Search
- Page Path
- HOME > Search
Review Articles
- Miscellaneous
- Toward Systems-Level Metabolic Analysis in Endocrine Disorders and Cancer
- Aliya Lakhani, Da Hyun Kang, Yea Eun Kang, Junyoung O. Park
- Endocrinol Metab. 2023;38(6):619-630. Published online November 21, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1814
- 2,522 View
- 111 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
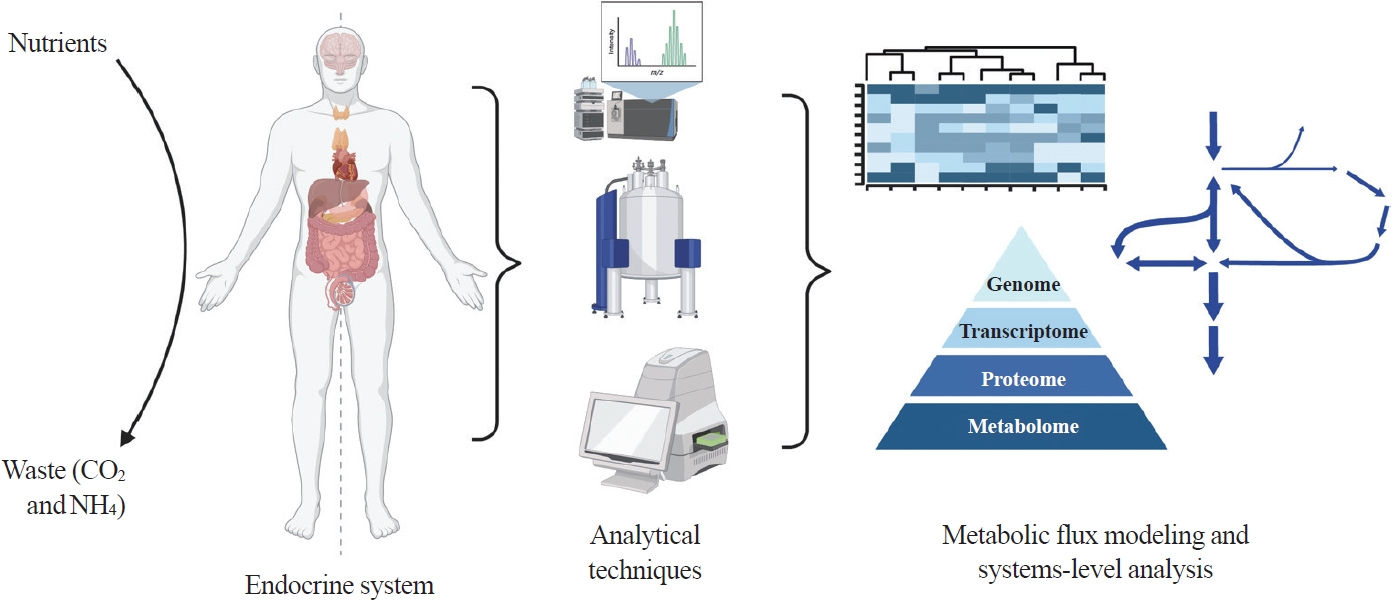
ePub - Metabolism is a dynamic network of biochemical reactions that support systemic homeostasis amidst changing nutritional, environmental, and physical activity factors. The circulatory system facilitates metabolite exchange among organs, while the endocrine system finely tunes metabolism through hormone release. Endocrine disorders like obesity, diabetes, and Cushing’s syndrome disrupt this balance, contributing to systemic inflammation and global health burdens. They accompany metabolic changes on multiple levels from molecular interactions to individual organs to the whole body. Understanding how metabolic fluxes relate to endocrine disorders illuminates the underlying dysregulation. Cancer is increasingly considered a systemic disorder because it not only affects cells in localized tumors but also the whole body, especially in metastasis. In tumorigenesis, cancer-specific mutations and nutrient availability in the tumor microenvironment reprogram cellular metabolism to meet increased energy and biosynthesis needs. Cancer cachexia results in metabolic changes to other organs like muscle, adipose tissue, and liver. This review explores the interplay between the endocrine system and systems-level metabolism in health and disease. We highlight metabolic fluxes in conditions like obesity, diabetes, Cushing’s syndrome, and cancers. Recent advances in metabolomics, fluxomics, and systems biology promise new insights into dynamic metabolism, offering potential biomarkers, therapeutic targets, and personalized medicine.
-
Citations
Citations to this article as recorded by- Editorial: Tumor metabolism and programmed cell death
Dan-Lan Pu, Qi-Nan Wu
Frontiers in Endocrinology.2024;[Epub] CrossRef
- Editorial: Tumor metabolism and programmed cell death

- Calcium & bone metabolism
- Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis
- Brendan F. Boyce, Jinbo Li, Zhenqiang Yao, Lianping Xing
- Endocrinol Metab. 2023;38(5):504-521. Published online September 26, 2023
- DOI: https://doi.org/10.3803/EnM.2023.501
- 2,205 View
- 101 Download
- 3 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
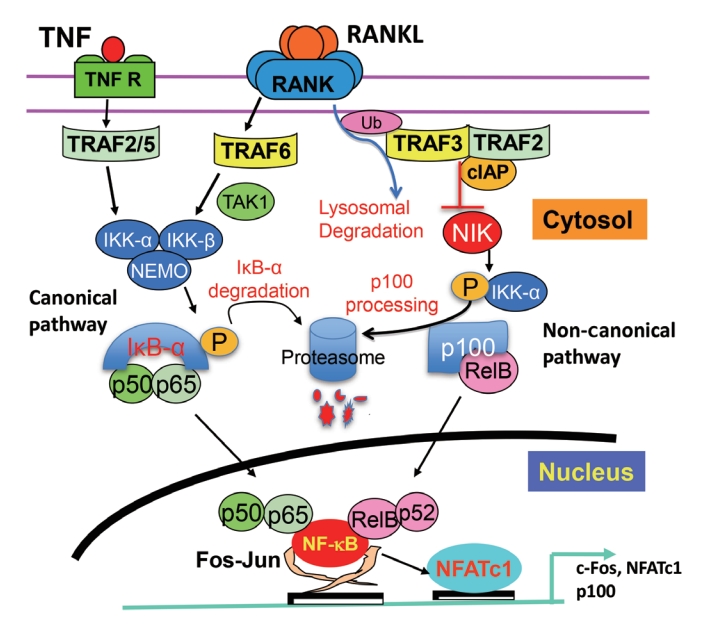
ePub - Maintenance of skeletal integrity requires the coordinated activity of multinucleated bone-resorbing osteoclasts and bone-forming osteoblasts. Osteoclasts form resorption lacunae on bone surfaces in response to cytokines by fusion of precursor cells. Osteoblasts are derived from mesenchymal precursors and lay down new bone in resorption lacunae during bone remodeling. Nuclear factorkappa B (NF-κB) signaling regulates osteoclast and osteoblast formation and is activated in osteoclast precursors in response to the essential osteoclastogenic cytokine, receptor activator of NF-κB ligand (RANKL), which can also control osteoblast formation through RANK-RANKL reverse signaling in osteoblast precursors. RANKL and some pro-inflammatory cytokines, including tumor necrosis factor (TNF), activate NF-κB signaling to positively regulate osteoclast formation and functions. However, these cytokines also limit osteoclast and osteoblast formation through NF-κB signaling molecules, including TNF receptor-associated factors (TRAFs). TRAF6 mediates RANKL-induced osteoclast formation through canonical NF-κB signaling. In contrast, TRAF3 limits RANKL- and TNF-induced osteoclast formation, and it restricts transforming growth factor β (TGFβ)-induced inhibition of osteoblast formation in young and adult mice. During aging, neutrophils expressing TGFβ and C-C chemokine receptor type 5 (CCR5) increase in bone marrow of mice in response to increased NF-κB-induced CC motif chemokine ligand 5 (CCL5) expression by mesenchymal progenitor cells and injection of these neutrophils into young mice decreased bone mass. TGFβ causes degradation of TRAF3, resulting in decreased glycogen synthase kinase-3β/β-catenin-mediated osteoblast formation and age-related osteoporosis in mice. The CCR5 inhibitor, maraviroc, prevented accumulation of TGFβ+/CCR5+ neutrophils in bone marrow and increased bone mass by inhibiting bone resorption and increasing bone formation in aged mice. This paper updates current understanding of how NF-κB signaling is involved in the positive and negative regulation of cytokine-mediated osteoclast and osteoblast formation and activation with a focus on the role of TRAF3 signaling, which can be targeted therapeutically to enhance bone mass.
-
Citations
Citations to this article as recorded by- The Role of Rosavin in the Pathophysiology of Bone Metabolism
Piotr Wojdasiewicz, Paweł Turczyn, Anna Lach-Gruba, Łukasz A. Poniatowski, Daryush Purrahman, Mohammad-Reza Mahmoudian-Sani, Dariusz Szukiewicz
International Journal of Molecular Sciences.2024; 25(4): 2117. CrossRef - The role of monocyte/macrophage chemokines in pathogenesis of osteoarthritis: A review
Hao Luo, Linfeng Li, Song Han, Tao Liu
International Journal of Immunogenetics.2024;[Epub] CrossRef - The effect of low-level laser therapy on osteoclast differentiation: Clinical implications for tooth movement and bone density
Chun-Yi Huang, Huynh Hoai Thuong Le, Hsiao-Chi Tsai, Chih-Hsin Tang, Jian-Hong Yu
Journal of Dental Sciences.2024;[Epub] CrossRef - Genetic Deficiency of the Long Pentraxin 3 Affects Osteogenesis and Osteoclastogenesis in Homeostatic and Inflammatory Conditions
Valentina Granata, Dario Strina, Maria Lucia Schiavone, Barbara Bottazzi, Alberto Mantovani, Antonio Inforzato, Cristina Sobacchi
International Journal of Molecular Sciences.2023; 24(23): 16648. CrossRef
- The Role of Rosavin in the Pathophysiology of Bone Metabolism

- Calcium & bone metabolism
- Skeletal Senescence with Aging and Type 2 Diabetes
- Joshua Nicholas Farr
- Endocrinol Metab. 2023;38(3):295-301. Published online June 14, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1727

- 2,727 View
- 127 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Osteoporosis and type 2 diabetes (T2D) are common diseases that often coexist. While both of these diseases are associated with poor bone quality and increased fracture risk, their pathogenesis of increased fracture risk differs and is multifactorial. Mounting evidence now indicates that key fundamental mechanisms that are central to both aging and energy metabolism exist. Importantly, these mechanisms represent potentially modifiable therapeutic targets for interventions that could prevent or alleviate multiple complications of osteoporosis and T2D, including poor bone quality. One such mechanism that has gained increasing momentum is senescence, which is a cell fate that contributes to multiple chronic diseases. Accumulating evidence has established that numerous boneresident cell types become susceptible to cellular senescence with old age. Recent work also demonstrates that T2D causes the premature accumulation of senescent osteocytes during young adulthood, at least in mice, although it remains to be seen which other bone-resident cell types become senescent with T2D. Given that therapeutically removing senescent cells can alleviate age-related bone loss and T2D-induced metabolic dysfunction, it will be important in future studies to rigorously test whether interventions that eliminate senescent cells can also alleviate skeletal dysfunction in context of T2D, as it does with aging.
-
Citations
Citations to this article as recorded by- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Cellular Neuroscience.2024;[Epub] CrossRef - The synergistic effect of diabetes mellitus and osteoporosis on the all-cause mortality: a cohort study of an American population
Weihua Li, Siyu Xie, Shengdong Zhong, Liting Lan
Frontiers in Endocrinology.2024;[Epub] CrossRef - Identification of systemic biomarkers and potential drug targets for age-related macular degeneration
Shizhen Lei, Mang Hu, Zhongtao Wei
Frontiers in Aging Neuroscience.2024;[Epub] CrossRef
- Single-cell sequencing reveals an important role of SPP1 and microglial activation in age-related macular degeneration

- Miscellaneous
- Brown Adipose Tissue: Activation and Metabolism in Humans
- Imane Hachemi, Mueez U-Din
- Endocrinol Metab. 2023;38(2):214-222. Published online March 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1659
- 6,131 View
- 454 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
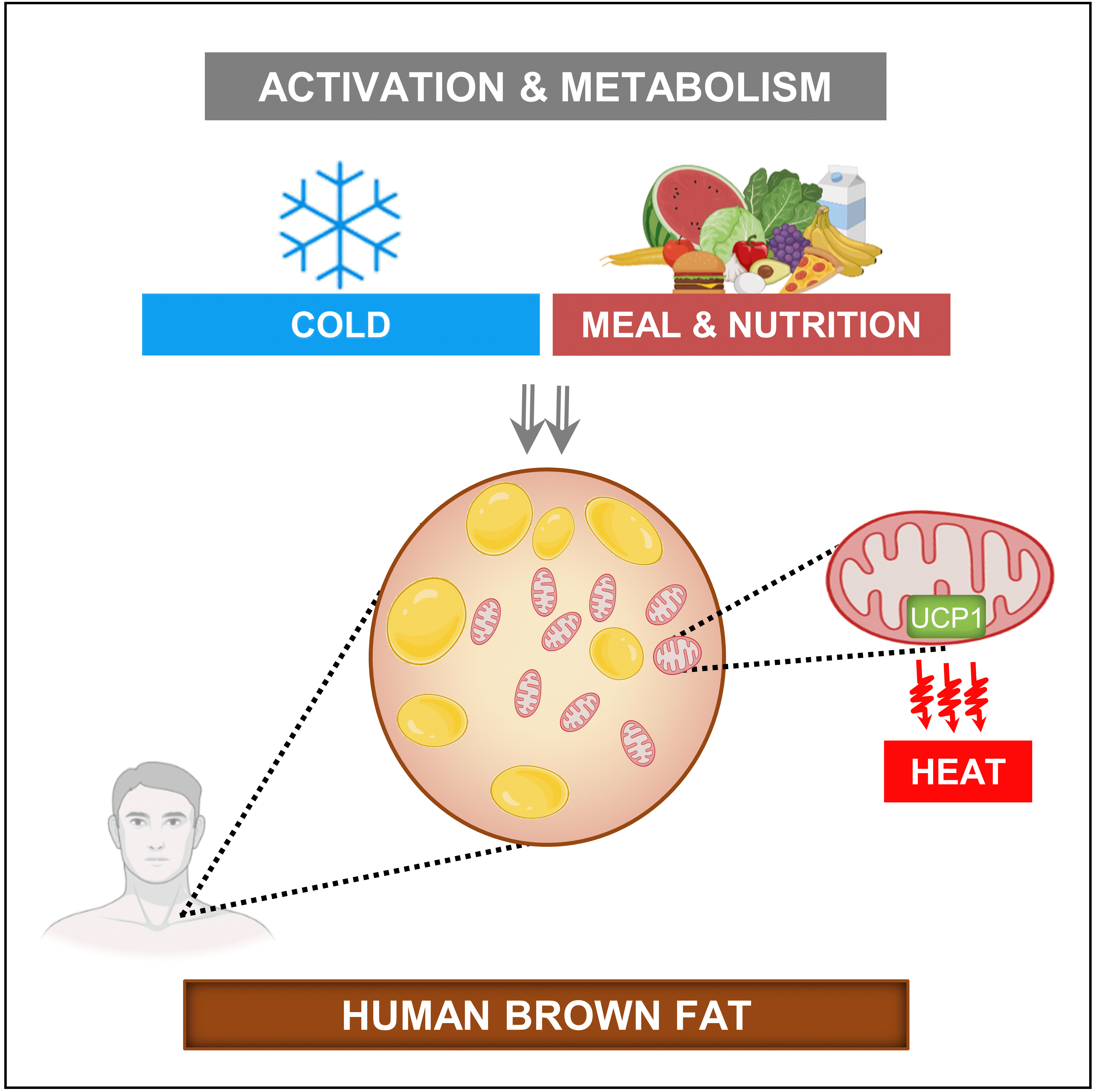
ePub - Brown adipose tissue (BAT) is a thermogenic organ contributing to non-shivering thermogenesis. BAT becomes active under cold stress via sympathetic nervous system activation. However, recent evidence has suggested that BAT may also be active at thermoneutrality and in a postprandial state. BAT has superior energy dissipation capacity compared to white adipose tissue (WAT) and muscles. Thus, it has been proposed that the recruitment and activation of additional BAT may increase the overall energy-expending capacity in humans, potentially improving current whole-body weight management strategies. Nutrition plays a central role in obesity and weight management. Thus, this review discusses human studies describing BAT hyper-metabolism after dietary interventions. Nutritional agents that can potentially recruit brown adipocytes via the process of BAT-WAT transdifferentiation are also discussed.
-
Citations
Citations to this article as recorded by- Spermidine activates adipose tissue thermogenesis through autophagy and fibroblast growth factor 21
Yinhua Ni, Liujie Zheng, Liqian Zhang, Jiamin Li, Yuxiang Pan, Haimei Du, Zhaorong Wang, Zhengwei Fu
The Journal of Nutritional Biochemistry.2024; 125: 109569. CrossRef - A natural sustained-intestinal release formulation of red chili pepper extracted capsaicinoids (Capsifen®) safely modulates energy balance and endurance performance: a randomized, double-blind, placebo-controlled study
N. Roopashree, Das S. Syam, I. M. Krishnakumar, K. N. Mala, Bradley S. Fleenor, Jestin Thomas
Frontiers in Nutrition.2024;[Epub] CrossRef - MRI Methods to Visualize and Quantify Adipose Tissue in Health and Disease
Katerina Nikiforaki, Kostas Marias
Biomedicines.2023; 11(12): 3179. CrossRef
- Spermidine activates adipose tissue thermogenesis through autophagy and fibroblast growth factor 21

- Calcium & Bone Metabolism
- Update on Preoperative Parathyroid Localization in Primary Hyperparathyroidism
- Hye-Sun Park, Namki Hong, Jong Ju Jeong, Mijin Yun, Yumie Rhee
- Endocrinol Metab. 2022;37(5):744-755. Published online October 25, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1589

- 4,126 View
- 357 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Parathyroidectomy is the treatment of choice for primary hyperparathyroidism when the clinical criteria are met. Although bilateral neck exploration is traditionally the standard method for surgery, minimally invasive parathyroidectomy (MIP), or focused parathyroidectomy, has been widely accepted with comparable curative outcomes. For successful MIP, accurate preoperative localization of parathyroid lesions is essential. However, no consensus exists on the optimal approach for localization. Currently, ultrasonography and technetium-99m-sestamibi–single photon emission computed tomography/computed tomography are widely accepted in most cases. However, exact localization cannot always be achieved, especially in cases with multiglandular disease, ectopic glands, recurrent disease, and normocalcemic primary hyperparathyroidism. Therefore, new modalities for preoperative localization have been developed and evaluated. Positron emission tomography/computed tomography and parathyroid venous sampling have demonstrated improvements in sensitivity and accuracy. Both anatomical and functional information can be obtained by combining these methods. As each approach has its advantages and disadvantages, the localization study should be deliberately chosen based on each patient’s clinical profile, costs, radiation exposure, and the availability of experienced experts. In this review, we summarize various methods for the localization of hyperfunctioning parathyroid tissues in primary hyperparathyroidism.
-
Citations
Citations to this article as recorded by- Expression of the Calcium-Sensing Receptor on Normal and Abnormal Parathyroid and Thyroid Tissue
Anne L. Worth, Mesrop Ayrapetyan, Susan J. Maygarden, Zibo Li, Zhanhong Wu, Chris B. Agala, Lawrence T. Kim
Journal of Surgical Research.2024; 293: 618. CrossRef - Use of [18F]fluorocholine PET/CT in the detection of primary hyperparathyroidism in paediatrics: a case report
Helena Martínez Sánchez, Francisca Moreno Macián, Sara León Cariñena, Carmen de Mingo Alemany, Lidia Blasco González, Raquel Sánchez Vañó
Journal of Pediatric Endocrinology and Metabolism.2024;[Epub] CrossRef - A Rare Case of Hyperfunctioning Lipoadenoma Presenting as a Cystic Pararthyroid Lesion
Jinyoung Kim, Ohjoon Kwon, Tae-Jung Kim, So Lyung Jung, Eun Ji Han, Ki-Ho Song
Journal of Bone Metabolism.2023; 30(2): 201. CrossRef - Role of 18F-Fluorocholine Positron Emission Tomography (PET)/Computed Tomography (CT) in Diagnosis of Elusive Parathyroid Adenoma
Janan R Badier, Pokhraj P Suthar, Jagadeesh S Singh, Miral D Jhaveri
Cureus.2023;[Epub] CrossRef - Pitfalls of DualTracer 99m-Technetium (Tc) Pertechnetate and Sestamibi Scintigraphy before Parathyroidectomy: Between Primary-Hyperparathyroidism-Associated Parathyroid Tumour and Ectopic Thyroid Tissue
Mara Carsote, Mihaela Stanciu, Florina Ligia Popa, Oana-Claudia Sima, Eugenia Petrova, Anca-Pati Cucu, Claudiu Nistor
Medicina.2023; 60(1): 15. CrossRef - Diagnostic Performance of Magnetic Resonance Imaging for Parathyroid Localization of Primary Hyperparathyroidism: A Systematic Review
Max H. M. C. Scheepers, Zaid Al-Difaie, Lloyd Brandts, Andrea Peeters, Bjorn Winkens, Mahdi Al-Taher, Sanne M. E. Engelen, Tim Lubbers, Bas Havekes, Nicole D. Bouvy, Alida A. Postma
Diagnostics.2023; 14(1): 25. CrossRef
- Expression of the Calcium-Sensing Receptor on Normal and Abnormal Parathyroid and Thyroid Tissue

Original Articles
- Diabetes, Obesity and Metabolism
- High Cardiorespiratory Fitness Protects against Molecular Impairments of Metabolism, Heart, and Brain with Higher Efficacy in Obesity-Induced Premature Aging
- Patcharapong Pantiya, Chanisa Thonusin, Natticha Sumneang, Benjamin Ongnok, Titikorn Chunchai, Sasiwan Kerdphoo, Thidarat Jaiwongkam, Busarin Arunsak, Natthaphat Siri-Angkul, Sirawit Sriwichaiin, Nipon Chattipakorn, Siriporn C. Chattipakorn
- Endocrinol Metab. 2022;37(4):630-640. Published online August 5, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1430
- 4,098 View
- 122 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
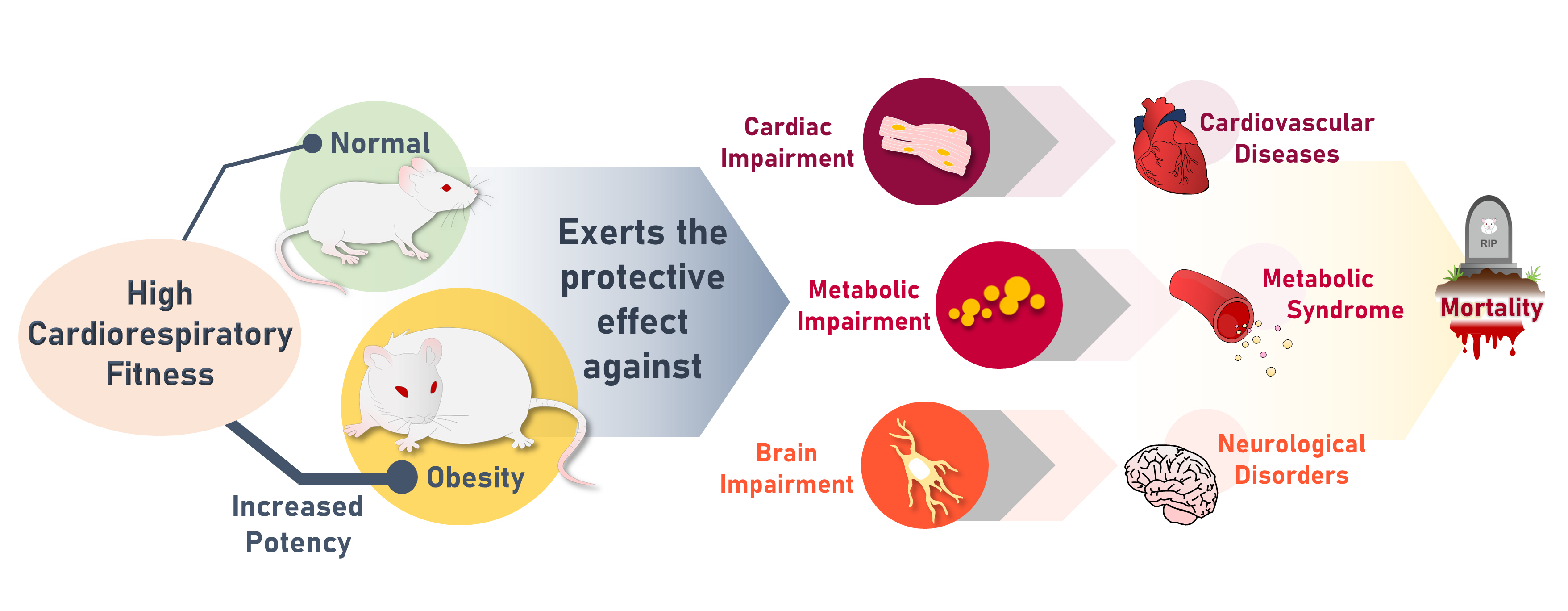
High cardiorespiratory fitness (CRF) protects against age-related diseases. However, the mechanisms mediating the protective effect of high intrinsic CRF against metabolic, cardiac, and brain impairments in non-obese versus obese conditions remain incompletely understood. We aimed to identify the mechanisms through which high intrinsic CRF protects against metabolic, cardiac, and brain impairments in non-obese versus obese untrained rats.
Methods
Seven-week-old male Wistar rats were divided into two groups (n=8 per group) to receive either a normal diet or a highfat diet (HFD). At weeks 12 and 28, CRF, carbohydrate and fatty acid oxidation, cardiac function, and metabolic parameters were evaluated. At week 28, behavior tests were performed. At the end of week 28, rats were euthanized to collect heart and brain samples for molecular studies.
Results
The obese rats exhibited higher values for aging-related parameters than the non-obese rats, indicating that they experienced obesity-induced premature aging. High baseline CRF levels were positively correlated with several favorable metabolic, cardiac, and brain parameters at follow-up. Specifically, the protective effects of high CRF against metabolic, cardiac, and brain impairments were mediated by the modulation of body weight and composition, the lipid profile, substrate oxidation, mitochondrial function, insulin signaling, autophagy, apoptosis, inflammation, oxidative stress, cardiac function, neurogenesis, blood-brain barrier, synaptic function, accumulation of Alzheimer’s disease-related proteins, and cognition. Interestingly, this effect was more obvious in HFD-fed rats.
Conclusion
The protective effect of high CRF is mediated by the modulation of several mechanisms. These effects exhibit greater efficacy under conditions of obesity-induced premature aging. -
Citations
Citations to this article as recorded by- Associations that Cardiorespiratory Fitness and Body Mass Index Loss Have with Deficit Accumulation Frailty
KAYLONI OLSON, DENISE K. HOUSTON, JOHNATHAN ROSS, RENA R. WING, FELICIA R. SIMPSON, AMBARISH PANDEY, MICHAEL P. WALKUP, MIA YANG, MARK A. ESPELAND
Medicine & Science in Sports & Exercise.2024; 56(4): 717. CrossRef - Interplay between obesity and aging on myocardial geometry and function: Role of leptin-STAT3-stress signaling
Wei Jin, Fei Tu, Feng Dong, Qinqin Deng, Miyesaier Abudureyimu, Wei Yu, Guo-jun Cai, Jian-ming Pei, Zhaohui Pei, Jun Ren
Biochimica et Biophysica Acta (BBA) - General Subjects.2023; 1867(2): 130281. CrossRef - Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings
Jaime A. Gallo-Villegas, Juan C. Calderón
European Journal of Applied Physiology.2023; 123(5): 945. CrossRef
- Associations that Cardiorespiratory Fitness and Body Mass Index Loss Have with Deficit Accumulation Frailty

- Diabetes, Obesity and Metabolism
- The Impact of Insulin Resistance on Hepatic Fibrosis among United States Adults with Non-Alcoholic Fatty Liver Disease: NHANES 2017 to 2018
- Ji Cheol Bae, Lauren A. Beste, Kristina M. Utzschneider
- Endocrinol Metab. 2022;37(3):455-465. Published online June 21, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1434
- 4,239 View
- 136 Download
- 9 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We aimed to investigate the association of hepatic steatosis with liver fibrosis and to assess the interactive effects of hepatic steatosis and insulin resistance on liver fibrosis in a nationally representative sample of United States adults.
Methods
We conducted a cross-sectional analysis using data from National Health and Nutrition Examination Survey 2017 to 2018, which for the first time included transient elastography to assess liver stiffness and hepatic steatosis. We evaluated the association between hepatic steatosis (using controlled attenuation parameter [CAP]) and clinically significant liver fibrosis (defined as liver stiffness ≥7.5 kPa) using logistic regression with an interaction term for hepatic steatosis and insulin resistance (defined as homeostatic model assessment of insulin resistance ≥3.0).
Results
Among adults undergoing transient elastography (n=2,023), 45.9% had moderate or greater hepatic steatosis and 11.3% had clinically significant liver fibrosis. After adjustment for demographic and metabolic factors, the odds of significant liver fibrosis increased as CAP score rose (odds ratio, 1.35 per standard deviation increment; 95% confidence interval, 1.11 to 1.64). We detected a significant interaction effect between CAP score and insulin resistance on the probability of significant liver fibrosis (P=0.016 for interaction). The probability of significant liver fibrosis increased in the presence of insulin resistance with increasing CAP score, while those without insulin resistance had low probability of significant liver fibrosis, even with high CAP scores.
Conclusion
Individuals with hepatic steatosis had higher odds of fibrosis when insulin resistance was present. Our findings emphasize the importance of the metabolic aspects of the disease on fibrosis risk and suggest a need to better identify patients with metabolic associated fatty liver disease. -
Citations
Citations to this article as recorded by- Association of insulin resistance indicators with hepatic steatosis and fibrosis in patients with metabolic syndrome
Tzu-chia Kuo, Yang-bor Lu, Chieh-lun Yang, Bin Wang, Lin-xin Chen, Ching-ping Su
BMC Gastroenterology.2024;[Epub] CrossRef - No More NAFLD: The Term Is Now MASLD
Ji Cheol Bae
Endocrinology and Metabolism.2024; 39(1): 92. CrossRef - Insulin Resistance/Sensitivity Measures as Screening Indicators of Metabolic-Associated Fatty Liver Disease and Liver Fibrosis
Mohammad E. Khamseh, Mojtaba Malek, Soodeh Jahangiri, Sohrab Nobarani, Azita Hekmatdoost, Marieh Salavatizadeh, Samira Soltanieh, Haleh Chehrehgosha, Hoda Taheri, Zeinab Montazeri, Fereshteh Attaran, Faramarz Ismail-Beigi, Fariba Alaei-Shahmiri
Digestive Diseases and Sciences.2024;[Epub] CrossRef - The association of Neuromedin U levels and non-alcoholic fatty liver disease: A comparative analysis
Murat Keskin, Sercan Avul, Aylin Beyaz, Nizameddin Koca
Heliyon.2024; 10(5): e27291. CrossRef - Oral Insulin Alleviates Liver Fibrosis and Reduces Liver Steatosis in Patients With Metabolic Dysfunction-associated Steatohepatitis and Type 2 Diabetes: Results of Phase II Randomized, Placebo-controlled Feasibility Clinical Trial
Yuval Ishay, Joel Neutel, Yotam Kolben, Ram Gelman, Orly Sneh Arbib, Oliver Lopez, Helena Katchman, Rizwana Mohseni, Miriam Kidron, Yaron Ilan
Gastro Hep Advances.2024; 3(3): 417. CrossRef - Comparative and Predictive Significance of Serum Leptin Levels in Non-alcoholic Fatty Liver Disease
Mehwish Qamar, Abeer Fatima, Ambreen Tauseef, Muhammad I Yousufzai, Ibrahim Liaqat, Qanbar Naqvi
Cureus.2024;[Epub] CrossRef - Greater Severity of Steatosis Is Associated with a Higher Risk of Incident Diabetes: A Retrospective Longitudinal Study
Ji Min Han, Jung Hwan Cho, Hye In Kim, Sunghwan Suh, Yu-Ji Lee, Jung Won Lee, Kwang Min Kim, Ji Cheol Bae
Endocrinology and Metabolism.2023; 38(4): 418. CrossRef - Hepatic T-cell senescence and exhaustion are implicated in the progression of fatty liver disease in patients with type 2 diabetes and mouse model with nonalcoholic steatohepatitis
Byeong Chang Sim, Yea Eun Kang, Sun Kyoung You, Seong Eun Lee, Ha Thi Nga, Ho Yeop Lee, Thi Linh Nguyen, Ji Sun Moon, Jingwen Tian, Hyo Ju Jang, Jeong Eun Lee, Hyon-Seung Yi
Cell Death & Disease.2023;[Epub] CrossRef - Familial clustering of nonalcoholic fatty liver disease in first‐degree relatives of adults with lean nonalcoholic fatty liver disease
Sorachat Niltwat, Chanin Limwongse, Natthinee Charatcharoenwitthaya, Duangkamon Bunditvorapoom, Wimolrak Bandidniyamanon, Phunchai Charatcharoenwitthaya
Liver International.2023; 43(12): 2713. CrossRef - Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease
Jun-Hyuk Lee, Yu-Jin Kwon, Kyongmin Park, Hye Sun Lee, Hoon-Ki Park, Jee Hye Han, Sang Bong Ahn
Nutrients.2022; 14(15): 3039. CrossRef - DPP-4 Inhibitor in Type 2 Diabetes Mellitus Patient with Non-Alcoholic Fatty Liver Disease: Achieving Two Goals at Once?
Ji Cheol Bae
Endocrinology and Metabolism.2022; 37(6): 858. CrossRef
- Association of insulin resistance indicators with hepatic steatosis and fibrosis in patients with metabolic syndrome

Review Article
- Thyroid
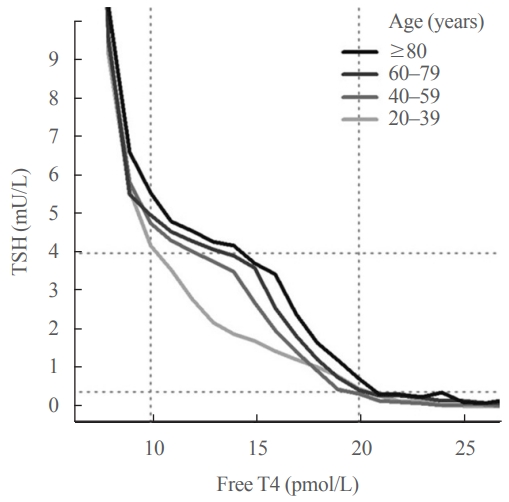
- Thyroid Function across the Lifespan: Do Age-Related Changes Matter?
- John P. Walsh
- Endocrinol Metab. 2022;37(2):208-219. Published online April 14, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1463
- 6,079 View
- 339 Download
- 12 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Circulating concentrations of thyrotropin (TSH) and thyroxine (T4) are tightly regulated. Each individual has setpoints for TSH and free T4 which are genetically determined, and subject to environmental and epigenetic influence. Pituitary-thyroid axis setpoints are probably established in utero, with maturation of thyroid function continuing until late gestation. From neonatal life (characterized by a surge of TSH and T4 secretion) through childhood and adolescence (when free triiodothyronine levels are higher than in adults), thyroid function tests display complex, dynamic patterns which are sexually dimorphic. In later life, TSH increases with age in healthy older adults without an accompanying fall in free T4, indicating alteration in TSH setpoint. In view of this, and evidence that mild subclinical hypothyroidism in older people has no health impact, a strong case can be made for implementation of age-related TSH reference ranges in adults, as is routine in children.
-
Citations
Citations to this article as recorded by- The ageing thyroid: implications for longevity and patient care
Diana van Heemst
Nature Reviews Endocrinology.2024; 20(1): 5. CrossRef - Incidence and Determinants of Spontaneous Normalization of Subclinical Hypothyroidism in Older Adults
Evie van der Spoel, Nicolien A van Vliet, Rosalinde K E Poortvliet, Robert S Du Puy, Wendy P J den Elzen, Terence J Quinn, David J Stott, Naveed Sattar, Patricia M Kearney, Manuel R Blum, Heba Alwan, Nicolas Rodondi, Tinh-Hai Collet, Rudi G J Westendorp,
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1167. CrossRef - Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications
Rosalie B. T. M. Sterenborg, Inga Steinbrenner, Yong Li, Melissa N. Bujnis, Tatsuhiko Naito, Eirini Marouli, Tessel E. Galesloot, Oladapo Babajide, Laura Andreasen, Arne Astrup, Bjørn Olav Åsvold, Stefania Bandinelli, Marian Beekman, John P. Beilby, Jette
Nature Communications.2024;[Epub] CrossRef - Evaluation of multiple organophosphate insecticide exposure in relation to altered thyroid hormones in NHANES 2007‐2008 adult population
Massira Ousseni Diawara, Songtao Li, Mingzhi Zhang, Francis Manyori Bigambo, Xu Yang, Xu Wang, Tianyu Dong, Di Wu, Chenghao Yan, Yankai Xia
Ecotoxicology and Environmental Safety.2024; 273: 116139. CrossRef - Thyroid-function reference ranges in the diagnosis of thyroid dysfunction in adults
Salman Razvi
Nature Reviews Endocrinology.2024; 20(5): 253. CrossRef - Association between exposure to chemical mixtures and epigenetic ageing biomarkers: Modifying effects of thyroid hormones and physical activity
Wanying Shi, Jianlong Fang, Huimin Ren, Peijie Sun, Juan Liu, Fuchang Deng, Shuyi Zhang, Qiong Wang, Jiaonan Wang, Shilu Tong, Song Tang, Xiaoming Shi
Journal of Hazardous Materials.2024; 469: 134009. CrossRef - DNA Methylation in Autoimmune Thyroid Disease
Nicole Lafontaine, Scott G Wilson, John P Walsh
The Journal of Clinical Endocrinology & Metabolism.2023; 108(3): 604. CrossRef - A Causality between Thyroid Function and Bone Mineral Density in Childhood: Abnormal Thyrotropin May Be Another Pediatric Predictor of Bone Fragility
Dongjin Lee, Moon Ahn
Metabolites.2023; 13(3): 372. CrossRef - Serum Lipidomic Analysis Reveals Biomarkers and Metabolic Pathways of Thyroid Dysfunction
Hua Dong, Wenjie Zhou, Xingxu Yan, Huan Zhao, Honggang Zhao, Yan Jiao, Guijiang Sun, Yubo Li, Zuncheng Zhang
ACS Omega.2023; 8(11): 10355. CrossRef - Developmental and environmental modulation of fecal thyroid hormone levels in wild Assamese macaques (Macaca assamensis)
Verena Behringer, Michael Heistermann, Suchinda Malaivijitnond, Oliver Schülke, Julia Ostner
American Journal of Primatology.2023;[Epub] CrossRef - Prevalence of Functional Alterations and the Effects of Thyroid
Autoimmunity on the Levels of TSH in an Urban Population of Colombia:
A Population-Based Study
Hernando Vargas-Uricoechea, Valentina Agredo-Delgado, Hernando David Vargas-Sierra, María V. Pinzón-Fernández
Endocrine, Metabolic & Immune Disorders - Drug Targets.2023; 23(6): 857. CrossRef - Genetic determinants of thyroid function in children
Tessa A Mulder, Purdey J Campbell, Peter N Taylor, Robin P Peeters, Scott G Wilson, Marco Medici, Colin Dayan, Vincent V W Jaddoe, John P Walsh, Nicholas G Martin, Henning Tiemeier, Tim I M Korevaar
European Journal of Endocrinology.2023; 189(2): 164. CrossRef - Relationship between Thyroid CT Density, Volume, and Future TSH Elevation: A 5-Year Follow-Up Study
Tomohiro Kikuchi, Shouhei Hanaoka, Takahiro Nakao, Yukihiro Nomura, Takeharu Yoshikawa, Md Ashraful Alam, Harushi Mori, Naoto Hayashi
Life.2023; 13(12): 2303. CrossRef - Thyroid Stimulating Hormone and Thyroid Hormones (Triiodothyronine and Thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status
Katleen Van Uytfanghe, Joel Ehrenkranz, David Halsall, Kelly Hoff, Tze Ping Loh, Carole A. Spencer, Josef Köhrle
Thyroid®.2023; 33(9): 1013. CrossRef - Blood hormones and suicidal behaviour: A systematic review and meta-analysis
Xue-Lei Fu, Xia Li, Jia-Mei Ji, Hua Wu, Hong-Lin Chen
Neuroscience & Biobehavioral Reviews.2022; 139: 104725. CrossRef
- The ageing thyroid: implications for longevity and patient care

Original Articles
- Calcium & Bone Metabolism
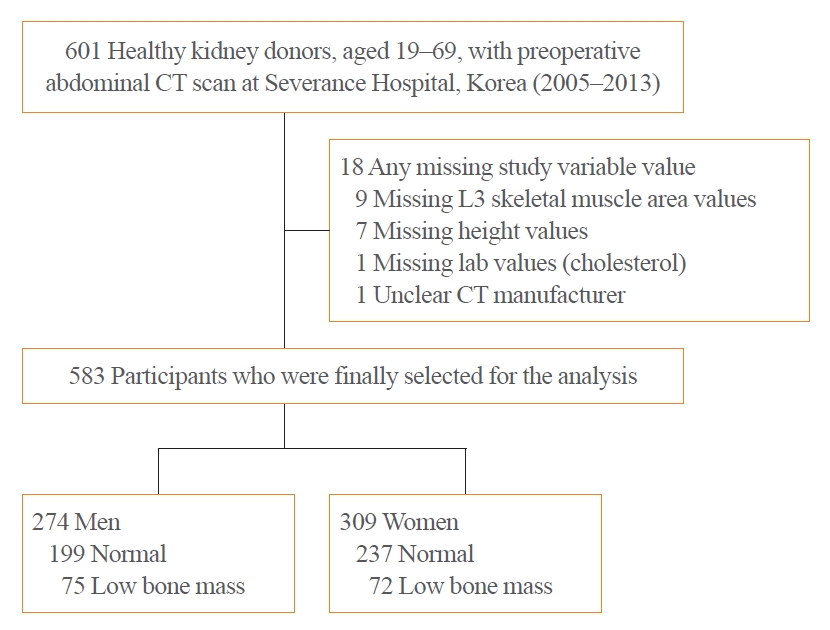
- Computed Tomography-Derived Skeletal Muscle Radiodensity Is an Early, Sensitive Marker of Age-Related Musculoskeletal Changes in Healthy Adults
- Yeon Woo Jung, Namki Hong, Joon Chae Na, Woong Kyu Han, Yumie Rhee
- Endocrinol Metab. 2021;36(6):1201-1210. Published online December 13, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1206
- 3,732 View
- 135 Download
- 1 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
A decrease in computed tomography (CT)-derived skeletal muscle radiodensity (SMD) reflects age-related ectopic fat infiltration of muscle, compromising muscle function and metabolism. We investigated the age-related trajectory of SMD and its association with vertebral trabecular bone density in healthy adults.
Methods
In a cohort of healthy adult kidney donors aged 19 to 69 years (n=583), skeletal muscle index (SMI, skeletal muscle area/height2), SMD, and visceral-to-subcutaneous fat (V/S) ratio were analyzed at the level of L3 from preoperative CT scans. Low bone mass was defined as an L1 trabecular Hounsfield unit (HU) <160 HU.
Results
L3SMD showed constant decline from the second decade (annual change –0.38% and –0.43% in men and women), whereas the decline of L3SMI became evident only after the fourth decade of life (–0.37% and –0.18% in men and women). One HU decline in L3SMD was associated with elevated odds of low bone mass (adjusted odds ratio, 1.07; 95% confidence interval, 1.02 to 1.13; P=0.003), independent of L3SMI, age, sex, and V/S ratio, with better discriminatory ability compared to L3SMI (area under the receiver-operating characteristics curve 0.68 vs. 0.53, P<0.001). L3SMD improved the identification of low bone mass when added to age, sex, V/S ratio, and L3SMI (category-free net reclassification improvement 0.349, P<0.001; integrated discrimination improvement 0.015, P=0.0165).
Conclusion
L3SMD can be an early marker for age-related musculoskeletal changes showing linear decline throughout life from the second decade in healthy adults, with potential diagnostic value for individuals with low bone mass. -
Citations
Citations to this article as recorded by- A review of radiological definitions of sarcopenia in cancer
James W. Wang, Jiarong Chen, Alison H. McGregor, Matthew Williams
JCSM Clinical Reports.2023; 8(2): 36. CrossRef - Weight‐adjusted waist as an integrated index for fat, muscle and bone health in adults
Kyoung Jin Kim, Serhim Son, Kyeong Jin Kim, Sin Gon Kim, Nam Hoon Kim
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(5): 2196. CrossRef
- A review of radiological definitions of sarcopenia in cancer

- Thyroid
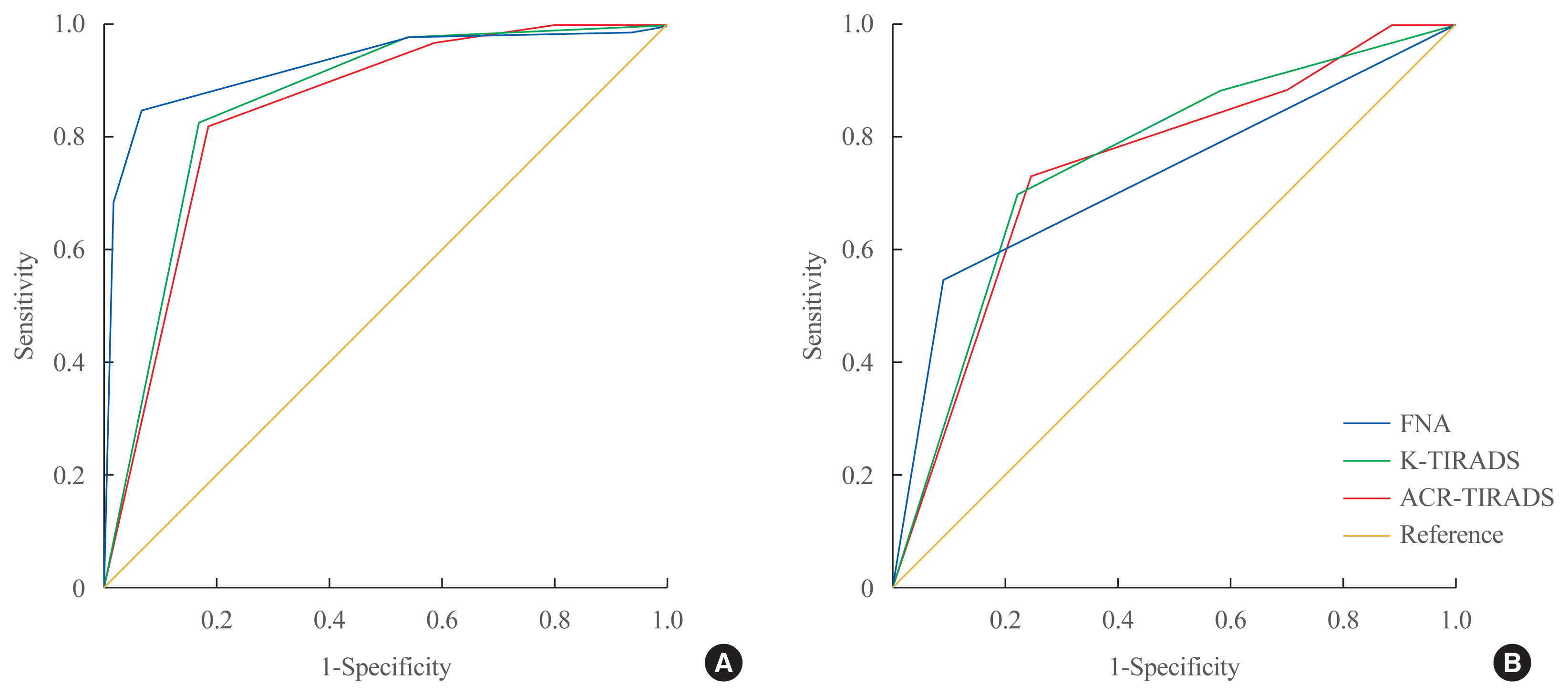
- Comparison of Korean vs. American Thyroid Imaging Reporting and Data System in Malignancy Risk Assessment of Indeterminate Thyroid Nodules
- Sunyoung Kang, Seul Ki Kwon, Hoon Sung Choi, Min Joo Kim, Young Joo Park, Do Joon Park, Sun Wook Cho
- Endocrinol Metab. 2021;36(5):1111-1120. Published online October 21, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1208
- 4,030 View
- 127 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The management of cytologically indeterminate thyroid nodules is challenging for clinicians. This study aimed to compare the diagnostic performance of the Korean Thyroid Imaging Reporting and Data Systems (K-TIRADS) with that of the American College of Radiology (ACR)-TIRADS for predicting the malignancy risk of indeterminate thyroid nodules.
Methods
Thyroid nodules diagnosed by fine-needle aspiration (FNA) followed by surgery or core needle biopsy at a single referral hospital were enrolled.
Results
Among 200 thyroid nodules, 78 (39.0%) nodules were classified as indeterminate by FNA (Bethesda category III, IV, and V), and 114 (57.0%) nodules were finally diagnosed as malignancy by surgery or core needle biopsy. The area under the curve (AUC) was higher for FNA than for either TIRADS system in all nodules, while all three methods showed similar AUCs for indeterminate nodules. However, for Bethesda category III nodules, applying K-TIRADS 5 significantly increased the risk of malignancy compared to a cytological examination alone (50.0% vs. 26.5%, P=0.028), whereas applying ACR-TIRADS did not lead to a change.
Conclusion
K-TIRADS and ACR-TIRADS showed similar diagnostic performance in assessing indeterminate thyroid nodules, and K-TIRADS had beneficial effects for malignancy prediction in Bethesda category III nodules. -
Citations
Citations to this article as recorded by- Is the nodule location a predictive risk factor for cancer in AUS/FLUS thyroid nodules? A retrospective cohort study
Saad M. Alqahtani, Bassam A. Altalhi, Yousef S. Alalawi, Saif S. Al-Sobhi
Asian Journal of Surgery.2024;[Epub] CrossRef - Diagnostic Performance of Various Ultrasound Risk Stratification Systems for Benign and Malignant Thyroid Nodules: A Meta-Analysis
Ji-Sun Kim, Byung Guk Kim, Gulnaz Stybayeva, Se Hwan Hwang
Cancers.2023; 15(2): 424. CrossRef - The impact of thyroid imaging reporting and data system on the management of Bethesda III thyroid nodules
Saad M. Alqahtani, Saif S. Al-Sobhi, Mohammed A. Alturiqy, Riyadh I. Alsalloum, Hindi N. Al-Hindi
Journal of Taibah University Medical Sciences.2023; 18(3): 506. CrossRef - Diagnostic Performance of Six Ultrasound Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Network Meta-Analysis
Do Hyun Kim, Sung Won Kim, Mohammed Abdullah Basurrah, Jueun Lee, Se Hwan Hwang
American Journal of Roentgenology.2023; 220(6): 791. CrossRef - Diagnostic efficiency among Eu-/C-/ACR-TIRADS and S-Detect for thyroid nodules: a systematic review and network meta-analysis
Longtao Yang, Cong Li, Zhe Chen, Shaqi He, Zhiyuan Wang, Jun Liu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Comparison of diagnostic performance of two ultrasound risk stratification systems for thyroid nodules: a systematic review and meta-analysis
Yun Jin Kang, Hee Sun Ahn, Gulnaz Stybayeva, Ju Eun Lee, Se Hwan Hwang
La radiologia medica.2023; 128(11): 1407. CrossRef - Diagnostic Performance of ACR and Kwak TI-RADS for Benign and Malignant Thyroid Nodules: An Update Systematic Review and Meta-Analysis
Yun Jin Kang, Gulnaz Stybayeya, Ju Eun Lee, Se Hwan Hwang
Cancers.2022; 14(23): 5961. CrossRef - Comparison of Thyroid Imaging Reporting and Data Systems in Malignancy Risk Stratification of Indeterminate Thyroid Nodules
Bo Hyun Kim
Endocrinology and Metabolism.2021; 36(5): 974. CrossRef
- Is the nodule location a predictive risk factor for cancer in AUS/FLUS thyroid nodules? A retrospective cohort study

Review Article
- Diabetes, Obesity and Metabolism
- Receptor-Mediated Muscle Homeostasis as a Target for Sarcopenia Therapeutics
- Jong Hyeon Yoon, Ki-Sun Kwon
- Endocrinol Metab. 2021;36(3):478-490. Published online June 28, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1081
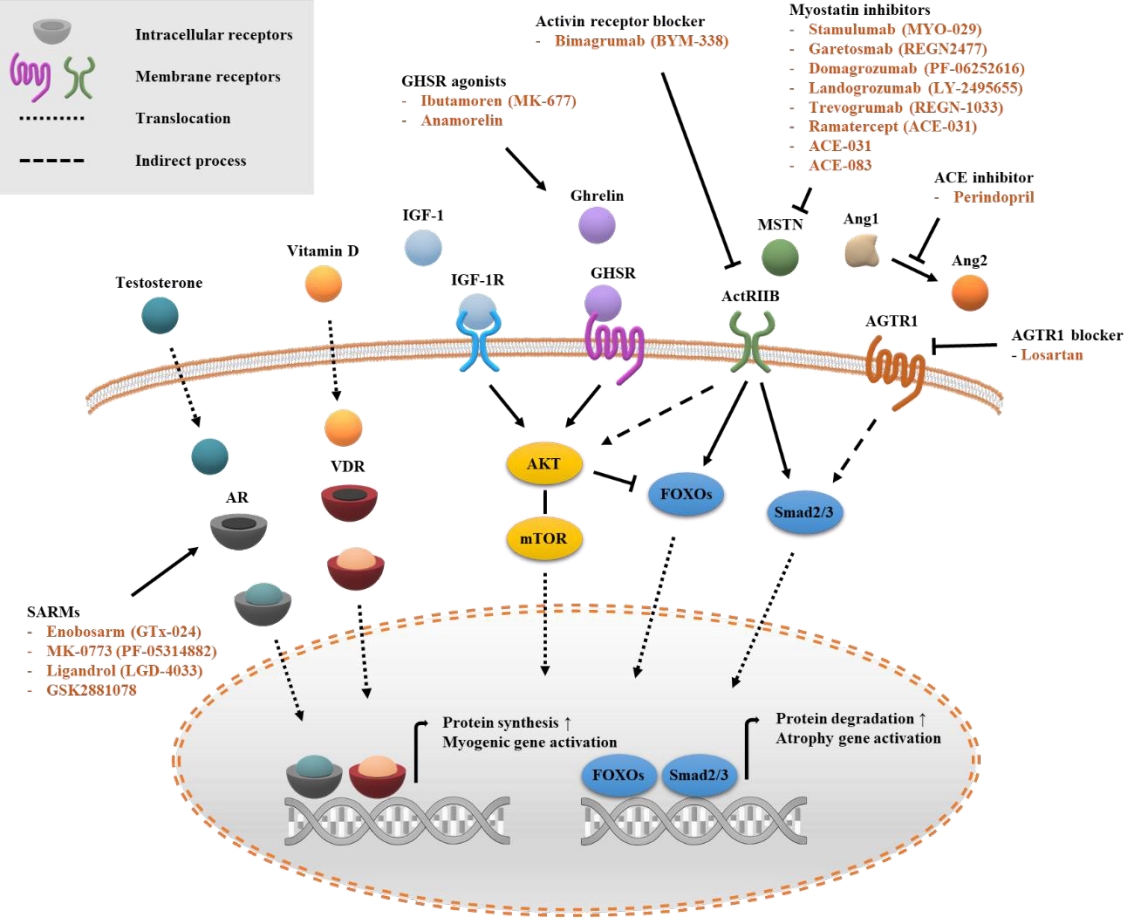
- 8,908 View
- 337 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Sarcopenia is a disease characterized by age-related decline of skeletal muscle mass and function. The molecular mechanisms of the pathophysiology of sarcopenia form a complex network due to the involvement of multiple interconnected signaling pathways. Therefore, signaling receptors are major targets in pharmacological strategies in general. To provide a rationale for pharmacological interventions for sarcopenia, we herein describe several druggable signaling receptors based on their role in skeletal muscle homeostasis and changes in their activity with aging. A brief overview is presented of the efficacy of corresponding drug candidates under clinical trials. Strategies targeting the androgen receptor, vitamin D receptor, Insulin-like growth factor-1 receptor, and ghrelin receptor primarily focus on promoting anabolic action using natural ligands or mimetics. Strategies involving activin receptors and angiotensin receptors focus on inhibiting catabolic action. This review may help to select specific targets or combinations of targets in the future.
-
Citations
Citations to this article as recorded by- The Current Landscape of Pharmacotherapies for Sarcopenia
Gulistan Bahat, Serdar Ozkok
Drugs & Aging.2024; 41(2): 83. CrossRef - Associations of micronutrient dietary patterns with sarcopenia among US adults: a population-based study
Yining Liu, Xiangliang Liu, Linnan Duan, Yixin Zhao, Yuwei He, Wei Li, Jiuwei Cui
Frontiers in Nutrition.2024;[Epub] CrossRef - Impact of Vitamin D Level on Sarcopenia in Elderly People: A Critical Review
Saniya Khan, Sunil Kumar, Sourya Acharya, Anil Wanjari
Journal of Health and Allied Sciences NU.2023; 13(04): 453. CrossRef - Novel Potential Targets for Function-Promoting Therapies: Orphan Nuclear Receptors, Anti-inflammatory Drugs, Troponin Activators, Mas Receptor Agonists, and Urolithin A
Waly Dioh, Vihang Narkar, Anurag Singh, Fady Malik, Luigi Ferrucci, Cendrine Tourette, Jean Mariani, Rob van Maanen, Roger A Fielding, Lewis A Lipsitz
The Journals of Gerontology: Series A.2023; 78(Supplement): 44. CrossRef - Alverine citrate promotes myogenic differentiation and ameliorates muscle atrophy
Jong Hyeon Yoon, Seung-Min Lee, Younglang Lee, Min Ju Kim, Jae Won Yang, Jeong Yi Choi, Ju Yeon Kwak, Kwang-Pyo Lee, Yong Ryoul Yang, Ki-Sun Kwon
Biochemical and Biophysical Research Communications.2022; 586: 157. CrossRef - Adeno-associated virus-mediated expression of an inactive CaMKIIβ mutant enhances muscle mass and strength in mice
Takahiro Eguchi, Yuji Yamanashi
Biochemical and Biophysical Research Communications.2022; 589: 192. CrossRef - Gastric Mobility and Gastrointestinal Hormones in Older Patients with Sarcopenia
Hsien-Hao Huang, Tse-Yao Wang, Shan-Fan Yao, Pei-Ying Lin, Julia Chia-Yu Chang, Li-Ning Peng, Liang-Kung Chen, David Hung-Tsang Yen
Nutrients.2022; 14(9): 1897. CrossRef - Molecular Mechanisms Underlying Intensive Care Unit-Acquired Weakness and Sarcopenia
Marcela Kanova, Pavel Kohout
International Journal of Molecular Sciences.2022; 23(15): 8396. CrossRef
- The Current Landscape of Pharmacotherapies for Sarcopenia

Original Articles
- Endocrine Research
- Effect of CCL11 on In Vitro Myogenesis and Its Clinical Relevance for Sarcopenia in Older Adults
- Da Ae Kim, So Jeong Park, Jin Young Lee, Jeoung Hee Kim, Seungjoo Lee, Eunju Lee, Il-Young Jang, Hee-Won Jung, Jin Hoon Park, Beom-Jun Kim
- Endocrinol Metab. 2021;36(2):455-465. Published online April 14, 2021
- DOI: https://doi.org/10.3803/EnM.2020.942

- 5,230 View
- 146 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
The C-C motif chemokine ligand 11 (CCL11) has been receiving attention as a potential pro-aging factor. Accordingly, it may be involved in muscle metabolism and sarcopenia, a key component of aging phenotypes. To clarify this potential, we investigated the effects of CCL11 on in vitro muscle biology and its clinical relevance for sarcopenia parameters in older adults.
Methods
Myogenesis was induced in mouse C2C12 myoblasts with 2% horse serum. Human blood samples were collected from 79 participants who underwent a functional assessment. Thereafter, CCL11 level was measured using a quantikine ELISA kit. Sarcopenia was defined using the Asian-specific guideline.
Results
Recombinant CCL11 treatment significantly stimulated myogenesis in a dose-dependent manner, and consistently increased the expression of myogenic differentiation markers. Among the C-C chemokine receptors (CCRs), CCR5, not CCR2 and CCR3, was predominantly expressed in muscle cells. Further, the CCR5 inhibitor blocked recombinant CCL11-stimulated myogenesis. In a clinical study, serum CCL11 level was not significantly different according to the status of sarcopenia, low muscle mass, weak muscle strength, and poor physical performance, and was not associated with skeletal muscle index, grip strength, short physical performance battery score, gait speed, and time to complete 5 chair stands, after adjusting for sex, age, and body mass index.
Conclusion
Contrary to expectations, CCL11 exerted beneficial effects on muscle metabolism at least in vitro system. However, its impact on human muscle health was not evident, suggesting that circulating CCL11 may not be a useful biomarker for sarcopenia risk assessment in older adults. -
Citations
Citations to this article as recorded by- Mapping the causal associations of cytokines with sarcopenia and aging traits: Evidence from bidirectional Mendelian randomization
Mingchong Liu, Xiao Fu, Daqian Yu, Meng Li, Yutao Pan, Chensong Yang, Guixin Sun
Journal of Cachexia, Sarcopenia and Muscle.2024;[Epub] CrossRef - C-C motif chemokine CCL11 is a novel regulator and a potential therapeutic target in non-alcoholic fatty liver disease
Zhiwen Fan, Xinyue Sun, Xuelian Chen, Huimin Liu, Xiulian Miao, Yan Guo, Yong Xu, Jie Li, Xiaoping Zou, Zilong Li
JHEP Reports.2023; : 100754. CrossRef - C–C motif chemokine CCL11 is a novel regulator and a potential therapeutic target in non-alcoholic fatty liver disease
Zhiwen Fan, Xinyue Sun, Xuelian Chen, Huimin Liu, Xiulian Miao, Yan Guo, Yong Xu, Jie Li, Xiaoping Zou, Zilong Li
JHEP Reports.2023; 5(9): 100805. CrossRef - Lumican Inhibits Osteoclastogenesis and Bone Resorption by Suppressing Akt Activity
Jin-Young Lee, Da-Ae Kim, Eun-Young Kim, Eun-Ju Chang, So-Jeong Park, Beom-Jun Kim
International Journal of Molecular Sciences.2021; 22(9): 4717. CrossRef - Aldosterone Inhibits In Vitro Myogenesis by Increasing Intracellular Oxidative Stress via Mineralocorticoid Receptor
Jin Young Lee, Da Ae Kim, Eunah Choi, Yun Sun Lee, So Jeong Park, Beom-Jun Kim
Endocrinology and Metabolism.2021; 36(4): 865. CrossRef
- Mapping the causal associations of cytokines with sarcopenia and aging traits: Evidence from bidirectional Mendelian randomization

- Clinical Study
- Development of a Non-Invasive Liver Fibrosis Score Based on Transient Elastography for Risk Stratification in Patients with Type 2 Diabetes
- Chi-Ho Lee, Wai-Kay Seto, Kelly Ieong, David T.W. Lui, Carol H.Y. Fong, Helen Y. Wan, Wing-Sun Chow, Yu-Cho Woo, Man-Fung Yuen, Karen S.L. Lam
- Endocrinol Metab. 2021;36(1):134-145. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.887

- 4,537 View
- 132 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
In non-alcoholic fatty liver disease (NAFLD), transient elastography (TE) is an accurate non-invasive method to identify patients at risk of advanced fibrosis (AF). We developed a diabetes-specific, non-invasive liver fibrosis score based on TE to facilitate AF risk stratification, especially for use in diabetes clinics where TE is not readily available.
Methods
Seven hundred sixty-six adults with type 2 diabetes and NAFLD were recruited and randomly divided into a training set (n=534) for the development of diabetes fibrosis score (DFS), and a testing set (n=232) for internal validation. DFS identified patients with AF on TE, defined as liver stiffness (LS) ≥9.6 kPa, based on a clinical model comprising significant determinants of LS with the lowest Akaike information criteria. The performance of DFS was compared with conventional liver fibrosis scores (NFS, FIB-4, and APRI), using area under the receiver operating characteristic curve (AUROC), sensitivity, specificity, positive and negative predictive values (NPV).
Results
DFS comprised body mass index, platelet, aspartate aminotransferase, high-density lipoprotein cholesterol, and albuminuria, five routine measurements in standard diabetes care. Derived low and high DFS cut-offs were 0.1 and 0.3, with 90% sensitivity and 90% specificity, respectively. Both cut-offs provided better NPVs of >90% than conventional fibrosis scores. The AUROC of DFS for AF on TE was also higher (P<0.01) than the conventional fibrosis scores, being 0.85 and 0.81 in the training and testing sets, respectively.
Conclusion
Compared to conventional fibrosis scores, DFS, with a high NPV, more accurately identified diabetes patients at-risk of AF, who need further evaluation by hepatologists. -
Citations
Citations to this article as recorded by- Implementation of a liver health check in people with type 2 diabetes
Kushala W M Abeysekera, Luca Valenti, Zobair Younossi, John F Dillon, Alina M Allen, Mazen Noureddin, Mary E Rinella, Frank Tacke, Sven Francque, Pere Ginès, Maja Thiele, Philip N Newsome, Indra Neil Guha, Mohammed Eslam, Jörn M Schattenberg, Saleh A Alqa
The Lancet Gastroenterology & Hepatology.2024; 9(1): 83. CrossRef - Sequential algorithm to stratify liver fibrosis risk in overweight/obese metabolic dysfunction-associated fatty liver disease
Chi-Ho Lee, David Tak-Wai Lui, Raymond Hang-Wun Li, Michele Mae-Ann Yuen, Carol Ho-Yi Fong, Ambrose Pak-Wah Leung, Justin Chiu-Man Chu, Loey Lung-Yi Mak, Tai-Hing Lam, Jean Woo, Yu-Cho Woo, Aimin Xu, Hung-Fat Tse, Kathryn Choon-Beng Tan, Bernard Man-Yung
Frontiers in Endocrinology.2023;[Epub] CrossRef - Non-Invasive Measurement of Hepatic Fibrosis by Transient Elastography: A Narrative Review
Luca Rinaldi, Chiara Giorgione, Andrea Mormone, Francesca Esposito, Michele Rinaldi, Massimiliano Berretta, Raffaele Marfella, Ciro Romano
Viruses.2023; 15(8): 1730. CrossRef - Metabolic dysfunction-associated fatty liver disease — How relevant is this to primary care physicians and diabetologists?
Chi-Ho Lee
Primary Care Diabetes.2022; 16(2): 245. CrossRef - Non‐alcoholic fatty liver disease and type 2 diabetes: An update
Chi‐H Lee, David TW Lui, Karen SL Lam
Journal of Diabetes Investigation.2022; 13(6): 930. CrossRef - Ultrasound-Based Hepatic Elastography in Non-Alcoholic Fatty Liver Disease: Focus on Patients with Type 2 Diabetes
Georgiana-Diana Cazac, Cristina-Mihaela Lăcătușu, Cătălina Mihai, Elena-Daniela Grigorescu, Alina Onofriescu, Bogdan-Mircea Mihai
Biomedicines.2022; 10(10): 2375. CrossRef
- Implementation of a liver health check in people with type 2 diabetes

Review Articles
- Miscellaneous
- Sarcopenia and Muscle Aging: A Brief Overview
- Tam Dao, Alexander E. Green, Yun A Kim, Sung-Jin Bae, Ki-Tae Ha, Karim Gariani, Mi-ra Lee, Keir J. Menzies, Dongryeol Ryu
- Endocrinol Metab. 2020;35(4):716-732. Published online December 23, 2020
- DOI: https://doi.org/10.3803/EnM.2020.405
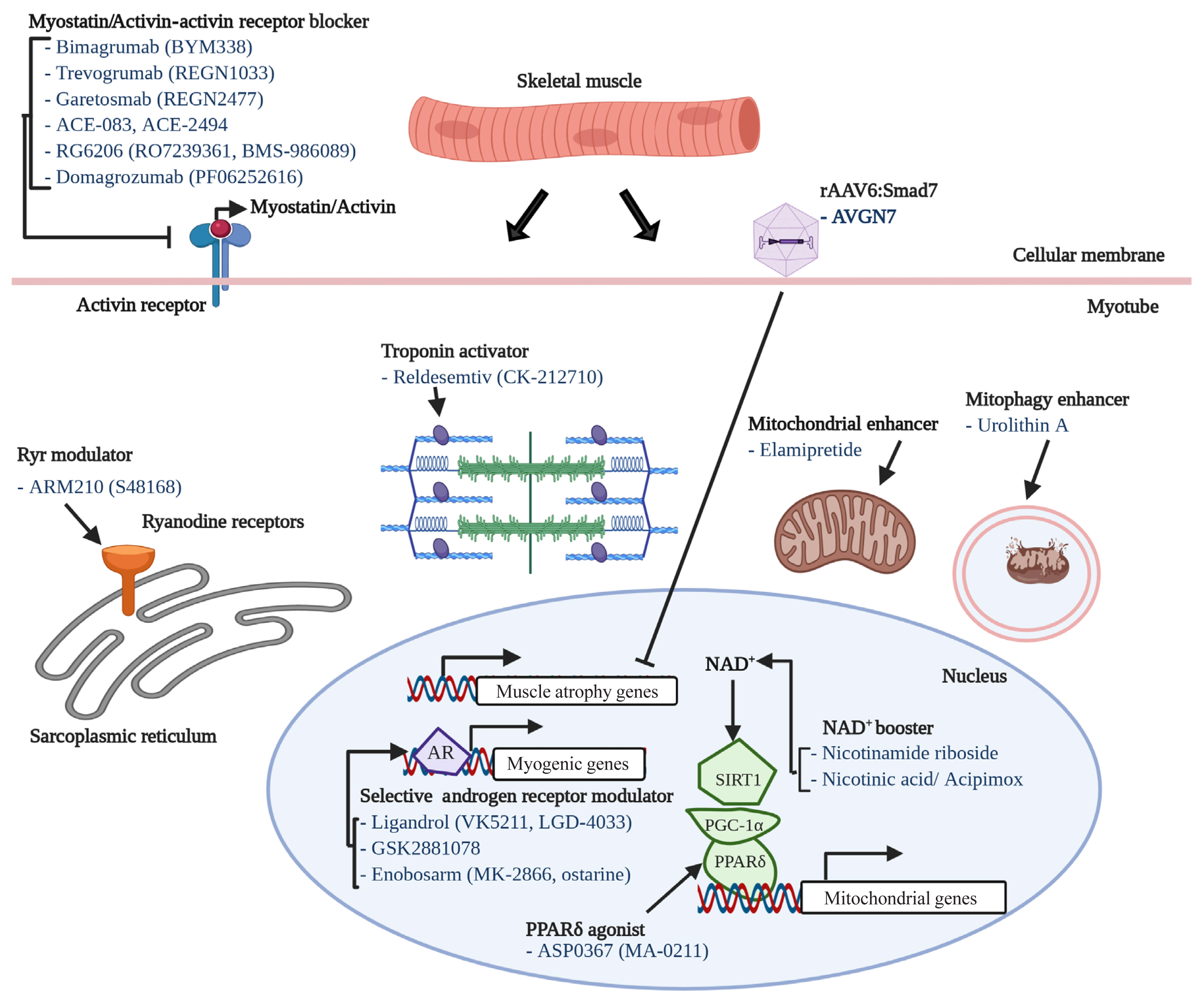
- 23,027 View
- 1,271 Download
- 74 Web of Science
- 79 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The world is facing the new challenges of an aging population, and understanding the process of aging has therefore become one of the most important global concerns. Sarcopenia is a condition which is defined by the gradual loss of skeletal muscle mass and function with age. In research and clinical practice, sarcopenia is recognized as a component of geriatric disease and is a current target for drug development. In this review we define this condition and provide an overview of current therapeutic approaches. We further highlight recent findings that describe key pathophysiological phenotypes of this condition, including alterations in muscle fiber types, mitochondrial function, nicotinamide adenine dinucleotide (NAD+) metabolism, myokines, and gut microbiota, in aged muscle compared to young muscle or healthy aged muscle. The last part of this review examines new therapeutic avenues for promising treatment targets. There is still no accepted therapy for sarcopenia in humans. Here we provide a brief review of the current state of research derived from various mouse models or human samples that provide novel routes for the development of effective therapeutics to maintain muscle health during aging.
-
Citations
Citations to this article as recorded by- Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age
Yuan Li, Laura Berliocchi, Zhiquan Li, Lene Juel Rasmussen
Aging Cell.2024;[Epub] CrossRef - Circulating lumican as a potential biomarker for osteosarcopenia in older adults
So Jeong Park, Eunhye Ji, Hyun Ju Yoo, Kyunggon Kim, Sunghwan Ji, Ji Yeon Baek, Jin Young Lee, Hee-Won Jung, Il-Young Jang, Eunju Lee, Namki Hong, Beom-Jun Kim
Bone.2024; 179: 116959. CrossRef - D‐galactose might mediate some of the skeletal muscle hypertrophy‐promoting effects of milk—A nutrient to consider for sarcopenia?
Jan Homolak, Ana Babic Perhoc, Davor Virag, Ana Knezovic, Jelena Osmanovic Barilar, Melita Salkovic‐Petrisic
BioEssays.2024;[Epub] CrossRef - Dark tea extract attenuates age-related muscle loss by suppressing oxidative stress and inflammation in skeletal muscle of mice
Ahyoung Yoo, Hyo Deok Seo, Jeong-Hoon Hahm, Chang Hwa Jung, Jiyun Ahn, Tae Youl Ha
Journal of Functional Foods.2024; 112: 105980. CrossRef - Moderate dietary restriction delays the onset of age-associated sarcopenia in Caenorhabditis elegans due to reduced myosin UNC-54 degradation
Sobha Tumbapo, Adam Strudwick, Jana J. Stastna, Simon C. Harvey, Marieke J. Bloemink
Mechanisms of Ageing and Development.2024; 217: 111900. CrossRef - Trop T, hand grip strength and waist circumference as markers of sarcopenic obesity in postmenopausal women: An analytical cross-sectional study
Sheetal Sarangi, Arul Senghor K. A., Vinodhini V. M.
Indian Journal of Physiology and Pharmacology.2024; 68: 57. CrossRef - Temporal Muscle Thickness: A Practical Approximation for Assessing Muscle Mass in Older Adults
Miguel German Borda, Jonathan Patricio Baldera, Jessica Samuelsson, Anna Zettergren, Lina Rydén, Eric Westman, Mario Ulises Pérez-Zepeda, Silke Kern, Luis Carlos Venegas, Gustavo Duque, Ingmar Skoog, Dag Aarsland
Journal of the American Medical Directors Association.2024; 25(4): 664. CrossRef - Undernutrition in obese older adults by fat percentage
Meris Esra Bozkurt, Tugba Erdogan, Nezahat Muge Catikkas, Serdar Ozkok, Cihan Kilic, Gulistan Bahat, Mehmet Akif Karan
Aging Clinical and Experimental Research.2024;[Epub] CrossRef - The BET inhibitor JQ1 targets fat metabolism and counteracts obesity
Claudia Fornelli, Alessia Sofia Cento, Lorenzo Nevi, Raffaella Mastrocola, Gustavo Ferreira Alves, Giuseppina Caretti, Massimo Collino, Fabio Penna
Journal of Advanced Research.2024;[Epub] CrossRef - Effects of Low-Load Blood Flow Restriction Training on Muscle Anabolism Biomarkers and Thrombotic Biomarkers Compared with Traditional Training in Healthy Adults Older Than 60 Years: Systematic Review and Meta-Analysis
Raúl Fabero-Garrido, Miguel Gragera-Vela, Tamara del Corral, Marta Hernández-Martín, Gustavo Plaza-Manzano, Ibai López-de-Uralde-Villanueva
Life.2024; 14(3): 411. CrossRef - Sarcopenia and non-alcoholic fatty liver disease
R. G. Myazin
Experimental and Clinical Gastroenterology.2024; (2): 120. CrossRef - THE ROLE OF DAIRY FOODS FOR HEALTHY AGING
Emine Kocyigit
Anti-Aging Eastern Europe.2024; 3(1): 23. CrossRef - Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy
Jun Ho Lee, Jung Yoon Jang, Young Hoon Kwon, Seung Ho Lee, Cheol Park, Yung Hyun Choi, Nam Deuk Kim
Applied Sciences.2023; 13(2): 986. CrossRef - Relationship between asthma and sarcopenia in the elderly: a nationwide study from the KNHANES
Ha-Kyeong Won, Yewon Kang, Jin An, Ji-Hyang Lee, Woo-Jung Song, Hyouk-Soo Kwon, You Sook Cho, Hee-Bom Moon, Il-Young Jang, Tae-Bum Kim
Journal of Asthma.2023; 60(2): 304. CrossRef - A Lignan from Alnus japonica Activates Myogenesis and Alleviates Dexamethasone-induced Myotube Atrophy
Hyejin Lee, Ji Hye Jeong, Seung Hwan Hwang, Sung Hum Yeon, Jae-Ha Ryu
Planta Medica.2023; 89(05): 484. CrossRef - A cross-talk between sestrins, chronic inflammation and cellular senescence governs the development of age-associated sarcopenia and obesity
Gregory Livshits, Alexander Kalinkovich
Ageing Research Reviews.2023; 86: 101852. CrossRef - Facilitators and Barriers of Tai Chi Practice in Community-Dwelling Older Adults: Qualitative Study
Yan Du, Penny Roberts, Wei Liu
Asian/Pacific Island Nursing Journal.2023; 7: e42195. CrossRef - Extract of Alnus japonica prevents dexamethasone-induced muscle atrophy in mice
Hyejin Lee, Kyeong Seon Lee, Ji Hye Jeong, Ji Soo Yoon, Seung Hwan Hwang, Sang-Yoon Kim, Sung Hum Yeon, Jae-Ha Ryu
Journal of Functional Foods.2023; 101: 105419. CrossRef - Axis “microbiota - muscle”
A. N. Zavyalova, V. P. Novikova, P. D. Ignatova
Experimental and Clinical Gastroenterology.2023; (11): 60. CrossRef - Comparison of the effects of commercial whey protein and native whey protein on muscle strength and muscle protein synthesis in rats
Jiyun Kim, Eun Woo Jeong, Youjin Baek, Gwang-woong Go, Hyeon Gyu Lee
Food Science and Biotechnology.2023; 32(3): 381. CrossRef - Prevalence of sarcopenia in patients with COPD through different musculature measurements: An updated meta-analysis and meta-regression
Jie He, Hezhi Li, Jun Yao, Yan Wang
Frontiers in Nutrition.2023;[Epub] CrossRef - Proteome network analysis of skeletal muscle in lignan-enriched nutmeg extract-fed aged mice
Je-Ho Lee, Hyuno Kang, Gyung-Tae Ban, Beom Kyu Kim, JaeHyeon Lee, Heeyoun Hwang, Hwa-Seung Yoo, Kun Cho, Jong-Soon Choi
Journal of Analytical Science and Technology.2023;[Epub] CrossRef - Aging, Physical Exercise, Telomeres, and Sarcopenia: A Narrative Review
David Hernández-Álvarez, Juana Rosado-Pérez, Graciela Gavia-García, Taide Laurita Arista-Ugalde, Itzen Aguiñiga-Sánchez, Edelmiro Santiago-Osorio, Víctor Manuel Mendoza-Núñez
Biomedicines.2023; 11(2): 598. CrossRef - Genetic and Probiotic Characteristics of Urolithin A Producing Enterococcus faecium FUA027
Mengjie Xia, Shuting Mu, Yaowei Fang, Xiaomeng Zhang, Guang Yang, Xiaoyue Hou, Fuxiang He, Yaling Zhao, Yichen Huang, Wei Zhang, Juan Shen, Shu Liu
Foods.2023; 12(5): 1021. CrossRef - A nomogram to predict the risk of sarcopenia in older people
Guangjiao Yin, Juanjuan Qin, Ziwei Wang, Fang Lv, Xujun Ye
Medicine.2023; 102(16): e33581. CrossRef - Air pollution weaken your muscle? Evidence from a cross-sectional study on sarcopenia in central China
Faxue Zhang, Tianzhou Li, Bingbing Chen, Nuoya Li, Xupeng Zhang, Shijie Zhu, Gaichan Zhao, Xiaowei Zhang, TingTing Ma, Fang Zhou, Hao Liu, Wei Zhu
Ecotoxicology and Environmental Safety.2023; 258: 114962. CrossRef - Safety and Risk Factors of Needle Thoracentesis Decompression in Tension Pneumothorax in Patients over 75 Years Old
Yanhu Wang, Lei Wang, Cheng Chen, Yifan Que, Yinyi Li, Jiang Luo, Ming Yin, Miao Lv, Guogang Xu, Anita Pye
Canadian Respiratory Journal.2023; 2023: 1. CrossRef - Allosteric modulation of ryanodine receptor RyR1 by nucleotide derivatives
Spencer Cholak, James W. Saville, Xing Zhu, Alison M. Berezuk, Katharine S. Tuttle, Omid Haji-Ghassemi, Francisco J. Alvarado, Filip Van Petegem, Sriram Subramaniam
Structure.2023; 31(7): 790. CrossRef - Sarcopenia and Cardiovascular Diseases
Abdulla A. Damluji, Maha Alfaraidhy, Noora AlHajri, Namit N. Rohant, Manish Kumar, Christina Al Malouf, Samira Bahrainy, Min Ji Kwak, Wayne B. Batchelor, Daniel E. Forman, Michael W. Rich, James Kirkpatrick, Ashok Krishnaswami, Karen P. Alexander, Gary Ge
Circulation.2023; 147(20): 1534. CrossRef - Mitochondrial-Encoded Peptide MOTS-c, Diabetes, and Aging-Related Diseases
Byung Soo Kong, Changhan Lee, Young Min Cho
Diabetes & Metabolism Journal.2023; 47(3): 315. CrossRef - Pathogenesis, Intervention, and Current Status of Drug Development for Sarcopenia: A Review
Jung Yoon Jang, Donghwan Kim, Nam Deuk Kim
Biomedicines.2023; 11(6): 1635. CrossRef - Polyamines and Physical Activity in Musculoskeletal Diseases: A Potential Therapeutic Challenge
Letizia Galasso, Annalisa Cappella, Antonino Mulè, Lucia Castelli, Andrea Ciorciari, Alessandra Stacchiotti, Angela Montaruli
International Journal of Molecular Sciences.2023; 24(12): 9798. CrossRef - Non-alcoholic fatty liver disease in women – Current knowledge and emerging concepts
Pei Chia Eng, Roberta Forlano, Tricia Tan, Pinelopi Manousou, Waljit S. Dhillo, Chioma Izzi-Engbeaya
JHEP Reports.2023; 5(10): 100835. CrossRef - Effects of polyphenols and their metabolites on age-related diseases
Chouari Zhor, Lounis Wafaa, Imen Ghzaiel, Khadidja Kessas, Amira Zarrouk, Mohamed Ksila, Taoufik Ghrairi, Norbert Latruffe, Olfa Masmoudi-Kouki, Adil El Midaoui, Dominique Vervandier-Fasseur, Mohamed Hammami, Gérard Lizard, Anne Vejux, Omar Kharoubi
Biochemical Pharmacology.2023; 214: 115674. CrossRef - Circadian regulation in aging: Implications for spaceflight and life on earth
Deeksha Malhan, Britt Schoenrock, Müge Yalçin, Dieter Blottner, Angela Relόgio
Aging Cell.2023;[Epub] CrossRef - Mitochondria-associated programmed cell death as a therapeutic target for age-related disease
Thanh T. Nguyen, Shibo Wei, Thu Ha Nguyen, Yunju Jo, Yan Zhang, Wonyoung Park, Karim Gariani, Chang-Myung Oh, Hyeon Ho Kim, Ki-Tae Ha, Kyu Sang Park, Raekil Park, In-Kyu Lee, Minho Shong, Riekelt H. Houtkooper, Dongryeol Ryu
Experimental & Molecular Medicine.2023; 55(8): 1595. CrossRef - Normative reference values, determinants and regression equations for the incremental shuttle walk test (ISWT) in healthy Asian population aged 21 to 80 years
Muhammad Zulhaziq Bin Azman, Katherin S. Huang, Wei Jun Koh, Sarah S. Leong, Benjamin Ong, Johanna L. Soon, Sherman W. Tan, Melissa Y. Chan, Mingxing Yang, Meredith T. Yeung, Hans-Peter Kubis
PLOS ONE.2023; 18(9): e0291132. CrossRef - Influence of sarcopenia on postoperative complications in patients undergoing autologous microsurgical breast reconstruction: an inverse probability of treatment weighting analysis
Seung-Jun Lee, Yun-Jung Yang, Dong-Won Lee, Seung-Yong Song, Dae-Hyun Lew, Eun-Jung Yang
Frontiers in Oncology.2023;[Epub] CrossRef - Stromal vascular fraction in the treatment of myositis
S. Gandolfi, B. Pileyre, L. Drouot, I. Dubus, I. Auquit-Auckbur, J. Martinet
Cell Death Discovery.2023;[Epub] CrossRef - Multidisciplinary approach to sarcopenia: a narrative review
Wook Tae Park, Oog-Jin Shon, Gi Beom Kim
Journal of Yeungnam Medical Science.2023; 40(4): 352. CrossRef - Metallosis after Hip Arthroplasty Damages Skeletal Muscle: A Case Report
Roberto Bonanni, Lorenzo Abbondante, Ida Cariati, Elena Gasbarra, Umberto Tarantino
Geriatrics.2023; 8(5): 92. CrossRef - Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men
Antoneta Granic, Karen Suetterlin, Tea Shavlakadze, Miranda D. Grounds, Avan A. Sayer
Clinical Science.2023; 137(22): 1721. CrossRef - Predicted lean body mass trajectories, and cancer risk and cancer‐specific and all‐cause mortality: A prospective cohort study
Chenan Liu, Qingsong Zhang, Tong Liu, Qi Zhang, Mengmeng Song, Guotian Ruan, Shiqi Lin, Ziwen Wang, Xin Zheng, Yue Chen, Heyang Zhang, Yizhong Ge, Hailun Xie, Jinyu Shi, Li Deng, Shouling Wu, Hanping Shi
Journal of Cachexia, Sarcopenia and Muscle.2023; 14(6): 2916. CrossRef - Cancer Cachexia: New Insights and Future Directions
Claudia Raluca Mariean, Oana Mirela Tiucă, Alexandru Mariean, Ovidiu Simion Cotoi
Cancers.2023; 15(23): 5590. CrossRef - Development and comparative analysis of protein-polyphenol-fibre bars as nutritional supplements suitable for healthy senior consumers
M. Jolji, B. Pecsenye, Z. Mposula, A. Aleya, T. Kiss, E. Mathé
Acta Universitatis Sapientiae, Alimentaria.2023; 16(1): 103. CrossRef - Zebrafish as an Emerging Model for Sarcopenia: Considerations, Current Insights, and Future Directions
Santiago Callegari, Foad Mirzaei, Lila Agbaria, Sanobar Shariff, Burhan Kantawala, Desmond Moronge, Brian M. O. Ogendi
International Journal of Molecular Sciences.2023; 24(23): 17018. CrossRef - Laboratory markers of osteosarcopenic obesity
O. V. Gritsenko, O. V. Gruzdeva, G. A. Chumakova, O. L. Barbarash
Russian Journal of Cardiology.2023; 28(12): 5563. CrossRef - Higher Plasma Stromal Cell-Derived Factor 1 Is Associated with Lower Risk for Sarcopenia in Older Asian Adults
Sunghwan Ji, Kyunggon Kim, So Jeong Park, Jin Young Lee, Hee-Won Jung, Hyun Ju Yoo, Il-Young Jang, Eunju Lee, Ji Yeon Baek, Beom-Jun Kim
Endocrinology and Metabolism.2023; 38(6): 701. CrossRef - Familial Correlation and Heritability of Hand Grip Strength in Korean Adults (Korea National Health and Nutrition Examination Survey 2014 to 2019)
Seong Hee Ahn, Eun Byeol Park, Seongha Seo, Yongin Cho, Da Hea Seo, So Hun Kim, Young Ju Suh, Seongbin Hong
Endocrinology and Metabolism.2023; 38(6): 709. CrossRef - Ageing with neuromuscular disease: Implications for a lifeworld‐led care through a humanising approach
Louise Abildgaard Møller, Bente Martinsen, Ulla Werlauf, Pia Dreyer
Journal of Clinical Nursing.2022; 31(17-18): 2507. CrossRef - CT-derived relationship between low relative muscle mass and bone damage in patients with multiple myeloma undergoing stem cells transplantation
Alberto Stefano Tagliafico, Federica Rossi, Bianca Bignotti, Lorenzo Torri, Alessandro Bonsignore, Liliana Belgioia, Alida Domineitto
The British Journal of Radiology.2022;[Epub] CrossRef - A new paradigm in sarcopenia: Cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction
Danbi Jo, Gwangho Yoon, Oh Yoen Kim, Juhyun Song
Biomedicine & Pharmacotherapy.2022; 147: 112636. CrossRef - Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle
James W. Daily, Sunmin Park
Cells.2022; 11(3): 338. CrossRef - Change of Computed Tomography-Based Body Composition after Adrenalectomy in Patients with Pheochromocytoma
Yousun Ko, Heeryoel Jeong, Seungwoo Khang, Jeongjin Lee, Kyung Won Kim, Beom-Jun Kim
Cancers.2022; 14(8): 1967. CrossRef - Acute bioenergetic insulin sensitivity of skeletal muscle cells: ATP-demand-provoked glycolysis contributes to stimulation of ATP supply
Rosie A. Donnell, Jane E. Carré, Charles Affourtit
Biochemistry and Biophysics Reports.2022; 30: 101274. CrossRef - The Hunt Is On! In Pursuit of the Ideal Stem Cell Population for Cartilage Regeneration
T. Mark Campbell, F. Jeffrey Dilworth, David S. Allan, Guy Trudel
Frontiers in Bioengineering and Biotechnology.2022;[Epub] CrossRef - Decreased Serum Level of Sclerostin in Older Adults with Sarcopenia
Seong Hee Ahn, Hee-Won Jung, Eunju Lee, Ji Yeon Baek, Il-Young Jang, So Jeong Park, Jin Young Lee, Eunah Choi, Yun Sun Lee, Seongbin Hong, Beom-Jun Kim
Endocrinology and Metabolism.2022; 37(3): 487. CrossRef - Effects of Muscles on Bone Metabolism—with a Focus on Myokines
Beom-Jun Kim
Annals of Geriatric Medicine and Research.2022; 26(2): 63. CrossRef - Sclerostin as a Putative Myokine in Sarcopenia
Hyon-Seung Yi
Endocrinology and Metabolism.2022; 37(3): 430. CrossRef - Slc12a8 in the lateral hypothalamus maintains energy metabolism and skeletal muscle functions during aging
Naoki Ito, Ai Takatsu, Hiromi Ito, Yuka Koike, Kiyoshi Yoshioka, Yasutomi Kamei, Shin-ichiro Imai
Cell Reports.2022; 40(4): 111131. CrossRef - Restoration of NAD+homeostasis protects C2C12 myoblasts and mouse levator ani muscle from mechanical stress-induced damage
Guotao Huang, Yong He, Li Hong, Min Zhou, Xiaohu Zuo, Zhihan Zhao
Animal Cells and Systems.2022; 26(4): 192. CrossRef - Machine learning-featured Secretogranin V is a circulating diagnostic biomarker for pancreatic adenocarcinomas associated with adipopenia
Yunju Jo, Min-Kyung Yeo, Tam Dao, Jeongho Kwon, Hyon‐Seung Yi, Dongryeol Ryu
Frontiers in Oncology.2022;[Epub] CrossRef - Development and Verification of a Combined Diagnostic Model for Sarcopenia with Random Forest and Artificial Neural Network
Shangjin Lin, Cong Chen, Xiaoxi Cai, Fengjian Yang, Yongqian Fan, Rajesh Kaluri
Computational and Mathematical Methods in Medicine.2022; 2022: 1. CrossRef - Bronchial Asthma and Sarcopenia: An Upcoming Potential Interaction
Nikolaos D. Karakousis, Ourania S. Kotsiou, Konstantinos I. Gourgoulianis
Journal of Personalized Medicine.2022; 12(10): 1556. CrossRef - Suppressive Effects of Turmeric Extract on Muscle Atrophy in Dexamethasone-Treated Mice and Myotubes
Kyohei Furukawa, Marika Kousaka, Huijuan Jia, Hisanori Kato
Nutrients.2022; 14(19): 3979. CrossRef - Normative reference values and regression equations to predict the 6-minute walk distance in the Asian adult population aged 21–80 years
Meredith T. Yeung, Melissa Y. Chan, Katherin S. Huang, Tian Jie Chen, Cyprian P. Chia, Meihiko M. Fong, Cherilyn S. Ho, Derek T. Koh, Mitchell J. Neo, Mark Tan
Hong Kong Physiotherapy Journal.2022; 42(02): 111. CrossRef - Food insecurity as a risk factor of sarcopenic obesity in older adults
Diana Fonseca-Pérez, Cecilia Arteaga-Pazmiño, Claudia P. Maza-Moscoso, Sara Flores-Madrid, Ludwig Álvarez-Córdova
Frontiers in Nutrition.2022;[Epub] CrossRef - Transcriptome Sequencing Analysis of circRNA in Skeletal Muscle between Fast- and Slow-Growing Chickens at Embryonic Stages
Genxi Zhang, Jin Zhang, Pengfei Wu, Xuanze Ling, Qifan Wang, Kaizhi Zhou, Peifeng Li, Li Zhang, Hongxin Ye, Qi Zhang, Qingyu Wei, Tao Zhang, Xinglong Wang
Animals.2022; 12(22): 3166. CrossRef - Amino Acids in Cancer and Cachexia: An Integrated View
Maurizio Ragni, Claudia Fornelli, Enzo Nisoli, Fabio Penna
Cancers.2022; 14(22): 5691. CrossRef - Determination of skeletal muscle mass by aspartate aminotransferase / alanine aminotransferase ratio, insulin and FSH in Chinese women with sarcopenia
Mengting Yin, He Zhang, Qianhui Liu, Fei Ding, Lisha Hou, Yiping Deng, Tao Cui, Yixian Han, Yijun Chen, Chen Huang, Jirong Yue, Yong He
BMC Geriatrics.2022;[Epub] CrossRef - The inflammatory response, a mixed blessing for muscle homeostasis and plasticity
Zineb Bouredji, Anteneh Argaw, Jérôme Frenette
Frontiers in Physiology.2022;[Epub] CrossRef - Effect of CCL11 on In Vitro Myogenesis and Its Clinical Relevance for Sarcopenia in Older Adults
Da Ae Kim, So Jeong Park, Jin Young Lee, Jeoung Hee Kim, Seungjoo Lee, Eunju Lee, Il-Young Jang, Hee-Won Jung, Jin Hoon Park, Beom-Jun Kim
Endocrinology and Metabolism.2021; 36(2): 455. CrossRef - Association of Water Intake with Hand Grip Strength in Community-Dwelling Older Adults
Hyeonmok Kim, Sun Hee Beom, Tae Ho Kim, Beom-Jun Kim
Nutrients.2021; 13(6): 1756. CrossRef - Receptor-Mediated Muscle Homeostasis as a Target for Sarcopenia Therapeutics
Jong Hyeon Yoon, Ki-Sun Kwon
Endocrinology and Metabolism.2021; 36(3): 478. CrossRef - Redox Signaling and Sarcopenia: Searching for the Primary Suspect
Nicholas A. Foreman, Anton S. Hesse, Li Li Ji
International Journal of Molecular Sciences.2021; 22(16): 9045. CrossRef - Aldosterone Inhibits In Vitro Myogenesis by Increasing Intracellular Oxidative Stress via Mineralocorticoid Receptor
Jin Young Lee, Da Ae Kim, Eunah Choi, Yun Sun Lee, So Jeong Park, Beom-Jun Kim
Endocrinology and Metabolism.2021; 36(4): 865. CrossRef - Artificial‐intelligence‐driven discovery of prognostic biomarker for sarcopenia
Heewon Chung, Yunju Jo, Dongryeol Ryu, Changwon Jeong, Seong‐Kyu Choe, Jinseok Lee
Journal of Cachexia, Sarcopenia and Muscle.2021; 12(6): 2220. CrossRef - Association between Neurologic Outcomes and Changes of Muscle Mass Measured by Brain Computed Tomography in Neurocritically Ill Patients
Yun Im Lee, Ryoung-Eun Ko, Joonghyun Ahn, Keumhee C. Carriere, Jeong-Am Ryu
Journal of Clinical Medicine.2021; 11(1): 90. CrossRef - Computed Tomography-Derived Skeletal Muscle Radiodensity Is an Early, Sensitive Marker of Age-Related Musculoskeletal Changes in Healthy Adults
Yeon Woo Jung, Namki Hong, Joon Chae Na, Woong Kyu Han, Yumie Rhee
Endocrinology and Metabolism.2021; 36(6): 1201. CrossRef
- Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age

- Hypothalamus and Pituitary gland
- Current National and International Guidelines for the Management of Male Hypogonadism: Helping Clinicians to Navigate Variation in Diagnostic Criteria and Treatment Recommendations
- Ahmed Al-Sharefi, Richard Quinton
- Endocrinol Metab. 2020;35(3):526-540. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.760

- 9,535 View
- 505 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Male hypogonadism—rebadged by some as testosterone deficiency syndrome—is a clinical and biochemical diagnosis of increasing worldwide interest. Organic male hypogonadism—usually permanent—is well-established, but aging men may also exhibit lower serum testosterone levels; principally due to burden of extra-gonadal comorbidities such as obesity, diabetes and metabolic syndrome, but with an underlying intact hypothalamo-pituitary-testicular (HPT) axis capable of springing back into operation once comorbidities are addressed. Despite encouraging observational data and plausible theoretical underpinning, evidence for efficacy and safety of testosterone in this “aging” group of men is lacking; addressing comorbid illnesses remains the key priority instead. Nevertheless, in recent years, accumulation of misleading information online has triggered a global tsunami of testosterone prescriptions. Despite this, many men with organic hypogonadism remain undiagnosed or untreated; many more face a diagnostic odyssey before achieving care by the appropriate specialist. As testosterone therapy is not without risk several clinical practice guidelines have been published specialist societies to guide physicians on best practice. However, these are heterogeneous in key areas, reflecting divergent approaches to the same evidence basis. Herein, we navigate the major clinical practice guidelines on male hypogonadism and test their respective recommendations against current best evidence.
-
Citations
Citations to this article as recorded by- Expert Opinion on the Diagnosis and Management of Male Hypogonadism in India
Sanjay Kalra, Jubbin Jacob, A. G. Unnikrishnan, Ganapathi Bantwal, Abhay Sahoo, Rakesh Sahay, Sushil Jindal, Madhu Sudan Agrawal, Nitin Kapoor, Banshi Saboo, Mangesh Tiwaskar, Kapil Kochhar, Henrik Falhammar
International Journal of Endocrinology.2023; 2023: 1. CrossRef - Management Outcomes in Males With Hypogonadotropic Hypogonadism Treated With Gonadotropins
Bahaa O Sahib, Ibrahim H Hussein, Nassar T Alibrahim, Abbas A Mansour
Cureus.2023;[Epub] CrossRef - The Association between Inflammation, Testosterone and SHBG in men: A cross‐sectional Multi‐Ethnic Study of Atherosclerosis
Amar Osmancevic, Bledar Daka, Erin D. Michos, Penelope Trimpou, Matthew Allison
Clinical Endocrinology.2023; 99(2): 190. CrossRef - The Illusory Case for Treatment of an Invented Disease
David J. Handelsman
Frontiers in Endocrinology.2022;[Epub] CrossRef - Effect of Chronic Heart Failure Complicated with Type 2 Diabetes Mellitus on Cognitive Function in the Elderly
Yang Liu, Rui Meng, Jianzeng Dong, Xiaonan Xi
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Different Factors Are Associated With Sex Hormones and Leydig Cell Function in Israelis and Palestinians in Jerusalem
Guy Vishnevsky, Ronit Sinnreich, Hisham Nassar, Dafna Merom, Maya Ish-Shalom, Jeremy D. Kark, Hagai Levine
American Journal of Men's Health.2022; 16(4): 155798832211060. CrossRef - Association of rs9939609 polymorphism in the FTO gene with features of androgen status in men
S. V. Yankovskaya, K. I. Mosalev, I. D. Ivanov, B. B. Pinkhasov, V. G. Selyatitskaya
Сибирский научный медицинский журнал.2022; 42(2): 18. CrossRef - Clinical and pharmacological basis of the use of testosterone drugs for hormonal replacement therapy for hypogonadism in men
N. I. Volkova, A. V. Safronenko, E. V. Gantsgorn, Yu. S. Degtyareva
Obesity and metabolism.2022; 19(2): 233. CrossRef - Monitoring and Management of Bardet-Biedl Syndrome: What the Multi-Disciplinary Team Can Do
Lavinia Caba, Laura Florea, Elena Emanuela Braha, Valeriu Vasile Lupu, Eusebiu Vlad Gorduza
Journal of Multidisciplinary Healthcare.2022; Volume 15: 2153. CrossRef - Non-alcoholic fatty liver disease across endocrinopathies: Interaction with sex hormones
Sara Arefhosseini, Mehrangiz Ebrahimi-Mameghani, Farzad Najafipour, Helda Tutunchi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men
Bruno Lunenfeld, George Mskhalaya, Michael Zitzmann, Giovanni Corona, Stefan Arver, Svetlana Kalinchenko, Yuliya Tishova, Abraham Morgentaler
The Aging Male.2021; 24(1): 119. CrossRef
- Expert Opinion on the Diagnosis and Management of Male Hypogonadism in India


 KES
KES

 First
First Prev
Prev



