Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(2); 2024 > Article
-
Original ArticleHypothalamus and pituitary gland Clinical Characteristics, Diagnosis, and Treatment of Thyroid Stimulating Hormone-Secreting Pituitary Neuroendocrine Tumor (TSH PitNET): A Single-Center Experience
 Keypoint
Keypoint
· This study focused on the characteristics, diagnostic methods, and outcomes of thyroid-stimulating hormone-secreting pituitary neuroendocrine tumors.
· Elevated serum alpha-subunit and blunted thyrotropin-releasing hormone stimulation were highly sensitive markers. Most cases were macroadenomas, and 23% showed hormone co-secretion.
· A rare mixed gangliocytoma-pituitary adenoma was also discovered, emphasizing the need for a careful diagnosis. -
Jung Heo1
 , Yeon-Lim Suh2, Se Hoon Kim3, Doo-Sik Kong4, Do-Hyun Nam4, Won-Jae Lee4, Sung Tae Kim5, Sang Duk Hong6, Sujin Ryu7, You-Bin Lee7,8, Gyuri Kim7,8, Sang-Man Jin7,8, Jae Hyeon Kim7,8, Kyu Yeon Hur7,8
, Yeon-Lim Suh2, Se Hoon Kim3, Doo-Sik Kong4, Do-Hyun Nam4, Won-Jae Lee4, Sung Tae Kim5, Sang Duk Hong6, Sujin Ryu7, You-Bin Lee7,8, Gyuri Kim7,8, Sang-Man Jin7,8, Jae Hyeon Kim7,8, Kyu Yeon Hur7,8
-
Endocrinology and Metabolism 2024;39(2):387-396.
DOI: https://doi.org/10.3803/EnM.2023.1877
Published online: February 5, 2024
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
4Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
5Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
6Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
7Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
8Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding author: Kyu Yeon Hur. Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-1232, Fax: +82-2-3410-6983, E-mail: ky.hur@samsung.com
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,464 Views
- 42 Download
ABSTRACT
-
Background
- Thyroid-stimulating hormone (TSH)-secreting pituitary neuroendocrine tumor (TSH PitNET) is a rare subtype of PitNET. We investigated the comprehensive characteristics and outcomes of TSH PitNET cases from a single medical center. Also, we compared diagnostic methods to determine which showed superior sensitivity.
-
Methods
- A total of 17 patients diagnosed with TSH PitNET after surgery between 2002 and 2022 in Samsung Medical Center was retrospectively reviewed. Data on comprehensive characteristics and treatment outcomes were collected. The sensitivities of diagnostic methods were compared.
-
Results
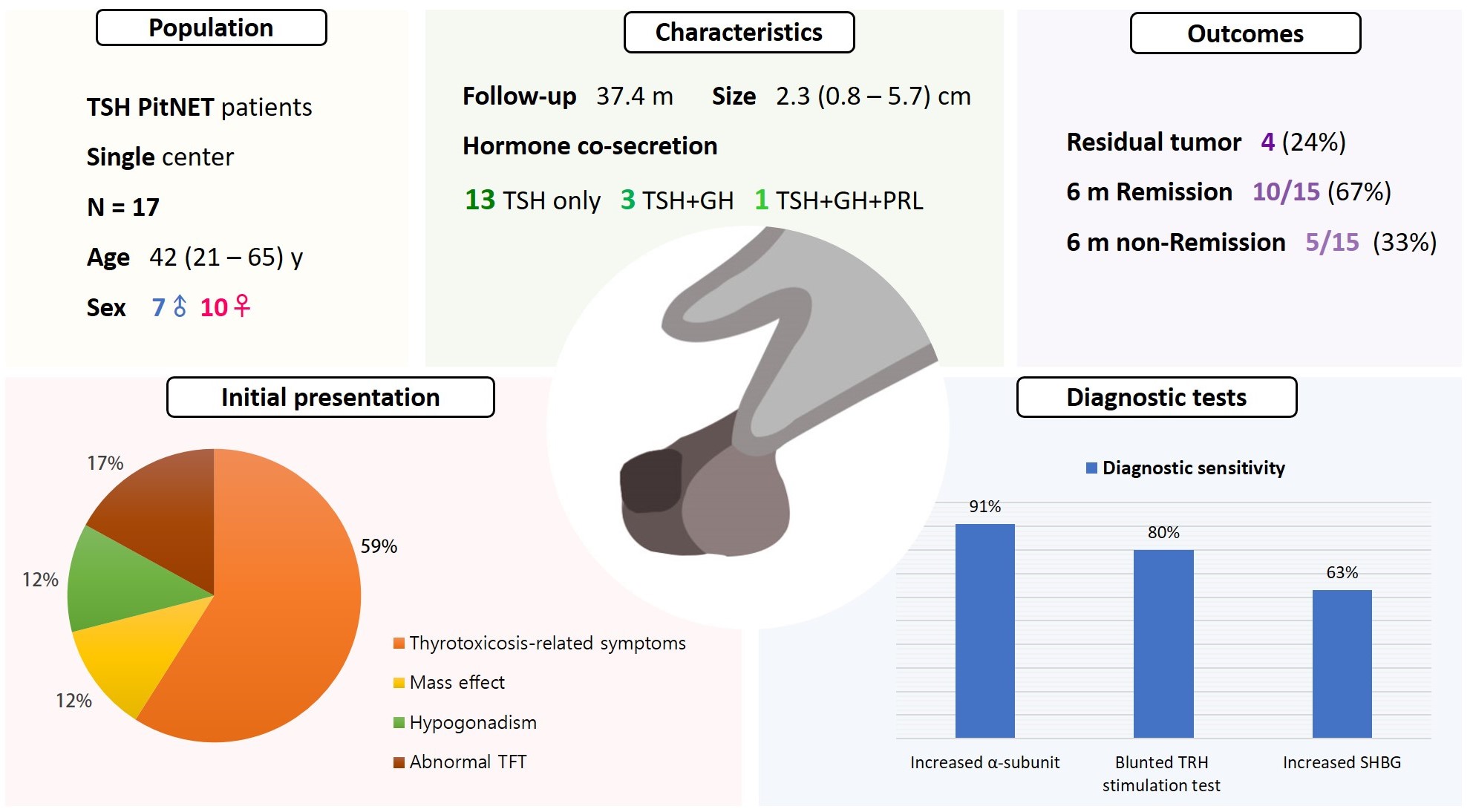
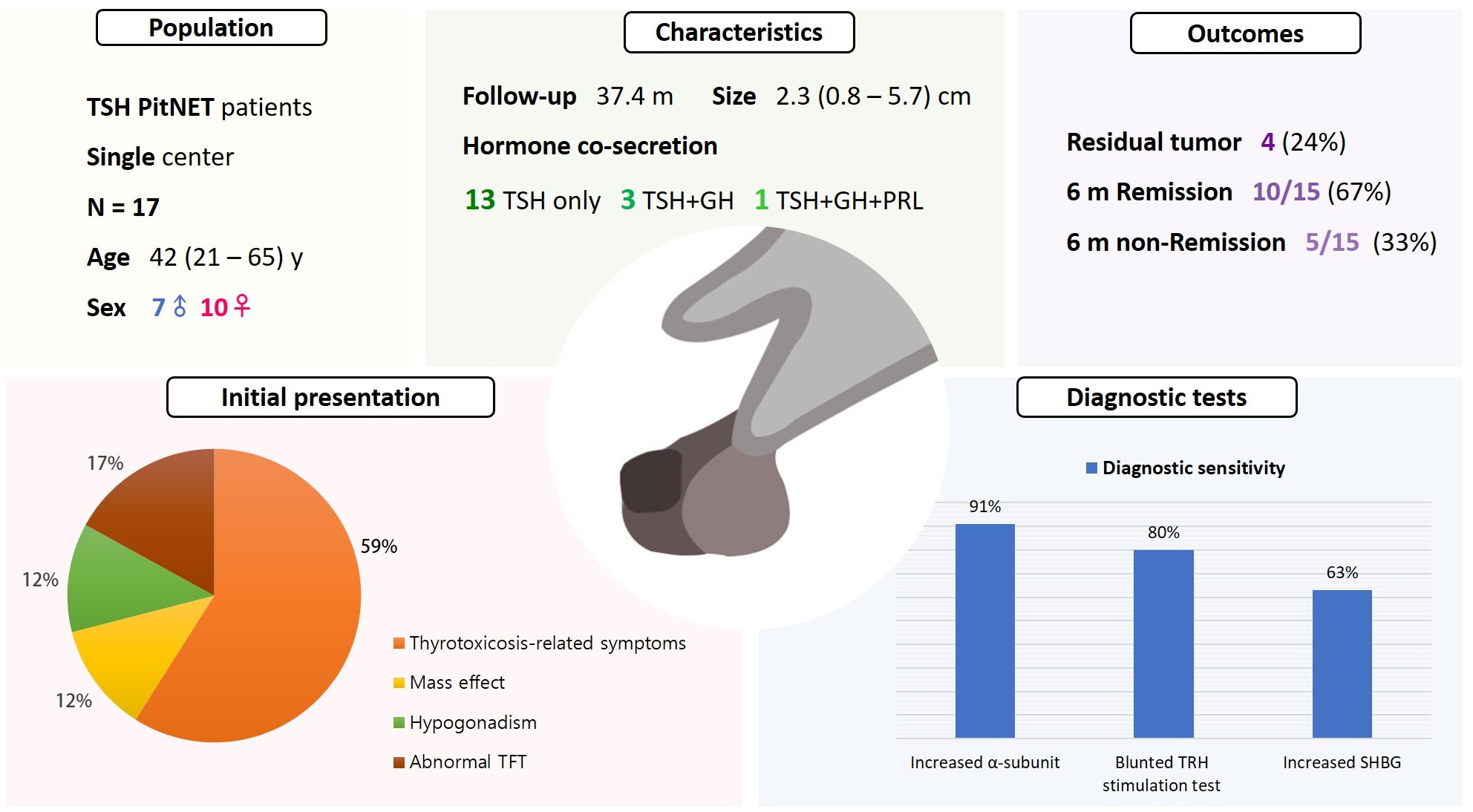
- Seven were male (41%), and the median age at diagnosis was 42 years (range, 21 to 65); the median follow-up duration was 37.4 months. The most common (59%) initial presentation was hyperthyroidism-related symptoms. Hormonal co-secretion was present in four (23%) patients. Elevated serum alpha-subunit (α-SU) showed the greatest diagnostic sensitivity (91%), followed by blunted response at thyrotropin-releasing hormone (TRH) stimulation (80%) and elevated sex hormone binding globulin (63%). Fourteen (82%) patients had macroadenoma, and a specimen of one patient with heavy calcification was negative for TSH. Among 15 patients who were followed up for more than 6 months, 10 (67%) achieved hormonal and structural remission within 6 months postoperatively. A case of growth hormone (GH)/TSH/prolactin (PRL) co-secreting mixed gangliocytoma-pituitary adenoma (MGPA) was discovered.
-
Conclusion
- The majority of the TSH PitNET cases was macroadenoma, and 23% showed hormone co-secretion. A rare case of GH/TSH/PRL co-secreting MGPA was discovered. Serum α-SU and TRH stimulation tests showed great diagnostic sensitivity. Careful consideration is needed in diagnosing TSH PitNET. Achieving remission requires complete tumor resection. In case of nonremission, radiotherapy or medical therapy can improve the long-term remission rate.
- Thyroid-stimulating hormone (TSH)-secreting pituitary neuroendocrine tumor (TSH PitNET) is a rare type of tumor [1-4]. TSH PitNET was first described by Jailer in 1960, and its prevalence has been reported as 0.5% to 2% of all pituitary adenomas. However, from the introduction of dynamic contrast-enhanced magnetic resonance imaging (MRI) and ultrasensitive TSH and the improved awareness of clinicians, the incidence in recent studies is reported to be as high as 4% [5-9].
- Owing to the improved sensitivity of TSH and routine assessment of free thyroxine (FT4) along with TSH, central hyperthyroidism is easy to diagnose, with simple thyroid function test (TFT) measurement presenting a peculiar pattern showing thyrotoxicosis and inappropriately nonsuppressed TSH [3,10]. However, challenges remain in diagnosing TSH PitNET because of complicated methods of diagnosis, which require a combination of clinical manifestations and hormonal status, image findings, and possible differential diagnosis [11,12]. These hurdles can result in delayed diagnosis or inaccurate diagnosis, leading to faulty treatment such as administration of antithyroid drugs, thyroidectomy, or thyroid ablative therapy. Due to the rarity of TSH PitNET, even the most recent systematic review contains fewer than 600 cases [13].
- The purpose of this study was to investigate and report the comprehensive characteristics and outcomes of TSH PitNET patients from a single medical center. We also discovered a rare case of accompanying TSH PitNET. Furthermore, we compared several diagnostic methods to determine which showed superior sensitivity.
INTRODUCTION
- Patients
- We retrospectively reviewed patients diagnosed both hormonally and pathologically with TSH PitNET after surgery at Samsung Medical Center between 2002 and 2022. All data were reviewed using information collected from the electronic medical record system. Patients who had insufficient hormonal or pathological information were excluded, and a total of 17 patients finally were analyzed. This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2023-02-074). Informed consent was waived by the board due to a retrospective nature of this study.
- Hormonal and biochemical evaluation
- Basal pituitary hormonal evaluation including adrenocorticotropic hormone (ACTH), cortisol, TSH, FT4, triiodothyronine (T3), prolactin (PRL), growth hormone (GH), insulin-like growth factor 1 (IGF-1), luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, and testosterone was performed for all subjects before and after surgery. An oral glucose tolerance test (OGTT) was performed when preoperative IGF-1 was above the age-adjusted reference range. Thyroid autoantibodies (thyroid peroxidase antibody, thyroglobulin antibody, and TSH receptor antibody) were evaluated in 16 patients.
- Serum alpha-subunit (α-SU) and sex hormone binding globulin (SHBG) were measured, and the thyrotropin-releasing hormone (TRH) stimulation test was performed in some subjects preoperatively to verify the diagnosis of TSH PitNET. Blunted response to TRH administration was defined as a TSH increase less than twice the baseline level or less than 5 IU/L.
- Pathologic review
- All surgical specimens of TSH PitNET underwent hematoxylineosin stain. There was a case showing extensive intratumoral calcification, which processed decalcification. Immunohistochemical (IHC) staining was performed on 4 μm-thick formalinfixed paraffin embedded sections using Ventana BenchMark XT against antibodies including β-TSH (DAKO, Glostrup, Denmark; 1:50), ACTH (DAKO; 1:200), GH (Cell Marque, Rocklin, CA, USA; 1:200), PRL (DAKO; 1:2,000), LH (DAKO; 1:100), and FSH (Cell Marque; 1:2,000). Immunohistochemistry for Ki-67 (DAKO; 1:200) was performed using Leica Bond III (Leica Biosystems, Heidelberg, Germany). The cytoplasmic staining of the pituitary hormones and nuclear staining of Ki-67 in tumor cells were interpreted as positive by an experienced neuropathologist in the same institution. Ki-67 labeling index was also accessed in the hot spot. The result of IHC staining on a heavily calcified tumor tissue could not be determined because the decalcification may affect immunoexpression of pituitary hormones. Four cases were initially negative for TSH and reassessed with Ventana BenchMark Ultra (Ventana, Oro Valley, AZ, USA) for pituitary hormones.
- Imaging studies
- Sellar MRI, including dynamic study, was conducted for all subjects preoperatively and immediately after and within 6 months after surgery. Tumor size was designated as the maximal diameter. A tumor <1 cm was defined as microadenoma, and a tumor ≥1 cm was defined as macroadenoma. Cases involving suprasellar, infrasellar, cavernous sinus (CS), or other brain parenchyma were described as tumors with invasion. Some of the subjects underwent thyroid ultrasonography and/or thyroid scan to evaluate the volume of thyroid glands and any nodularity.
- Criteria for remission
- Remission was defined as resolution of the symptoms of hyperthyroidism, normalized TFT profile, and no evidence of residual or recurrent tumor on MRI within 6 months postoperatively.
- Statistical analysis
- Continuous variables are presented as median (range), and categorical variables are shown as number (percentage). Statistical analyses were performed using IBM SPSS Statistics for Windows version 25.0 (IBM Co., Armonk, NY, USA). Categorical variables were compared using a chi-square test or Fisher’s exact test. The Mann-Whitney test was used to compare continuous variables. Correlation analysis was performed to identify the association between size of adenoma and α-SU. P<0.05 was regarded as statistically significant.
- Data availability
- The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
METHODS
- Clinical characteristics
- The patients’ baseline characteristics are listed in Table 1. The median age at diagnosis of the 17 subjects was 42 years (range, 21 to 65), and seven (41%) were male. The most common initial presentation was hyperthyroidism-related symptoms (59%), and the second most common presentation was asymptomatic abnormal TFT on routine health examination (17%), followed by hypogonadism (12%) and mass effect (12%). Two patients were not diagnosed promptly. Among patients who underwent thyroid ultrasonography or thyroid scintigraphy, 11 of 13 (85%) had goiter and eight of nine (89%) had thyroid nodules, respectively. One patient presented coexisting Graves’ disease and TSH PitNET. Sixteen (94%) patients underwent endoscopic transsphenoidal approach; tumor removal via craniotomy was necessary in one patient because of the huge size of the mass.
- Biochemical characteristics
- The median initial TSH, FT4, and T3 were 5.46 μIU/mL (range, 1.64 to 13.31), 2.74 ng/dL (range, 1.16 to 4.85), and 236.5 ng/dL (range, 92.4 to 347.1), respectively (Table 2). Four (25%) patients exhibited positive thyroid peroxidase antibody or thyroglobulin antibody, and the one patient with concomitant Graves’ disease and TSH PitNET was positive for TSH receptor antibody. Evidence of hormonal co-secretion was present in four (23%) patients, one of whom showed triple co-secretion of TSH, GH, and PRL. The other three patients exhibited secretion of TSH and GH. A brief course of short-acting somatostatin analogue (SSA) was administered to five patients, and four (80%) achieved euthyroidism before surgery.
- Radiologic and pathologic characteristics
- The median size of TSH PitNET was 2.3 cm (range, 0.8 to 5.7). Among the 17 cases, 14 (82%) were macroadenomas and three (18%) were microadenomas. The median size of GH/TSH co-secreting PitNET was greater than that of single-secreting TSH PitNET (2.6 cm vs. 1.5 cm, P=0.073). The percentage of macroadenoma tended to be higher among GH/TSH co-secreting PitNET cases than in single-secreting TSH PitNET cases (100% vs. 77%, P=0.582), but the difference was not statistically significant (Supplemental Table S1).
- A total of 13 (76%) patients showed invasion on sellar MRI imaging, and the most common pattern was suprasellar extension of tumor as observed in nine (9/13, 70%) patients. In a case with a 7.1 cm, large mass, the tumor extended to the parenchyma of the right frontal lobe (Table 3). Regarding IHC staining, five patients showed immuno-negativity on TSH at first assessment. Repeated IHC staining with another instrument revealed that four of five TSH-negative specimens were TSH-positive. Ultimately, 16 patients (94%) were positive for TSH on IHC staining; one surgical specimen harboring heavy calcification in almost the entire mass was negative for TSH.
- Diagnostic tests
- Serum α-SU, TRH stimulation test, and SHBG were tested at varying frequencies in patients with TSH PitNET. Ten of 17 (59%) patients underwent α-SU test, eight (47%) underwent SHBG, and TRH stimulation test was conducted in 15 patients (88%). The results of the diagnostic tests showed elevated α-SU in 91% of cases (10/11) and increased SHBG in 63% (5/8). Following TRH stimulation, 12 of 15 (80%) patients showed a blunted response. α-SU testing showed the greatest sensitivity among the diagnostic tools (Table 2).
- We then performed correlation analyses to evaluation whether α-SU is affected by tumor size and compared the responsiveness in TRH stimulation test. Serum α-SU was positively correlated with increasing tumor size (rho=0.711, P=0.014 [Spearman]). Patients who showed blunted response at TRH stimulation tend to have a larger tumor size compared with the tumor size of those with normal responsiveness (2.2 cm vs. 1.1 cm, P=0.420). Among the patients with blunted TRH response, 83% had macroadenomas, whereas 67% of those who had normal TRH response were macroadenomas (P=1.000) (Supplemental Table S2).
- Treatment outcomes
- Immediate postoperative sellar MRI revealed residual tumors in four (24%) patients. Among 14 patients who were followed for more than 6 months, 10 (71%) achieved hormonal and structural remission within 6 months postoperatively. Two of the four remaining patients did not only show a biochemically hyperthyroid state, but also had residual tumor mass on MRI.
- A case of GH/TSH/PRL co-secreting mixed gangliocytoma-pituitary adenoma
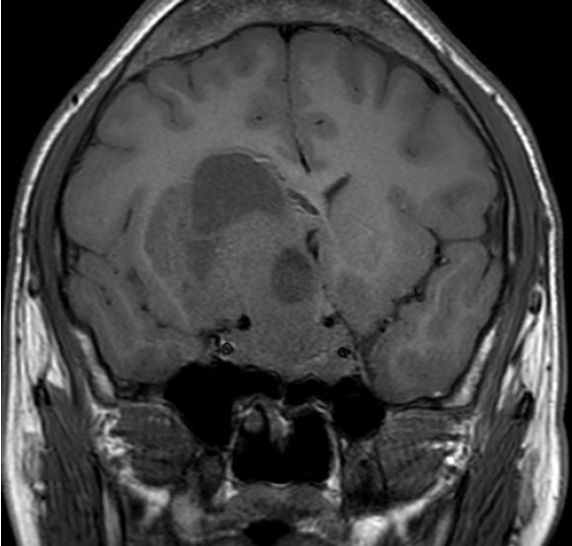
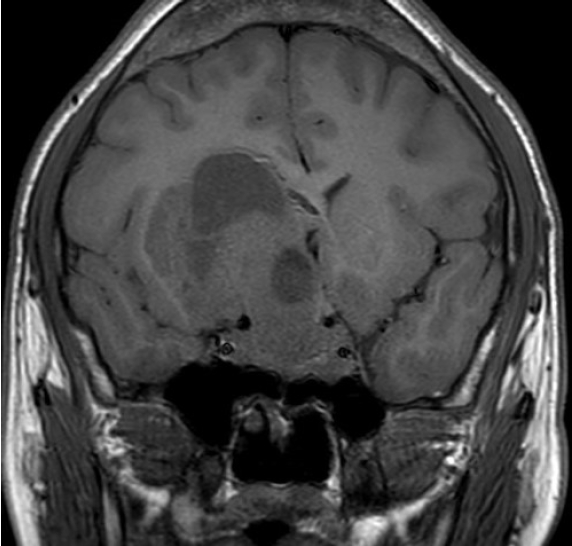
- A 21-year-old male with right visual impairment underwent brain computed tomography after head contusion. A 7-cm-sized sellar mass was detected, and he was referred to the department of neurosurgery. Sellar MRI revealed a 7.1 cm mass invading both the CS and suprasellar area extending toward the right frontal lobe of the cerebrum (Fig. 1). At initial pituitary hormonal screening, T3 and FT4 were elevated at 347 and 4.85 ng/dL, respectively, and TSH was 6.26 μIU/mL. IGF-1 was 1,137 ng/mL, and 75 g OGTT showed a nadir GH of 9.19 ng/mL. PRL level was 1,429 ng/mL, and ACTH, LH, and FSH were within normal range. Serum α-SU was elevated to 10.90 IU/L, and SHBG was within the normal range at 41.5 nmol/L. TRH stimulation test showed a blunted response. The patient underwent tumor removal through craniotomy, and the pathologic diagnosis revealed mixed gangliocytoma-pituitary adenoma (MGPA). Hyper-secreted hormones were all normalized postoperatively; however, remnant tumor was detected on sellar MRI. Adjuvant gamma-knife surgery was planned.
RESULTS
- In the present study, we reviewed 20 years of data of patients with TSH PitNET from a single medical center, including clinical, biochemical, radiological, and pathological characteristics and treatment outcomes. As for the baseline characteristics, the majority (82%) of cases was macroadenoma, and invasion or extension was found in more than half of the patients on sellar MRI, where suprasellar extension was the most common pattern (53%). These findings were generally consistent with those of previous studies [9,13]. Additionally, 23% of tumors showed plurihormonal secretion, especially TSH with GH, which is slightly less frequent than in previous studies [9,13]. The most common presentation leading to diagnosis of TSH PitNET was hyperthyroidism-related symptoms such as palpitation or dyspnea (59%). Regarding surgical outcome, 71% of patients achieved hormonal and structural remission within 6 months postoperatively, consistent with previous findings [8,14-16]. We further evaluated the sensitivities of diagnostic methods of TSH PitNET. Elevated serum α-SU showed the greatest sensitivity (91%), followed by blunted response at TRH stimulation (80%) and elevated SHBG (63%). These results verified the robustness of α-SU and TRH stimulation test in diagnosing TSH PitNET.
- In this study, we focused on clinically functioning TSH PitNET; however, the cohorts of previous studies on TSH PitNET are heterogeneous; Wang et al. [17] addressed IHC-positive and clinically non-functioning TSH PitNET, Yu et al. [18] covered GH-TSH co-secreting pituitary adenomas, and Azzalin et al. [16] included both active and silent cases. Similarly, not all studies used the same diagnostic tools. Azzalin et al. [16] used α-SU/ TSH molar ratio, while Nazato and Abucham [19] conducted sequencing of thyroid hormone receptor (THR)-β gene. Kim et al. [20] conducted the T3 suppression test in some cases for diagnosis, but we used α-SU, SHBG, and TRH stimulation.
- Remission rate has been reported heterogeneously in previous studies. Kim et al. [20] reported the remission rate as 84%, and similarly Yamada et al. [8] reported as 84.4%. In a study by Byun et al. [21], the remission rate was reported as 93%, but it’s worth noting that only postoperative achievement of a euthyroid state was considered in defining remission. On the other hand, similarly to our study, Azzalin et al. [16] reported 66%, and Cossu et al. [9] reported pooled remission rate as 66% and 76% after adjuvant radiotherapy and medical therapy respectively, in their systematic review and meta-analysis on TSH PitNET. Relatively recent studies have shown greater remission rate, and this may be attributed to early detection and treatment which consequently allow lower prevalence of macroadenoma and tumor invasion [9]. Six-month remission rate from our study was 67%, which is lesser than expectation. Patients with short follow-up duration were excluded, while a case of large MGPA was included in evaluating the remission rate, and additionally, the proportion of remnant tumors in postoperative MRI, significantly associated with remission (P=0.004), was high in our cohort. Furthermore, subsequent treatment was not uniformly performed in cases where a remnant tumor was present in our study. However, in non-remission cases, adjuvant treatment with radiotherapy or medical treatment with SSA or dopamine agonist is known to improve the long-term remission rate [9]. Hence, improving complete resection rate and prompt adjuvant therapies, if indicated, could potentially lead to a better remission rate.
- Since diagnosis of TSH PitNET is challenging, physicians have to conduct a series of pertinent diagnostic tests, particularly in cases of suspected microadenoma. Previous guidelines and publications recommend repeat TFT testing when FT4 and T3 are high with measurable TSH to exclude assay interference and examination of thyroid autoantibodies [11,22-25]. After eliminating the confounders, several diagnostic methods to verify hyperthyroid state and centrality and to rule out resistance to thyroid hormone (RTH) should be conducted. The common biochemical finding of TSH PitNET is elevated serum level of α-SU, which has been observed in approximately 30% to 70% of cases [11,12]. In the 1990s, elevated α-SU was considered a highly specific marker of TSH PitNET; however, the robustness of α-SU has been undermined recently [2]. Serum α-SU has been observed within the normal range in some microadenoma cases [26,27], and better and earlier detection of TSH PitNET resulted in increased prevalence of microadenoma and consequently decreased sensitivity of α-SU in diagnosing TSH PitNET [2]. In our study, α-SU level was positively correlated with tumor size. Of note, the observed sensitivity of α-SU in our study was high, at 91% (10/11), which is greater than previous data, reflecting the large proportion of macroadenoma (82%) in our study cohort. Serum α-SU of all macroadenoma patients was elevated, and only one patient with a 0.8-cm microadenoma showed a normal α-SU level. This result supports the finding that elevated α-SU exhibits great sensitivity, especially in macroadenoma.
- The diagnostic sensitivity of blunted response in the TRH stimulation test (80%, 12/15) was second to that of elevated α-SU, and it was consistent with previous reports [12]. Among three patients who showed a normal TRH response, two exhibited elevated serum α-SU level and one with a 0.8-cm microadenoma showed a normal range of serum α-SU. In previous publications, the sensitivity of TRH stimulation test was described as being superior to that of α-SU [1,11,12,23,26,28,29]. Only nine patients underwent both TRH stimulation test and serum α-SU. Thus, direct comparison of diagnostic sensitivity between those two methods in this study lacks statistical power.
- To successfully discriminate microadenoma from RTH, the use of markers that reflect tissue hyperthyroidism as SHBG or carboxy-terminal cross-linked telopeptide of type-I collagen (ITCP) adjuvant to α-SU or TRH stimulation test may be beneficial. ITCP, a bone resorption marker, is elevated when bone is over-exposed to thyroid hormone [30,31], and increased SHBG level represents stimulated hepatic production because of hyperthyroidism [32-34]. RTH, the foremost condition to be differentiated from TSH PitNET, barely undergoes tissue hyperthyroidism owing to the resistance of thyroid hormone receptors, so SHBG and/or ICTP are effective markers to distinguish TSH PitNET from RTH [24]. Tjornstrand and Nystrom [12] proposed that SHBG and/or ITCP be measured preferentially. In contrast, some publications did not prioritize SHBG and/or ITCP over other biochemical markers [11,24,35]. The results of our study revealed a sensitivity of 63% (5/8), which is inferior to that of other methods. Moreover, SHBG may be not elevated in GH/TSH co-secreting PitNET because GH lowers serum SHBG level [36,37]. Of note, an MGPA patient with co-secretion of GH/TSH/PRL showed a substantially elevated FT4 of 4.85 ng/7dL and α-SU of 10.9 IU/L, but SHBG was within the normal range at 41.5 nmol/L. Considering that GH is the most commonly co-secreted hormone in TSH PitNET [9], along with the relatively low sensitivity of SHBG in our study, SHBG may not be appropriate as the first diagnostic step.
- Nonetheless, SHBG may be important in microadenoma, in which the role of diagnostic methods dependent on tumor volume can be weakened. SHBG level was elevated in both patients with microadenoma who underwent SHBG test. In a patient with microadenoma who showed non-elevated α-SU and responsiveness in TRH stimulation test, elevated SHBG was the only biochemical clue of TSH PitNET. Hence, when TSH PitNET is suspected after excluding confounders, evaluation of biochemical evidence should be initiated with serum α-SU, and TRH stimulation test may be performed if necessary. In cases of microadenoma where serum α-SU and TRH stimulation tests are both inconclusive, and especially when the tumor is single-secreting, the SHBG test may be helpful. The T3 suppression test is regarded as the most useful method of diagnosing TSH PitNET, showing almost 100% sensitivity. However, the T3 suppression test is rarely conducted because it takes 10 days to complete and is contraindicated in elderly patients and patients with pulmonary, cardiovascular, or psychiatric disease due to heavy loading of liothyronine [12]. As the detection rate of microadenoma increases, revision of stepwise diagnostic tests is necessary.
- Surgical specimens of five patients initially were negative in TSH IHC staining. Repeated staining with another instrument revealed positive TSH results in four of five patients, except one patient whose specimen exhibited extensive calcification, hindering adequate staining. Notably, TSH immuno-negativity has been reported in few previous articles. Yamada et al. [8] reviewed 90 cases of TSH PitNET from a single-center, and three were TSH immuno-negative. Subsequent pretreatment with proteinase K revealed positive immunostaining. The proteinase K method is used to break down the protein cross-links that inhibit antibody from binding to tissue antigen, reducing falsenegative cases [17,38]. Yu et al. [18] examined 65 cases of GH/TSH PitNET, and 23 were negative for TSH immunostaining. The authors suggested several possible mechanisms of the phenomenon: immature and not fully differentiated progenitor cells may be difficult to stain; abnormal biological structure and/or behavior including synthesis, storage, and secretion can result in immuno-negativity; and tissue fibrosis of tumor may prevent antigen-antibody binding when staining. The heavily calcified specimen of a patient in our study failed to be stained for all immunostaining markers and even Ki-67. This case supports that tissue deformation may interrupt the process of immunostaining. Diagnosis of TSH PitNET is attainable as long as MRI imaging and several biochemical tests are confirmative, even though IHC is negative for TSH. Nevertheless, when clinically, biochemically, and radiologically evident TSH PitNET is negative on TSH IHC, repeat IHC staining with a more sensitive method, e.g., including pretreatment with proteinase K, can be considered. In addition, staining for the Pit-1 transcription factor can resolve the discordance between the biochemical activity of TSH PitNET and immuno-negativity [39].
- A 7.1-cm–sized mass invading extensively and secreting GH, TSH, and PRL simultaneously was identified as MGPA through pathologic confirmation. The tumor was resected via craniotomy due to its large volume, and immediate postoperative MRI revealed remnant tumor. MGPA is an extremely rare tumor composed of adenohypophyseal and ganglion cell proliferation [40,41], with only about 150 cases reported so far [42]. There is no specific method to discriminate MGPA from PitNET on MRI [43], and diagnosis relies on pathological findings. Approximately 75% of MGPA is secretory [44], and the most common form is GH hypersecretion, followed by PRL and ACTH [45]. Some MGPAs produced paired hypothalamic and pituitary hormones, e.g., GH/growth hormone-releasing hormone (GHRH) or TSH/TRH, while some cases secreted two different pituitary hormones [42,46,47]. Notably, the MGPA case of the current study secreted all Pit-1-originated pituitary hormones of GH, TSH, and PRL; to our knowledge, this is the first study to report a triple co-secreting MGPA. The prognosis of MGPA is not well established due to lack of experience [42], and the responsiveness to adjuvant therapy or recurrence rate is not clear. More cases of this rare disease entity need to be accumulated for further understanding of disease behavior and for drawing a consensus for patient management.
- There are several limitations in this study. The major limitation is the small number of subjects, which results in low statistical power. Second, since the study was conducted retrospectively, few diagnostic tests were examined and not uniformly among patients. ITCP, T3 suppression test, α-SU increment after TRH stimulation, or classic octreotide suppression test were not performed in our institution. Third, the cohort mostly showed macroadenoma, and the diagnostic performances of α-SU, TRH stimulation test, and SHBG were not generalizable reliably to microadenoma. Nevertheless, this study not only offers insights from a of 20-year experience of TSH PitNET patients at a single medical center but also complements the data from hundreds of previous cases. Additionally, this study also reports an extremely rare case of GH/TSH/PRL co-secreting MGPA. We also compared the sensitivities of commonly used diagnostic tests in TSH PitNET and evaluated whether the sensitivities varied according to tumor size. We found that TSH immunostaining of TSH PitNET is sometimes falsely negative depending on the intrinsic property of tissue or staining method. Reexamination with a more sensitive method or staining for the Pit-1 transcription factor can resolve the contradictory results between biochemical activity and negative immunostaining in TSH PitNET.
- In conclusion, the prevalence of TSH PitNET has increased because of the development of accurate laboratory testing and MRI imaging. For diagnosis of macroadenoma, α-SU and TRH stimulation test exhibited considerable sensitivity, whereas an adjuvant method may be needed in diagnosing microadenoma. TSH immuno-negativity is rare, and a more sensitive staining method or transcription factor staining can resolve discordance. A rare case of GH/TSH/PRL co-secreting MGPA was discovered. Delayed diagnosis or misdiagnosis of TSH PitNET can result in tumor aggravation and related consequences. A detailed diagnostic method is necessary for TSH PitNET, particularly microadenoma. Complete resection of the tumor is crucial for remission, and postoperative radiotherapy or medical therapy with SSA or dopamine receptor agonist can improve the long-term remission rate.
DISCUSSION
Supplementary Material
Supplemental Table S1.
Supplemental Table S2.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.H., K.Y.H. Acquisition, analysis, or interpretation of data: J.H., Y.L.S., S.H.K., K.Y.H. Drafting the work or revising: J.H., Y.L.S., S.R., K.Y.H. Final approval of the manuscript: J.H., Y.L.S., S.H.K., D.S.K., D.H.N., W.J.L., S.T.K., S.D.H., S.R., Y.B.L., G.K., S.M.J., J.H.K., K.Y.H.
Article information
Values are expressed as median (range) or number (%).
TSH, thyroid-stimulating hormone; FT4, free thyroxine; T3, triiodothyronine; TPO Ab, thyroid peroxidase antibody; Tg Ab, thyroglobulin antibody; α-SU, alpha-subunit; SHBG, sex hormone binding globulin; TRH, thyrotropin-releasing hormone; GH, growth hormone; PRL, prolactin.
- 1. Beck-Peccoz P, Brucker-Davis F, Persani L, Smallridge RC, Weintraub BD. Thyrotropin-secreting pituitary tumors. Endocr Rev 1996;17:610–38.ArticlePubMed
- 2. Socin HV, Chanson P, Delemer B, Tabarin A, Rohmer V, Mockel J, et al. The changing spectrum of TSH-secreting pituitary adenomas: diagnosis and management in 43 patients. Eur J Endocrinol 2003;148:433–42.ArticlePubMed
- 3. Beck-Peccoz P, Persani L, Mannavola D, Campi I. Pituitary tumours: TSH-secreting adenomas. Best Pract Res Clin Endocrinol Metab 2009;23:597–606.PubMed
- 4. Amlashi FG, Tritos NA. Thyrotropin-secreting pituitary adenomas: epidemiology, diagnosis, and management. Endocrine 2016;52:427–40.ArticlePubMedPDF
- 5. Kucharczyk W, Davis DO, Kelly WM, Sze G, Norman D, Newton TH. Pituitary adenomas: high-resolution MR imaging at 1.5 T. Radiology 1986;161:761–5.ArticlePubMed
- 6. Caldwell G, Kellett HA, Gow SM, Beckett GJ, Sweeting VM, Seth J, et al. A new strategy for thyroid function testing. Lancet 1985;1:1117–9.ArticlePubMed
- 7. Ross DS, Ardisson LJ, Meskell MJ. Measurement of thyrotropin in clinical and subclinical hyperthyroidism using a new chemiluminescent assay. J Clin Endocrinol Metab 1989;69:684–8.ArticlePubMed
- 8. Yamada S, Fukuhara N, Horiguchi K, Yamaguchi-Okada M, Nishioka H, Takeshita A, et al. Clinicopathological characteristics and therapeutic outcomes in thyrotropin-secreting pituitary adenomas: a single-center study of 90 cases. J Neurosurg 2014;121:1462–73.ArticlePubMed
- 9. Cossu G, Daniel RT, Pierzchala K, Berhouma M, Pitteloud N, Lamine F, et al. Thyrotropin-secreting pituitary adenomas: a systematic review and meta-analysis of postoperative outcomes and management. Pituitary 2019;22:79–88.ArticlePubMedPDF
- 10. Gershengorn MC, Weintraub BD. Thyrotropin-induced hyperthyroidism caused by selective pituitary resistance to thyroid hormone: a new syndrome of “inappropriate secretion of TSH”. J Clin Invest 1975;56:633–42.ArticlePubMedPMC
- 11. Beck-Peccoz P, Lania A, Beckers A, Chatterjee K, Wemeau JL. 2013 European Thyroid Association guidelines for the diagnosis and treatment of thyrotropin-secreting pituitary tumors. Eur Thyroid J 2013;2:76–82.ArticlePubMedPMC
- 12. Tjornstrand A, Nystrom HF. Diagnosis of endocrine disease: diagnostic approach to TSH-producing pituitary adenoma. Eur J Endocrinol 2017;177:R183–97.ArticlePubMed
- 13. De Herdt C, Philipse E, De Block C. Endocrine tumours: thyrotropin-secreting pituitary adenoma: a structured review of 535 adult cases. Eur J Endocrinol 2021;185:R65–74.ArticlePubMed
- 14. Kirkman MA, Jaunmuktane Z, Brandner S, Khan AA, Powell M, Baldeweg SE. Active and silent thyroid-stimulating hormone-expressing pituitary adenomas: presenting symptoms, treatment, outcomes, and recurrence. World Neurosurg 2014;82:1224–31.ArticlePubMed
- 15. Rotermund R, Riedel N, Burkhardt T, Matschke J, Schmidt NO, Aberle J, et al. Surgical treatment and outcome of TSH-producing pituitary adenomas. Acta Neurochir (Wien) 2017;159:1219–26.ArticlePubMedPDF
- 16. Azzalin A, Appin CL, Schniederjan MJ, Constantin T, Ritchie JC, Veledar E, et al. Comprehensive evaluation of thyrotropinomas: single-center 20-year experience. Pituitary 2016;19:183–93.ArticlePubMedPDF
- 17. Wang EL, Qian ZR, Yamada S, Rahman MM, Inosita N, Kageji T, et al. Clinicopathological characterization of TSH-producing adenomas: special reference to TSH-immunoreactive but clinically non-functioning adenomas. EndocrPathol 2009;20:209–20.ArticlePubMedPDF
- 18. Yu N, Duan L, Hu F, Yang S, Liu J, Chen M, et al. Clinical features and therapeutic outcomes of GH/TSH cosecreting pituitary adenomas: experience of a single pituitary center. Front Endocrinol (Lausanne) 2023;14:1197244.ArticlePubMedPMC
- 19. Nazato DM, Abucham J. Diagnosis and treatment of TSHsecreting adenomas: review of a longtime experience in a reference center. J Endocrinol Invest 2018;41:447–54.ArticlePubMedPDF
- 20. Kim SH, Ku CR, Na M, Yoo J, Kim W, Jung IH, et al. Immediate postoperative measurement of thyroid-stimulating hormone as an early predictor of remission in thyroid-stimulating hormone-secreting pituitary adenomas. J Neurosurg 2020;134:794–800.ArticlePubMed
- 21. Byun J, Kim JH, Kim YH, Cho YH, Hong SH, Kim CJ. Thyroid-stimulating hormone-secreting pituitary adenomas:single institutional experience of 14 consecutive cases. J Korean Neurosurg Soc 2020;63:495–503.ArticlePubMedPMCPDF
- 22. Campi I, Covelli D, Moran C, Fugazzola L, Cacciatore C, Orlandi F, et al. The differential diagnosis of discrepant thyroid function tests: insistent pitfalls and updated flow-chart based on a long-standing experience. Front Endocrinol (Lausanne) 2020;11:432.ArticlePubMedPMC
- 23. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013;27:745–62.ArticlePubMedPMC
- 24. Beck-Peccoz P, Giavoli C, Lania A. A 2019 update on TSHsecreting pituitary adenomas. J Endocrinol Invest 2019;42:1401–6.ArticlePubMedPDF
- 25. Gurnell M, Halsall DJ, Chatterjee VK. What should be done when thyroid function tests do not make sense? Clin Endocrinol (Oxf) 2011;74:673–8.ArticlePubMed
- 26. Larry Jameson J, De Groot LJ, de Kretser DM, Giudice LC, Grossman AB, Melmed S, et al. Endocrinology. 7th ed. Philadelphia: Elsevier/Saunders; 2016. Chapter 15, TSH-producing adenomas; p. 266-74.
- 27. Beck-Peccoz P, Persani L, Faglia G. Glycoprotein hormone alpha-subunit in pituitary adenomas. Trends Endocrinol Metab 1992;3:41–5.PubMed
- 28. Faglia G, Beck-Peccoz P, Piscitelli G, Medri G. Inappropriate secretion of thyrotropin by the pituitary. Horm Res 1987;26:79–99.ArticlePubMed
- 29. Faglia G. The clinical impact of the thyrotropin-releasing hormone test. Thyroid 1998;8:903–8.ArticlePubMed
- 30. Loviselli A, Mastinu R, Rizzolo E, Massa GM, Velluzzi F, Sammartano L, et al. Circulating telopeptide type I is a peripheral marker of thyroid hormone action in hyperthyroidism and during levothyroxine suppressive therapy. Thyroid 1997;7:561–6.ArticlePubMed
- 31. Persani L, Preziati D, Matthews CH, Sartorio A, Chatterjee VK, Beck-Peccoz P. Serum levels of carboxyterminal crosslinked telopeptide of type I collagen (ICTP) in the differential diagnosis of the syndromes of inappropriate secretion of TSH. Clin Endocrinol (Oxf) 1997;47:207–14.ArticlePubMedPDF
- 32. Beck-Peccoz P, Roncoroni R, Mariotti S, Medri G, Marcocci C, Brabant G, et al. Sex hormone-binding globulin measurement in patients with inappropriate secretion of thyrotropin (IST): evidence against selective pituitary thyroid hormone resistance in nonneoplastic IST. J Clin Endocrinol Metab 1990;71:19–25.ArticlePubMed
- 33. Sarne DH, Refetoff S, Rosenfield RL, Farriaux JP. Sex hormone-binding globulin in the diagnosis of peripheral tissue resistance to thyroid hormone: the value of changes after short term triiodothyronine administration. J Clin Endocrinol Metab 1988;66:740–6.ArticlePubMed
- 34. Akande EO, Anderson DC. Role of sex-hormone-binding globulin in hormonal changes and amenorrhoea in thyrotoxic women. Br J ObstetGynaecol 1975;82:557–61.ArticlePubMed
- 35. Briet C, Suteau V, Illouz F, Rodien P. Thyrotropin-secreting tumor “TSH-PitNET”: from diagnosis to treatment. Ann Endocrinol (Paris) 2023;84:407–12.ArticlePubMed
- 36. Rudd BT, Rayner PH, Thomas PH. Observations on the role of GH/IGF-1 and sex hormone binding globulin (SHBG) in the pubertal development of growth hormone deficient (GHD) children. Acta Endocrinol Suppl (Copenh) 1986;279:164–9.ArticlePubMed
- 37. Gafny M, Silbergeld A, Klinger B, Wasserman M, Laron Z. Comparative effects of GH, IGF-I and insulin on serum sex hormone binding globulin. Clin Endocrinol (Oxf) 1994;41:169–75.ArticlePubMed
- 38. Ramos-Vara JA, Beissenherz ME. Optimization of immunohistochemical methods using two different antigen retrieval methods on formalin-fixed paraffin-embedded tissues: experience with 63 markers. J Vet Diagn Invest 2000;12:307–11.ArticlePubMedPDF
- 39. Yoon JH, Choi W, Park JY, Hong AR, Kim SS, Kim HK, et al. A challenging TSH/GH co-secreting pituitary adenoma with concomitant thyroid cancer; a case report and literature review. BMC EndocrDisord 2021;21:177.ArticlePubMedPMCPDF
- 40. Xiao P, Xue L, Peng JJ, Feng ST, Liao B, Wen JM. An intrasellar mixed gangliocytoma-adenoma including ependymal component, and review of the literature. BMJ Case Rep 2009;2009:bcr11.2008.1200.ArticlePubMedPMC
- 41. Lopes MB, Sloan E, Polder J. Mixed gangliocytoma-pituitary adenoma: insights on the pathogenesis of a rare Sellar tumor. Am J Surg Pathol 2017;41:586–95.PubMed
- 42. Sakata K, Fujimori K, Komaki S, Furuta T, Sugita Y, Ashida K, et al. Pituitary gangliocytoma producing TSH and TRH: a review of “gangliocytomas of the Sellar region”. J Clin Endocrinol Metab 2020;105:3109–21.ArticlePubMedPMCPDF
- 43. Chen D, Xu J, Zhong P, Huang X, Xu M. Pituitary adenoma with gangliocytoma: report of two cases. Oncol Lett 2014;8:781–4.ArticlePubMedPMC
- 44. Puchner MJ, Ludecke DK, Saeger W, Riedel M, Asa SL. Gangliocytomas of the Sellar region: a review. Exp Clin Endocrinol Diabetes 1995;103:129–49.ArticlePubMed
- 45. Yang B, Yang C, Sun Y, Du J, Liu P, Jia G, et al. Mixed gangliocytoma-pituitary adenoma in the Sellar region: a largescale single-center experience. Acta Neurochir (Wien) 2018;160:1989–99.ArticlePubMedPDF
- 46. Teramoto S, Tange Y, Ishii H, Goto H, Ogino I, Arai H. Mixed gangliocytoma-pituitary adenoma containing GH and GHRH co-secreting adenoma cells. Endocrinol Diabetes Metab Case Rep 2019;2019:19–0099.ArticlePubMedPMC
- 47. Balci S, Saglam A, Oruckaptan H, Erbas T, Soylemezoglu F. Pituitary adenoma with gangliocytic component: report of 5 cases with focus on immunoprofile of gangliocytic component. Pituitary 2015;18:23–30.ArticlePubMedPDF
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite