Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(6); 2022 > Article
-
Review ArticleThyroid Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Maternal Thyroid Dysfunction, and Child Autism Spectrum Disorder
 Keypoint
Keypoint
Increasing epidemiological evidence indicates that abnormal maternal thyroid function during pregnancy is associated with an increased risk of child autism spectrum disorder (ASD) and other neurodevelopmental disorders. The aim of this review is to evaluate and summarize reports with regard to potential mechanisms in this pathway regarding prenatal exposure to per- and polyfluoroalkyl substances in the relationship between thyroid dysfunction and ASD. -
Hyeong-Moo Shin1
 , Jiwon Oh2, Rebecca J. Schmidt2,3, Elizabeth N. Pearce4
, Jiwon Oh2, Rebecca J. Schmidt2,3, Elizabeth N. Pearce4 -
Endocrinology and Metabolism 2022;37(6):819-829.
DOI: https://doi.org/10.3803/EnM.2022.1598
Published online: November 23, 2022
1Department of Environmental Science, Baylor University, Waco, TX, USA
2Department of Public Health Sciences, University of California, Davis, CA, USA
3UC Davis MIND (Medical Investigations of Neurodevelopmental Disorders) Institute, Sacramento, CA, USA
4Section of Endocrinology, Diabetes, and Nutrition, Department of Medicine, Boston University School of Medicine, Boston, MA, USA
- Corresponding author: Hyeong-Moo Shin. Department of Environmental Science, Baylor University, One Bear Place #97266, Waco, TX 76798, USA Tel: +1-254-710-7627, Fax: +1-254-710-3409 E-mail: hyeongmoo_shin@baylor.edu
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Autism spectrum disorder (ASD), with its high economic and societal costs, is a growing public health concern whose prevalence has risen steadily over the last two decades. Although actual increased incidence versus improved diagnosis remains controversial, the increased prevalence of ASD suggests non-inherited factors as likely contributors. There is increasing epidemiologic evidence that abnormal maternal thyroid function during pregnancy is associated with increased risk of child ASD and other neurodevelopmental disorders. Prenatal exposure to endocrine-disrupting chemicals such as per- and polyfluoroalkyl substances (PFAS) is known to disrupt thyroid function and can affect early brain development; thus, thyroid dysfunction is hypothesized to mediate this relationship. The concept of a potential pathway from prenatal PFAS exposure through thyroid dysfunction to ASD etiology is not new; however, the extant literature on this topic is scant. The aim of this review is to evaluate and summarize reports with regard to potential mechanisms in this pathway.
- Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized by limited interests, repetitive behaviors, and impaired social interaction and communication [1]. ASD is a growing public health concern in part due to its high economic and societal costs, especially in developed countries [2]. Annual costs (direct medical, direct non-medical, and productivity combined) of ASD in 2025 are projected to reach nearly one-half trillion dollars in the United States [3]. The prevalence of ASD has risen steadily in the last two decades [4]; in the United States in 2018, one of every 44 children (3 to 8 years old) was estimated to have ASD [5]. Although actual increased incidence versus improved diagnosis remains controversial [6-8], the rapid rise in ASD prevalence suggests that environmental factors may contribute to ASD etiology [9,10]. In the last decade, environmental research linking modifiable factors to ASD has proliferated, with replication or meta-analysis covering pesticides [11,12], air pollution [13,14], maternal fever during pregnancy [15,16], periconceptional nutrition [17-19], maternal diabetes or obesity [20,21], preeclampsia [22], and interpregnancy interval [23-25].
- Thyroid hormones (THs) are essential for brain development and influence brain function throughout life [26,27]. Animal studies have shown that THs regulate crucial processes of brain development in mammals, including proliferation, migration, and differentiation of neuronal cells [28-30]. There is epidemiologic evidence that abnormal maternal thyroid function during pregnancy is associated with increased risk of child ASD and other neurodevelopmental disorders [31]. In addition, the prevalence of thyroid peroxidase antibody (TPO-Ab), a marker for thyroid autoimmunity, was reported to be higher in families with autism probands than in comparison subjects [32].
- Simultaneous with a growing understanding of the importance of maternal thyroid homeostasis for fetal brain development, chemical production volumes have increased 300-fold since the 1970s [33], leading to widespread human exposure to compounds known as endocrine-disrupting chemicals (EDCs) [34-36]. EDCs are defined as exogenous chemicals that interfere with hormone actions, resulting in increased risk of adverse health effects [37]. A wide range of EDCs disrupt thyroid homeostasis in laboratory animal studies [38]. Hundreds of synthetic chemicals interfere with the production, transport, and metabolism of THs [39]. Studies have shown that a broad range of EDCs can bind to TH receptors, may produce complex effects on TH signaling [40-42], and either alone or in combination, act at many levels in the thyroid system [43].
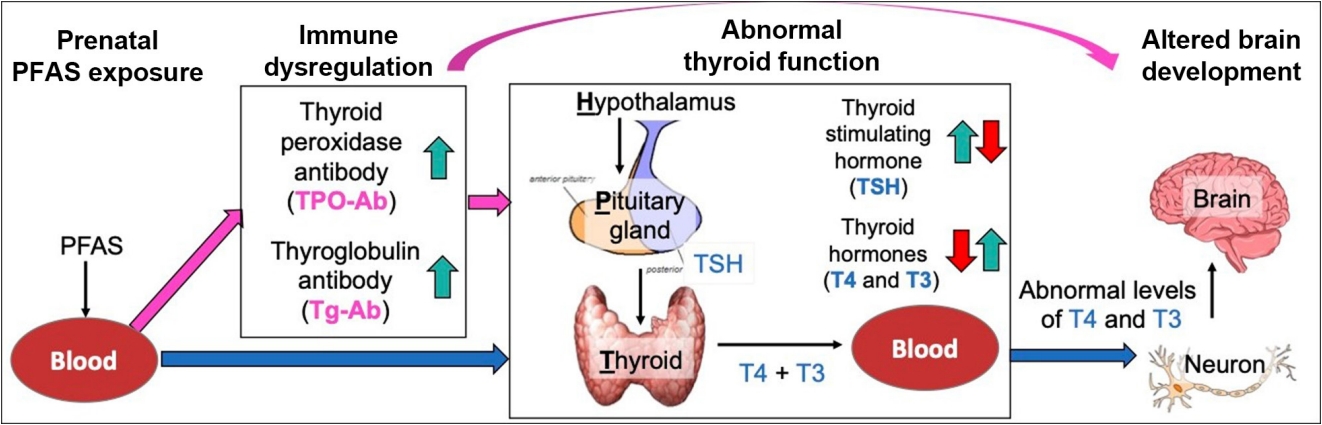
- Among a large number of EDCs, this review focuses on per-and polyfluoroalkyl substances (PFAS), a class of synthetic chemicals widely used in consumer (e.g., cookware, dental floss) and industrial (e.g., lining of gas pipes, surfactant) applications [44]. Recently PFAS have received significant public attention due to increasing evidence of their widespread environmental contamination and adverse health effects. As PFAS-containing products are widely used in daily life, many common PFAS compounds have been detected in the blood of most of the United States general population [45]. PFAS have also been detected in cord blood [46,47] and in amniotic fluid [48,49]. Importantly, both animal studies [50-52] and epidemiologic studies [53-55] have shown that prenatal exposure to PFAS disrupts thyroid function and immune systems, which can alter early brain development (Fig. 1). Moreover, there is epidemiologic evidence that PFAS exposure is associated with child neurodevelopmental disorders such as attention-deficit/hyperactivity disorder [56,57], indicating that PFAS may adversely affect child brain development.
- The aim of this review is to assess evidence for a potential pathway from prenatal PFAS exposure through abnormal thyroid function to ASD etiology. Hypothesizing that maternal thyroid dysfunction mediates a relationship between prenatal PFAS exposure and child ASD, we have focused on potential mechanisms related to thyroid dysfunction. This review also discusses antibody-mediated immune dysregulation that may cause thyroid dysfunction [58,59]. Building on the current report, subsequent research may help set the stage in support of prenatal thyroid treatment and strategies to prevent or reduce PFAS exposure.
INTRODUCTION
- Thyroid function is assessed with thyroid stimulating hormone (TSH) and free thyroxine (FT4). When TSH is high and FT4 is low or within a normal range, hypothyroidism is diagnosed. According to the American Thyroid Association guidelines, the population-based trimester-specific normal reference range for serum TSH should be used when assessing thyroid function during pregnancy [60]. However, reference ranges which are assay-, laboratory-, cohort-, and population-specific are preferred when available. Some studies have defined maternal abnormal thyroid function based on hospital diagnosis codes for hyperthyroidism or hypothyroidism or on prescriptions for THs or anti-thyroid drugs [61,62].
THYROID FUNCTION
- Abnormal thyroid function and ASD
- Epidemiologic studies have suggested that maternal gestational hypothyroidism, hyperthyroidism, and hypothyroxinemia were associated with increased risk of ASD in children (Table 1) [61-66]. In addition, low FT4 levels in cord blood were associated with increased ASD risk [67,68]. Overt maternal hypothyroidism is associated with impaired offspring cognition, which reflects that placental transfer of maternal THs to the fetus is essential for the regulation of fetal brain development [69,70]. Severe iodine deficiency (in which inadequate substrate for TH synthesis causes both maternal and fetal TH levels to be low) may cause cretinism, a syndrome of profoundly impaired growth and neurodevelopment. Maternal thyroid dysfunction during pregnancy is also known to be associated with adverse maternal and fetal outcomes such as preterm delivery, preeclampsia, and low birth weight [71-73], which are known risk factors for ASD [22,74-77].
- Antibody-mediated immune dysregulation, ASD, and thyroid dysfunction
- There is substantial evidence that autoimmunity and immune system dysfunction likely play a role in the development of ASD [78]. A body of epidemiologic evidence has shown that autoimmune disorders are significantly more frequent in families of autism probands than in those of comparison subjects [79-81]. More mothers of children with ASD had ASD-specific autoantibodies to proteins in the developing brain, compared with mothers of typically developing children [82-87]. In addition, a higher prevalence of maternal ASD-specific autoantibodies during pregnancy is associated with increased risk of child ASD and other neurodevelopmental disorders [88-90].
- In a case-control Finnish study, maternal TPO-Ab positivity during pregnancy was associated with increased risk of child ASD (odds ratio, 1.78; 95% confidence interval, 1.16 to 2.75) (Table 1) [32], implying that there is a potential role of thyroid autoimmunity in ASD etiology, although in that analysis maternal FT4 and TSH levels were not independently associated with ASD. It has been demonstrated that women with TPO-Ab positivity have a blunting of the typical thyroidal response to human chorionic gonadotropin in early gestation [91,92], resulting in lower serum FT4 levels and higher serum TSH levels. Thus, thyroid autoimmunity (high TPO-Ab and/or thyroglobulin antibody [Tg-Ab]) may be a secondary intermediate outcome (1) between PFAS exposure and ASD or (2) between PFAS exposure and thyroid dysfunction (Fig. 1).
- Prenatal PFAS exposure and ASD
- To our knowledge, seven epidemiologic studies to date have examined associations between maternal PFAS exposure and child ASD [49,93-98]. Although results differed, three studies showed that higher prenatal exposure to perfluorohexane sulfonate, perfluorononanoate, perfluorooctanoate (PFOA), or perfluorooctane sulfonate was associated with increased risk of child ASD (Table 2) [96-98]. Potential reasons for inconsistent results among these studies include differences in timing of exposure measures in pregnancy, characteristics of study populations, methods of identification or confirmation of ASD cases, and genetic factors. In addition, because PFAS were moderately correlated with each other and one PFAS may confound another, consideration of a single compound in the model may explain, at least in part, the inconsistent findings. The number of ASD cases is relatively small in three prospective birth cohorts [49, 93,97], potentially resulting in inadequate power to detect associations.
- Prenatal PFAS exposure, thyroid dysfunction, and immune dysregulation
- Many epidemiologic studies have shown that higher prenatal PFAS concentrations in maternal blood are associated with altered TH levels in maternal blood or cord blood (Table 3) [99-110]. Although study results are not entirely consistent, increases or decreases in THs indicate that prenatal PFAS exposure may disrupt maternal or neonatal thyroid homeostasis. In addition, three studies have reported that higher exposure to a mixture of PFAS was associated with increased or decreased THs [107,109,110], implying that PFAS can disrupt thyroid either alone or in combination. Some PFAS levels were significantly higher in infants with congenital hypothyroidism compared with healthy infants [111]. Two studies reported the relationship between prenatal PFAS exposure and thyroid autoimmunity; PFOA was inversely associated with TPO-Ab [106], whereas perfluorododecanoic acid was positively associated with TPO-Ab [110].
- Studies have shown that PFAS exposure alone was not associated with TH levels among those with normal TPO-Ab but was associated with increases and decreases in THs among those with high TPO-Ab levels or low iodine concentrations. In a prospective birth cohort study, higher prenatal PFAS levels were associated with increased TSH levels only among pregnant women with high TPO-Ab (≥9 IU/mL) [112]. Another prospective birth cohort showed that higher prenatal PFOA levels were associated with lower prevalence of TPO-Ab in maternal blood and that PFAS-induced thyroid disruption and susceptibility may vary by the presence of two maternal TPO-Ab and Tg-Ab [106]. In a subset of United States adults, PFAS exposure was more likely to be associated with thyroid disruption in individuals with both TPO-Ab positivity and a urinary iodine concentration (UIC) <100 µg/L than in individuals with TPO-Ab positivity or low UIC alone, or TPO-Ab negative individuals with UIC ≥100 µg/L [113].
POTENTIAL MECHANISMS
- To date, no studies have examined a potential pathway from prenatal PFAS exposure through thyroid dysfunction and/or thyroid autoimmunity to ASD etiology within a well-characterized ASD population. Iodine deficiency is associated with increased risk of hypothyroidism [114] and known to cause brain damage [65,115]. However, most studies included in Tables 1, 3 have failed to measure important biomarkers that might affect maternal thyroid function, such as iodine status or thyroid antibodies. Thus, this review highlights that more rigorous studies are needed to yield robust and generalizable information about this potential pathway. Moreover, the evidence on mechanisms of this pathway summarized in this review suggests that thyroid dysfunction could mediate a relationship between prenatal PFAS exposure and child ASD, and this potential mediation effect could help explain significant findings from only three of the seven studies on an association between PFAS exposure and child ASD [96-98]. Therefore, future studies need to carefully disentangle the relationships among all potential mechanisms through mediation analysis [116-118] to help explain the underlying mechanism of any relationship between PFAS exposure and child ASD.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
-
Acknowledgements
- This work was supported by the grant from the National Institute of Environmental Health Sciences (R21-ES033389).
| Study | Population (countries) | Sample size, n | ASD diagnosis method | Thyroid or antibody measurement | Key thyroid dysfunctions or antibody positivitya | Estimate (95% CI)b |
|---|---|---|---|---|---|---|
| Roman et al. (2013) [64] | Population-based cohort (The Netherlands) | 4,309 | PDP-CBCL SRS | TSH | Severe hypothyroxinemia | OR, 3.89 (1.83–8.20) |
| FT4 | ||||||
| TPO-Ab | ||||||
| Andersen et al. (2014) [61] | Population-based cohort (Denmark) | 857,014 | ICD-10 | Not measuredc | Hyperthyroidism | HR, 1.34 (1.14–1.59) |
| Hypothyroidism | ||||||
| Andersen et al. (2018) [66] | Population-based cohort (Denmark) | 7,624 | ICD-10 | TSH | Hypothyroidism | HR, 1.75 (1.12–2.73) |
| FT4 | Overt hyperthyroidism | HR, 2.18 (1.08–4.39) | ||||
| Getahun et al. (2018) [63] | Retrospective cohort (USA) | 397,201 | DSM-IV | TSH | Hypothyroidism | HR, 1.31 (1.13–1.53) |
| FT4 | ||||||
| Levie et al. (2018) [65] | Population-based cohort (Spain, the Netherlands, the United Kingdom) | 9,036 | CAST | TSH | FT4 <5th percentile | OR, 1.5 (1.0–2.3) |
| PDP-CBCL | FT4 | FT4 >95th percentile | OR, 1.2 (0.7–2.1) | |||
| SCDC | TPO-Ab | FT4 <2.5th percentile | OR, 1.3 (0.7–2.5) | |||
| FT4 >97.5th percentile | OR, 1.9 (1.0–3.4) | |||||
| Rotem et al. (2020) [62] | Population-based cohort (Israel) | 437,222 | ICD-9 | TSH | Hypothyroidism | OR, 1.28 (1.11–1.49) |
| FT4 | Hyperthyroidism | OR, 1.39 (0.88–2.18) | ||||
| Other thyroid conditions | OR, 1.22 (1.05–1.42) | |||||
| Brown et al. (2015) [32] | Nested case-control study (Finland) | 960 | ICD-10 | TPO-Ab | TPO-Ab+ | OR, 1.78 (1.16–2.75) |
ASD, autism spectrum disorder; CI, confidence interval; PDP-CBCL, the Pervasive Developmental Problems Subscale of the Child Behavior Checklist for Toddlers; SRS, social responsiveness scale; TSH, thyroid stimulating hormone; FT4, free thyroxine; TPO-Ab, thyroid peroxidase antibody; OR, odds ratio; ICD-9 or 10, the ninth or tenth revision of the International Classification of Diseases; HR, hazard ratio; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; CAST, Childhood Autism Spectrum Test; SCDC, Social Communication Disorder Checklist; TPO-Ab+, positive to TPO-Ab.
a Each study defined thyroid dysfunction using various levels of TSH and/or FT4;
b Adjusted for various confounders and covariates in each study;
c Hyperthyroidism and hypothyroidism were defined by the various combinations of first hospital diagnosis of hyperthyroidism or hypothyroidism, number of anti-thyroid medications, and number of prescriptions of thyroid hormones.
| Study | Population (country) | Sample size, na | ASD diagnosis method | Blood sample type | PFAS with notable findings | Estimate (95% CI)b |
|---|---|---|---|---|---|---|
| Braun et al. (2014) [93] | Prospective birth cohort (USA) | 175 | SRS | Prenatal maternal serum | PFOS | β, –2.0 (–4.4 to 0.4) |
| Liew et al. (2015) [94] | Nested case-control study (Denmark) | 770 | ICD-10 | Prenatal maternal plasma | PFOA | RR, 0.98 (0.82–1.16) |
| PFOS | RR, 0.87 (0.74–1.02) | |||||
| Lyall et al. (2018) [95] | Nested case-control study (USA) | 986 | DSM-IV | Prenatal maternal serum | PFOA | OR, 0.92 (0.74–1.15) |
| PFOS | OR, 0.92 (0.73–1.17) | |||||
| Long et al. (2019) [49] | Retrospective cohort (Denmark) | 210 | ICD-8 | Amniotic fluid | PFOS | OR, 0.41 (0.17–0.97) |
| ICD-10 | ||||||
| Shin et al. (2020) [96] | Case-control study (USA) | 453 | ADI-R | Postnatal maternal serumc | PFHxS | OR, 1.46 (0.98–2.18) |
| ADOS-G | PFOS | OR, 1.03 (0.99–1.08) | ||||
| Oh et al. (2021) [97] | Prospective birth cohort (USA) | 173 | ADOS | Prenatal maternal serum | PFOA | RR, 1.31 (1.04–1.65) |
| PFNA | RR, 1.79 (1.13–2.85) | |||||
| Skogheim et al. (2021) [98] | Prospective birth cohort (Norway) | 1,380 | ICD-10 | Prenatal maternal plasma | PFOA | OR, 1.71 (1.20–2.45) |
PFAS, per- and polyfluoroalkyl substances; ASD, autism spectrum disorder; CI, confidence interval; SRS, social responsiveness scale; PFOS, perfluorooctane sulfonate; ICD-8 or 10, the eighth or tenth revision of the International Classification of Diseases; PFOA, perfluorooctanoate; RR, relative risk; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; OR, odds ratio; ADI-R, Autism Diagnostic Interview-Revised; ADOSG, Autism Diagnostic Observation Schedules-Generic; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate.
a Number of mother-child pairs in which the child has SRS or a final diagnosis of ASD;
b Adjusted for various confounders and covariates in each study;
c Reconstructed maternal PFAS serum concentrations at the time of pregnancy using a simple pharmacokinetic model and maternal blood samples collected when the child was 2 to 5 years old in a case-control study.
| Study | Population (country) | Sample size, na | Blood sample type | Thyroid or antibody measurement | Relationships between PFAS and thyroidb |
|---|---|---|---|---|---|
| Kim et al. (2011) [99] | Prospective birth cohort (South Korea) | 44 mothers | Prenatal maternal plasma | TT3, TT4, TSH | No relationshipc |
| 43 infants | Cord serum | ||||
| de Cock et al. (2014) [100] | Prospective birth cohort (The Netherlands) | 83 | Cord plasma | TT4 | PFOA (↑) → TT4 (↑) |
| Wang et al. (2014) [101] | Prospective birth cohort (Taiwan) | 285 mothers | Prenatal maternal plasma | TT3, TT4 | PFNA (↑), PFUnDA (↑), PFDoA (↑) → FT4 (↓), TT4 (↓) |
| 116 neonates | Cord serum | FT4, TSH | |||
| Berg et al. (2015) [102] | Prospective birth cohort (Norway) | 515 | Prenatal maternal serum | TT3, TT4 | PFOS (↑) → TSH (↑) |
| FT4, TSH | |||||
| Shah-Kulkarni et al. (2016) [103] | Retrospective birth cohort (South Korea) | 279 | Cord serum | TT3, TT4, TSH | PFPeA (↑) → TT4 (↑) |
| Berg et al. (2017) [104] | Prospective birth cohort (Norway) | 391 | Prenatal maternal serum | TT3, TT4 | PFOS (↑) → TSH (↑) |
| FT4, TSH, TPO-Ab | |||||
| Preston et al. (2018) [105] | Prospective birth cohort (USA) | 732 mothers | Prenatal maternal serum Cord serum | TT4, FT4I | PFOA (↑), PFHxS (↑), MeFOSAA (↑) → FT4I (↓) |
| 480 neonates | T3U, TSH | ||||
| Itoh et al. (2019) [106] | Prospective birth cohort (Japan) | 701 | Prenatal maternal serum Cord serum | FT3, FT4, TSH, | PFOS (↑) → TSH (↑) |
| TPO-Ab, Tg-Ab | PFOA (↑) → TPO-Ab (↓) | ||||
| Lebeaux et al. (2020) [108] | Prospective birth cohort (USA) | 468 | Prenatal maternal serum Cord serum | FT3, TT3, TT4 | PFOA (↑), PFOS (↑), PFHxS (↑) → FT4 (↓) |
| FT4, TSH | TPO-Ab (↑) → FT4 (↓) | ||||
| Preston et al. (2020) [107] | Prospective birth cohort (USA) | 726 mothers | Prenatal maternal plasma Cord serum | TT4, FT4I | PFAS mixture (↑) → FT4I (↓) |
| 465 neonates | T3U, TSH | ||||
| Liang et al. (2020) [109] | Prospective birth cohort (China) | 300 | Cord plasma | FT3, TT4 | PFAS mixture (↑) → FT3 (↑) |
| FT4, TSH | |||||
| Guo et al. (2021) [110] | Prospective birth cohort (China) | 490 | Cord serum | FT3, TT3, TT4 | PFHpA (↑), PFNA (↑) → TSH (↓) |
| FT4, TSH, TPO-Ab, Tg-Ab | PFOA (↑), PFOS (↑), PFNA (↑), PFUnDA (↑) → TT4 (↑) | ||||
| PFOS (↑), PFUnDA (↑), PFDoA (↑) → FT4 (↑) | |||||
| PFDoA (↑) → TT3 (↑), TPO-Ab (↑) | |||||
| PFAS mixture (↑) → TT4 (↑), FT4 (↑) |
PFAS, per- and polyfluoroalkyl substances; TT3, total triiodothyronine; TT4, total thyroxine; TSH, thyroid stimulating hormone; PFOA, perfluorooctanoate; FT4, free thyroxine; PFNA, perfluorononanoate; PFUnDA, perfluoroundecanoic acid; PFDoA, perfluorododecanoic acid; PFOS, perfluorooctane sulfonate; PFPeA, perfluoro-n-pentanoic acid; TPO-Ab, thyroid peroxidase antibody; FT4I, free thyroxine index; PFHxS, perfluorohexane sulfonate; MeFOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetate; FT3, free triiodothyronine; Tg-Ab, thyroglobulin antibody; T3U, triiodothyronine resin uptake; PFHpA, perfluoroheptanoic acid.
a Number of mother-child pairs, unless otherwise noted;
b Adjusted for various confounders and covariates in each study;
c Results were not statistically significant even after adjusting for major covariates.
- 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington: American Psychiatric Association; 2013.
- 2. Rogge N, Janssen J. The economic costs of autism spectrum disorder: a literature review. J Autism Dev Disord 2019;49:2873–900.ArticlePubMedPDF
- 3. Leigh JP, Du J. Brief report: forecasting the economic burden of autism in 2015 and 2025 in the United States. J Autism Dev Disord 2015;45:4135–9.ArticlePubMedPDF
- 4. Myers SM, Voigt RG, Colligan RC, Weaver AL, Storlie CB, Stoeckel RE, et al. Autism spectrum disorder: incidence and time trends over two decades in a populationbased birth cohort. J Autism Dev Disord 2019;49:1455–74.ArticlePubMedPMCPDF
- 5. Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ 2021;70:1–16.
- 6. Grinker RR, Leventhal BL. Estimating the incidence of autism. Epidemiology 2009;20:622–3.Article
- 7. Hertz-Picciotto I, Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology 2009;20:84–90.ArticlePubMedPMC
- 8. King M, Bearman P. Diagnostic change and the increased prevalence of autism. Int J Epidemiol 2009;38:1224–34.ArticlePubMedPMC
- 9. Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011;68:1095–102.ArticlePubMedPMC
- 10. Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA 2014;311:1770–7.ArticlePubMedPMC
- 11. Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California central valley. Environ Health Perspect 2007;115:1482–9.ArticlePubMedPMC
- 12. Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 2014;122:1103–9.ArticlePubMedPMC
- 13. Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect 2011;119:873–7.ArticlePubMedPMC
- 14. Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013;70:71–7.ArticlePubMedPMC
- 15. Brucato M, Ladd-Acosta C, Li M, Caruso D, Hong X, Kaczaniuk J, et al. Prenatal exposure to fever is associated with autism spectrum disorder in the Boston Birth Cohort. Autism Res 2017;10:1878–90.ArticlePubMedPMCPDF
- 16. Croen LA, Qian Y, Ashwood P, Zerbo O, Schendel D, Pinto-Martin J, et al. Infection and fever in pregnancy and autism spectrum disorders: findings from the study to explore early development. Autism Res 2019;12:1551–61.ArticlePubMedPMCPDF
- 17. Schmidt RJ. Maternal folic acid supplements associated with reduced autism risk in the child. Evid Based Med 2013;18:e53.ArticlePubMedPMC
- 18. Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 2011;22:476–85.ArticlePubMedPMC
- 19. Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr 2012;96:80–9.ArticlePubMedPMC
- 20. Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012;129:e1121–8.ArticlePubMedPMC
- 21. Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 2016;137:e20152206.ArticlePubMedPMCPDF
- 22. Mann JR, McDermott S, Bao H, Hardin J, Gregg A. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord 2010;40:548–54.ArticlePubMedPDF
- 23. Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 2011;127:246–53.ArticlePubMedPMCPDF
- 24. Gunnes N, Suren P, Bresnahan M, Hornig M, Lie KK, Lipkin WI, et al. Interpregnancy interval and risk of autistic disorder. Epidemiology 2013;24:906–12.ArticlePubMed
- 25. Zerbo O, Yoshida C, Gunderson EP, Dorward K, Croen LA. Interpregnancy interval and risk of autism spectrum disorders. Pediatrics 2015;136:651–7.ArticlePubMedPMCPDF
- 26. Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al. Endotext. South Dartmouth: MDText.com Inc; 2000 Chapter, Thyroid hormones in brain development and function [cited 2022 Oct 27]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK285549.
- 27. Rovet JF. The role of thyroid hormones for brain development and cognitive function. Endocr Dev 2014;26:26–43.ArticlePubMed
- 28. Bernal J. Thyroid hormones and brain development. Vitam Horm 2005;71:95–122.ArticlePubMed
- 29. Lavado-Autric R, Auso E, Garcia-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 2003;111:1073–82.ArticlePubMedPMC
- 30. Stepien BK, Huttner WB. Transport, metabolism, and function of thyroid hormones in the developing mammalian brain. Front Endocrinol (Lausanne) 2019;10:209.ArticlePubMedPMC
- 31. Ge GM, Leung MT, Man KK, Leung WC, Ip P, Li GH, et al. Maternal thyroid dysfunction during pregnancy and the risk of adverse outcomes in the offspring: a systematic review and meta-analysis. J Clin Endocrinol Metab 2020;105:dgaa555.ArticlePubMedPDF
- 32. Brown AS, Surcel HM, Hinkka-Yli-Salomaki S, CheslackPostava K, Bao Y, Sourander A. Maternal thyroid autoantibody and elevated risk of autism in a national birth cohort. Prog Neuropsychopharmacol Biol Psychiatry 2015;57:86–92.ArticlePubMedPMC
- 33. UN Environment Programme. Global chemicals outlook: towards sound management of chemicals. Nairobi: UNEP; 2012. p. 44.
- 34. Shin HM, Moschet C, Young TM, Bennett DH. Measured concentrations of consumer product chemicals in California house dust: implications for sources, exposure, and toxicity potential. Indoor Air 2020;30:60–75.ArticlePubMedPMCPDF
- 35. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 2009;30:293–342.ArticlePubMedPMCPDF
- 36. Mughal BB, Fini JB, Demeneix BA. Thyroid-disrupting chemicals and brain development: an update. Endocr Connect 2018;7:R160–86.ArticlePubMedPMC
- 37. La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol 2020;16:45–57.ArticlePubMedPMCPDF
- 38. Brucker-Davis F. Effects of environmental synthetic chemicals on thyroid function. Thyroid 1998;8:827–56.ArticlePubMed
- 39. Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect 2002;110 Suppl 3:337–48.ArticlePubMedPMC
- 40. Talsness CE. Overview of toxicological aspects of polybrominated diphenyl ethers: a flame-retardant additive in several consumer products. Environ Res 2008;108:158–67.ArticlePubMed
- 41. Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab 2002;87:5185–90.ArticlePubMedPDF
- 42. Fritsche E, Cline JE, Nguyen NH, Scanlan TS, Abel J. Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environ Health Perspect 2005;113:871–6.ArticlePubMedPMC
- 43. Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. Environmental chemicals and thyroid function. Eur J Endocrinol 2006;154:599–611.ArticlePubMed
- 44. Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 2006;40:32–44.ArticlePubMed
- 45. Centers for Disease Control and Prevention. Fourth national report on human exposure to environmental chemicals. Atlanta: CDC; 2015 [cited 2022 Oct 28]. Available from: https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_feb2015.pdf.Article
- 46. Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol 2010;44:7123–9.ArticlePubMed
- 47. Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, et al. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res 2008;108:56–62.ArticlePubMed
- 48. Jensen MS, Norgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, et al. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ Health Perspect 2012;120:897–903.ArticlePubMedPMC
- 49. Long M, Ghisari M, Kjeldsen L, Wielsoe M, NorgaardPedersen B, Mortensen EL, et al. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Mol Autism 2019;10:1.ArticlePubMedPMCPDF
- 50. Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 2012;355:240–8.ArticlePubMed
- 51. Torres L, Redko A, Limper C, Imbiakha B, Chang S, August A. Effect of perfluorooctanesulfonic acid (PFOS) on immune cell development and function in mice. Immunol Lett 2021;233:31–41.ArticlePubMedPMC
- 52. Guillette TC, McCord J, Guillette M, Polera ME, Rachels KT, Morgeson C, et al. Elevated levels of per- and polyfluoroalkyl substances in cape fear river striped bass (Morone saxatilis) are associated with biomarkers of altered immune and liver function. Environ Int 2020;136:105358.ArticlePubMedPMC
- 53. Chang ET, Adami HO, Boffetta P, Wedner HJ, Mandel JS. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit Rev Toxicol 2016;46:279–331.ArticlePubMedPMCPDF
- 54. Osuna CE, Grandjean P, Weihe P, El-Fawal HA. Autoantibodies associated with prenatal and childhood exposure to environmental chemicals in Faroese children. Toxicol Sci 2014;142:158–66.ArticlePubMedPMC
- 55. Coperchini F, Awwad O, Rotondi M, Santini F, Imbriani M, Chiovato L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J Endocrinol Invest 2017;40:105–21.ArticlePubMedPDF
- 56. Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12-15 years of age. Environ Health Perspect 2010;118:1762–7.ArticlePubMedPMC
- 57. Stein CR, Savitz DA. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5-18 years of age. Environ Health Perspect 2011;119:1466–71.ArticlePubMedPMC
- 58. Ushijima J, Furukawa S, Sameshima H. The presence of thyroid peroxidase antibody is associated with lower placental weight in maternal thyroid dysfunction. Tohoku J Exp Med 2019;249:231–6.ArticlePubMed
- 59. Iddah MA, Macharia BN. Autoimmune thyroid disorders. ISRN Endocrinol 2013;2013:509764.ArticlePubMedPMCPDF
- 60. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017;27:315–89.ArticlePubMed
- 61. Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 2014;121:1365–74.ArticlePubMed
- 62. Rotem RS, Chodick G, Shalev V, Davidovitch M, Koren G, Hauser R, et al. Maternal thyroid disorders and risk of autism spectrum disorder in progeny. Epidemiology 2020;31:409–17.ArticlePubMed
- 63. Getahun D, Jacobsen SJ, Fassett MJ, Wing DA, Xiang AH, Chiu VY, et al. Association between maternal hypothyroidism and autism spectrum disorders in children. Pediatr Res 2018;83:580–8.ArticlePubMedPDF
- 64. Roman GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB, et al. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol 2013;74:733–42.ArticlePubMedPDF
- 65. Levie D, Korevaar TI, Bath SC, Dalmau-Bueno A, Murcia M, Espada M, et al. Thyroid function in early pregnancy, child IQ, and autistic traits: a meta-analysis of individual participant data. J Clin Endocrinol Metab 2018;103:2967–79.ArticlePubMedPDF
- 66. Andersen SL, Andersen S, Vestergaard P, Olsen J. Maternal thyroid function in early pregnancy and child neurodevelopmental disorders: a Danish nationwide case-cohort study. Thyroid 2018;28:537–46.ArticlePubMed
- 67. Hoshiko S, Grether JK, Windham GC, Smith D, Fessel K. Are thyroid hormone concentrations at birth associated with subsequent autism diagnosis? Autism Res 2011;4:456–63.ArticlePubMed
- 68. Lyall K, Anderson M, Kharrazi M, Windham GC. Neonatal thyroid hormone levels in association with autism spectrum disorder. Autism Res 2017;10:585–92.ArticlePubMedPDF
- 69. Korevaar TI, Tiemeier H, Peeters RP. Clinical associations of maternal thyroid function with foetal brain development: epidemiological interpretation and overview of available evidence. Clin Endocrinol (Oxf) 2018;89:129–38.ArticlePubMedPDF
- 70. Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB. Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr 2016;104 Suppl 3:918S–23S.ArticlePubMedPMC
- 71. Tingi E, Syed AA, Kyriacou A, Mastorakos G, Kyriacou A. Benign thyroid disease in pregnancy: a state of the art review. J Clin Transl Endocrinol 2016;6:37–49.ArticlePubMedPMC
- 72. Consortium on Thyroid and Pregnancy-Study Group on Preterm Birth, Korevaar TI, Derakhshan A, Taylor PN, Meima M, Chen L, et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA 2019;322:632–41.ArticlePubMedPMC
- 73. Derakhshan A, Peeters RP, Taylor PN, Bliddal S, Carty DM, Meems M, et al. Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol 2020;8:501–10.ArticlePubMedPMC
- 74. Harel-Gadassi A, Friedlander E, Yaari M, Bar-Oz B, Eventov-Friedman S, Mankuta D, et al. Risk for ASD in preterm infants: a three-year follow-up study. Autism Res Treat 2018;2018:8316212.ArticlePubMedPMCPDF
- 75. Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparen P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics 2009;124:e817–25.ArticlePubMedPDF
- 76. Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can 2010;30:125–34.PubMed
- 77. Lampi KM, Lehtonen L, Tran PL, Suominen A, Lehti V, Banerjee PN, et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr 2012;161:830–6.ArticlePubMedPMC
- 78. Hughes HK, Mills Ko E, Rose D, Ashwood P. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front Cell Neurosci 2018;12:405.ArticlePubMedPMC
- 79. Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol 1999;14:388–94.ArticlePubMedPDF
- 80. Spann MN, Timonen-Soivio L, Suominen A, CheslackPostava K, McKeague IW, Sourander A, et al. Proband and familial autoimmune diseases are associated with proband diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 2019;58:496–505.ArticlePubMedPMC
- 81. Chen SW, Zhong XS, Jiang LN, Zheng XY, Xiong YQ, Ma SJ, et al. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: a systematic review and meta-analysis. Behav Brain Res 2016;296:61–9.ArticlePubMed
- 82. Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry 2013;3:e277.ArticlePubMedPMCPDF
- 83. Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology 2008;29:226–31.ArticlePubMedPMC
- 84. Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, et al. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord 2012;42:1435–45.ArticlePubMedPMCPDF
- 85. Rossi CC, Fuentes J, Van de Water J, Amaral DG. Brief report: antibodies reacting to brain tissue in Basque Spanish children with autism spectrum disorder and their mothers. J Autism Dev Disord 2014;44:459–65.ArticlePubMedPMCPDF
- 86. Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain Behav Immun 2007;21:351–7.ArticlePubMed
- 87. Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol 2008;194:165–72.ArticlePubMed
- 88. Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry 2008;64:583–8.ArticlePubMedPMC
- 89. Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res 2008;1:275–83.ArticlePubMedPMC
- 90. Grether JK, Ashwood P, Van de Water J, Yolken RH, Anderson MC, Torres AR, et al. Prenatal and newborn immunoglobulin levels from mother-child pairs and risk of autism spectrum disorders. Front Neurosci 2016;10:218.ArticlePubMedPMC
- 91. Korevaar TI, Steegers EA, Pop VJ, Broeren MA, Chaker L, de Rijke YB, et al. Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: two population-based prospective cohort studies. J Clin Endocrinol Metab 2017;102:69–77.PubMed
- 92. Osinga JA, Derakhshan A, Palomaki GE, Ashoor G, Mannisto T, Maraka S, et al. TSH and FT4 reference intervals in pregnancy: a systematic review and individual participant data meta-analysis. J Clin Endocrinol Metab 2022;107:2925–33.ArticlePubMedPMCPDF
- 93. Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect 2014;122:513–20.ArticlePubMedPMC
- 94. Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish national birth cohort. Environ Health Perspect 2015;123:367–73.ArticlePubMedPMC
- 95. Lyall K, Yau VM, Hansen R, Kharrazi M, Yoshida CK, Calafat AM, et al. Prenatal maternal serum concentrations of per- and polyfluoroalkyl substances in association with autism spectrum disorder and intellectual disability. Environ Health Perspect 2018;126:017001.ArticlePubMedPMC
- 96. Shin HM, Bennett DH, Calafat AM, Tancredi D, HertzPicciotto I. Modeled prenatal exposure to per- and polyfluoroalkyl substances in association with child autism spectrum disorder: a case-control study. Environ Res 2020;186:109514.ArticlePubMedPMC
- 97. Oh J, Bennett DH, Calafat AM, Tancredi D, Roa DL, Schmidt RJ, et al. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ Int 2021;147:106328.ArticlePubMedPMC
- 98. Skogheim TS, Weyde KV, Aase H, Engel SM, Suren P, Oie MG, et al. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environ Res 2021;202:111692.ArticlePubMed
- 99. Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, et al. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol 2011;45:7465–72.ArticlePubMed
- 100. de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. Prenatal exposure to endocrine disrupting chemicals in relation to thyroid hormone levels in infants: a Dutch prospective cohort study. Environ Health 2014;13:106.PubMedPMC
- 101. Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, et al. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect 2014;122:529–34.ArticlePubMedPMC
- 102. Berg V, Nost TH, Hansen S, Elverland A, Veyhe AS, Jorde R, et al. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ Int 2015;77:63–9.ArticlePubMed
- 103. Shah-Kulkarni S, Kim BM, Hong YC, Kim HS, Kwon EJ, Park H, et al. Prenatal exposure to perfluorinated compounds affects thyroid hormone levels in newborn girls. Environ Int 2016;94:607–13.ArticlePubMed
- 104. Berg V, Nost TH, Pettersen RD, Hansen S, Veyhe AS, Jorde R, et al. Persistent organic pollutants and the association with maternal and infant thyroid homeostasis: a multipollutant assessment. Environ Health Perspect 2017;125:127–33.ArticlePubMedPMC
- 105. Preston EV, Webster TF, Oken E, Claus Henn B, McClean MD, Rifas-Shiman SL, et al. Maternal plasma per- and polyfluoroalkyl substance concentrations in early pregnancy and maternal and neonatal thyroid function in a prospective birth cohort: Project Viva (USA). Environ Health Perspect 2018;126:027013.ArticlePubMedPMC
- 106. Itoh S, Araki A, Miyashita C, Yamazaki K, Goudarzi H, Minatoya M, et al. Association between perfluoroalkyl substance exposure and thyroid hormone/thyroid antibody levels in maternal and cord blood: the Hokkaido study. Environ Int 2019;133(Pt A):105139.ArticlePubMed
- 107. Preston EV, Webster TF, Claus Henn B, McClean MD, Gennings C, Oken E, et al. Prenatal exposure to per- and polyfluoroalkyl substances and maternal and neonatal thyroid function in the project viva cohort: a mixtures approach. Environ Int 2020;139:105728.ArticlePubMedPMC
- 108. Lebeaux RM, Doherty BT, Gallagher LG, Zoeller RT, Hoofnagle AN, Calafat AM, et al. Maternal serum perfluoroalkyl substance mixtures and thyroid hormone concentrations in maternal and cord sera: the HOME study. Environ Res 2020;185:109395.ArticlePubMedPMC
- 109. Liang H, Wang Z, Miao M, Tian Y, Zhou Y, Wen S, et al. Prenatal exposure to perfluoroalkyl substances and thyroid hormone concentrations in cord plasma in a Chinese birth cohort. Environ Health 2020;19:127.ArticlePubMedPMCPDF
- 110. Guo J, Zhang J, Wang Z, Zhang L, Qi X, Zhang Y, et al. Umbilical cord serum perfluoroalkyl substance mixtures in relation to thyroid function of newborns: findings from sheyang mini birth cohort study. Chemosphere 2021;273:129664.ArticlePubMed
- 111. Kim DH, Kim UJ, Kim HY, Choi SD, Oh JE. Perfluoroalkyl substances in serum from South Korean infants with congenital hypothyroidism and healthy infants: its relationship with thyroid hormones. Environ Res 2016;147:399–404.ArticlePubMed
- 112. Webster GM, Venners SA, Mattman A, Martin JW. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res 2014;133:338–47.ArticlePubMed
- 113. Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP, Venners SA. Cross-sectional associations of serum perfluoroalkyl acids and thyroid hormones in U.S. adults: variation according to TPOAB and iodine status (NHANES 2007-2008). Environ Health Perspect 2016;124:935–42.ArticlePubMedPMC
- 114. de Escobar GM, Obregon MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr 2007;10:1554–70.ArticlePubMed
- 115. Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013;382:331–7.ArticlePubMed
- 116. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–50.ArticlePubMedPMC
- 117. Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods 2002;7:422–45.ArticlePubMed
- 118. Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: The Guilford Press; 2013.
References
Figure & Data
References
Citations

- Endocrine Disruptors and Thyroid Health
Elizabeth N. Pearce
Endocrine Practice.2024; 30(2): 172. CrossRef - Maternal Thyroid Dysfunction During Pregnancy as an Etiologic Factor in Autism Spectrum Disorder: Challenges and Opportunities for Research
Zoe B. Kaplan, Elizabeth N. Pearce, Sun Y. Lee, Hyeong-Moo Shin, Rebecca J. Schmidt
Thyroid®.2024; 34(2): 144. CrossRef - Effects of Endocrine-Disrupting Chemicals on Human Health
Jun Hyung Lee, Sung-Eun Cho
Laboratory Medicine Online.2023; 13(3): 129. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite


