Search
- Page Path
- HOME > Search
- Calcium & bone metabolism
- Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis
- Brendan F. Boyce, Jinbo Li, Zhenqiang Yao, Lianping Xing
- Endocrinol Metab. 2023;38(5):504-521. Published online September 26, 2023
- DOI: https://doi.org/10.3803/EnM.2023.501
- 2,140 View
- 101 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
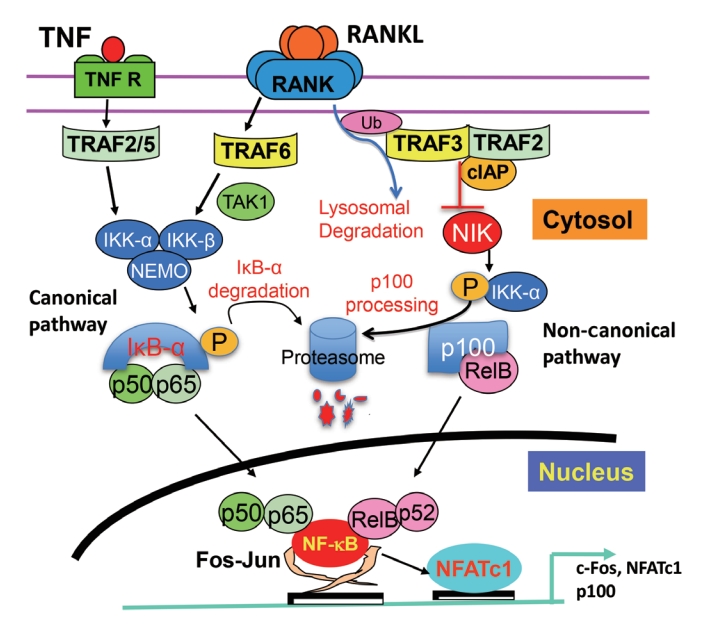
ePub - Maintenance of skeletal integrity requires the coordinated activity of multinucleated bone-resorbing osteoclasts and bone-forming osteoblasts. Osteoclasts form resorption lacunae on bone surfaces in response to cytokines by fusion of precursor cells. Osteoblasts are derived from mesenchymal precursors and lay down new bone in resorption lacunae during bone remodeling. Nuclear factorkappa B (NF-κB) signaling regulates osteoclast and osteoblast formation and is activated in osteoclast precursors in response to the essential osteoclastogenic cytokine, receptor activator of NF-κB ligand (RANKL), which can also control osteoblast formation through RANK-RANKL reverse signaling in osteoblast precursors. RANKL and some pro-inflammatory cytokines, including tumor necrosis factor (TNF), activate NF-κB signaling to positively regulate osteoclast formation and functions. However, these cytokines also limit osteoclast and osteoblast formation through NF-κB signaling molecules, including TNF receptor-associated factors (TRAFs). TRAF6 mediates RANKL-induced osteoclast formation through canonical NF-κB signaling. In contrast, TRAF3 limits RANKL- and TNF-induced osteoclast formation, and it restricts transforming growth factor β (TGFβ)-induced inhibition of osteoblast formation in young and adult mice. During aging, neutrophils expressing TGFβ and C-C chemokine receptor type 5 (CCR5) increase in bone marrow of mice in response to increased NF-κB-induced CC motif chemokine ligand 5 (CCL5) expression by mesenchymal progenitor cells and injection of these neutrophils into young mice decreased bone mass. TGFβ causes degradation of TRAF3, resulting in decreased glycogen synthase kinase-3β/β-catenin-mediated osteoblast formation and age-related osteoporosis in mice. The CCR5 inhibitor, maraviroc, prevented accumulation of TGFβ+/CCR5+ neutrophils in bone marrow and increased bone mass by inhibiting bone resorption and increasing bone formation in aged mice. This paper updates current understanding of how NF-κB signaling is involved in the positive and negative regulation of cytokine-mediated osteoclast and osteoblast formation and activation with a focus on the role of TRAF3 signaling, which can be targeted therapeutically to enhance bone mass.
-
Citations
Citations to this article as recorded by- The Role of Rosavin in the Pathophysiology of Bone Metabolism
Piotr Wojdasiewicz, Paweł Turczyn, Anna Lach-Gruba, Łukasz A. Poniatowski, Daryush Purrahman, Mohammad-Reza Mahmoudian-Sani, Dariusz Szukiewicz
International Journal of Molecular Sciences.2024; 25(4): 2117. CrossRef - The role of monocyte/macrophage chemokines in pathogenesis of osteoarthritis: A review
Hao Luo, Linfeng Li, Song Han, Tao Liu
International Journal of Immunogenetics.2024;[Epub] CrossRef - The effect of low-level laser therapy on osteoclast differentiation: Clinical implications for tooth movement and bone density
Chun-Yi Huang, Huynh Hoai Thuong Le, Hsiao-Chi Tsai, Chih-Hsin Tang, Jian-Hong Yu
Journal of Dental Sciences.2024;[Epub] CrossRef - Genetic Deficiency of the Long Pentraxin 3 Affects Osteogenesis and Osteoclastogenesis in Homeostatic and Inflammatory Conditions
Valentina Granata, Dario Strina, Maria Lucia Schiavone, Barbara Bottazzi, Alberto Mantovani, Antonio Inforzato, Cristina Sobacchi
International Journal of Molecular Sciences.2023; 24(23): 16648. CrossRef
- The Role of Rosavin in the Pathophysiology of Bone Metabolism

- Calcium & Bone Metabolism
- A Key Metabolic Regulator of Bone and Cartilage Health
- Elizabeth Pérez-Hernández, Jesús Javier Pastrana-Carballo, Fernando Gómez-Chávez, Ramesh C. Gupta, Nury Pérez-Hernández
- Endocrinol Metab. 2022;37(4):559-574. Published online August 8, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1443
- 7,860 View
- 340 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
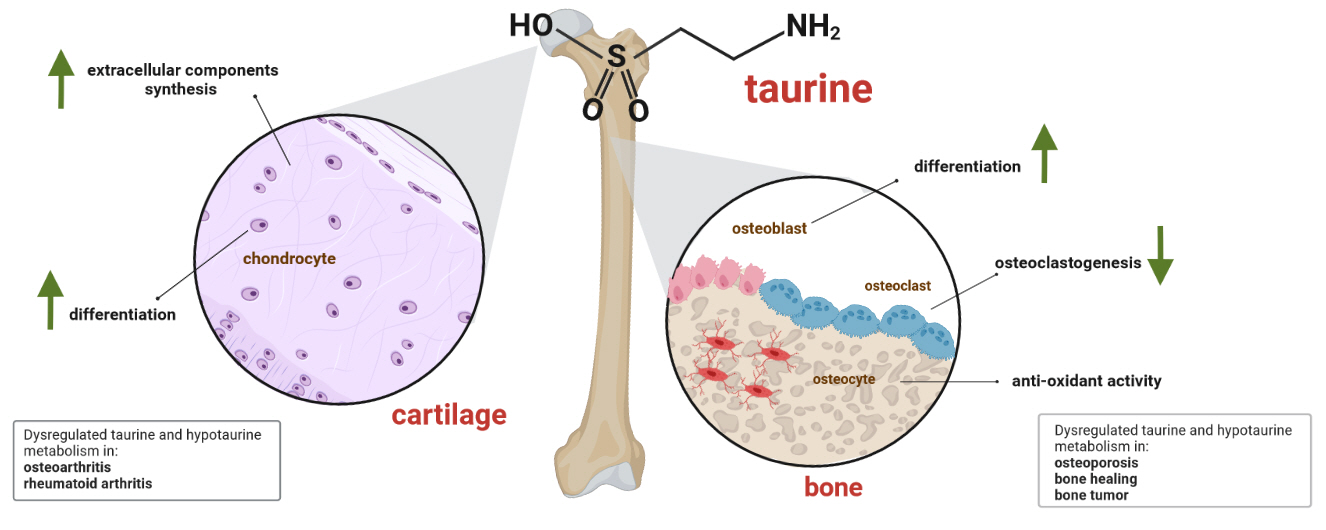
ePub - Taurine, a cysteine-derived zwitterionic sulfonic acid, is a common ingredient in energy drinks and is naturally found in fish and other seafood. In humans, taurine is produced mainly in the liver, and it can also be obtained from food. In target tissues, such as the retina, heart, and skeletal muscle, it functions as an essential antioxidant, osmolyte, and antiapoptotic agent. Taurine is also involved in energy metabolism and calcium homeostasis. Taurine plays a considerable role in bone growth and development, and high-profile reports have demonstrated the importance of its metabolism for bone health. However, these reports have not been collated for more than 10 years. Therefore, this review focuses on taurine–bone interactions and covers recently discovered aspects of taurine’s effects on osteoblastogenesis, osteoclastogenesis, bone structure, and bone pathologies (e.g., osteoporosis and fracture healing), with due attention to the taurine–cartilage relationship.
-
Citations
Citations to this article as recorded by- Metabolomics analysis of the potential mechanism of Yi-Guan-Jian decoction to reverse bone loss in glucocorticoid-induced osteoporosis
Mengxing Yin, Dezhi Zhou, Fu Jia, Xiaosan Su, Xiufang Li, Ruifen Sun, Junmin Li
Journal of Orthopaedic Surgery and Research.2023;[Epub] CrossRef - An in-silico approach to the potential modulatory effect of taurine on sclerostin (SOST) and its probable role during osteoporosis
Mazumder Adhish, I. Manjubala
Journal of Biomolecular Structure and Dynamics.2023; : 1. CrossRef - Flattening the biological age curve by improving metabolic health: to taurine or not to taurine, that’ s the question
Kwok M. Ho, Anna Lee, William Wu, Matthew T.V. Chan, Lowell Ling, Jeffrey Lipman, Jason Roberts, Edward Litton, Gavin M. Joynt, Martin Wong
Journal of Geriatric Cardiology.2023; 20(11): 813. CrossRef
- Metabolomics analysis of the potential mechanism of Yi-Guan-Jian decoction to reverse bone loss in glucocorticoid-induced osteoporosis

- Bone Metabolism
- Regulation of Osteoblast Metabolism by Wnt Signaling
- Megan C. Moorer, Ryan C. Riddle
- Endocrinol Metab. 2018;33(3):318-330. Published online August 14, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.3.318
- 6,374 View
- 117 Download
- 39 Web of Science
- 38 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Wnt/β-catenin signaling plays a critical role in the achievement of peak bone mass, affecting the commitment of mesenchymal progenitors to the osteoblast lineage and the anabolic capacity of osteoblasts depositing bone matrix. Recent studies suggest that this evolutionarily-conserved, developmental pathway exerts its anabolic effects in part by coordinating osteoblast activity with intermediary metabolism. These findings are compatible with the cloning of the gene encoding the low-density lipoprotein related receptor-5 (LRP5) Wnt co-receptor from a diabetes-susceptibility locus and the now well-established linkage between Wnt signaling and metabolism. In this article, we provide an overview of the role of Wnt signaling in whole-body metabolism and review the literature regarding the impact of Wnt signaling on the osteoblast's utilization of three different energy sources: fatty acids, glucose, and glutamine. Special attention is devoted to the net effect of nutrient utilization and the mode of regulation by Wnt signaling. Mechanistic studies indicate that the utilization of each substrate is governed by a unique mechanism of control with β-catenin-dependent signaling regulating fatty acid β-oxidation, while glucose and glutamine utilization are β-catenin-independent and downstream of mammalian target of rapamycin complex 2 (mTORC2) and mammalian target of rapamycin complex 1 (mTORC1) activation, respectively. The emergence of these data has provided a new context for the mechanisms by which Wnt signaling influences bone development.
-
Citations
Citations to this article as recorded by- Aseptic loosening around total joint replacement in humans is regulated by miR-1246 and miR-6089 via the Wnt signalling pathway
Yi Deng, Kate Phillips, Zhi-Ping Feng, Paul N. Smith, Rachel W. Li
Journal of Orthopaedic Surgery and Research.2024;[Epub] CrossRef - Cardamonin inhibits osteogenic differentiation by downregulating Wnt/beta‐catenin signaling and alleviates subchondral osteosclerosis in osteoarthritic mice
Fanding Meng, Pengchong Zhu, Xiaoli Ren, Limei Wang, Dong Ding, Jiangbo Yan, Ying Zhang, Shang‐You Yang, Bin Ning
Journal of Orthopaedic Research.2024;[Epub] CrossRef - Common Regulators of Lipid Metabolism and Bone Marrow Adiposity in Postmenopausal Women
Dae-Yong Kim, Seong-Hee Ko
Pharmaceuticals.2023; 16(2): 322. CrossRef - Novel mutation in LRP5 gene cause rare osteosclerosis: cases studies and literature review
Dichen Zhao, Lei Sun, Wenbin Zheng, Jing Hu, Bingna Zhou, Ou Wang, Yan Jiang, Weibo Xia, Xiaoping Xing, Mei Li
Molecular Genetics and Genomics.2023; 298(3): 683. CrossRef - RUNX Family as a Promising Biomarker and a Therapeutic Target in Bone Cancers: A Review on Its Molecular Mechanism(s) behind Tumorigenesis
Selvaraj Vimalraj, Saravanan Sekaran
Cancers.2023; 15(12): 3247. CrossRef - FGF19 protects against obesity-induced bone loss by promoting osteogenic differentiation
Ai Guo, Kai Li, Hong-Chuan Tian, Bai-Long Tao, Qian Xiao, Dian-Ming Jiang
Biomedicine & Pharmacotherapy.2022; 146: 112524. CrossRef - Estrogen receptor alpha and NFATc1 bind to a bone mineral density-associated SNP to repress WNT5B in osteoblasts
Sarocha Suthon, Jianjian Lin, Rachel S. Perkins, John R. Crockarell, Gustavo A. Miranda-Carboni, Susan A. Krum
The American Journal of Human Genetics.2022; 109(1): 97. CrossRef - Exposure of primary osteoblasts to combined magnetic and electric fields induced spatiotemporal endochondral ossification characteristic gene- and protein expression profiles
Klaus H. Dittmann, Claus Mayer, Heribert Stephan, Christin Mieth, Michael Bonin, Beat Lechmann, H. Peter Rodemann
Journal of Experimental Orthopaedics.2022;[Epub] CrossRef - Roxadustat promotes osteoblast differentiation and prevents estrogen deficiency-induced bone loss by stabilizing HIF-1α and activating the Wnt/β-catenin signaling pathway
Luyao Li, Afang Li, Li Zhu, Liangying Gan, Li Zuo
Journal of Orthopaedic Surgery and Research.2022;[Epub] CrossRef - T-cell factor 7L2 is a novel regulator of osteoblast functions that acts in part by modulation of hypoxia signaling
Subburaman Mohan, Chandrasekhar Kesavan
American Journal of Physiology-Endocrinology and Metabolism.2022; 322(6): E528. CrossRef - Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications
Ioannis Akoumianakis, Murray Polkinghorne, Charalambos Antoniades
Nature Reviews Cardiology.2022; 19(12): 783. CrossRef - Osteoporosis pathogenesis and treatment: existing and emerging avenues
Bo Liang, George Burley, Shu Lin, Yan-Chuan Shi
Cellular & Molecular Biology Letters.2022;[Epub] CrossRef - QingreHuoxue decoction protects joint and toe bone morphology and structure in rats with active RA through bidirectional regulation of bone metabolism
Hui Yang, Zhenyu Wu, Xun Gong, Bo Li, Guangjun Wu, Quan Jiang
Pharmacological Research - Modern Chinese Medicine.2022; 4: 100156. CrossRef - Regulation of Wnt signaling by non-coding RNAs during osteoblast differentiation
I. Saranya, R.L. Akshaya, N. Selvamurugan
Differentiation.2022; 128: 57. CrossRef - Looking at Mountains: Role of Sustained Hypoxia in Regulating Bone Mineral Homeostasis in Relation to Wnt Pathway and Estrogen
Lijy K. Babu, Dishari Ghosh
Clinical Reviews in Bone and Mineral Metabolism.2022; 20(1-4): 18. CrossRef - Effect of the Pulsed Electromagnetic Field Treatment in a Rat Model of Senile Osteoporosis In Vivo
Jun Zhou, Jinling Wang, Mengjian Qu, Xiarong Huang, Linwei Yin, Yang Liao, Fujin Huang, Pengyun Ning, Peirui Zhong, Yahua Zeng
Bioelectromagnetics.2022; 43(7): 438. CrossRef - Arm race between Rift Valley fever virus and host
Xiao Wang, Yupei Yuan, Yihan Liu, Leiliang Zhang
Frontiers in Immunology.2022;[Epub] CrossRef - Differential bone metabolism and protein expression in mice fed a high-fat diet versus Daurian ground squirrels following natural pre-hibernation fattening
Xuli Gao, Shenyang Shen, Qiaohua Niu, Weilan Miao, Yuting Han, Ziwei Hao, Ning An, Yingyu Yang, Yu Zhang, Han Zhang, Kenneth B. Storey, Hui Chang
Journal of Zhejiang University-SCIENCE B.2022; 23(12): 1042. CrossRef - miR‐142a‐5p promoted osteoblast differentiation via targeting nuclear factor IA
Hairui Yuan, Mengyue Li, Xue Feng, Endong Zhu, Baoli Wang
Journal of Cellular Physiology.2021; 236(3): 1810. CrossRef - Serum sclerostin and glucose homeostasis: No association in healthy men. Cross-sectional and prospective data from the EGIR-RISC study
Jens-Jacob L. Lauterlein, Pernille Hermann, Thomas Konrad, Peter Wolf, Peter Nilsson, Rafael Gabriel Sánchez, Ele Ferrannini, Beverley Balkau, Kurt Højlund, Morten Frost
Bone.2021; 143: 115681. CrossRef - Energy Metabolism and Ketogenic Diets: What about the Skeletal Health? A Narrative Review and a Prospective Vision for Planning Clinical Trials on this Issue
Daniela Merlotti, Roberta Cosso, Cristina Eller-Vainicher, Fabio Vescini, Iacopo Chiodini, Luigi Gennari, Alberto Falchetti
International Journal of Molecular Sciences.2021; 22(1): 435. CrossRef - Therapeutic potential of iron chelators on osteoporosis and their cellular mechanisms
Jian Zhang, Hai Zhao, Gang Yao, Penghai Qiao, Longfei Li, Shuguang Wu
Biomedicine & Pharmacotherapy.2021; 137: 111380. CrossRef - Insertion of gallic acid onto chitosan promotes the differentiation of osteoblasts from murine bone marrow-derived mesenchymal stem cells
Yunok Oh, Chang-Bum Ahn, M.P.C.K. Marasinghe, Jae-Young Je
International Journal of Biological Macromolecules.2021; 183: 1410. CrossRef - Biological Mechanisms of Paeonoside in the Differentiation of Pre-Osteoblasts and the Formation of Mineralized Nodules
Kyung-Ran Park, Joon Yeop Lee, Myounglae Cho, Jin Tae Hong, Hyung-Mun Yun
International Journal of Molecular Sciences.2021; 22(13): 6899. CrossRef - Enamel matrix derivative (EMD) enhances the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs)
Lu Cheng, Ying Li, Qian Xia, MaoHua Meng, ZhaoYang Ye, ZhengLong Tang, HongChao Feng, Xin Chen, HeLin Chen, Xiao Zeng, Yi Luo, Qiang Dong
Bioengineered.2021; 12(1): 7033. CrossRef - Synthesized Nanorods Hydroxyapatite by Microwave-Assisted Technology for In Vitro Osteoporotic Bone Regeneration through Wnt/β-Catenin Pathway
Nadia Z. Shaban, Marwa Y. Kenawy, Nahla A. Taha, Mona M. Abd El-Latif, Doaa A. Ghareeb
Materials.2021; 14(19): 5823. CrossRef - Chlorogenic Acids Inhibit Adipogenesis: Implications of Wnt/β-Catenin Signaling Pathway
Mengting Liu, Jian Qin, Jing Cong, Yubin Yang, Muhittin Yurekli
International Journal of Endocrinology.2021; 2021: 1. CrossRef - Regulation of the mesenchymal stem cell fate by interleukin-17: Implications in osteogenic differentiation
Jelena Krstić, Slavko Mojsilović, Sonja S Mojsilović, Juan F Santibanez
World Journal of Stem Cells.2021; 13(11): 1699. CrossRef - Regulation of the mesenchymal stem cell fate by interleukin-17: Implications in osteogenic differentiation
Jelena Krstić, Slavko Mojsilović, Sonja S Mojsilović, Juan F Santibanez
World Journal of Stem Cells.2021; 13(11): 1696. CrossRef - The Skeletal Cellular and Molecular Underpinning of the Murine Hindlimb Unloading Model
Priyanka Garg, Maura Strigini, Laura Peurière, Laurence Vico, Donata Iandolo
Frontiers in Physiology.2021;[Epub] CrossRef - Changes in Serum Dickkopf-1, RANK Ligand, Osteoprotegerin, and Bone Mineral Density after Allogeneic Hematopoietic Stem Cell Transplantation Treatment
Eunhee Jang, Jeonghoon Ha, Ki-Hyun Baek, Moo Il Kang
Endocrinology and Metabolism.2021; 36(6): 1211. CrossRef - Amyloid β peptide promotes bone formation by regulating Wnt/β‐catenin signaling and the OPG/RANKL/RANK system
Bu Yang, Shangfu Li, Zheng Chen, Feng Feng, Lei He, Bin Liu, Tianwei He, Xuan Wang, Ruiqiang Chen, Zihao Chen, Peigen Xie, Limin Rong
The FASEB Journal.2020; 34(3): 3583. CrossRef - Evaluation of chondrogenesis and osteogenesis via Wnt/β-Catenin, S100 immunoexpression and histomorphometry in fetal rats following maternal uterine artery ligation
Serap USLU, Gülperi ÖKTEM, Fatih OLTULU, Kenan DEMİR, Arzu İRBAN, Gülçin BAŞDEMİR, Ümit İNCE, Ayşegül UYSAL
Ege Tıp Dergisi.2020; 59(1): 39. CrossRef - Dual Effects of Lipid Metabolism on Osteoblast Function
Nathalie S. Alekos, Megan C. Moorer, Ryan C. Riddle
Frontiers in Endocrinology.2020;[Epub] CrossRef - Mesenchymal stem cells: amazing remedies for bone and cartilage defects
Parisa Kangari, Tahereh Talaei-Khozani, Iman Razeghian-Jahromi, Mahboobeh Razmkhah
Stem Cell Research & Therapy.2020;[Epub] CrossRef - Blue Mussel-Derived Peptides PIISVYWK and FSVVPSPK Trigger Wnt/β-Catenin Signaling-Mediated Osteogenesis in Human Bone Marrow Mesenchymal Stem Cells
Yunok Oh, Chang-Bum Ahn, Jae-Young Je
Marine Drugs.2020; 18(10): 510. CrossRef - Interleukin-35 stimulates tumor necrosis factor-α activated osteoblasts differentiation through Wnt/β-catenin signaling pathway in rheumatoid arthritis
Yuxuan Li, Lin Yuan, Shenyi Jiang, Siyan Liu, Liping Xia, Hui Shen, Jing Lu
International Immunopharmacology.2019; 75: 105810. CrossRef - The roles of Orai and Stim in bone health and disease
Lisa J. Robinson, Harry C. Blair, John B. Barnett, Jonathan Soboloff
Cell Calcium.2019; 81: 51. CrossRef
- Aseptic loosening around total joint replacement in humans is regulated by miR-1246 and miR-6089 via the Wnt signalling pathway

- Endocrine Research
- Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
- Kyoung Min Kim, Hyun-Jin Jin, Seo Yeon Lee, Hyo Jin Maeng, Gha Young Lee, Tae Jung Oh, Sung Hee Choi, Hak Chul Jang, Soo Lim
- Endocrinol Metab. 2017;32(3):389-395. Published online September 18, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.3.389
- 4,760 View
- 50 Download
- 11 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Bone strength is impaired in patients with type 2 diabetes mellitus despite an increase in bone mineral density (BMD). Thiazolidinedione (TZD), a peroxisome proliferator activated receptor γ agonist, promotes adipogenesis, and suppresses osteoblastogenesis. Therefore, its use is associated with an increased risk of fracture. The aim of this study was to examine the
in vitro andin vivo effects of lobeglitazone, a new TZD, on bone.Methods MC3T3E1 and C3H10T1/2 cells were cultured in osteogenic medium and exposed to lobeglitazone (0.1 or 1 µM), rosiglitazone (0.4 µM), or pioglitazone (1 µM) for 10 to 14 days. Alkaline phosphatase (ALP) activity, Alizarin red staining, and osteoblast marker gene expression were analyzed. For
in vivo experiments, 6-month-old C57BL/6 mice were treated with vehicle, one of two doses of lobeglitazone, rosiglitazone, or pioglitazone. BMD was assessed using a PIXImus2 instrument at the baseline and after 12 weeks of treatment.Results As expected,
in vitro experiments showed that ALP activity was suppressed and the mRNA expression of osteoblast marker genes RUNX2 (runt-related transcription factor 2) and osteocalcin was significantly attenuated after rosiglitazone treatment. By contrast, lobeglitazone at either dose did not inhibit these variables. Rosiglitazone-treated mice showed significantly accelerated bone loss for the whole bone and femur, but BMD did not differ significantly between the lobeglitazone-treated and vehicle-treated mice.Conclusion These findings suggest that lobeglitazone has no detrimental effects on osteoblast biology and might not induce side effects in the skeletal system.
-
Citations
Citations to this article as recorded by- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis
Deep Dutta, Saptarshi Bhattacharya, Manoj Kumar, Priyankar K. Datta, Ritin Mohindra, Meha Sharma
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102697. CrossRef - Efficacy and safety of lobeglitazone, a new Thiazolidinedione, as compared to the standard of care in type 2 diabetes mellitus: A systematic review and meta-analysis
Shashank R. Joshi, Saibal Das, Suja Xaviar, Shambo Samrat Samajdar, Indranil Saha, Sougata Sarkar, Shatavisa Mukherjee, Santanu Kumar Tripathi, Jyotirmoy Pal, Nandini Chatterjee
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(1): 102703. CrossRef - A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Y
Diabetes & Metabolism Journal.2022; 46(6): 855. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
Jaehyun Bae, Taegyun Park, Hyeyoung Kim, Minyoung Lee, Bong-Soo Cha
Diabetes & Metabolism Journal.2021; 45(3): 326. CrossRef - Effect of lobeglitazone on motor function in rat model of Parkinson’s disease with diabetes co-morbidity
Kambiz Hassanzadeh, Arman Rahimmi, Mohammad Raman Moloudi, Rita Maccarone, Massimo Corbo, Esmael Izadpanah, Marco Feligioni
Brain Research Bulletin.2021; 173: 184. CrossRef - Comparison of the Effects of Various Antidiabetic Medication on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus
Jeonghoon Ha, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Ki-Ho Song, Seung Hyun Ko, Moo Il Kang, Sung Dae Moon, Ki-Hyun Baek
Endocrinology and Metabolism.2021; 36(4): 895. CrossRef - Xenogeneic native decellularized matrix carrying PPARγ activator RSG regulating macrophage polarization to promote ligament-to-bone regeneration
Xue Han, Lijun Liao, Tian Zhu, Yuchan Xu, Fei Bi, Li Xie, Hui Li, Fangjun Huo, Weidong Tian, Weihua Guo
Materials Science and Engineering: C.2020; 116: 111224. CrossRef - Diabetes pharmacotherapy and effects on the musculoskeletal system
Evangelia Kalaitzoglou, John L. Fowlkes, Iuliana Popescu, Kathryn M. Thrailkill
Diabetes/Metabolism Research and Reviews.2019;[Epub] CrossRef - The effects of diabetes therapy on bone: A clinical perspective
Karim G. Kheniser, Carmen M. Polanco Santos, Sangeeta R. Kashyap
Journal of Diabetes and its Complications.2018; 32(7): 713. CrossRef
- Efficacy and safety of novel thiazolidinedione lobeglitazone for managing type-2 diabetes a meta-analysis

- Bone Metabolism
- Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
- Hyo Min Jeong, Sun Wook Cho, Serk In Park
- Endocrinol Metab. 2016;31(4):485-492. Published online December 20, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.4.485
- 3,867 View
- 47 Download
- 13 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The tumor microenvironment is comprised of diverse stromal cell populations in addition to tumor cells. Increasing evidence now clearly supports the role of microenvironment stromal cells in tumor progression and metastasis, yet the regulatory mechanisms and interactions among tumor and stromal cells remain to be elucidated. Bone metastasis is the major problem in many types of human malignancies including prostate, breast and lung cancers, and the biological basis of bone metastasis let alone curative approaches are largely undetermined. Among the many types of stromal cells in bone, osteoblasts are shown to be an important player. In this regard, osteoblasts are a key target cell type in the development of bone metastasis, but there are currently no drugs or therapeutic approaches are available that specifically target osteoblasts. This review paper summarizes the current knowledge on osteoblasts in the metastatic tumor microenvironment, aiming to provide clues and directions for future research endeavor.
-
Citations
Citations to this article as recorded by- Bone marrow adipocytes and lung cancer bone metastasis: unraveling the role of adipokines in the tumor microenvironment
Jian Li, Jialu Wu, Yanni Xie, Xijie Yu
Frontiers in Oncology.2024;[Epub] CrossRef - Circulating biomarkers for diagnosis and therapeutic monitoring in bone metastasis
Min-Kyoung Song, Serk In Park, Sun Wook Cho
Journal of Bone and Mineral Metabolism.2023; 41(3): 337. CrossRef - Mobilization of monocytic myeloid-derived suppressor cells is regulated by PTH1R activation in bone marrow stromal cells
Eun Jung Lee, Kyoung Jin Lee, Seungpil Jung, Kyong Hwa Park, Serk In Park
Bone Research.2023;[Epub] CrossRef - 2E‐Decene‐4,6‐diyn‐1‐ol‐acetate inhibits osteoclastogenesis through mitogen‐activated protein kinase‐c‐Fos‐NFATc1 signalling pathways
Young Ran Park, Xiang‐Dong Su, Saroj Kumar Shrestha, Seo Young Yang, Yunjo Soh
Clinical and Experimental Pharmacology and Physiology.2022; 49(3): 341. CrossRef - The let-7f-5p–Nme4 pathway mediates tumor necrosis factor α-induced impairment in osteogenesis of bone marrow-derived mesenchymal stem cells
Ying-Jie Zhao, Zheng-Chao Gao, Xi-Jing He, Jing Li
Biochemistry and Cell Biology.2021; 99(4): 488. CrossRef - Circulating Osteocalcin-Positive Cells as a Novel Diagnostic Biomarker for Bone Metastasis in Breast Cancer Patients
Kyung-Hun Lee, Kyoung Jin Lee, Tae-Yong Kim, Febby Hutomo, Hyun Jin Sun, Gi Jeong Cheon, Serk In Park, Sun Wook Cho, Seock-Ah Im
Journal of Bone and Mineral Research.2020; 35(10): 1838. CrossRef - The Early Results of Vertebral Pathological Compression Fracture of Extra- nodal Lymphoma with HIV-positive Patients Treated by Percutaneous Kyphoplasty
Sheng Sun, Biao Xu, Qiang Zhang, Chang-song Zhao, Rui Ma, Jie He, Yao Zhang
Current HIV Research.2020; 18(4): 248. CrossRef - Sema4D expression and secretion are increased by HIF-1α and inhibit osteogenesis in bone metastases of lung cancer
Wu-gui Chen, Jing Sun, Wei-wei Shen, Si-zhen Yang, Ying Zhang, Xu Hu, Hao Qiu, Shang-cheng Xu, Tong-wei Chu
Clinical & Experimental Metastasis.2019; 36(1): 39. CrossRef - Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis
Changki Lee, Young Mi Whang, Preston Campbell, Patrick L. Mulcrone, Florent Elefteriou, Sun Wook Cho, Serk In Park
Cancer Letters.2018; 414: 205. CrossRef - Targeting the tumour stroma to improve cancer therapy
Kenneth C. Valkenburg, Amber E. de Groot, Kenneth J. Pienta
Nature Reviews Clinical Oncology.2018; 15(6): 366. CrossRef - Bone Microenvironment and Role of Rank-Rankl-Opg in Breast Cancer Metastasis in Bone
Hongwei Zhang
Journal of Cancer Prevention & Current Research.2017;[Epub] CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Bone marrow adipocytes and lung cancer bone metastasis: unraveling the role of adipokines in the tumor microenvironment

- Bone Metabolism
- Dissecting Tumor-Stromal Interactions in Breast Cancer Bone Metastasis
- Yibin Kang
- Endocrinol Metab. 2016;31(2):206-212. Published online May 13, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.206
- 4,962 View
- 54 Download
- 34 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Bone metastasis is a frequent occurrence in breast cancer, affecting more than 70% of late stage cancer patients with severe complications such as fracture, bone pain, and hypercalcemia. The pathogenesis of osteolytic bone metastasis depends on cross-communications between tumor cells and various stromal cells residing in the bone microenvironment. Several growth factor signaling pathways, secreted micro RNAs (miRNAs) and exosomes are functional mediators of tumor-stromal interactions in bone metastasis. We developed a functional genomic approach to systemically identified molecular pathways utilized by breast cancer cells to engage the bone stroma in order to generate osteolytic bone metastasis. We showed that elevated expression of vascular cell adhesion molecule 1 (VCAM1) in disseminated breast tumor cells mediates the recruitment of pre-osteoclasts and promotes their differentiation to mature osteoclasts during the bone metastasis formation. Transforming growth factor β (TGF-β) is released from bone matrix upon bone destruction, and signals to breast cancer to further enhance their malignancy in developing bone metastasis. We furthered identified Jagged1 as a TGF-β target genes in tumor cells that engaged bone stromal cells through the activation of Notch signaling to provide a positive feedback to promote tumor growth and to activate osteoclast differentiation. Substantially change in miRNA expression was observed in osteoclasts during their differentiation and maturation, which can be exploited as circulating biomarkers of emerging bone metastasis and therapeutic targets for the treatment of bone metastasis. Further research in this direction may lead to improved diagnosis and treatment strategies for bone metastasis.
-
Citations
Citations to this article as recorded by- Osteoclast Cancer Cell Metabolic Cross-talk Confers PARP Inhibitor Resistance in Bone Metastatic Breast Cancer
Huijuan Fan, Zhanao Xu, Ke Yao, Bingxin Zheng, Yuan Zhang, Xuxiang Wang, Tengjiang Zhang, Xuan Li, Haitian Hu, Bin Yue, Zeping Hu, Hanqiu Zheng
Cancer Research.2024; 84(3): 449. CrossRef - Bone Marrow Mesenchymal Stem Cells Restrain the Migration and Invasion of Breast Cancer Cells by Up-Regulating miR-2158 and Inactivating RAI2/NLRP3 Pathway
Meiyu Xu, Shen Ye, Zhiqiang Tang, Shuai Gong
Journal of Biomaterials and Tissue Engineering.2023; 13(1): 162. CrossRef - Association of RANKL and EGFR gene expression with bone metastases in patients with metastatic non-small cell lung cancer
Anita J.W.M. Brouns, Lizza E.L. Hendriks, Iris J. Robbesom-van den Berge, Annemariek J.H.M. Driessen, Guido M.J.M. Roemen, Britt L.J. van Herpen, Zoë Dekkers, Bas Heitzer, Daphne J.G. Leunissen, Laura Moonen, Ragnar Lunde, Marcel Westenend, Marjolein van
Frontiers in Oncology.2023;[Epub] CrossRef - BMAL1 induces colorectal cancer metastasis by stimulating exosome secretion
Pengjuan Dong, Yaping Wang, Yutong Liu, Chunting Zhu, Jiaxin Lin, Ruizhe Qian, Luchun Hua, Chao Lu
Molecular Biology Reports.2022; 49(1): 373. CrossRef - Chemokines network in bone metastasis: Vital regulators of seeding and soiling
Gunjan Sharma, Ramesh Pothuraju, Ranjana Kumari Kanchan, Surinder Kumar Batra, Jawed Akhtar Siddiqui
Seminars in Cancer Biology.2022; 86: 457. CrossRef - The Signaling Pathways Associated With Breast Cancer Bone Metastasis
Xuelian Song, Changran Wei, Xiangqi Li
Frontiers in Oncology.2022;[Epub] CrossRef - Effects of 8-week noncontinuous aerobic exercise on the levels of CCL2, CCL5, and their respective receptors in female BALB/C mice suffering from breast cancer
Mehrnoosh Esmailiyan, Mehdi Kargarfard, Fahimeh Esfarjani, Golnaz Vaseghi
International Journal of Preventive Medicine.2022; 13(1): 55. CrossRef - Non‐coding RNAs in bone remodelling and bone metastasis: Mechanisms of action and translational relevance
Margherita Puppo, Hanna Taipaleenmäki, Eric Hesse, Philippe Clézardin
British Journal of Pharmacology.2021; 178(9): 1936. CrossRef - Sympathetic activity in breast cancer and metastasis: partners in crime
Francisco Conceição, Daniela M. Sousa, Joana Paredes, Meriem Lamghari
Bone Research.2021;[Epub] CrossRef - Extracellular Vesicles in Tumors: A Potential Mediator of Bone Metastasis
Shenglong Li, Wei Wang
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - Bone marrow niches in the regulation of bone metastasis
Fenfang Chen, Yujiao Han, Yibin Kang
British Journal of Cancer.2021; 124(12): 1912. CrossRef - Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer
Kerui Wu, Jiamei Feng, Feng Lyu, Fei Xing, Sambad Sharma, Yin Liu, Shih-Ying Wu, Dan Zhao, Abhishek Tyagi, Ravindra Pramod Deshpande, Xinhong Pei, Marco Gabril Ruiz, Hiroyuki Takahashi, Shunsuke Tsuzuki, Takahiro Kimura, Yin-yuan Mo, Yusuke Shiozawa, Ravi
Nature Communications.2021;[Epub] CrossRef - Circulating Osteocalcin-Positive Cells as a Novel Diagnostic Biomarker for Bone Metastasis in Breast Cancer Patients
Kyung-Hun Lee, Kyoung Jin Lee, Tae-Yong Kim, Febby Hutomo, Hyun Jin Sun, Gi Jeong Cheon, Serk In Park, Sun Wook Cho, Seock-Ah Im
Journal of Bone and Mineral Research.2020; 35(10): 1838. CrossRef - Polymer nanomedicines
Jindřich Kopeček, Jiyuan Yang
Advanced Drug Delivery Reviews.2020; 156: 40. CrossRef - Osteolytic metastasis in breast cancer: effective prevention strategies
Chandi C Mandal
Expert Review of Anticancer Therapy.2020; 20(9): 797. CrossRef - The Tumor Microenvironment of Primitive and Metastatic Breast Cancer: Implications for Novel Therapeutic Strategies
Giovanni Zarrilli, Gianluca Businello, Maria Vittoria Dieci, Silvia Paccagnella, Valentina Carraro, Rocco Cappellesso, Federica Miglietta, Gaia Griguolo, Valentina Guarneri, Marcello Lo Mele, Matteo Fassan
International Journal of Molecular Sciences.2020; 21(21): 8102. CrossRef - Identification and validation of DOCK4 as a potential biomarker for risk of bone metastasis development in patients with early breast cancer
Jules A Westbrook, Steven L Wood, David A Cairns, Kathryn McMahon, Renu Gahlaut, Helene Thygesen, Mike Shires, Stephanie Roberts, Helen Marshall, Maria R Oliva, Mark J Dunning, Andrew M Hanby, Peter J Selby, Valerie Speirs, Georgia Mavria, Robert E Colema
The Journal of Pathology.2019; 247(3): 381. CrossRef - The Relationship between Exosomes and Cancer: Implications for Diagnostics and Therapeutics
Wendy W. Weston, Timothy Ganey, H. Thomas Temple
BioDrugs.2019; 33(2): 137. CrossRef - In Vivo Assessment of VCAM-1 Expression by SPECT/CT Imaging in Mice Models of Human Triple Negative Breast Cancer
Montemagno, Dumas, Cavaillès, Ahmadi, Bacot, Debiossat, Soubies, Djaïleb, Leenhardt, Leiris, Dufies, Pagès, Hernot, Devoogdt, Perret, Riou, Fagret, Ghezzi, Broisat
Cancers.2019; 11(7): 1039. CrossRef - Notch and breast cancer metastasis: Current knowledge, new sights and targeted therapy (Review)
Yu Zhang, Zi‑Yan Xie, Xuan‑Tong Guo, Xing‑Hua Xiao, Li‑Xia Xiong
Oncology Letters.2019;[Epub] CrossRef - NPNT promotes early-stage bone metastases in breast cancer by regulation of the osteogenic niche
Dongsheng Wang, Chenglong Zhao, Liangliang Gao, Yao Wang, Xin Gao, Liang Tang, Kun Zhang, Zhenxi Li, Jing Han, Jianru Xiao
Journal of Bone Oncology.2018; 13: 91. CrossRef - Placental exosomes: A proxy to understand pregnancy complications
Jin Jin, Ramkumar Menon
American Journal of Reproductive Immunology.2018;[Epub] CrossRef - Understanding the Bone in Cancer Metastasis
Jaime Fornetti, Alana L Welm, Sheila A Stewart
Journal of Bone and Mineral Research.2018; 33(12): 2099. CrossRef - Role of Tumor-Derived Chemokines in Osteolytic Bone Metastasis
Salvatore J. Coniglio
Frontiers in Endocrinology.2018;[Epub] CrossRef - The role of exosomes in cancer metastasis
Teresa Bernadette Steinbichler, József Dudás, Herbert Riechelmann, Ira-Ida Skvortsova
Seminars in Cancer Biology.2017; 44: 170. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Impaired Bone Matrix Alignment Induced by Breast Cancer Metastasis
Aiko Sekita, Aira Matsugaki, Takayoshi Nakano
Journal of the Japan Institute of Metals.2017; 81(6): 308. CrossRef - SMURF2 regulates bone homeostasis by disrupting SMAD3 interaction with vitamin D receptor in osteoblasts
Zhan Xu, Matthew B. Greenblatt, Guang Yan, Heng Feng, Jun Sun, Sutada Lotinun, Nicholas Brady, Roland Baron, Laurie H. Glimcher, Weiguo Zou
Nature Communications.2017;[Epub] CrossRef - Bone Microenvironment and Role of Rank-Rankl-Opg in Breast Cancer Metastasis in Bone
Hongwei Zhang
Journal of Cancer Prevention & Current Research.2017;[Epub] CrossRef - Tumor–Stroma Interactions in Bone Metastasis: Molecular Mechanisms and Therapeutic Implications
Hanqiu Zheng, Wenyang Li, Yibin Kang
Cold Spring Harbor Symposia on Quantitative Biology.2016; 81: 151. CrossRef - Breast cancer cells obtain an osteomimetic featureviaepithelial-mesenchymal transition that have undergone BMP2/RUNX2 signaling pathway induction
Cong-Cong Tan, Gui-Xi Li, Li-Duan Tan, Xin Du, Xiao-Qing Li, Rui He, Qing-Shan Wang, Yu-Mei Feng
Oncotarget.2016; 7(48): 79688. CrossRef - Heterotypic models of osteosarcoma recapitulate tumor heterogeneity and biological behavior
Milcah C. Scott, Hirotaka Tomiyasu, John R. Garbe, Ingrid Cornax, Clarissa Amaya, M Gerard O'Sullivan, Subbaya Subramanian, Brad A. Bryan, Jaime F. Modiano
Disease Models & Mechanisms.2016;[Epub] CrossRef - Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
Hyo Min Jeong, Sun Wook Cho, Serk In Park
Endocrinology and Metabolism.2016; 31(4): 485. CrossRef
- Osteoclast Cancer Cell Metabolic Cross-talk Confers PARP Inhibitor Resistance in Bone Metastatic Breast Cancer

- Endocrine Research
- The Role of Nuclear Factor-E2-Related Factor 1 in the Oxidative Stress Response in MC3T3-E1 Osteoblastic Cells
- So Young Park, Sung Hoon Kim, Hyun Koo Yoon, Chang Hoon Yim, Sung-Kil Lim
- Endocrinol Metab. 2016;31(2):336-342. Published online April 25, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.336
- 3,947 View
- 61 Download
- 10 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Reactive oxygen species (ROS) and antioxidants are associated with maintenance of cellular function and metabolism. Nuclear factor-E2-related factor 1 (NFE2L1, Nrf1) is known to regulate the expression of a number of genes involved in oxidative stress and inflammation. The purpose of this study was to examine the effects of NFE2L1 on the response to oxidative stress in osteoblastic MC3T3-E1 cells.
Methods The murine calvaria-derived MC3T3-E1 cell line was exposed to lipopolysaccharide (LPS) for oxidative stress induction. NFE2L1 effects were evaluated using small interfering RNA (siRNA) for
NFE2L1 mRNA. ROS generation and the levels of known antioxidant enzyme genes were assayed.Results NFE2L1 expression was significantly increased 2.4-fold compared to the control group at 10 µg/mL LPS in MC3T3-E1 cells (P <0.05). LPS increased formation of intracellular ROS in MC3T3-E1 cells.NFE2L1 knockdown led to an additional increase of ROS (20%) in the group transfected withNFE2L1 siRNA compared with the control group under LPS stimulation (P <0.05). RNA interference ofNFE2L1 suppressed the expression of antioxidant genes including metallothionein 2, glutamatecysteine ligase catalytic subunit, and glutathione peroxidase 1 in LPS-treated MC3T3-E1 cells.Conclusion Our results suggest that NFE2L1 may have a distinct role in the regulation of antioxidant enzymes under inflammation-induced oxidative stress in MC3T3-E1 osteoblastic cells.
-
Citations
Citations to this article as recorded by- SDH5 down-regulation mitigates the damage of osteoporosis via inhibiting the MyD88/NF-κB signaling pathway
Hongzi Wu, Dehua Zhang, Haijun Xia, Yongqi Li, Feng Mao, Yi Liao
Immunopharmacology and Immunotoxicology.2023; 45(3): 317. CrossRef - N-acetyl Cysteine Inhibits Cell Proliferation and Differentiation of LPSInduced MC3T3-E1 Cells Via Regulating Inflammatory Cytokines
Wangyang Li, Hui Zhang, Junchi Chen, Yujie Tan, Ailing Li, Ling Guo
Current Pharmaceutical Biotechnology.2023; 24(3): 450. CrossRef - Unravelling the role of NFE2L1 in stress responses and related diseases
Xingzhu Liu, Chang Xu, Wanglong Xiao, Nianlong Yan
Redox Biology.2023; 65: 102819. CrossRef - Nfe2l1 deficiency mitigates streptozotocin-induced pancreatic β-cell destruction and development of diabetes in male mice
Simeng Bao, Hongzhi Zheng, Chengjie Chen, Yuhang Zhang, Lina Bao, Bei Yang, Yongyong Hou, Yanyan Chen, Qiang Zhang, Jingbo Pi, Jingqi Fu
Food and Chemical Toxicology.2021; 158: 112633. CrossRef - Long isoforms of NRF1 negatively regulate adipogenesis via suppression of PPARγ expression
Peng Xue, Yongyong Hou, Zhuo Zuo, Zhendi Wang, Suping Ren, Jian Dong, Jingqi Fu, Huihui Wang, Melvin E. Andersen, Qiang Zhang, Yuanyuan Xu, Jingbo Pi
Redox Biology.2020; 30: 101414. CrossRef - Protracted rosiglitazone treatment exacerbates inflammation in white adipose tissues of adipocyte-specific Nfe2l1 knockout mice
Suping Ren, Yongyong Hou, Zhuo Zuo, Zhiyuan Liu, Huihui Wang, Yuanyuan Xu, Masayuki Yamamoto, Qiang Zhang, Jingqi Fu, Jingbo Pi
Food and Chemical Toxicology.2020; 146: 111836. CrossRef - Nrf1 is paved as a new strategic avenue to prevent and treat cancer, neurodegenerative and other diseases
Jianxin Yuan, Shuwei Zhang, Yiguo Zhang
Toxicology and Applied Pharmacology.2018; 360: 273. CrossRef - Silencing of long isoforms of nuclear factor erythroid 2 like 1 primes macrophages towards M1 polarization
Huihui Wang, Jiayu Zhu, Zhiyuan Liu, Hang Lv, Peng Lv, Feng Chen, Jingqi Fu, Yongyong Hou, Rui Zhao, Yuanyuan Xu, Qiang Zhang, Jingbo Pi
Free Radical Biology and Medicine.2018; 117: 37. CrossRef - Costunolide increases osteoblast differentiation via ATF4-dependent HO-1 expression in C3H10T1/2 cells
Wan-Jin Jeon, Kyeong-Min Kim, Eun-Jung Kim, Won-Gu Jang
Life Sciences.2017; 178: 94. CrossRef
- SDH5 down-regulation mitigates the damage of osteoporosis via inhibiting the MyD88/NF-κB signaling pathway


 KES
KES

 First
First Prev
Prev



