience of the surgeon performing the surgery has an effect on the complication rate, a center with experience of >25 transsphenoidal operations for pituitary adenomas per year provides a high likelihood of safe transsphenoidal surgery [158].
- Radiation
- Radiation has been shown to be an effective treatment after surgical resection of the tumor to prevent regrowth or recurrence. However, it is often not used as a primary treatment except for those patients who are unfit for surgery. Although the efficacy of radiotherapy as first-line therapy is sparse, three studies have reported reduced tumor size in 38% to 83% of patients [159-161]. However, Lee et al. [159] reported that 24% of patients developed new or worsened hypopituitarism after gamma knife radiosurgery and reported that the number of patients that developed hypopituitarism significantly correlated with the dose of radiation given [160]. These studies suggest that radiotherapy as a primary surgery can be beneficial for patients who are unable to have surgical resection; however, the inability to obtain a histological classification is a considerable limitation for this treatment [160,161].
- In general, nfPitNETs require lower doses of radiation than fPitNETs, increasing its utility in nfPitNETs as a treatment while reducing the risk of developing hypopituitarism [162]. After radiation, the somatotroph axis is the most vulnerable to damage, resulting in isolated GH deficiency [163]. The incidence of the deficiency increases with increasing radiation doses. The most common sequence of deficiencies begins with GH, then LH/ FSH, ACTH and TSH, with the incidence of each at 100%, 91%, 77%, and 42%, respectively, after 8 years of follow-up [164]. This specific sequence was seen in 61% of patients with nfPitNETs [164].
- Currently, the most common method of radiation used is stereotactic radiation (SRS). The most used SRS techniques include the Cobalt-60 gamma radiation-emitting sources gamma knife, the robotic SRS system CyberKnife (Accuray, Madison, WI, USA), and the use of a modified linear accelerator. The most reported in the literature is gamma knife radiotherapy, although the clinical efficacy and toxicity are similar among the three modalities, with tumor shrinkage occurring in 20% to 60% of patients [165] and tumor control rates of 85%–95% at 5–10 years [166]. Additionally, low radiation doses, larger tumor volumes and suprasellar extension were associated with worse outcomes. Visual function also improved in 25% of patients, since patients with tumors close to the optic apparatus were excluded in treatment. Overall, the risk of severe complications with SRS radiation is low, with hypopituitarism being the most common long-term side effect occurring in 10% to 40% of patients within 5 years of treatment [165]. The factors increasing the risk of developing hypopituitarism were pre-existing hormonal pituitary deficits, suprasellar invasion and higher radiation doses targeted to the pituitary gland and stalk. Therefore, follow-up for hypopituitarism should be done every 6 months after radiation therapy. Other side effects include neurological complications, such as visual disturbances and cranial nerve compression due to cavernous sinus invasion, which have been reported in 1% to 4% of patients [165]. Other complications are quite rare and the risk of a second tumor after treatment is significantly lower than conventional radiotherapy. Due to the high incidence of complications, radiation should only be used on tumors with a high likelihood of progression [167].
- Medical therapy
- Due to the high occurrence of regrowth and recurrence of nfPitNETs and the complications that arise from radiotherapy, medical therapy provides another treatment option to manage these recurrent cases. The three medical therapies that have shown the most promise are DAs, somatostatin analogs and TMZ.
- Dopamine receptor 2 (DR2) expression is found in most pituitary tumors, as in normal pituitary glands the receptor binds with dopamine to inhibit the release of PRL. DR2 expression has been reported in nfPitNETs specifically [168-170], with one study reporting DR2 expression was similar between nfPitNETs and fPitNETs [171]. Due to the relationship between dopamine and PRL, DA therapy is the first-line treatment for prolactinomas, and the theory is that it would work similarly for other types of pituitary tumors like nfPitNETs. A few studies have found that in nfPitNETs there was no association between DR2 expression and tumor shrinkage from DA therapy, even though the majority of tumors were positive for DR2 expression [170,172]. In contrast, another study reported a correlation between DR2 expression and cabergoline-induced tumor shrinkage, with the short isoform of the receptor associated with more favorable outcomes [173]. Therefore, it is unclear if one can determine tumor susceptibility to DA therapy by analyzing DR2 expression.
- Bromocriptine therapy was the original DA therapy used in earlier studies; however, cabergoline therapy is more potent and is the DA therapy of choice. Bromocriptine therapy in patients with nfPitNETs is associated with decreased prevalence of residual tumor enlargement and suppression of cell proliferation in a small number of patients [168,170]. However, other studies have reported that the drug is ineffective in reducing tumor size or preventing tumor growth in nfPitNETs [174,175]. Cabergoline therapy in patients with nfPitNETs is associated with decreased prevalence of residual tumor enlargement, residual and primary tumor shrinkage, and stabilization in some patients, although some patients did exhibit tumor growth or no change in tumor size [169,170,172,173,176-178]. A recent meta-analysis showed cabergoline-induced tumor shrinkage in 19% of patients with nfPitNETs and prevented tumor progression after surgery in 50% of patients with nfPitNETs [179]. Larger controlled studies with long-term follow-up are needed before DA therapy can be incorporated into the routine practice for nfPitNETs.
- Medical therapy with somatoatatin analogs targeting somatostatin receptors is indicated in GH-secreting tumors and ACTH-secreting tumors; however, their role in nfPitNETs is still not fully assessed [180,181]. Expression profiles of somatostatin receptor 1–5 in nfPitNETs have been reported [182-184], suggesting the potential use of somatostatin analogs for nfPItNETs. Octreotide therapy in patients with nfPitNETs is associated with stabilization of post-surgical tumor remnants but has had limited effect on tumor shrinkage [99,171,185-187] and in vitro data indicate pasireotide, a multiple somatostatin receptors ligand, may be useful [186]. However, a phase 2 clinical trial has reported that pasireotide induced a tumor size reduction of at least 20% in only 16.7% patients with nonfunctioning gonadotroph tumor [188]. In a review article, tumor size reduction was reported in 12% of patients, increase in 5% and no change in 83% of patients [189]. These studies suggest the limited effectiveness of somatostatin analogs for nfPitNETs.
- Temozolomide
- TMZ is an oral alkylating agent with a growing role in treating carcinomas and aggressive pituitary tumors. Due to most of the studies looking at TMZ effects on aggressive pituitary tumors having small sample sizes and varying follow-up durations, the European Society of Endocrinology has labeled the amount of evidence as very low [190]. In a meta-analysis conducted by the European Society of Endocrinology, the tumor response after TMZ was approximately 47% (95% confidence interval, 36% to 58%) [190]. Even though the evidence is low, a 3-month trial of TMZ is recommended as first-line chemotherapy for carcinomas and aggressive PitNETs by the society [190]. Aggressive PitNETs are defined as having invasive growth and unusually rapid growth rate or showing persistent growth after standard treatment. Another review found similar results, reporting partial or complete response achieved in 22% of patients and stable disease in 48% [191]. This study also reported that prolactinomas and corticotroph tumors respond best to TMZ, showing approximately a 50% response rate, with nfPitNETs responding only half as frequently. Another review also reported that nfPitNETs exhibit lower TMZ responses at 40% compared to fPitNETs, which ranged from 60% to 73% [109]. It is suggested TMZ might not be as effective in treating nfPitNETs compared to fPitNETs due to the high expression of MGMT. The lack of randomized control trials in nfPitNETs and varying results between studies indicated that the role of TMZ in treating nfPitNETs remains unclear, although it seems reasonable to have a therapeutic trial of TMZ in patients with aggressive and invasive nfPitNETs [192].
- Capecitabine (pro-drug of 5-fluorouracil)+temozolomide
- Capecitabine is a systemic pro-drug of 5-Fluorouracil, which attenuates the activity of MGMT, promoting the apoptotic effect of TMZ in PitNET cells. Recent studies, including ours, suggested that TMZ combined with capecitabine (CAPTEM) can be more effective compared to TMZ monotherapy for the treatment of aggressive PitNETs, including nfPitNETs (pituitary carcinomas, silent corticotroph PitNETs, null cell adenomas, a poorly differentiated PIT1 tumors, and prolactinomas) [193-198]. Capecitabine 1,500 mg/m2/day (maximum daily dose of 2,500 mg on days 1 through 14 divided into two doses) and TMZ 150 to 200 mg/m2/day (divided into two doses) are given orally on days 10 through 14. This 2-week protocol is followed by 2 weeks off-treatment [193] and the treatment is well tolerated by patients. Although cases with nfPitNETS treated with CAPTEM are limited, promising outcomes are sometimes seen. Further studies are required to determine if CAPTEM is superior to TMZ monotherapy and can be a novel treatment option for aggressive nfPitNETs as in other neuroendocrine tumors.
MANAGEMENT
Asymptomatic tumor
Symptomatic tumor
Dopamine agonists
Somatostatin analogs
- Limited studies of experimental medical therapy are available partly due to the lack of proper in vitro and in vivo models of nfPitNETs. Genetically engineered mouse models of PitNETs share some biochemical and molecular features of PitNETs. However, no current mouse model fully recapitulates pituitary tumorigenesis [199,200]. Our mouse model with neuron-glial antigen 2 (NG2)-driven retinoblastoma protein (pRb) inactivation develop PIT1 linage PitNETs with high penetrance [201]. The tumors in the mice have pathological features that are similar to those of human aggressive PIT1-lineage nfPitNETs previously referred to as silent subtype 3 adenomas. However, the role of Rb gene for human PitNETs remains elusive.
- The mammalian target of rapamycin (mTOR) pathway is a recent treatment target for various tumor types, including endocrine tumors. The pathway is thought to be responsible for proliferation, survival, and drug resistance in various cancers [202]. Specifically, two mTOR regulatory kinases, Akt and extracellular signal-regulated kinase (Erk), show increased activity in nfPitNETs [55]. Sajjad et al. [203], therefore, evaluated whether an mTOR inhibitor, rapamycin, would induce mTOR inhibition in mTOR-active nfPitNET cells. The authors reported that all cell lines showed mTOR inhibition in response to rapamycin, while inhibition of Erk and Akt did not affect mTOR activity. However, cell viability or proliferation was not evaluated. Zatelli et al. [204] evaluated a different mTOR inhibitor, everolimus, and reported reduced cell viability in nfPitNET cells. Additionally, Lee et al. [205] evaluated the antitumor effects of NVPBEZ235, a dual PI3K/mTOR inhibitor, on nfPitNETs both in vitro and in rats. The authors reported antiproliferative, apoptosis and PI3K inhibition in the nfPitNETs both in vitro and in vivo in response to NVP-BEZ235. All of these studies together show evidence that PI3K/mTOR inhibition might prove beneficial for the treatment of nfPitNETs.
- A class of chimeric drugs combining somatostatin and dopamine receptor binding activity in one molecule has shown promising results in GH-secreting tumors by proving to be more effective than both DA and somatostatin analogs [206]. Therefore, Florio et al. [207] evaluated the efficacy of BIM-23A760, a dopamine-somatostatin chimeric, in controlling cell growth in nfPitNETs in vitro. BIM-23A760 significantly inhibited 3 H-thymidine incorporation, a marker of cell growth, in 60% of nfPitNETs analyzed. This was comparable to cabergoline, which showed significant inhibition in 66% of nfPitNETs and octreotide which was effective in 48% of nfPitNETs whereas combined cabergoline and octreotide was effective in 54% of nfPitNETs studied. The in vitro effectiveness of the chimeric drug in inhibiting cell growth in nfPitNETs was complemented by a recent in vivo study by Halem et al. [208]. After 8 weeks of BIM23A760 (also known as TBR-760) treatment, tumor growth in an animal model of nfPitNETs was nearly completely inhibited, compared to vehicle-treated mice who experienced 0.7% tumor growth. Treatment with DA and somatostatin analogs, either alone or in combination, resulted in no significant effect on tumor growth, except for a modest suppression by low-dose DA therapy. Furthermore, not only did the TBR-760 arrest tumor growth, but tumor shrinkage was seen in 20% of the nfPitNETs analyzed.
- Bromodomain-containing protein 4 (BRD4) is an important molecule that regulates transcription initiation and elongation and plays a key role in cell cycle progression. The expression of BDR4 in nfPitNET and GH-secreting PitNETs and the effects of ZBC-260, a BRD4 inhibitor, on cell cycle progression, apoptosis and expression of downstream genes were investigated by Shi et al. [209], and BRD4 expression was significantly higher in the PitNETs compared to normal pituitary tissue. However, there were no significant differences in expression between the nfPitNET subtypes. Additionally, ZBC-260 significantly inhibited cell proliferation in both PitNET subtypes and downregulated the expression of multiple viral factors in pituitary tumorigenesis, including c-Myc, B-cell lymphoma 2, and others.
TARGETS OF THERAPY IDENTIFIED BY EXPERIMENTAL FINDINGS
- There are other molecules, including signal transducer and activator of transcription 3 (STAT3), macrophage migratory inhibitory factor (MIF), and L-type amino acid transporter 1 (LAT1), that show promise in various cancers and could be potential therapeutic targets for nfPitNETs. STAT3 can upregulate specific genes related to cancer progression. Our group has recently reviewed STAT3’s role in tumorigenesis and hormone regulation in PitNETs [210]. With only one study analyzed nfPitNETs specifically and reported a significant association between interleukin-6 receptor (IL-6R)/Janus kinase 2 (JAK2)/STAT3/ MMP9 signaling pathway activation and invasiveness of null cell nfPitNETs in 52 patients [211], additional studies are required to confirm STAT3 as a potential therapeutic target. MIF is a pro-inflammatory cytokine expressed in a variety of tumors and cancers, including nfPitNETs [212,213], and plays a crucial role in cancer development. Even though MIF is overexpressed in many solid cancer tumors and is closely associated with tumor cell proliferation, angiogenesis and tumorigenesis as reported in a recent review [214], its role as a potential therapeutic biomarker in reducing tumor growth after incomplete surgical resection still needs to be assessed. LAT assist tumor growth by enabling the influx of amino acids, which are essential for cell proliferation [215]. LAT1 specifically, has been shown to be overexpressed in various types of solid cancers and associated with significantly shorter survival time. Furthermore, downregulation of LAT1 has been shown to impair tumor growth in different cancers [215], and LAT1 inhibition decreased cell proliferation in GH-producing PitNETs [216].
POTENTIAL TARGETS AND EMERGING THERAPIES FOR THE MANAGEMENT OF nfPitNETs
- After reviewing the current literature, it is evident that more research needs to be conducted to screen for and treat nfPitNETs effectively. Additional studies need to be performed to identify biomarkers as potential therapeutic targets and early screening tools. It is imperative to improve screening to reduce the delay in diagnosis that results in tumor growth. Once the tumor increases over 1 cm in size, mass effects are usually present which can cause reduced quality of life and irreparable damage. Due to this tumor growth, invasion into the cavernous sinus is more likely in nfPitNETs making complete tumor resection often not possible. Therefore, there is a need for effective adjunctive treatments to minimize residual tumor growth after incomplete tumor resection and decrease tumor recurrence that presents after complete surgical resection. Currently, radiation, repeat surgery and medical therapy are treatment options; however, no one treatment has proven superior or been extensively studied in large, randomized trials. The new biomarkers and novel treatment options for nfPitNETs suggested are based on small-scale in vivo and/or in vitro studies, and further studies are required to validate the findings. To improve patient care and their quality of life, large trials with long-term follow-up are essential in the future management of patients with nfPitNETs.
CONCLUSIONS
-
CONFLICTS OF INTEREST
Elizabeth Whyte is a recipient of Summer Research Studentship from Alberta Innovates. Masahiro Nezu is a recipient of fellowship from Daiichi Sankyo Foundation of Life Science. No potential conflict of interest relevant to this article was reported.
Article information
-
Acknowledgements
- This study is supported by a grant from the University Hospital Foundation.
| PitNET type and subtype | Hormonal IHC | Transcription factors | ||
|---|---|---|---|---|
| PIT1 lineage | ||||
| Somatotroph tumors | ||||
| Densely granulated | GH, α-subunit | PIT1 | ||
| Sparsely granulated | GH | PIT1 | ||
| Lactotroph tumors | ||||
| Sparsely granulated | PRL | PIT1, ERα | ||
| Densely granulated | PRL | PIT1, ERα | ||
| Mammosomatotroph tumor | GH, PRL, α-subunit | PIT1, ERα | ||
| Mixed somatotroph lactotroph | GH, PRL±α-subunit | PIT1, ERα | ||
| Thyrotroph tumor | β-TSH, α-subunit | PIT1, GATA3a | ||
| Mature plurihormonal PIT1-lineage tumor | GH±PRL, β-TSH, α-subunit | PIT1, ERα, GATA3a | ||
| Immature PIT1-lineage tumor | None or GH±PRL± β-TSH±α-subunit | PIT1, ERα | ||
| Acidophil stem cell tumor | PRL, GH | PIT1, ERα | ||
| TPIT lineage | ||||
| Corticotroph tumors | ||||
| Densely granulated | ACTH and other POMC derivatives | TPIT | ||
| Sparsely granulated | ACTH and other POMC derivatives | TPIT | ||
| Crooke’s cell | ACTH and other POMC derivatives | TPIT | ||
| SF1 lineage | ||||
| Gonadotroph tumor | ||||
| Sparsely granulated | β-FSH, β-LH, α-subunit, or none | SF1, GATA3a, ERα | ||
| No distinct cell lineage | ||||
| Plurihormonal tumor | Multiple combinations | Multiple combinations | ||
| Null cell | None | None | ||
Adapted from Asa et al. [2], with permission from Springer Nature.
PitNET, pituitary neuroendocrine tumor; IHC, immunohistochemistry; PIT1, pituitary specific transcription factor 1; GH, growth hormone; PRL, prolactin; ERα, estrogen receptor alpha; TSH, thyroid-stimulating hormone; GATA3, GATA binding protein 3; TPIT, T-box transcription factor; ACTH, adrenocorticotropic hormone; POMC, proopiomelanocortin; SF1, steroidogenic factor 1; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
a GATA3 is a paralog of GATA2, and GATA3 immunostaining can detect GATA2-positive cells that is important for development of gonadotrophs and thyrotrophs.
- 1. Chen Y, Wang CD, Su ZP, Chen YX, Cai L, Zhuge QC, et al. Natural history of postoperative nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Neuroendocrinology 2012;96:333–42.ArticlePubMedPDF
- 2. Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO classification of pituitary tumors. Endocr Pathol 2022;33:6–26.ArticlePubMedPDF
- 3. Ntali G, Wass JA. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 2018;21:111–8.ArticlePubMedPDF
- 4. Lamback EB, Wildemberg LE, Gadelha MR. Current opinion on the diagnosis and management of non-functioning pituitary adenomas. Expert Rev Endocrinol Metab 2021;16:309–20.ArticlePubMed
- 5. Brochier S, Galland F, Kujas M, Parker F, Gaillard S, Raftopoulos C, et al. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol 2010;163:193–200.ArticlePubMed
- 6. Rai A, Das L, Mukherjee KK, Dhandapani S, Tripathi M, Ahuja CK, et al. Phosphorylated EGFR (pEGFR T693) as a novel predictor of recurrence in non-functioning pituitary adenomas. Front Endocrinol (Lausanne) 2021;12:708111.ArticlePubMedPMC
- 7. Drange MR, Fram NR, Herman-Bonert V, Melmed S. Pituitary tumor registry: a novel clinical resource. J Clin Endocrinol Metab 2000;85:168–74.ArticlePubMed
- 8. Raappana A, Koivukangas J, Ebeling T, Pirila T. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab 2010;95:4268–75.ArticlePubMed
- 9. Oh JS, Kim HJ, Hann HJ, Kang TU, Kim DS, Kang MJ, et al. Incidence, mortality, and cardiovascular diseases in pituitary adenoma in Korea: a nationwide population-based study. Pituitary 2021;24:38–47.ArticlePubMedPDF
- 10. Osorio RC, Pereira MP, Joshi RS, Donohue KC, Sneed P, Braunstein S, et al. Socioeconomic predictors of case presentations and outcomes in 225 nonfunctional pituitary adenoma resections. J Neurosurg 2021;136:1325–36.Article
- 11. Cote DJ, Ruzevick JJ, Kang KM, Pangal DJ, Bove I, Carmichael JD, et al. Association between socioeconomic status and presenting characteristics and extent of disease in patients with surgically resected nonfunctioning pituitary adenoma. J Neurosurg 2022;137:1699–706.ArticlePubMed
- 12. Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol 2017;28:228–43.ArticlePubMedPDF
- 13. Nishioka H, Inoshita N, Mete O, Asa SL, Hayashi K, Takeshita A, et al. The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr Pathol 2015;26:349–55.ArticlePubMedPDF
- 14. Oystese KA, Casar-Borota O, Normann KR, Zucknick M, Berg JP, Bollerslev J. Estrogen receptor α, a sex-dependent predictor of aggressiveness in nonfunctioning pituitary adenomas: SSTR and sex hormone receptor distribution in NFPA. J Clin Endocrinol Metab 2017;102:3581–90.ArticlePubMed
- 15. Drummond J, Roncaroli F, Grossman AB, Korbonits M. Clinical and pathological aspects of silent pituitary adenomas. J Clin Endocrinol Metab 2019;104:2473–89.ArticlePubMedPMC
- 16. Ben-Shlomo A, Cooper O. Silent corticotroph adenomas. Pituitary 2018;21:183–93.ArticlePubMedPDF
- 17. Saeger W, Ludecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 2007;156:203–16.ArticlePubMed
- 18. Langlois F, Lim DS, Varlamov E, Yedinak CG, Cetas JS, McCartney S, et al. Clinical profile of silent growth hormone pituitary adenomas; higher recurrence rate compared to silent gonadotroph pituitary tumors, a large single center experience. Endocrine 2017;58:528–34.ArticlePubMedPDF
- 19. Fountas A, Lavrentaki A, Subramanian A, Toulis KA, Nirantharakumar K, Karavitaki N. Recurrence in silent corticotroph adenomas after primary treatment: a systematic review and meta-analysis. J Clin Endocrinol Metab 2019;104:1039–48.Article
- 20. Park P, Chandler WF, Barkan AL, Orrego JJ, Cowan JA, Griffith KA, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery 2004;55:100–7.ArticlePubMedPDF
- 21. Cho HY, Cho SW, Kim SW, Shin CS, Park KS, Kim SY. Silent corticotroph adenomas have unique recurrence characteristics compared with other nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf) 2010;72:648–53.ArticlePubMed
- 22. Erickson D, Scheithauer B, Atkinson J, Horvath E, Kovacs K, Lloyd RV, et al. Silent subtype 3 pituitary adenoma: a clinicopathologic analysis of the Mayo Clinic experience. Clin Endocrinol (Oxf) 2009;71:92–9.ArticlePubMed
- 23. Mete O, Gomez-Hernandez K, Kucharczyk W, Ridout R, Zadeh G, Gentili F, et al. Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod Pathol 2016;29:131–42.ArticlePubMedPDF
- 24. Fealey ME, Scheithauer BW, Horvath E, Erickson D, Kovacs K, McLendon R, et al. MGMT immunoexpression in silent subtype 3 pituitary adenomas: possible therapeutic implications. Endocr Pathol 2010;21:161–5.ArticlePubMedPDF
- 25. Salehi F, Scheithauer BW, Kros JM, Lau Q, Fealey M, Erickson D, et al. MGMT promoter methylation and immunoexpression in aggressive pituitary adenomas and carcinomas. J Neurooncol 2011;104:647–57.ArticlePubMedPDF
- 26. Lenders NF, Inder WJ, McCormack AI. Towards precision medicine for clinically non-functioning pituitary tumours. Clin Endocrinol (Oxf) 2021;95:398–409.ArticlePubMedPDF
- 27. Balogun JA, Monsalves E, Juraschka K, Parvez K, Kucharczyk W, Mete O, et al. Null cell adenomas of the pituitary gland: an institutional review of their clinical imaging and behavioral characteristics. Endocr Pathol 2015;26:63–70.ArticlePubMedPDF
- 28. Almeida JP, Stephens CC, Eschbacher JM, Felicella MM, Yuen KC, White WL, et al. Clinical, pathologic, and imaging characteristics of pituitary null cell adenomas as defined according to the 2017 World Health Organization criteria: a case series from two pituitary centers. Pituitary 2019;22:514–9.ArticlePubMedPDF
- 29. Mete O, Cintosun A, Pressman I, Asa SL. Epidemiology and biomarker profile of pituitary adenohypophysial tumors. Mod Pathol 2018;31:900–9.ArticlePubMedPDF
- 30. Tampourlou M, Ntali G, Ahmed S, Arlt W, Ayuk J, Byrne JV, et al. Outcome of nonfunctioning pituitary adenomas that regrow after primary treatment: a study from two large UK centers. J Clin Endocrinol Metab 2017;102:1889–97.ArticlePubMed
- 31. Chinezu L, Vasiljevic A, Trouillas J, Lapoirie M, Jouanneau E, Raverot G. Silent somatotroph tumour revisited from a study of 80 patients with and without acromegaly and a review of the literature. Eur J Endocrinol 2017;176:195–201.ArticlePubMed
- 32. Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, et al. A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 2007;61:580–5.ArticlePubMed
- 33. Ntali G, Capatina C, Grossman A, Karavitaki N. Clinical review: functioning gonadotroph adenomas. J Clin Endocrinol Metab 2014;99:4423–33.PubMed
- 34. Cote DJ, Smith TR, Sandler CN, Gupta T, Bale TA, Bi WL, et al. Functional gonadotroph adenomas: case series and report of literature. Neurosurgery 2016;79:823–31.PubMed
- 35. Hwang J, Seol HJ, Nam DH, Lee JI, Lee MH, Kong DS. Therapeutic strategy for cavernous sinus-invading nonfunctioning pituitary adenomas based on the modified Knosp grading system. Brain Tumor Res Treat 2016;4:63–9.ArticlePubMedPMCPDF
- 36. Hardy J, Vezina JL. Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol 1976;15:261–73.PubMed
- 37. Wilson CB. A decade of pituitary microsurgery: the Herbert Olivecrona lecture. J Neurosurg 1984;61:814–33.PubMed
- 38. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993;33:610–8.ArticlePubMed
- 39. Yuhan L, Zhiqun W, Jihui T, Renlong P. Ki-67 labeling index and Knosp classification of pituitary adenomas. Br J Neurosurg 2021 Apr 27 [Epub]. https://doi.org/10.1080/02688697.2021.1884186.ArticlePubMedPMC
- 40. Micko AS, Wohrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg 2015;122:803–11.ArticlePubMed
- 41. Araujo-Castro M, Acitores Cancela A, Vior C, Pascual-Corrales E, Rodriguez Berrocal V. Radiological Knosp, revisedKnosp, and Hardy-Wilson classifications for the prediction of surgical outcomes in the endoscopic endonasal surgery of pituitary adenomas: study of 228 cases. Front Oncol 2022;11:807040.ArticlePubMedPMC
- 42. Berkmann S, Lattmann J, Schuetz P, Diepers M, Remonda L, Fandino J, et al. The Shape grading system: a classification for growth patterns of pituitary adenomas. Acta Neurochir (Wien) 2021;163:3181–9.ArticlePubMedPDF
- 43. Mooney MA, Sarris CE, Zhou JJ, Barkhoudarian G, Chicoine MR, Fernandez-Miranda JC, et al. Proposal and validation of a simple grading scale (TRANSSPHER Grade) for predicting gross total resection of nonfunctioning pituitary macroadenomas after transsphenoidal surgery. Oper Neurosurg (Hagerstown) 2019;17:460–9.ArticlePubMed
- 44. Trouillas J, Roy P, Sturm N, Dantony E, Cortet-Rudelli C, Viennet G, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol 2013;126:123–35.ArticlePubMedPDF
- 45. Raverot G, Dantony E, Beauvy J, Vasiljevic A, Mikolasek S, Borson-Chazot F, et al. Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. J Clin Endocrinol Metab 2017;102:3368–74.ArticlePubMed
- 46. Lelotte J, Mourin A, Fomekong E, Michotte A, Raftopoulos C, Maiter D. Both invasiveness and proliferation criteria predict recurrence of non-functioning pituitary macroadenomas after surgery: a retrospective analysis of a monocentric cohort of 120 patients. Eur J Endocrinol 2018;178:237–46.ArticlePubMed
- 47. Sahakian N, Appay R, Resseguier N, Graillon T, Piazzola C, Laure C, et al. Real-life clinical impact of a five-tiered classification of pituitary tumors. Eur J Endocrinol 2022;187:893–904.ArticlePubMedPDF
- 48. Wierinckx A, Raverot G, Nazaret N, Jouanneau E, Auger C, Lachuer J, et al. Proliferation markers of human pituitary tumors: contribution of a genome-wide transcriptome approach. Mol Cell Endocrinol 2010;326:30–9.ArticlePubMed
- 49. Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, et al. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 2002;111:673–85.PubMed
- 50. Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 2012;97:2990–3011.ArticlePubMed
- 51. Alrezk R, Hannah-Shmouni F, Stratakis CA. MEN4 and CDKN1B mutations: the latest of the MEN syndromes. Endocr Relat Cancer 2017;24:T195–208.ArticlePubMedPMC
- 52. Daly AF, Beckers A. The role of AIP mutations in pituitary adenomas: 10 years on. Endocrine 2017;55:333–5.ArticlePubMedPDF
- 53. Bi WL, Horowitz P, Greenwald NF, Abedalthagafi M, Agarwalla PK, Gibson WJ, et al. Landscape of genomic alterations in pituitary adenomas. Clin Cancer Res 2017;23:1841–51.ArticlePubMedPMCPDF
- 54. Zhan X, Desiderio DM. Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med Genomics 2010;3:13.ArticlePubMedPMCPDF
- 55. Ewing I, Pedder-Smith S, Franchi G, Ruscica M, Emery M, Vax V, et al. A mutation and expression analysis of the oncogene BRAF in pituitary adenomas. Clin Endocrinol (Oxf) 2007;66:348–52.ArticlePubMed
- 56. Lin Y, Jiang X, Shen Y, Li M, Ma H, Xing M, et al. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer 2009;16:301–10.ArticlePubMed
- 57. Simpson DJ, Bicknell JE, McNicol AM, Clayton RN, Farrell WE. Hypermethylation of the p16/CDKN2A/MTSI gene and loss of protein expression is associated with nonfunctional pituitary adenomas but not somatotrophinomas. Genes Chromosomes Cancer 1999;24:328–36.PubMed
- 58. Zhang X, Sun H, Danila DC, Johnson SR, Zhou Y, Swearingen B, et al. Loss of expression of GADD45 gamma, a growth inhibitory gene, in human pituitary adenomas: implications for tumorigenesis. J Clin Endocrinol Metab 2002;87:1262–7.PubMed
- 59. Zhang X, Zhou Y, Mehta KR, Danila DC, Scolavino S, Johnson SR, et al. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab 2003;88:5119–26.ArticlePubMed
- 60. Chhabra R, Rai A, Dutta P. Phosphorylation of Nucks1 on serine 79: a prognostic marker of recurrent nonfunctioning pituitary adenoma. J Neurol Surg B Skull Base 2019;80(S 01):S1–244.Article
- 61. Yu S, Wang XS, Cao KC, Bao XJ, Yu J. Identification of CDK6 and RHOU in serum exosome as biomarkers for the invasiveness of non-functioning pituitary adenoma. Chin Med Sci J 2019;34:168–76.ArticlePubMed
- 62. Komori T. Regulation of Rb family proteins by Cdk6/Ccnd1 in growth plates. Cell Cycle 2013;12:2161–2.ArticlePubMedPMC
- 63. Cheng S, Li C, Xie W, Miao Y, Guo J, Wang J, et al. Integrated analysis of DNA methylation and mRNA expression profiles to identify key genes involved in the regrowth of clinically non-functioning pituitary adenoma. Aging (Albany NY) 2020;12:2408–27.ArticlePubMedPMC
- 64. Aydin B, Beklen H, Arga KY, Bayrakli F, Turanli B. Epigenomic and transcriptomic landscaping unraveled candidate repositioned therapeutics for non-functioning pituitary neuroendocrine tumors. J Endocrinol Invest 2023;46:727–47.ArticlePubMedPDF
- 65. Zatelli MC. Pathogenesis of non-functioning pituitary adenomas. Pituitary 2018;21:130–7.ArticlePubMedPDF
- 66. Gentilin E, Degli Uberti E, Zatelli MC. Strategies to use microRNAs as therapeutic targets. Best Pract Res Clin Endocrinol Metab 2016;30:629–39.PubMed
- 67. Butz H, Liko I, Czirjak S, Igaz P, Khan MM, Zivkovic V, et al. Down-regulation of Wee1 kinase by a specific subset of microRNA in human sporadic pituitary adenomas. J Clin Endocrinol Metab 2010;95:E181–91.ArticlePubMed
- 68. Wu S, Gu Y, Huang Y, Wong TC, Ding H, Liu T, et al. Novel biomarkers for non-functioning invasive pituitary adenomas were identified by using analysis of microRNAs expression profile. Biochem Genet 2017;55:253–67.ArticlePubMedPDF
- 69. Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov 2013;12:130–46.ArticlePubMedPMCPDF
- 70. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800.ArticlePubMedPDF
- 71. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to antiPD-1 therapy. Clin Cancer Res 2014;20:5064–74.ArticlePubMedPMCPDF
- 72. Mei Y, Bi WL, Greenwald NF, Du Z, Agar NY, Kaiser UB, et al. Increased expression of programmed death ligand 1 (PD-L1) in human pituitary tumors. Oncotarget 2016;7:76565–76.ArticlePubMedPMC
- 73. Brilli L, Danielli R, Ciuoli C, Calabro L, Di Giacomo AM, Cerase A, et al. Prevalence of hypophysitis in a cohort of patients with metastatic melanoma and prostate cancer treated with ipilimumab. Endocrine 2017;58:535–41.ArticlePubMedPDF
- 74. Manoranjan B, Mahendram S, Almenawer SA, Venugopal C, McFarlane N, Hallett R, et al. The identification of human pituitary adenoma-initiating cells. Acta Neuropathol Commun 2016;4:125.ArticlePubMedPMCPDF
- 75. Carreno G, Gonzalez-Meljem JM, Haston S, Martinez-Barbera JP. Stem cells and their role in pituitary tumorigenesis. Mol Cell Endocrinol 2017;445:27–34.ArticlePubMed
- 76. Donangelo I, Ren SG, Eigler T, Svendsen C, Melmed S. Sca1⁺ murine pituitary adenoma cells show tumor-growth advantage. Endocr Relat Cancer 2014;21:203–16.ArticlePubMedPMC
- 77. Martinez-Barbera JP, Andoniadou CL. Concise review: paracrine role of stem cells in pituitary tumors: a focus on adamantinomatous craniopharyngioma. Stem Cells 2016;34:268–76.ArticlePubMedPMCPDF
- 78. Peverelli E, Giardino E, Treppiedi D, Meregalli M, Belicchi M, Vaira V, et al. Dopamine receptor type 2 (DRD2) and somatostatin receptor type 2 (SSTR2) agonists are effective in inhibiting proliferation of progenitor/stem-like cells isolated from nonfunctioning pituitary tumors. Int J Cancer 2017;140:1870–80.PubMed
- 79. Lu R, Gao H, Wang H, Cao L, Bai J, Zhang Y. Overexpression of the Notch3 receptor and its ligand Jagged1 in human clinically non-functioning pituitary adenomas. Oncol Lett 2013;5:845–51.ArticlePubMedPMC
- 80. Freda PU, Bruce JN, Khandji AG, Jin Z, Hickman RA, Frey E, et al. Presenting features in 269 patients with clinically nonfunctioning pituitary adenomas enrolled in a prospective study. J Endocr Soc 2020;4:bvaa021.ArticlePubMedPMCPDF
- 81. Jahangiri A, Wagner JR, Chin AT, Han SW, Tran MT, Miller LM, et al. Incidence of headache as a presenting complaint in over 1000 patients with sellar lesions and factors predicting postoperative improvement. Clin Neurol Neurosurg 2015;132:16–20.ArticlePubMed
- 82. Bujawansa S, Thondam SK, Steele C, Cuthbertson DJ, Gilkes CE, Noonan C, et al. Presentation, management and outcomes in acute pituitary apoplexy: a large single-centre experience from the United Kingdom. Clin Endocrinol (Oxf) 2014;80:419–24.ArticlePubMed
- 83. Rizzoli P, Iuliano S, Weizenbaum E, Laws E. Headache in patients with pituitary lesions: a longitudinal cohort study. Neurosurgery 2016;78:316–23.PubMed
- 84. Greenman Y, Melmed S. Diagnosis and management of nonfunctioning pituitary tumors. Ann Rev Med 1996;47:95–106.ArticlePubMed
- 85. Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris) 2015;76:210–9.ArticlePubMed
- 86. Ogra S, Nichols AD, Stylli S, Kaye AH, Savino PJ, DaneshMeyer HV. Visual acuity and pattern of visual field loss at presentation in pituitary adenoma. J Clin Neurosci 2014;21:735–40.ArticlePubMed
- 87. Jahangiri A, Lamborn KR, Blevins L, Kunwar S, Aghi MK. Factors associated with delay to pituitary adenoma diagnosis in patients with visual loss. J Neurosurg 2012;116:283–9.ArticlePubMed
- 88. Rey-Dios R, Payner TD, Cohen-Gadol AA. Pituitary macroadenoma causing symptomatic internal carotid artery compression: surgical treatment through transsphenoidal tumor resection. J Clin Neurosci 2014;21:541–6.ArticlePubMed
- 89. Landeiro JA, Fonseca EO, Monnerat AL, Taboada GF, Cabral GA, Antunes F. Nonfunctioning giant pituitary adenomas: invasiveness and recurrence. Surg Neurol Int 2015;6:179.ArticlePubMedPMC
- 90. Arita K, Tominaga A, Sugiyama K, Eguchi K, Iida K, Sumida M, et al. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J Neurosurg 2006;104:884–91.ArticlePubMed
- 91. Nishizawa S, Ohta S, Yokoyama T, Uemura K. Therapeutic strategy for incidentally found pituitary tumors (“pituitary incidentalomas”). Neurosurgery 1998;43:1344–50.ArticlePubMed
- 92. Wildemberg LE, Glezer A, Bronstein MD, Gadelha MR. Apoplexy in nonfunctioning pituitary adenomas. Pituitary 2018;21:138–44.ArticlePubMedPDF
- 93. Vargas G, Gonzalez B, Ramirez C, Ferreira A, Espinosa E, Mendoza V, et al. Clinical characteristics and treatment outcome of 485 patients with nonfunctioning pituitary macroadenomas. Int J Endocrinol 2015;2015:756069.ArticlePubMedPMCPDF
- 94. Karavitaki N, Collison K, Halliday J, Byrne JV, Price P, Cudlip S, et al. What is the natural history of nonoperated nonfunctioning pituitary adenomas? Clin Endocrinol (Oxf) 2007;67:938–43.ArticlePubMed
- 95. Fernandez-Balsells MM, Murad MH, Barwise A, GallegosOrozco JF, Paul A, Lane MA, et al. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab 2011;96:905–12.ArticlePubMed
- 96. Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al. Endotext. South Dartmouth: MDText.com, Inc; 2022 Chapter, Non-functioning pituitary adenomas [cited 2023 Nov 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534880.
- 97. Karavitaki N, Thanabalasingham G, Shore HC, Trifanescu R, Ansorge O, Meston N, et al. Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition?: a study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin Endocrinol (Oxf) 2006;65:524–9.ArticlePubMed
- 98. Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 1996;38:99–107.ArticlePubMed
- 99. Ramírez C, Cheng S, Vargas G, Asa SL, Ezzat S, Gonzalez B, et al. Expression of Ki-67, PTTG1, FGFR4, and SSTR 2, 3, and 5 in nonfunctioning pituitary adenomas: a high throughput TMA, immunohistochemical study. J Clin Endocrinol Metab 2012;97:1745–51.ArticlePubMed
- 100. Widhalm G, Wolfsberger S, Preusser M, Fischer I, Woehrer A, Wunderer J, et al. Residual nonfunctioning pituitary adenomas: prognostic value of MIB-1 labeling index for tumor progression. J Neurosurg 2009;111:563–71.ArticlePubMed
- 101. Steno A, Bocko J, Rychly B, Chorvath M, Celec P, Fabian M, et al. Nonfunctioning pituitary adenomas: association of Ki-67 and HMGA-1 labeling indices with residual tumor growth. Acta Neurochir (Wien) 2014;156:451–61.ArticlePubMedPDF
- 102. Tanaka Y, Hongo K, Tada T, Sakai K, Kakizawa Y, Kobayashi S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg 2003;98:359–65.ArticlePubMed
- 103. Saeger W, Ludecke B, Ludecke DK. Clinical tumor growth and comparison with proliferation markers in non-functioning (inactive) pituitary adenomas. Exp Clin Endocrinol Diabetes 2008;116:80–5.ArticlePubMed
- 104. Jaffrain-Rea ML, Di Stefano D, Minniti G, Esposito V, Bultrini A, Ferretti E, et al. A critical reappraisal of MIB-1 labelling index significance in a large series of pituitary tumours: secreting versus non-secreting adenomas. Endocr Relat Cancer 2002;9:103–13.ArticlePubMed
- 105. Yokoyama S, Hirano H, Moroki K, Goto M, Imamura S, Kuratsu JI. Are nonfunctioning pituitary adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery 2001;49:857–63.ArticlePubMed
- 106. Widhalm G, Wolfsberger S, Preusser M, Woehrer A, Kotter MR, Czech T, et al. O(6)-methylguanine DNA methyltransferase immunoexpression in nonfunctioning pituitary adenomas: are progressive tumors potential candidates for temozolomide treatment? Cancer 2009;115:1070–80.ArticlePubMed
- 107. Lau Q, Scheithauer B, Kovacs K, Horvath E, Syro LV, Lloyd R. MGMT immunoexpression in aggressive pituitary adenoma and carcinoma. Pituitary 2010;13:367–79.ArticlePubMedPDF
- 108. McCormack A, Kaplan W, Gill AJ, Little N, Cook R, Robinson B, et al. MGMT expression and pituitary tumours: relationship to tumour biology. Pituitary 2013;16:208–19.ArticlePubMedPDF
- 109. McCormack AI, Wass JA, Grossman AB. Aggressive pituitary tumours: the role of temozolomide and the assessment of MGMT status. Eur J Clin Invest 2011;41:1133–48.ArticlePubMed
- 110. Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology 2006;147:4831–42.PubMed
- 111. Zhou W, Song Y, Xu H, Zhou K, Zhang W, Chen J, et al. In nonfunctional pituitary adenomas, estrogen receptors and slug contribute to development of invasiveness. J Clin Endocrinol Metab 2011;96:E1237–45.ArticlePubMed
- 112. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 1999;5:1317–21.ArticlePubMedPDF
- 113. Heaney AP, Fernando M, Melmed S. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest 2002;109:277–83.ArticlePubMedPMC
- 114. Chamaon K, Kanakis D, Mawrin C, Dietzmann K, Kirches E. Transcripts of PTTG and growth factors bFGF and IGF1 are correlated in pituitary adenomas. Exp Clin Endocrinol Diabetes 2010;118:121–6.ArticlePubMed
- 115. Minematsu T, Suzuki M, Sanno N, Takekoshi S, Teramoto A, Osamura RY. PTTG overexpression is correlated with angiogenesis in human pituitary adenomas. Endocr Pathol 2006;17:143–53.ArticlePubMed
- 116. Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, et al. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol (Oxf) 2006;65:536–43.ArticlePubMed
- 117. Jia W, Lu R, Jia G, Ni M, Xu Z. Expression of pituitary tumor transforming gene (PTTG) in human pituitary macroadenomas. Tumour Biol 2013;34:1559–67.ArticlePubMedPDF
- 118. Trott G, Ongaratti BR, de Oliveira Silva CB, Abech GD, Haag T, Rech CG, et al. PTTG overexpression in non-functioning pituitary adenomas: correlation with invasiveness, female gender and younger age. Ann Diagn Pathol 2019;41:83–9.ArticlePubMed
- 119. Wierzbicka-Tutka I, Sokolowski G, Baldys-Waligorska A, Adamek D, Radwanska E, Golkowski F. PTTG and Ki-67 expression in pituitary adenomas. Przegl Lek 2016;73:53–8.PubMed
- 120. Dai C, Liang S, Sun B, Li Y, Kang J. Anti-VEGF therapy in refractory pituitary adenomas and pituitary carcinomas: a review. Front Oncol 2021;11:773905.ArticlePubMedPMC
- 121. Cristina C, Perez-Millan MI, Luque G, Dulce RA, Sevlever G, Berner SI, et al. VEGF and CD31 association in pituitary adenomas. Endocr Pathol 2010;21:154–60.ArticlePubMedPDF
- 122. Wang Y, Li J, Tohti M, Hu Y, Wang S, Li W, et al. The expression profile of dopamine D2 receptor, MGMT and VEGF in different histological subtypes of pituitary adenomas: a study of 197 cases and indications for the medical therapy. J Exp Clin Cancer Res 2014;33:56.ArticlePubMedPMCPDF
- 123. Lloyd RV, Scheithauer BW, Kuroki T, Vidal S, Kovacs K, Stefaneanu L. Vascular endothelial growth factor (VEGF) expression in human pituitary adenomas and carcinomas. Endocr Pathol 1999;10:229–35.ArticlePubMedPDF
- 124. Sato M, Tamura R, Tamura H, Mase T, Kosugi K, Morimoto Y, et al. Analysis of tumor angiogenesis and immune microenvironment in non-functional pituitary endocrine tumors. J Clin Med 2019;8:695.ArticlePubMedPMC
- 125. He W, Huang L, Shen X, Yang Y, Wang D, Yang Y, et al. Relationship between RSUME and HIF-1α/VEGF-A with invasion of pituitary adenoma. Gene 2017;603:54–60.ArticlePubMed
- 126. McCabe CJ, Boelaert K, Tannahill LA, Heaney AP, Stratford AL, Khaira JS, et al. Vascular endothelial growth factor, its receptor KDR/Flk-1, and pituitary tumor transforming gene in pituitary tumors. J Clin Endocrinol Metab 2002;87:4238–44.ArticlePubMed
- 127. Uraki S, Ariyasu H, Doi A, Kawai S, Takeshima K, Morita S, et al. Reduced expression of mismatch repair genes MSH6/MSH2 directly promotes pituitary tumor growth via the ATR-Chk1 pathway. J Clin Endocrinol Metab 2018;103:1171–9.ArticlePubMedPDF
- 128. Uraki S, Ariyasu H, Doi A, Takeshima K, Morita S, Inaba H, et al. MSH6/2 and PD-L1 expressions are associated with tumor growth and invasiveness in silent pituitary adenoma subtypes. Int J Mol Sci 2020;21:2831.ArticlePubMedPMC
- 129. Pecina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 2003;3:17.PubMedPMC
- 130. Oystese KA, Berg JP, Normann KR, Zucknick M, CasarBorota O, Bollerslev J. The role of E and N-cadherin in the postoperative course of gonadotroph pituitary tumours. Endocrine 2018;62:351–60.ArticlePubMedPDF
- 131. Gong J, Zhao Y, Abdel-Fattah R, Amos S, Xiao A, Lopes MB, et al. Matrix metalloproteinase-9, a potential biological marker in invasive pituitary adenomas. Pituitary 2008;11:37–48.ArticlePubMedPDF
- 132. Kawamoto H, Kawamoto K, Mizoue T, Uozumi T, Arita K, Kurisu K. Matrix metalloproteinase-9 secretion by human pituitary adenomas detected by cell immunoblot analysis. Acta Neurochir (Wien) 1996;138:1442–8.ArticlePubMedPDF
- 133. Turner HE, Nagy Z, Esiri MM, Harris AL, Wass JA. Role of matrix metalloproteinase 9 in pituitary tumor behavior. J Clin Endocrinol Metab 2000;85:2931–5.ArticlePubMed
- 134. Liu H, Zhang S, Wu T, Lv Z, Ba J, Gu W, et al. Expression and clinical significance of cathepsin K and MMPs in invasive non-functioning pituitary adenomas. Front Oncol 2022;12:901647.ArticlePubMedPMC
- 135. Rai A, Yelamanchi SD, Radotra BD, Gupta SK, Mukherjee KK, Tripathi M, et al. Phosphorylation of β-catenin at Serine552 correlates with invasion and recurrence of non-functioning pituitary neuroendocrine tumours. Acta Neuropathol Commun 2022;10:138.ArticlePubMedPMCPDF
- 136. Peverelli E, Giardino E, Treppiedi D, Locatelli M, Vaira V, Ferrero S, et al. Dopamine receptor type 2 (DRD2) inhibits migration and invasion of human tumorous pituitary cells through ROCK-mediated cofilin inactivation. Cancer Lett 2016;381:279–86.ArticlePubMed
- 137. Cooper O, Bonert V, Mamelak AN, Bannykh S, Melmed S. Dural invasion as a marker of aggressive pituitary adenomas. Neurosurgery 2022;90:775–83.ArticlePubMedPMC
- 138. Freda PU, Beckers AM, Katznelson L, Molitch ME, Montori VM, Post KD, et al. Pituitary incidentaloma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011;96:894–904.ArticlePubMedPMCPDF
- 139. Fleseriu M, Bodach ME, Tumialan LM, Bonert V, Oyesiku NM, Patil CG, et al. Congress of neurological surgeons systematic review and evidence-based guideline for pretreatment endocrine evaluation of patients with nonfunctioning pituitary adenomas. Neurosurgery 2016;79:E527–9.ArticlePubMed
- 140. Hong JW, Lee MK, Kim SH, Lee EJ. Discrimination of prolactinoma from hyperprolactinemic non-functioning adenoma. Endocrine 2010;37:140–7.ArticlePubMedPDF
- 141. Bashari WA, Senanayake R, Fernandez-Pombo A, Gillett D, Koulouri O, Powlson AS, et al. Modern imaging of pituitary adenomas. Best Pract Res Clin Endocrinol Metab 2019;33:101278.ArticlePubMed
- 142. Newman SA, Turbin RE, Bodach ME, Tumialan LM, Oyesiku NM, Litvack Z, et al. Congress of neurological surgeons systematic review and evidence-based guideline on pretreatment ophthalmology evaluation in patients with suspected nonfunctioning pituitary adenomas. Neurosurgery 2016;79:E530–2.ArticlePubMed
- 143. Lucas JW, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, Litvack Z, et al. Congress of neurological surgeons systematic review and evidence-based guideline on primary management of patients with nonfunctioning pituitary adenomas. Neurosurgery 2016;79:E533–5.ArticlePubMed
- 144. Tresoldi AS, Carosi G, Betella N, Del Sindaco G, Indirli R, Ferrante E, et al. Clinically nonfunctioning pituitary incidentalomas: characteristics and natural history. Neuroendocrinology 2020;110:595–603.ArticlePubMedPDF
- 145. Han AJ, Varlamov EV, Fleseriu M. Nonfunctioning pituitary microadenomas: should imaging interval be extended?: a large single-center cohort study. J Clin Endocrinol Metab 2022;107:e1231. –41.ArticlePubMedPDF
- 146. Dekkers OM, Hammer S, de Keizer RJ, Roelfsema F, Schutte PJ, Smit JW, et al. The natural course of non-functioning pituitary macroadenomas. Eur J Endocrinol 2007;156:217–24.ArticlePubMed
- 147. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am 2008;37:151–71.ArticlePubMed
- 148. Murad MH, Fernandez-Balsells MM, Barwise A, GallegosOrozco JF, Paul A, Lane MA, et al. Outcomes of surgical treatment for nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2010;73:777–91.ArticlePubMed
- 149. Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. The clinical and endocrine outcome to transsphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer 1991;68:860–6.ArticlePubMed
- 150. Esposito D, Olsson DS, Ragnarsson O, Buchfelder M, Skoglund T, Johannsson G. Non-functioning pituitary adenomas: indications for pituitary surgery and post-surgical management. Pituitary 2019;22:422–34.ArticlePubMedPMCPDF
- 151. Penn DL, Burke WT, Laws ER. Management of non-functioning pituitary adenomas: surgery. Pituitary 2018;21:145–53.ArticlePubMedPDF
- 152. de Divitiis E, Laws ER, Giani U, Iuliano SL, de Divitiis O, Apuzzo ML. The current status of endoscopy in transsphenoidal surgery: an international survey. World Neurosurg 2015;83:447–54.ArticlePubMed
- 153. Almutairi RD, Muskens IS, Cote DJ, Dijkman MD, Kavouridis VK, Crocker E, et al. Gross total resection of pituitary adenomas after endoscopic vs. microscopic transsphenoidal surgery: a meta-analysis. Acta Neurochir (Wien) 2018;160:1005–21.ArticlePubMedPMCPDF
- 154. Yu SY, Du Q, Yao SY, Zhang KN, Wang J, Zhu Z, et al. Outcomes of endoscopic and microscopic transsphenoidal surgery on non-functioning pituitary adenomas: a systematic review and meta-analysis. J Cell Mol Med 2018;22:2023–7.ArticlePubMedPMCPDF
- 155. Coburger J, Konig R, Seitz K, Bazner U, Wirtz CR, Hlavac M. Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J Neurosurg 2014;120:346–56.ArticlePubMed
- 156. Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M. Intraoperative high-field MRI for transsphenoidal reoperations of nonfunctioning pituitary adenoma. J Neurosurg 2014;121:1166–75.ArticlePubMed
- 157. Tandon V, Raheja A, Suri A, Chandra PS, Kale SS, Kumar R, et al. Randomized trial for superiority of high field strength intra-operative magnetic resonance imaging guided resection in pituitary surgery. J Clin Neurosci 2017;37:96–103.ArticlePubMed
- 158. Honegger J, Grimm F. The experience with transsphenoidal surgery and its importance to outcomes. Pituitary 2018;21:545–55.ArticlePubMedPDF
- 159. Lee CC, Kano H, Yang HC, Xu Z, Yen CP, Chung WY, et al. Initial gamma knife radiosurgery for nonfunctioning pituitary adenomas. J Neurosurg 2014;120:647–54.PubMed
- 160. Park KJ, Kano H, Parry PV, Niranjan A, Flickinger JC, Lunsford LD, et al. Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery 2011;69:1188–99.ArticlePubMedPDF
- 161. Mingione V, Yen CP, Vance ML, Steiner M, Sheehan J, Laws ER, et al. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg 2006;104:876–83.ArticlePubMed
- 162. Sheehan JP, Niranjan A, Sheehan JM, Jane JA Jr, Laws ER, Kondziolka D, et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg 2005;102:678–91.ArticlePubMed
- 163. Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al. Endotext. South Dartmouth: MDText.com, Inc; 2021 Chapter, Hypopituitarism following cranial radiotherapy [cited 2023 Nov 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532082.
- 164. Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Applegate G, Sutton ML. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med 1989;70:145–60.PubMed
- 165. Minniti G, Flickinger J, Tolu B, Paolini S. Management of nonfunctioning pituitary tumors: radiotherapy. Pituitary 2018;21:154–61.ArticlePubMedPDF
- 166. Kotecha R, Sahgal A, Rubens M, De Salles A, Fariselli L, Pollock BE, et al. Stereotactic radiosurgery for non-functioning pituitary adenomas: meta-analysis and International Stereotactic Radiosurgery Society practice opinion. Neuro Oncol 2020;22:318–32.ArticlePubMedPDF
- 167. Even-Zohar N, Greenman Y. Management of NFAs: medical treatment. Pituitary 2018;21:168–75.ArticlePubMedPDF
- 168. Renner U, Arzberger T, Pagotto U, Leimgruber S, Uhl E, Muller A, et al. Heterogeneous dopamine D2 receptor subtype messenger ribonucleic acid expression in clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 1998;83:1368–75.PubMed
- 169. Vieira Neto L, Wildemberg LE, Moraes AB, Colli LM, Kasuki L, Marques NV, et al. Dopamine receptor subtype 2 expression profile in nonfunctioning pituitary adenomas and in vivo response to cabergoline therapy. Clin Endocrinol (Oxf) 2015;82:739–46.ArticlePubMed
- 170. Greenman Y, Cooper O, Yaish I, Robenshtok E, Sagiv N, Jonas-Kimchi T, et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol 2016;175:63–72.ArticlePubMed
- 171. Martinez-Lopez S, Garcia-Martinez A, Torregrosa-Quesada ME, Lopez-Munoz B, Camara R, Fajardo C, et al. Is somatostatin receptor and dopamine receptor profiling useful in the management of silent somatotroph tumors? J Endocrinol Invest 2020;43:859–63.ArticlePubMedPDF
- 172. Batista RL, Musolino NR, Cescato VA, da Silva GO, Medeiros RS, Herkenhoff CG, et al. Cabergoline in the management of residual nonfunctioning pituitary adenoma: a single-center, open-label, 2-year randomized clinical trial. Am J Clin Oncol 2019;42:221–7.PubMed
- 173. Pivonello R, Matrone C, Filippella M, Cavallo LM, Di Somma C, Cappabianca P, et al. Dopamine receptor expression and function in clinically nonfunctioning pituitary tumors: comparison with the effectiveness of cabergoline treatment. J Clin Endocrinol Metab 2004;89:1674–83.ArticlePubMedPDF
- 174. Verde G, Oppizzi G, Chiodini PG, Dallabonzana D, Luccarelli G, Liuzzi A. Effect of chronic bromocriptine administration on tumor size in patients with “nonsecreting” pituitary adenomas. J Endocrinol Invest 1985;8:113–5.ArticlePubMedPDF
- 175. van Schaardenburg D, Roelfsema F, van Seters AP, Vielvoye GJ. Bromocriptine therapy for non-functioning pituitary adenoma. Clin Endocrinol (Oxf) 1989;30:475–84.ArticlePubMed
- 176. Lohmann T, Trantakis C, Biesold M, Prothmann S, Guenzel S, Schober R, et al. Minor tumour shrinkage in nonfunctioning pituitary adenomas by long-term treatment with the dopamine agonist cabergoline. Pituitary 2001;4:173–8.PubMed
- 177. de Herder WW, Reijs AE, Feelders RA, van Aken MO, Krenning EP, Tanghe HL, et al. Dopamine agonist therapy of clinically non-functioning pituitary macroadenomas. Is there a role for 123I-epidepride dopamine D2 receptor imaging? Eur J Endocrinol 2006;155:717–23.ArticlePubMed
- 178. Garcia EC, Naves LA, Silva AO, de Castro LF, Casulari LA, Azevedo MF. Short-term treatment with cabergoline can lead to tumor shrinkage in patients with nonfunctioning pituitary adenomas. Pituitary 2013;16:189–94.ArticlePubMedPDF
- 179. Botelho MS, Franzini IA, Nunes-Nogueira VD, Boguszewski CL. Treatment of non-functioning pituitary adenoma with cabergoline: a systematic review and meta-analysis. Pituitary 2022;25:810–8.ArticlePubMedPDF
- 180. Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med 2012;366:914–24.ArticlePubMed
- 181. Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:3933–51.ArticlePubMed
- 182. Lee M, Lupp A, Mendoza N, Martin N, Beschorner R, Honegger J, et al. SSTR3 is a putative target for the medical treatment of gonadotroph adenomas of the pituitary. Endocr Relat Cancer 2015;22:111–9.ArticlePubMed
- 183. Ibanez-Costa A, Rivero-Cortes E, Vazquez-Borrego MC, Gahete MD, Jimenez-Reina L, Venegas-Moreno E, et al. Octreotide and pasireotide (dis)similarly inhibit pituitary tumor cells in vitro. J Endocrinol 2016;231:135–45.ArticlePubMed
- 184. Tateno T, Kato M, Tani Y, Oyama K, Yamada S, Hirata Y. Differential expression of somatostatin and dopamine receptor subtype genes in adrenocorticotropin (ACTH)-secreting pituitary tumors and silent corticotroph adenomas. Endocr J 2009;56:579–84.ArticlePubMed
- 185. Fusco A, Giampietro A, Bianchi A, Cimino V, Lugli F, Piacentini S, et al. Treatment with octreotide LAR in clinically non-functioning pituitary adenoma: results from a case-control study. Pituitary 2012;15:571–8.ArticlePubMedPDF
- 186. Zawada NB, Kunert-Radek J, Pawlikowski M, Pisarek H, Radek M. An evaluation of the effects of somatostatin analogue therapy in non-functioning pituitary adenomas in comparison to acromegaly. Endokrynol Pol 2016;67:292–8.PubMed
- 187. Zatelli MC, Piccin D, Vignali C, Tagliati F, Ambrosio MR, Bondanelli M, et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr Relat Cancer 2007;14:91–102.ArticlePubMed
- 188. ClinicalTrials.gov. Evaluate the efficacy and safety of pasireotide LAR (long acting release) on the treatment of patients with clinically non-functioning pituitary adenoma [Internet]. Bethesda: National Library of Medicine; 2019 [cited 2023 Nov 2]. Available from: https://clinicaltrials.gov/study/NCT01283542.
- 189. Colao A, Di Somma C, Pivonello R, Faggiano A, Lombardi G, Savastano S. Medical therapy for clinically non-functioning pituitary adenomas. Endocr Relat Cancer 2008;15:905–15.ArticlePubMed
- 190. Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic V, et al. European Society of Endocrinology clinical practice guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol 2018;178:G1–24.ArticlePubMed
- 191. Halevy C, Whitelaw BC. How effective is temozolomide for treating pituitary tumours and when should it be used? Pituitary 2017;20:261–6.ArticlePubMedPDF
- 192. Deutschbein T, Jaursch-Hancke C, Knappe UJ, Saeger W, Flitsch J, Bojunga J, et al. First German guideline on diagnostics and therapy of clinically non-functioning pituitary tumors. Exp Clin Endocrinol Diabetes 2021;129:250–64.ArticlePubMed
- 193. Nakano-Tateno T, Lau KJ, Wang J, McMahon C, Kawakami Y, Tateno T, et al. Multimodal non-surgical treatments of aggressive pituitary tumors. Front Endocrinol (Lausanne) 2021;12:624686.ArticlePubMedPMC
- 194. Nakano-Tateno T, Satou M, Inoshita N, van Landeghem FK, Easaw J, Mehta V, et al. Effects of CAPTEM (capecitabine and temozolomide) on a corticotroph carcinoma and an aggressive corticotroph tumor. Endocr Pathol 2021;32:418–26.ArticlePubMedPDF
- 195. Santos-Pinheiro F, Penas-Prado M, Kamiya-Matsuoka C, Waguespack SG, Mahajan A, Brown PD, et al. Treatment and long-term outcomes in pituitary carcinoma: a cohort study. Eur J Endocrinol 2019;181:397–407.ArticlePubMed
- 196. Alshaikh OM, Asa SL, Mete O, Ezzat S. An institutional experience of tumor progression to pituitary carcinoma in a 15-year cohort of 1055 consecutive pituitary neuroendocrine tumors. Endocr Pathol 2019;30:118–27.ArticlePubMedPDF
- 197. Ishida A, Shichi H, Fukuoka H, Shiramizu H, Inoshita N, Yamada S. Temozolomide and capecitabine treatment for an aggressive somatotroph pituitary tumor: a case report and literature review. Front Oncol 2022;12:916982.ArticlePubMedPMC
- 198. Ishida A, Shichi H, Fukuoka H, Inoshita N, Ogawa W, Yamada S. Efficacy of temozolomide combined with capecitabine (CAPTEM) on refractory prolactinomas as assessed using an ex vivo 3D spheroid assay. Pituitary 2022;25:238–45.ArticlePubMedPDF
- 199. Cano DA, Soto-Moreno A, Leal-Cerro A. Genetically engineered mouse models of pituitary tumors. Front Oncol 2014;4:203.ArticlePubMedPMC
- 200. Gahete MD, Jimenez-Vacas JM, Alors-Perez E, HerreroAguayo V, Fuentes-Fayos AC, Pedraza-Arevalo S, et al. Mouse models in endocrine tumors. J Endocrinol 2019;240:R73–96.
- 201. Tateno T, Nakano-Tateno T, Ezzat S, Asa SL. NG2 targets tumorigenic Rb inactivation in Pit1-lineage pituitary cells. Endocr Relat Cancer 2016;23:445–56.ArticlePubMed
- 202. Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4:335–48.ArticlePubMedPDF
- 203. Sajjad EA, Zielinski G, Maksymowicz M, Hutnik L, Bednarczuk T, Wlodarski P. mTOR is frequently active in GHsecreting pituitary adenomas without influencing their morphopathological features. Endocr Pathol 2013;24:11–9.ArticlePubMedPDF
- 204. Zatelli MC, Minoia M, Filieri C, Tagliati F, Buratto M, Ambrosio MR, et al. Effect of everolimus on cell viability in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 2010;95:968–76.ArticlePubMed
- 205. Lee M, Wiedemann T, Gross C, Leinhauser I, Roncaroli F, Braren R, et al. Targeting PI3K/mTOR signaling displays potent antitumor efficacy against nonfunctioning pituitary adenomas. Clin Cancer Res 2015;21:3204–15.ArticlePubMedPDF
- 206. Jaquet P, Gunz G, Saveanu A, Dufour H, Taylor J, Dong J, et al. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur J Endocrinol 2005;153:135–41.ArticlePubMed
- 207. Florio T, Barbieri F, Spaziante R, Zona G, Hofland LJ, van Koetsveld PM, et al. Efficacy of a dopamine-somatostatin chimeric molecule, BIM-23A760, in the control of cell growth from primary cultures of human non-functioning pituitary adenomas: a multi-center study. Endocr Relat Cancer 2008;15:583–96.ArticlePubMed
- 208. Halem HA, Hochgeschwender U, Rih JK, Nelson R, Johnson GA, Thiagalingam A, et al. TBR-760, a dopamine-somatostatin compound, arrests growth of aggressive nonfunctioning pituitary adenomas in mice. Endocrinology 2020;161:bqaa101.ArticlePubMedPMCPDF
- 209. Shi C, Ye Z, Han J, Ye X, Lu W, Ji C, et al. BRD4 as a therapeutic target for nonfunctioning and growth hormone pituitary adenoma. Neuro Oncol 2020;22:1114–25.ArticlePubMedPMCPDF
- 210. Liu C, Nakano-Tateno T, Satou M, Chik C, Tateno T. Emerging role of signal transducer and activator of transcription 3 (STAT3) in pituitary adenomas. Endocr J 2021;68:1143–53.ArticlePubMed
- 211. Feng J, Yu SY, Li CZ, Li ZY, Zhang YZ. Integrative proteomics and transcriptomics revealed that activation of the IL-6R/JAK2/STAT3/MMP9 signaling pathway is correlated with invasion of pituitary null cell adenomas. Mol Cell Endocrinol 2016;436:195–203.ArticlePubMed
- 212. Tampanaru-Sarmesiu A, Stefaneanu L, Thapar K, Kovacs K, Donnelly T, Metz CN, et al. Immunocytochemical localization of macrophage migration inhibitory factor in human hypophysis and pituitary adenomas. Arch Pathol Lab Med 1997;121:404–10.PubMed
- 213. Pyle ME, Korbonits M, Gueorguiev M, Jordan S, Kola B, Morris DG, et al. Macrophage migration inhibitory factor expression is increased in pituitary adenoma cell nuclei. J Endocrinol 2003;176:103–10.ArticlePubMed
- 214. Kindt N, Journe F, Laurent G, Saussez S. Involvement of macrophage migration inhibitory factor in cancer and novel therapeutic targets. Oncol Lett 2016;12:2247–53.ArticlePubMedPMC
- 215. Hafliger P, Charles RP. The L-type amino acid transporter LAT1: an emerging target in cancer. Int J Mol Sci 2019;20:2428.ArticlePubMedPMC
- 216. Satou M, Wang J, Nakano-Tateno T, Teramachi M, Suzuki T, Hayashi K, et al. L-type amino acid transporter 1, LAT1, in growth hormone-producing pituitary tumor cells. Mol Cell Endocrinol 2020;515:110868.ArticlePubMed
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite