Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(2); 2024 > Article
-
Review ArticleCalcium & bone metabolism Bone Loss after Solid Organ Transplantation: A Review of Organ-Specific Considerations
 Keypoint
Keypoint
· This review article explores the often overlooked issue of solid organ transplantation-induced osteoporosis, emphasizing its significance in post-transplant care.
· It delves into the prevalence and multifactorial causes of this condition, including post-transplant health decline, hormonal changes, and the effects of immunosuppressive medication.
· The review also highlights the importance of organ-specific approaches in understanding and managing transplantation osteoporosis and aims to raise awareness among clinicians and researchers, ultimately contributing to improved care for transplant recipients. -
Kyoung Jin Kim1*
 , Jeonghoon Ha2*
, Jeonghoon Ha2* , Sang Wan Kim3, Jung-Eun Kim4, Sihoon Lee5, Han Seok Choi6, Namki Hong7, Sung Hye Kong8,9, Seong Hee Ahn10, So Young Park11, Ki-Hyun Baek12
, Sang Wan Kim3, Jung-Eun Kim4, Sihoon Lee5, Han Seok Choi6, Namki Hong7, Sung Hye Kong8,9, Seong Hee Ahn10, So Young Park11, Ki-Hyun Baek12 , on Behalf of Metabolic Bone Disease Study Group of Korean Endocrine Society
, on Behalf of Metabolic Bone Disease Study Group of Korean Endocrine Society -
Endocrinology and Metabolism 2024;39(2):267-282.
DOI: https://doi.org/10.3803/EnM.2024.1939
Published online: April 25, 2024
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
3Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
4Department of Molecular Medicine, Cell and Matrix Research Institute, School of Medicine, Kyungpook National University, Daegu, Korea
5Department of Internal Medicine, Gachon University College of Medicine, Incheon, Korea
6Department of Internal Medicine, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea
7Department of Internal Medicine, Endocrine Research Institute, Yonsei University College of Medicine, Seoul, Korea
8Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
9Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
10Department of Endocrinology and Metabolism, Inha University Hospital, Inha University College of Medicine, Incheon, Korea
11Department of Endocrinology and Metabolism, College of Medicine, Kyung Hee University, Seoul, Korea
12Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding author: Ki-Hyun Baek. Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 10 63-ro, Yeongdeungpo-gu, Seoul 07345, Korea Tel: +82-2-3779-1400, Fax: +82-2-780-3132, E-mail: drbkh@catholic.ac.kr
- *These authors contributed equally to this work.
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 478 Views
- 23 Download
ABSTRACT
- This review article investigates solid organ transplantation-induced osteoporosis, a critical yet often overlooked issue, emphasizing its significance in post-transplant care. The initial sections provide a comprehensive understanding of the prevalence and multifactorial pathogenesis of transplantation osteoporosis, including factors such as deteriorating post-transplantation health, hormonal changes, and the impact of immunosuppressive medications. Furthermore, the review is dedicated to organ-specific considerations in transplantation osteoporosis, with separate analyses for kidney, liver, heart, and lung transplantations. Each section elucidates the unique challenges and management strategies pertinent to transplantation osteoporosis in relation to each organ type, highlighting the necessity of an organ-specific approach to fully understand the diverse manifestations and implications of transplantation osteoporosis. This review underscores the importance of this topic in transplant medicine, aiming to enhance awareness and knowledge among clinicians and researchers. By comprehensively examining transplantation osteoporosis, this study contributes to the development of improved management and care strategies, ultimately leading to improved patient outcomes in this vulnerable group. This detailed review serves as an essential resource for those involved in the complex multidisciplinary care of transplant recipients.
- Solid organ transplantation has revolutionized the landscape of medical therapeutics, offering a lifeline for patients with end-stage renal, liver, cardiac, and pulmonary diseases [1,2]. Over the past two decades, remarkable advances in immunosuppressive agents and surgical techniques have significantly improved patient and graft survival rates [3]. The success of solid organ transplantation has led to a steady rise in transplant procedures, with the number nearly doubling since 1988 and surpassing 25,000 annually in the United States alone [3]. However, as patients increasingly benefit from transplantation, certain major complications should be considered with significant attention, particularly transplantation-induced osteoporosis [4,5].
- Osteoporosis, a condition characterized by compromised bone strength and increased susceptibility to fracture, is a pervasive concern in the transplant community [6]. Approximately half of all transplant recipients exhibit signs of osteoporosis, and some centers report vertebral fractures in nearly one-third of their patients [7]. Organ dysfunction is associated with pronounced adverse effects on skeletal integrity, as evidenced by marked decreases in bone mineral density (BMD), deterioration of microstructural quality, and a consequent reduction in biomechanical strength, cumulatively increasing the risk of osteoporotic fractures even before transplantation [8]. The most precipitous decline in BMD typically occurs within the first 6 to 12 months following transplantation, marked by a striking surge in fracture risk [9,10]. Recent research underscores the gravity of this issue, revealing that the hazard ratio for osteoporosis after solid organ transplantation is approximately five times higher than that for non-transplant patients [9]. Several key risk factors contribute to the development of transplantation osteoporosis, with pre-transplant bone disease and post-transplant immunosuppressive therapy emerging as the primary culprit [7,11,12]. Aging, tobacco use, alcohol consumption, nutritional deficiencies, immobility, and hypogonadism are additional factors that compound the risk [2].
- This review article aims to expound on the significant problems associated with transplantation-related osteoporosis. This study provides a comprehensive overview of the prevalence of osteoporosis and fractures in transplant recipients, elucidates the underlying etiological factors, and examines the natural history of this condition. Furthermore, this review focuses on the latest advancements in prevention and treatment strategies, offering a beacon of hope for transplant recipients at risk of this debilitating complication.
INTRODUCTION
- Bone loss after solid organ transplantation
- Cross-sectional studies have provided comprehensive insights into osteoporosis prevalence, the rate of bone loss, and the frequency of fractures following various types of organ transplantation, highlighting the significant bone health challenges in these patients (Table 1) [1,10]. In kidney transplant recipients, osteoporosis prevalence is reported to be 17% to 49% at the lumbar spine, 11% to 56% at the femoral neck, and 22% to 52% at the radius [13]. Bone loss rates are highest in the first 6 to 18 months post-transplantation, ranging from 4% to 10% in the lumbar spine and 5% to 8% in the femoral neck [14]. Despite not being consistently linked to factors such as sex, patient age, or cumulative glucocorticoid (GC) dose, BMD remains low even up to 20 years after transplantation [10]. Lung transplant recipients exhibit a high osteoporosis prevalence (up to 73%) [10]. During the first year after transplantation, bone loss in the lumbar spine and femoral neck ranges from 2% to 5% [13]. Cardiac transplant recipients undergo the most rapid bone loss within the first year post-transplant, with spinal BMD declining by 6% to 10% in the first 6 months and femoral neck BMD decreasing by 6% to 11% in the first year [15,16]. Bone loss is associated with vitamin D and testosterone deficiency in men [16]. After liver transplantation, the highest rates of bone loss and fractures are observed during the first 6 to 12 months [13]. Earlier studies reported a spine BMD decline of 2% to 24% in the first year, although more recent studies have revealed lower rates of bone loss [10]. After solid organ transplantation, large decreases in BMD at the lumbar spine and femoral neck are observed during the first year, mainly in the first 3 to 6 months, likely related to large doses of GCs used immediately after grafting [14-18]. Rates of lumbar spine bone loss slow thereafter, with stabilization by 6 to 12 months and even some recovery after liver, lung, and heart transplantation [7].
- Fracture outcomes after solid organ transplantation
- Fractures are more common in appendicular sites than in axial sites and are especially prevalent within the first 3 years post-transplant, with women and patients transplanted for diabetic nephropathy at an increased risk [19]. The fracture rates during this period after lung transplantation are high, between 18% and 37% [15]. Vertebral fracture incidence in cardiac transplant recipient ranges from 33% to 36% in the first 1 to 3 years pos-ttransplant [20]. The fracture rates after liver transplantation range from 24% to 65%, primarily affecting the ribs and vertebrae [10]. Women with primary biliary cirrhosis are at the greatest risk of pre-liver transplantation spinal and femoral neck BMD, with preexisting vertebral fractures being predictive of post-transplant fractures [21]. For heart and liver transplant recipients, the occurrence of fractures is most common in the year following transplantation, closely mirroring the periods of most significant BMD loss [20,22,23]. This indicates a critical phase where bone health is notably at risk. In kidney transplant recipients, the risk of fractures remains high, aligned with a continuous decrease in BMD [24].
- The nature of fractures varies by the type of transplant: lung, heart, and liver transplant patients are more prone to fractures in the spine and ribs, while kidney transplant patients frequently experience fractures in the hip and ankle/foot [15,19,23]. Key factors increasing fracture risk post-transplant include older age, a history of fractures prior to the transplant, postmenopausal status, and a lower body mass index [20,22-24]. For kidney transplant patients, additional risks involve diabetes mellitus and extended dialysis [25]. This highlights the need for tailored approaches to manage and mitigate fracture risks in transplant recipients.
PREVALENCE OF TRANSPLANTATION OSTEOPOROSIS
- Various factors play a role in the development of osteoporosis following solid organ transplantation (Fig. 1) [7,26]. Contributors to this condition include pre-transplant bone disorders, use of immunosuppressive drugs, dietary and lifestyle habits, and imbalances in the parathyroid-calcium-vitamin D and pituitar-ygonadal axes [7]. Patients awaiting organ transplantation frequently experience fractures and/or osteoporosis. Factors such as advanced age, inadequate nutrition, limited mobility, hypogonadism, cachexia, and lifestyle habits such as smoking and excessive alcohol consumption often play a significant role in the compromised bone health observed in patients with organ failure requiring transplantation. Moreover, specific pathophysiological conditions associated with liver, lung, heart, and kidney failure uniquely affect bone health before transplantation.
- Declining health conditions
- As patients endure longer waiting periods for organ transplantation, they frequently experience worsening health conditions characterized by reduced physical activity, inadequate nutrition, and muscle loss [7,27]. If not properly managed, these factors can detrimentally affect bone health. A notable observation is the association between the extent of bone loss and the duration of post-transplant hospital stay.
- Hormonal changes after transplantation
- Regarding the effects of the hypothalamic-pituitary-gonadal (HPG) axis related to transplantation, an initial decline occurs in testosterone levels, which typically normalizes within the first year, specifically in cardiac transplant recipients [16]. Despite experiencing significant changes in the HPG axis and sex hormone metabolism, liver transplant recipients usually regain normal physiological functions post-transplantation [28]. In women, particularly in those treated for acute liver failure, regular menstrual cycles resume in most cases [29]. Kidney transplants effectively remedy hyperprolactinemia caused by uremia, leading to normalization of the HPG axis in many male and premenopausal female patients [30].
- Changes in serum 25-hydroxyvitamin D and parathyroid hormone after transplantation
- Further insights from prospective studies on lung, heart, and liver transplant recipients have revealed that serum 25-hydroxyvitamin D (25OHD) levels generally increase from low pre-transplant levels to normal levels, a trend possibly linked to vitamin D supplementation [16,31,32]. Liver transplant recipients exhibit a progressive rise in serum parathyroid hormone (PTH) concentration, whereas cardiac transplant recipients usually exhibit no significant change in their mildly elevated PTH levels [16,32]. The causes of these elevated PTH levels remain unclear but could be related to a decrease in renal function observed in some transplant recipients. In kidney transplant recipients, a progressive decrease in serum PTH concentration is observed in the first 6 months post-transplantation [33]. However, persistent hyperparathyroidism is still observed, possibly because of the slow regression of hyperplastic parathyroid glands [34]. Factors influencing ongoing hyperparathyroidism include the duration of pre-transplant dialysis, higher pre-transplant PTH levels, and post-transplant conditions such as reduced glomerular filtration rate, cyclosporine usage, and low serum 25OHD levels [34].
- Immunosuppressive medications
- Immunosuppressive medications, including GCs, calcineurin inhibitors, and mammalian target of rapamycin (mTOR) inhibitors, may influence bone metabolism (Table 2) [35]. Assessing the direct impact of these agents on bone health is more complicated because the severity of post-transplantation bone disorder is also correlated with the debilitating nature of the disease before transplantation [36]. Moreover, immunosuppressants may have long-term negative effects on bone health [37].
- In the post-transplant setting, GCs are essential but vary in dosage depending on the organ transplanted and the number of rejection episodes [3]. Initially administered at high doses immediately after transplantation, these doses are rapidly tapered off but may be increased during periods of graft rejection [3,10]. The most significant bone loss, predominantly in trabecular bone sites, occurs within the first 3 to 12 months post-transplantation [10]. Recent advances in immunosuppressive therapies have curtailed the use of GCs, consequently reducing the rate of post-transplant bone loss [10]. However, epidemiological studies have demonstrated that even small doses of GCs are linked to a marked increase in fracture risk because of their impact on bone metabolism [38,39]. The pathogenesis of GC-induced osteoporosis involves direct and indirect mechanisms that affect bone tissue [39]. GCs disrupt the balance between bone resorption and formation, leading to a net loss of bone mass and deterioration of bone strength. At the cellular level, GCs enhance the activity of osteoclasts by stimulating the production of receptor activators of nuclear factor kappa B ligand while simultaneously decreasing the production of its decoy receptor, osteoprotegerin. This results in the increased differentiation, activation, and survival of osteoclasts. Additionally, GCs prolong the lifespan of osteoclasts by inducing the production of macrophage colony-stimulating factors. GCs also severely affect osteoblasts, significantly reducing their number and function, leading to impaired bone formation. Furthermore, GCs inhibit the function of mature osteoblasts, suppress the synthesis of type I collagen, and disrupt insulin-like growth factors 1 (IGF-1) critical for bone formation. They also inhibit Wnt-β-catenin signaling and promote apoptosis in osteoblasts and osteocytes, contributing to a disproportionate reduction in bone strength relative to BMD loss. GCs counteract the effects of vitamin D, reducing intestinal calcium absorption and renal tubular calcium reabsorption [40]. This disruption of calcium metabolism is exacerbated by GC-induced alterations in hormonal pathways, including those of growth hormones, IGF-1, gonadotropins, and PTH [41]. The immediate post-transplantation period is characterized by enhanced bone remodeling with increased levels of bone resorption markers, often accompanied by secondary hyperparathyroidism [10]. This condition can be further aggravated by low vitamin D levels commonly observed in patients before transplantation. The negative effects of GCs on muscle and sex hormone levels indirectly affect bone health, muscle strength, and balance, thereby increasing the risk of fractures [42]. Current therapeutic approaches in transplant medicine emphasize the use of the lowest effective dose of GCs to ensure graft survival. The overall reduction in GCs post-transplantation appears to be beneficial for skeletal health. However, further prospective studies are needed to confirm this and evaluate the long-term effects of such treatment strategies.
- Cyclosporine and tacrolimus, key calcineurin inhibitors, are central to immunosuppressive therapy post-solid organ transplantation. These drugs bind to cytoplasmic proteins and selectively inhibit calcineurin, impeding the transcription of cytokines such as interleukin-2, tumor necrosis factor-alpha, and interferon-gamma in T lymphocytes [43]. Earlier studies have suggested that cyclosporine may reduce bone resorption and boost osteoblastic function [44]. Kidney transplant recipients who followed a post-transplantation regimen including cyclosporine but excluding GCs reportedly experienced less bone loss and a reduced fracture rate [45]. However, a significant reduction in BMD was initially associated with tacrolimus [46]. Tacrolimus-based regimens, which may limit GC use, are believed to cause a more modest reduction in BMD than cyclosporine [47]. Animal studies have demonstrated that tacrolimus increases bone volume and reduces osteoclast numbers [48]. However, comparative studies in rodents have indicated that, while cyclosporine may enhance both bone formation and resorption, leading to high-turnover bone loss, tacrolimus predominantly enhances bone resorption, resulting in bone loss. In light of these findings, BMD monitoring is recommended for patients receiving calcineurin inhibitors, as these agents have demonstrated dose-dependent effects on bones in preclinical models [49].
- Sirolimus and everolimus are macrolide immunosuppressants that inhibit mTOR and affect T- and B-lymphocyte responses to cytokines, unlike earlier immunosuppressants such as cyclosporine and tacrolimus [50]. These mTOR inhibitors have demonstrated potential benefits for bone health in preclinical studies. They upregulate osteoprotegerin in bone marrow cells and reduce cathepsin K levels in human osteoclasts, suggesting decreased bone resorption [51]. However, high mTOR inhibitor levels may negatively affect osteoblast differentiation [52]. Clinical studies, particularly in oncology, suggest that mTOR inhibition with everolimus suppresses bone turnover irrespective of prior treatments or bone metastases [53]. In kidney transplant recipients, sirolimus-based immunosuppression is linked to reduced bone turnover markers and inhibition of osteoclast formation, offering an advantage over calcineurin inhibitors [54]. Further research is needed to fully understand the impact of mTOR inhibitors on the skeleton; however, current evidence suggests their potential in reducing osteoporosis risk in transplant patients and lessening the dependence on GCs. Particularly, sirolimus has been identified as a bone-sparing agent, with everolimus exhibiting promise in suppressing bone resorption and exerting bone-protective effects.
- Mycophenolic acid (MPA) and azathioprine are the key immunosuppressants used in transplant medicine. MPA, used for its ability to inhibit B- and T-lymphocyte proliferation, is available as mycophenolate mofetil and enteric-coated mycophenolate sodium and has fewer gastrointestinal side effects and potential bone marrow suppression [55]. Azathioprine, a purine metabolism antagonist, also reduces lymphocyte numbers and immunoglobulin synthesis, with similar risks of bone marrow suppression [56]. The precise effect of these agents on bone fragility in transplant recipients is not well defined; however, they are hypothesized to indirectly protect bone health by reducing the need for concurrent GC administration [10]. However, this protective effect requires confirmation in future studies.
MULTIFACTORIAL PATHOGENESIS OF TRANSPLANTATION OSTEOPOROSIS
- Kidney transplantation
- In patients with chronic kidney disease (CKD), an imbalance in bone remodeling leads to predominant bone resorption influenced by the uremic environment and upregulation of receptor activator of nuclear factor kappa-b ligand (RANKL), contributing to renal osteodystrophy or CKD-metabolic bone disease (CKD-MBD), which is associated with a high fracture risk, particularly end-stage renal disease (ESRD) [57]. This risk is elevated in Caucasian and female dialysis patients and is correlated with increased mortality [58]. Following renal transplantation, this high fracture risk persists, with incidence rates ranging from 7% to 44% in the post-transplant period, particularly within the first 3 years and predominantly at appendicular sites such as hips and ankles [2,25,59,60]. Studies indicate a 3.6- to 3.8-fold increase in fracture risk compared to the general population, with improvements in immunosuppressive therapy contributing to a decline in hip fracture rates since 1997 [60].
- Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend BMD assessments in patients with CKD, as these patients have a heightened risk of fractures and mortality [57]. CKD-MBD typically involves cortical bone loss associated with peripheral fractures. The pharmacological management for CKD-MBD is not well defined; however, a period of observation is suggested before initiating anti-fracture treatments and post-mineral disorder correction. The efficacy of treatments such as bisphosphonates in reducing fracture risk in CKD is not well established, and most antiosteoporotic medications are not recommended for patients with an epidermal growth factor receptor below 30 mL/min [61]. Denosumab is promising in improving BMD in patients with CKD but requires monitoring for the occurrence of hypocalcemia [62].
- The high fracture risk in patients with CKD results from a combination of traditional osteoporosis risk factors and those specific to CKD, such as age, sex, diabetes, and GC use [58]. The fracture risk assessment tool can assess fracture risk in nondialysis patients with CKD; however, conditions such as scoliosis or osteoarthritis may overestimate lumbar spine BMD, necessitating spine radiography or vertebral fracture assessment by dual X-ray absorptiometry for accurate evaluation [63]. The trabecular bone score (TBS) is also useful in enhancing fracture risk prediction in both ESRD and kidney transplant recipients [64].
- Bone turnover markers are negatively correlated with volumetric hip BMD; however, they are limited by biological variability, retention in worsening kidney disease, and other factors [65]. Bone-specific alkaline phosphatase, procollagen type 1 N-terminal propeptide, and tartrate-resistant acid phosphatase may be useful in differentiating bone turnover rates [66]. Additionally, the correlation between PTH levels and fracture rates, which are indicative of different bone disorders, is significant. PTH values less than 150 pg/mL are markers of adynamic bone disease, and those above 600 pg/mL denote high-turnover hyperparathyroid bone disease, each linked to an increased likelihood of fractures [58]. Effectively controlling PTH levels can enhance bone mineralization and reduce the levels of bone turnover markers. Emerging biomarkers such as sclerostin and fibroblast growth factor 23 are still under investigation [58]. Histomorphometric analysis of bone biopsies remains the gold standard for diagnosing CKD-MBD; however, its practical implementation in clinical settings remains challenging [67].
- In kidney transplant recipients, a range of bone and mineral abnormalities, including hypophosphatemia, hypercalcemia, hyperparathyroidism, osteomalacia, osteopenia, and osteoporosis, are commonly observed post-transplantation [68]. Despite transplantation, bone disease often does not improve. The first year post-transplantation, especially the initial 3 to 6 months, is critical for bone loss, predominantly in trabecular bone-rich areas [14]. This loss is influenced by high doses of GC and cyclosporine during the early post-transplant months and can be more substantial than the typical annual rate of decline in postmenopausal women [69]. Factors such as lower body weight, older age, lower glomerular filtration rate, GC treatment, and pretransplantation diabetes are associated with lower BMD, which is associated with fracture rates [70]. Long-term, post-transplant recipients continue to exhibit lower BMD than healthy controls, but the trabecular microarchitecture and bone mechanical properties may not differ significantly [71]. Persistent secondary hyperparathyroidism remains a risk factor for bone loss in transplant recipients, with elevated baseline levels of PTH and phosphate improving post-transplant [14]. Bone turnover decreases over time, and recipients often develop low bone turnover or adynamic bone disease, which is potentially exacerbated by bisphosphonates [72]. Bone biopsies indicate reduced turnover and impaired mineralization, with changes in trabecular number and separation and improvements in cortical thickness. Overall, conventional laboratory tests, imaging, and fracture risk assessment tools should be interpreted cautiously in the post-transplantation setting owing to the lack of standardization [68].
- Various treatments, including calcitriol, alfacalcidol, and bisphosphonates such as clodronate, alendronate, risedronate, pamidronate, ibandronate, zoledronic acid, denosumab, and teriparatide have been explored for managing bone diseases in kidney transplant recipients [1]. However, randomized controlled trials evaluating these treatments are underpowered to assess their impact on fracture risk reduction, with fracture data often not reported or only captured as adverse events. Consequently, the efficacy of these agents is primarily inferred from their effects on BMD as a surrogate marker of bone strength. Most studies have demonstrated preservation or an increase in BMD with antiresorptive agents; however, their anti-fracture efficacy in transplant recipients remains controversial [73]. Treatments with denosumab improve areal BMD, TBS, and volumetric BMD [74]. However, the impact on trabecular bone loss and connectivity varies, with some agents, such as zoledronic acid, only partially attenuating trabecular bone loss [75]. Despite their potential benefits, safety concerns exist regarding the use of anti-osteoporotic agents in transplant recipients. Bisphosphonates should be used with caution because of the risk of nephrotoxicity and the exacerbation of adynamic bone disease. A risk of hypocalcemia and secondary hyperparathyroidism also exists with bisphosphonate administration, and denosumab is associated with an increased risk of urinary tract infections and hypocalcemia [76,77]. Additionally, long-term use of bisphosphonates may be associated with atypical femur fractures and jaw osteonecrosis [78]. Vitamin D supplementation, including calcitriol and alfacalcidol, has been demonstrated to improve posttransplantation bone loss [76]. However, their utility may be limited owing to persistent hyperparathyroidism and hypercalcemia.
- The 2017 KDIGO CKD-MBD guidelines recommend BMD testing in kidney transplant recipients if it influences treatment decisions [57]. Treatment options for the first 12 months post-transplantation include vitamin D, calcitriol/alpha-calcidol, and/ or antiresorptive agents. However, data to guide treatment after the first year are insufficient, and bone biopsy should be considered before starting antiresorptive or other osteoporosis therapies to determine the most appropriate treatment based on the type of bone disease [1].
- Liver transplantation
- Managing and preventing osteoporosis and bone fractures are paramount in liver transplant recipients. Before liver transplantation, cirrhosis can lead to bone loss due to various underlying causes, such as alcoholic liver disease, viral infections, non-alcoholic steatohepatitis, or autoimmune disorders [79]. Moreover, several medications prescribed for managing liver diseases, such as GCs, loop diuretics, ribavirin, and tenofovir, adversely affect bone health. Additionally, malnutrition and vitamin D deficiency are frequently observed in patients with liver disease, which further contribute to bone-related issues [79]. Following transplantation, calcineurin inhibitors and GCs are the primary contributors to bone loss [79]. After liver transplantation, BMD decreases rapidly, primarily within the first 3 to 6 months [2,80]. Bone histology studies suggest that bone loss halts at approximately 6 months post-transplantation, with a subsequent increase in BMD, especially in the lumbar spine [81]. This leads to recovery of bone mass within 2 years post-surgery [2,81]. The increase in BMD is significantly more pronounced in premenopausal women than in perimenopausal and postmenopausal women, possibly due to the protective effects of estrogen on the skeleton [82].
- The incidence of post-liver transplantation fractures remains high, ranging from 10% to 43% [20,22]. Most fractures occur within the first 2 years post-transplant, predominantly affecting the lumbar spine and ribs [8,83]. Women with primary biliary cirrhosis and those with severe bone conditions before liver transplantation are at the highest risk [8]. A major risk factor for post-transplant fractures is preexisting low BMD. Additionally, pre-transplant fractures, older age, type of liver disease, and retransplantation also contribute to fracture risk [84]. Immunosuppressive drugs, including tacrolimus, cyclosporine, and corticosteroids, are essential for suppressing organ rejection after transplantation. However, these medications have been associated with negative outcomes for bone health [2,85]. Tacrolimus and cyclosporine have been identified as contributors to rapid trabecular bone loss in liver transplant recipients [2].
- Calcium and vitamin D supplementation is essential for treating osteopenia in patients after liver transplantation. Therefore, all patients are recommended to have adequate daily calcium intake along with vitamin D supplementation to maintain serum calcium levels above 20 to 30 ng/mL [8]. Specific recommendations for calcium and vitamin D intake have not yet been established for Korean transplant recipients. However, the general Korean population is recommended to consume 800–1,000 mg of calcium and 800–1,000 IU of vitamin D daily to improve bone health [86,87]. In transplant recipients, owing to potential changes in dietary calcium intake and absorption, a higher intake of calcium, possibly >1,200 mg, may be recommended [2,8]. Transplant recipients are required to consult their healthcare providers for personalized nutritional advice, considering their unique health needs and conditions. Calcitriol, an active vitamin D metabolite, is often preferred because of its efficacy in patients with liver diseases and is typically prescribed at a dose of 0.25 μg twice daily [88]. Alendronate (70 mg weekly) has demonstrated positive effects on BMD in liver transplant recipients, significantly more than treatment with calcium and calcitriol alone [89]. Studies have also highlighted the benefits of ibandronate (150 mg monthly) combined with vitamin D and calcium for improving BMD and reducing fracture rates in liver transplant patients [90]. The antiresorptive action of zoledronic acid (4 mg monthly infusion) has been recognized for its beneficial effects on bone mineralization and fracture reduction [91]. Raloxifene, a selective estrogen receptor modulator, can increase BMD; however, its efficacy in patients with liver disease requires further investigation [92]. Hormonal therapy for women and testosterone therapy for men are typically not advised because of potential risks, including malignancy and other adverse effects [79]. Moreover, the effectiveness of hormonal and testosterone therapies after transplantation has not been documented.
- In a retrospective study by Brunova et al. [93] involving 63 post-solid organ transplant patients, denosumab (60 mg every 6 months) significantly reduced the prevalence of osteoporosis and improved bone density over an average treatment period of 1.65 years. Particularly effective in transplant recipients with kidney failure or bisphosphonate intolerance, denosumab presents risks such as hypocalcemia or osteonecrosis of the jaw [93]. Zavatta et al. [94] reported that denosumab significantly reduced bone turnover markers in post-liver transplantation osteoporosis, although only a modest increase in the total hip BMD was observed during the observation period. Further studies are required to determine the efficacy of denosumab in liver transplant recipients. Similarly, evidence for the use of anabolic agents, such as teriparatide or romosozumab, in this patient group is lacking, underscoring the need for further research to confirm their efficacy.
- Cardiac transplantation
- Osteoporosis is in patients with end-stage heart disease and is often marked by a significant decrease in BMD before cardiac transplantation [36]. This bone loss accelerates in the first year post-transplantation, begins to stabilize, and potentially increases from the second year onwards [95]. Fragility fractures are common in cardiac transplant recipients, with the incidence ranging between 22% and 44% within the first 3 years posttransplantation, and the risk of vertebral fractures is particularly high in the first year [20,96]. BMD and fractures are not strongly correlated because fractures can occur even at normal BMD levels [23]. Therefore, diagnostic challenges persist in accurately assessing post-transplantation bone fragility, with cross-sectional studies suggesting that the standard densitometric criteria may not be reliable [97].
- In cardiac transplantation candidates with end-stage heart failure, a higher prevalence of impaired bone strength is observed [36,98], largely due to heart failure-associated factors such as immobilization, reduced renal function, secondary hyperparathyroidism, nutritional deficiencies, and the use of certain medications such as loop diuretics, angiotensin-converting enzyme inhibitors, or proton pump inhibitors [99-101]. Longitudinal studies have highlighted that pre-transplant BMD (particularly in the lumbar spine), history of fractures, and age are significant predictors of bone loss or fracture after transplantation [20,102]. The pathophysiology of bone loss following cardiac transplantation is characterized by the disruption of bone remodeling, a delicate process typically balanced between boneresorbing and bone-forming activities. This imbalance is primarily driven by immunosuppressive treatments, notably corticosteroids, and calcineurin inhibitors (such as cyclosporine, tacrolimus, or rapamycin), which are key factors in diminished bone strength [99]. Earlier studies have suggested that within the first year after transplantation, BMD tends to stabilize or slowly improve, although this occurs less rapidly than in the initial months following surgery [8,103]. The decrease in the rate of bone loss after cardiac transplantation is likely due to the gradual lowering of corticosteroid doses in the immunosuppressive therapy regimen [104].
- Studies on pharmacological interventions for bone loss after heart transplantation are limited. Specific guidelines for calcium and vitamin D intake for cardiac transplant recipients have not yet been established. Generally, daily consumption of 800–1,000 mg calcium and 800–1,000 IU vitamin D is suggested to enhance bone health in the Korean population [86,87]. Transplant recipients may need to consume more than 1,200 mg of calcium because of possible alterations in the daily dietary calcium intake and absorption capacity of calcium [2,8]. Resistance exercises have been suggested to play a beneficial role in preventing bone loss after cardiac transplantation [105]. In cardiac transplant recipients, low doses of calcitriol (0.25 μg/day) have been found to be ineffective in preventing lumbar spine bone loss [106]. Sambrook et al. [107] discovered that in lung or cardiac transplant patients, doses of calcitriol between 0.5 and 0.75 μg/day were effective in reducing bone loss in the femoral neck compared with a placebo. Studies have highlighted the effectiveness of bisphosphonates in reducing bone loss in patients undergoing heart transplantation. Shane et al. [108] discovered that administering alendronate at a dose of 10 mg/day resulted in lower bone loss in both the lumbar spine and total hip compared with treatments involving only calcitriol (0.5 μg/day). In a study on male cardiac transplant patients, intravenous ibandronate was found to preserve bone mass, reduce the risk of vertebral fracture, and maintain balanced bone turnover [109]. In a 1-year study, cardiac transplant patients receiving either a single 5-mg infusion of zoledronic acid or alendronate (70 mg weekly) were compared for their effect on BMD. Both treatments prevented bone loss at the hip; however, while zoledronic acid maintained stable spine BMD, alendronate decreased spine BMD [110]. Nevertheless, evidence regarding the efficacy of denosumab in cardiac transplant recipients is limited. Uzquiano et al. [111] observed that denosumab (60 mg every 6 months) effectively improved lumbar spine BMD, suggesting that it is a viable option for treating bone loss in cardiac transplant recipients. The risk of hypocalcemia during denosumab administration can be reduced by adjusting calcium levels before initiating treatment [111]. In a phase 3 study, romosozumab exhibited a higher risk of cardiovascular events than alendronate [112]. Consequently, romosozumab has not undergone clinical trials for treating bone loss in heart transplant recipients, and its use in this group appears inadvisable until more definitive conclusions are drawn regarding its impact on cardiovascular events. The use of teriparatide in cardiac transplant recipients is limited. Reports on its use in patients with low bone turnover and concurrent renal disease have been made [113]; however, additional evidence is required to fully support its application in cardiac transplant recipients.
- Lung transplantation
- Osteoporosis is prevalent among lung transplantation candidates, particularly those with chronic obstructive pulmonary disease (COPD) [114]. The prevalence of osteoporosis and osteopenia in patients with COPD ranges from 9%–69% and 27%–67%, respectively [115]. Factors contributing to this include older age, female sex, low body mass index, malnutrition, history of fractures, tobacco exposure, and steroid consumption [115,116]. After lung transplantation, bone loss in the lumbar spine and femoral neck varies between 2% and 5% in the first year, with pretransplant BMD being an important predictor of subsequent bone loss [102]. However, research on fracture risk after lung transplantation is limited. The incidence of fractures after lung transplantation is estimated to be between 5% and 37% in the first year and can reach up to 53% within 5 years [117-119]. However, the long-term implications of fractures in lung transplant recipients have not been thoroughly investigated.
- Bone loss following lung transplantation is attributed to factors such as a low body mass index, frailty, diabetes, and chronic hypoxia, along with the ongoing use of immunosuppressive therapy [120,121]. GCs and calcineurin inhibitors (cyclosporine or tacrolimus), integral to immunosuppressive regimens, adversely affect bone homeostasis [6,7]. Consequently, osteoporosis prevention, including the use of calcium, vitamin D, and bisphosphonates, is a standard component of lung transplantation protocols [122]. In the realm of solid organ transplantation, patients undergoing lung transplantation are particularly susceptible to low BMD, underscoring the importance of addressing bone loss and fractures both before and after the transplant procedure [123].
- Similar to other solid organ transplants, maintaining proper calcium and vitamin D levels in patients undergoing lung transplantation is crucial. The optimal calcium and vitamin D intake levels in patients who have undergone lung transplantation remain unknown. Generally, an adequate daily calcium intake of 800 to 1,000 mg or more, along with vitamin D supplementation (800 to 1,000 IU), is recommended to maintain serum calcium levels above 20 to 30 ng/mL [2,8]. Bisphosphonates are commonly used to manage bone loss after lung transplantation. Intravenous pamidronate has been effective in preventing bone loss in both the lumbar spine and femoral neck in lung transplant recipients [124]. Additionally, the use of alendronate (10 mg/day), either alone or in combination with mechanical loading, increases spinal BMD post-transplantation [125]. Owing to the more pronounced bone loss observed after lung transplantation than after other organ transplants, prophylactic zoledronic acid is occasionally used before transplantation [126]. Ng et al. [126] studied 60 patients who were administered zoledronic acid 6 months before transplantation and discovered that the prophylactic use of zoledronic acid effectively prevented bone loss post-transplantation. A few reports have suggested the effectiveness of denosumab post-lung transplantation. However, caution is advised owing to the possibility of hypocalcemia after denosumab administration [127]. As in other post-organ transplant settings, the use of anabolic agents, such as teriparatide or romosozumab, is still not well supported by evidence and should be reserved for future studies.
ORGAN-SPECIFIC CONSIDERATIONS
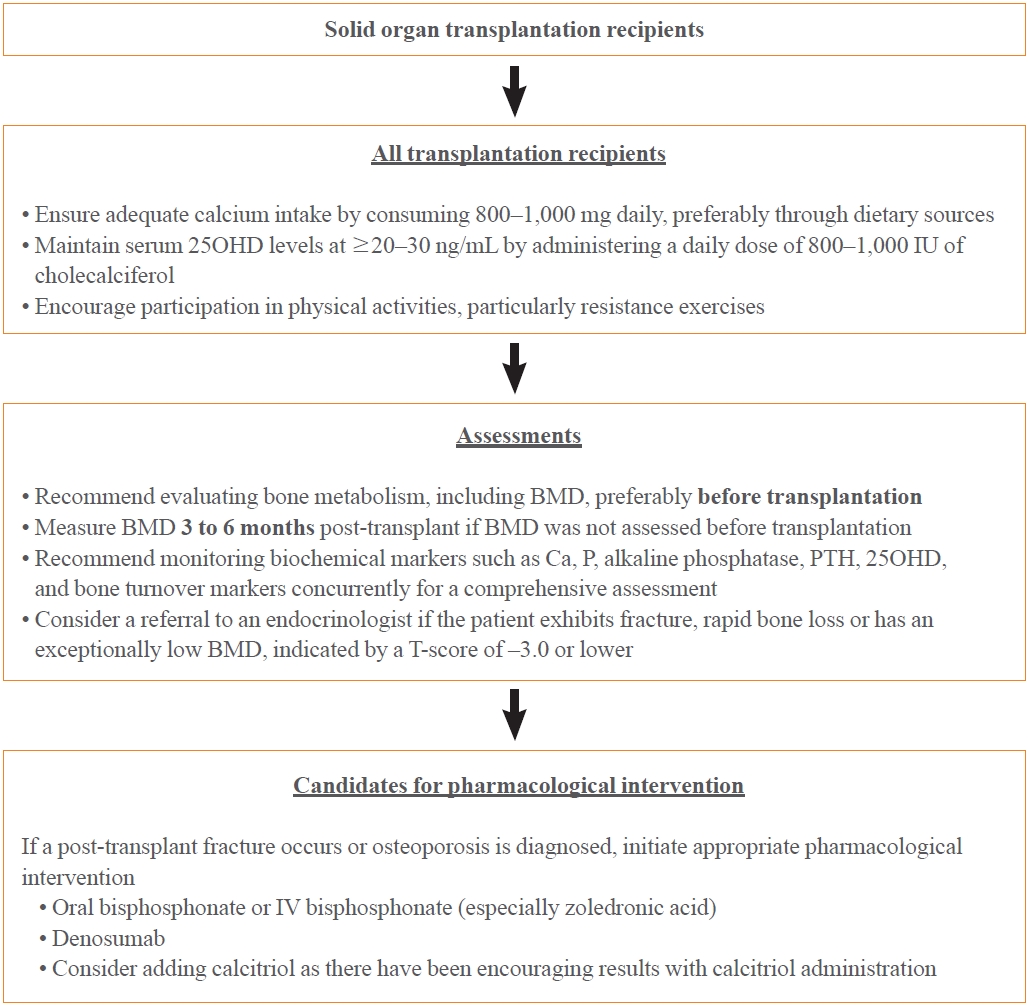
- Transplant recipients face a significant risk of rapid bone loss and high fracture rates, particularly during the early post-transplantation period. This critical phase is often characterized by high doses of GCs and calcineurin inhibitors, leading to uncoupled bone turnover, with increased bone resorption and decreased bone formation. Owing to the lack of reliable clinical predictors of post-transplant fractures and the possibility of fractures in patients with normal pre-transplant BMD, all transplant recipients should be considered for early preventive osteoporosis therapy, particularly long-term recipients with osteoporosis on densitometry evaluation or those who have already experienced fractures.
- The pathogenesis of this condition is not completely understood; however, preexisting bone disorders and transplant-specific therapies play a significant role. Moreover, the rate of bone loss or fracture post-transplantation varies according to gender, ethnicity, and clinical factors; however, the current evidence base is insufficient. This underscores the need for further investigations to explore these potential variations. No definitive standard exists for preventing and treating osteoporosis and fractures after transplantation; however, early intervention with vitamin D and/or bisphosphonates has been associated with a reduced incidence of these conditions. Calcium and vitamin D supplements are essential in transplant recipients; however, the specific requirements for each transplant context remain unclear owing to limited evidence. Considering the unique clinical needs of each patient, a personalized approach should be adopted. The optimal calcium and vitamin D levels in transplant recipients can vary among individuals. Owing to the absence of definitive evidence guiding the exact dosage, adhering to the general guidelines for calcium and vitamin D intake is recommended to maintain bone health until further targeted research provides more specific directions (Fig. 2). Oral or parenteral bisphosphonates are currently the most effective agents for preventing and treating post-transplant osteoporosis. Additionally, active vitamin D metabolites have demonstrated the potential to reduce hyperparathyroidism, especially after kidney transplantation. Denosumab has also been under consideration. However, further research is vital to verify the effectiveness of these various therapeutic approaches and develop comprehensive clinical guidelines for managing bone disorders in organ transplant recipients. Given their mechanisms of action, the anabolic agents teriparatide and romosozumab are expected to be effective in some post-transplant patients. However, evidence supporting their efficacy and safety in this specific patient population remains unclear, necessitating further studies (Fig. 2).
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
-
Acknowledgements
- This research is a component of an initiative by the Metabolic Bone Disease Study Group of the Korean Endocrine Society, marking the accomplishments and efforts of the group during their 2022–2023 tenure. We extend our gratitude to the members of the Metabolic Bone Disease Study Group for their unwavering commitment and significant contributions to the advancement of the group. The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the program year of 2023.
- 1. Anastasilakis AD, Tsourdi E, Makras P, Polyzos SA, Meier C, McCloskey EV, et al. Bone disease following solid organ transplantation: a narrative review and recommendations for management from the European Calcified Tissue Society. Bone 2019;127:401–18.ArticlePubMed
- 2. Lan GB, Xie XB, Peng LK, Liu L, Song L, Dai HL. Current status of research on osteoporosis after solid organ transplantation: pathogenesis and management. Biomed Res Int 2015;2015:413169.ArticlePubMedPMCPDF
- 3. Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21st century. Ann Transl Med 2018;6:409.ArticlePubMedPMC
- 4. Delmas PD. Osteoporosis in patients with organ transplants: a neglected problem. Lancet 2001;357:325–6.ArticlePubMed
- 5. Kulak CA, Cochenski Borba VZ, Kulak J, Ribeiro Custodio M. Osteoporosis after solid organ transplantation. Minerva Endocrinol 2012;37:221–31.PubMed
- 6. Kulak CA, Borba VZ, Kulak J Jr, Custodio MR. Osteoporosis after transplantation. Curr Osteoporos Rep 2012;10:48–55.ArticlePubMedPDF
- 7. Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab 2005;90:2456–65.ArticlePubMed
- 8. Ebeling PR. Approach to the patient with transplantation-related bone loss. J Clin Endocrinol Metab 2009;94:1483–90.ArticlePubMed
- 9. Chen H, Lai YR, Yang Y, Gau SY, Huang CY, Tsai TH, et al. High risk of osteoporosis and fracture following solid organ transplantation: a population-based study. Front Endocrinol (Lausanne) 2023;14:1167574.ArticlePubMedPMC
- 10. Clifford J, Rosen MD. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. Ames: Wiley-Blackwell; 2013. Chapter 61, Transplantation osteoporosis; p. 495-507.
- 11. Shane E, Epstein S. Transplantation osteoporosis. Transplant Rev 2001;15:11–32.Article
- 12. Kulak CA, Borba VZ, Kulak Junior J, Custodio MR. Bone disease after transplantation: osteoporosis and fractures risk. Arq Bras Endocrinol Metabol 2014;58:484–92.ArticlePubMed
- 13. Cohen A, Sambrook P, Shane E. Management of bone loss after organ transplantation. J Bone Miner Res 2004;19:1919–32.ArticlePubMedPDF
- 14. Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 1991;325:544–50.ArticlePubMed
- 15. Shane E, Papadopoulos A, Staron RB, Addesso V, Donovan D, McGregor C, et al. Bone loss and fracture after lung transplantation. Transplantation 1999;68:220–7.ArticlePubMed
- 16. Shane E, Rivas M, McMahon DJ, Staron RB, Silverberg SJ, Seibel MJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997;82:1497–506.ArticlePubMed
- 17. Floreani A, Mega A, Tizian L, Burra P, Boccagni P, Baldo V, et al. Bone metabolism and gonad function in male patients undergoing liver transplantation: a two-year longitudinal study. Osteoporos Int 2001;12:749–54.ArticlePubMedPDF
- 18. Guo CY, Johnson A, Locke TJ, Eastell R. Mechanisms of bone loss after cardiac transplantation. Bone 1998;22:267–71.ArticlePubMed
- 19. Ramsey-Goldman R, Dunn JE, Dunlop DD, Stuart FP, Abecassis MM, Kaufman DB, et al. Increased risk of fracture in patients receiving solid organ transplants. J Bone Miner Res 1999;14:456–63.ArticlePubMedPDF
- 20. Leidig-Bruckner G, Hosch S, Dodidou P, Ritschel D, Conradt C, Klose C, et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet 2001;357:342–7.ArticlePubMed
- 21. Compston JE. Osteoporosis after liver transplantation. Liver Transpl 2003;9:321–30.ArticlePubMed
- 22. Ninkovic M, Skingle SJ, Bearcroft PW, Bishop N, Alexander GJ, Compston JE. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol 2000;12:931–5.ArticlePubMed
- 23. Shane E, Rivas M, Staron RB, Silverberg SJ, Seibel MJ, Kuiper J, et al. Fracture after cardiac transplantation: a prospective longitudinal study. J Clin Endocrinol Metab 1996;81:1740–6.ArticlePubMed
- 24. Vautour LM, Melton LJ 3rd, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: a population-based study. Osteoporos Int 2004;15:160–7.ArticlePubMedPDF
- 25. Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA 2002;288:3014–8.ArticlePubMed
- 26. Kovvuru K, Kanduri SR, Vaitla P, Marathi R, Gosi S, Garcia Anton DF, et al. Risk factors and management of osteoporosis post-transplant. Medicina (Kaunas) 2020;56:302.ArticlePubMedPMC
- 27. Dienemann T, Ziolkowski SL, Bender S, Goral S, Long J, Baker JF, et al. Changes in body composition, muscle strength, and fat distribution following kidney transplantation. Am J Kidney Dis 2021;78:816–25.ArticlePubMedPMC
- 28. Madersbacher S, Ludvik G, Stulnig T, Grunberger T, Maier U. The impact of liver transplantation on endocrine status in men. Clin Endocrinol (Oxf) 1996;44:461–6.ArticlePubMedPDF
- 29. Mass K, Quint EH, Punch MR, Merion RM. Gynecological and reproductive function after liver transplantation. Transplantation 1996;62:476–9.ArticlePubMed
- 30. Saha MT, Saha HH, Niskanen LK, Salmela KT, Pasternack AI. Time course of serum prolactin and sex hormones following successful renal transplantation. Nephron 2002;92:735–7.ArticlePubMedPDF
- 31. Aris RM, Neuringer IP, Weiner MA, Egan TM, Ontjes D. Severe osteoporosis before and after lung transplantation. Chest 1996;109:1176–83.ArticlePubMed
- 32. Feller RB, McDonald JA, Sherbon KJ, McCaughan GW. Evidence of continuing bone recovery at a mean of 7 years after liver transplantation. Liver Transpl Surg 1999;5:407–13.ArticlePubMed
- 33. Julian BA, Quarles LD, Niemann KM. Musculoskeletal complications after renal transplantation: pathogenesis and treatment. Am J Kidney Dis 1992;19:99–120.ArticlePubMed
- 34. Bouquegneau A, Salam S, Delanaye P, Eastell R, Khwaja A. Bone disease after kidney transplantation. Clin J Am Soc Nephrol 2016;11:1282–96.ArticlePubMedPMC
- 35. Epstein S. Post-transplantation bone disease: the role of immunosuppressive agents and the skeleton. J Bone Miner Res 1996;11:1–7.ArticlePubMedPDF
- 36. Dolgos S, Hartmann A, Isaksen GA, Simonsen S, Bjortuft O, Boberg KM, et al. Osteoporosis is a prevalent finding in patients with solid organ failure awaiting transplantation: a population based study. Clin Transplant 2010;24:E145–52.ArticlePubMed
- 37. Yu TM, Lin CL, Chang SN, Sung FC, Huang ST, Kao CH. Osteoporosis and fractures after solid organ transplantation: a nationwide population-based cohort study. Mayo Clin Proc 2014;89:888–95.PubMed
- 38. Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med 2018;379:2547–56.ArticlePubMed
- 39. Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int 2019;30:1145–56.ArticlePubMedPDF
- 40. Rubin MR, Bilezikian JP. Clinical review 151: the role of parathyroid hormone in the pathogenesis of glucocorticoidinduced osteoporosis: a re-examination of the evidence. J Clin Endocrinol Metab 2002;87:4033–41.PubMed
- 41. Bonadonna S, Burattin A, Nuzzo M, Bugari G, Rosei EA, Valle D, et al. Chronic glucocorticoid treatment alters spontaneous pulsatile parathyroid hormone secretory dynamics in human subjects. Eur J Endocrinol 2005;152:199–205.ArticlePubMed
- 42. Klein GL. The effect of glucocorticoids on bone and muscle. Osteoporos Sarcopenia 2015;1:39–45.ArticlePubMed
- 43. Siekierka JJ, Sigal NH. FK-506 and cyclosporin A: immunosuppressive mechanism of action and beyond. Curr Opin Immunol 1992;4:548–52.ArticlePubMed
- 44. Orcel P, Bielakoff J, Modrowski D, Miravet L, de Vernejoul MC. Cyclosporin A induces in vivo inhibition of resorption and stimulation of formation in rat bone. J Bone Miner Res 1989;4:387–91.ArticlePubMedPDF
- 45. McIntyre HD, Menzies B, Rigby R, Perry-Keene DA, Hawley CM, Hardie IR. Long-term bone loss after renal transplantation: comparison of immunosuppressive regimens. Clin Transplant 1995;9:20–4.PubMed
- 46. Stempfle HU, Werner C, Echtler S, Assum T, Meiser B, Angermann CE, et al. Rapid trabecular bone loss after cardiac transplantation using FK506 (tacrolimus)-based immunosuppression. Transplant Proc 1998;30:1132–3.ArticlePubMed
- 47. Monegal A, Navasa M, Guanabens N, Peris P, Pons F, Martinez de Osaba MJ, et al. Bone mass and mineral metabolism in liver transplant patients treated with FK506 or cyclosporine A. Calcif Tissue Int 2001;68:83–6.ArticlePubMedPDF
- 48. Kanda J, Izumo N, Furukawa M, Shimakura T, Yamamoto N, Takahashi HE, et al. Effects of the calcineurin inhibitors cyclosporine and tacrolimus on bone metabolism in rats. Biomed Res 2018;39:131–9.ArticlePubMed
- 49. Movsowitz C, Epstein S, Fallon M, Ismail F, Thomas S. Cyclosporin-A in vivo produces severe osteopenia in the rat: effect of dose and duration of administration. Endocrinology 1988;123:2571–7.ArticlePubMed
- 50. Hardinger KL, Koch MJ, Brennan DC. Current and future immunosuppressive strategies in renal transplantation. Pharmacotherapy 2004;24:1159–76.ArticlePubMed
- 51. Mogi M, Kondo A. Down-regulation of mTOR leads to upregulation of osteoprotegerin in bone marrow cells. Biochem Biophys Res Commun 2009;384:82–6.ArticlePubMed
- 52. Kneissel M, Luong-Nguyen NH, Baptist M, Cortesi R, Zumstein-Mecker S, Kossida S, et al. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone 2004;35:1144–56.ArticlePubMed
- 53. Gnant M, Baselga J, Rugo HS, Noguchi S, Burris HA, Piccart M, et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J Natl Cancer Inst 2013;105:654–63.PMC
- 54. Campistol JM, Holt DW, Epstein S, Gioud-Paquet M, Rutault K, Burke JT, et al. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transpl Int 2005;18:1028–35.ArticlePubMed
- 55. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000;47:85–118.ArticlePubMed
- 56. Trotter JL, Rodey GE, Gebel HM. Azathioprine decreases suppressor T cells in patients with multiple sclerosis. N Engl J Med 1982;306:365–6.Article
- 57. Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int 2017;92:26–36.ArticlePubMed
- 58. Pimentel A, Urena-Torres P, Zillikens MC, Bover J, CohenSolal M. Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 2017;92:1343–55.ArticlePubMed
- 59. Naylor KL, Li AH, Lam NN, Hodsman AB, Jamal SA, Garg AX. Fracture risk in kidney transplant recipients: a systematic review. Transplantation 2013;95:1461–70.PubMed
- 60. Durieux S, Mercadal L, Orcel P, Dao H, Rioux C, Bernard M, et al. Bone mineral density and fracture prevalence in long-term kidney graft recipients. Transplantation 2002;74:496–500.ArticlePubMed
- 61. Wilson LM, Rebholz CM, Jirru E, Liu MC, Zhang A, Gayleard J, et al. Benefits and harms of osteoporosis medications in patients with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2017;166:649–58.ArticlePubMed
- 62. Jamal SA, Ljunggren O, Stehman-Breen C, Cummings SR, McClung MR, Goemaere S, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011;26:1829–35.ArticlePubMedPDF
- 63. Whitlock RH, Leslie WD, Shaw J, Rigatto C, Thorlacius L, Komenda P, et al. The Fracture Risk Assessment Tool (FRAX®) predicts fracture risk in patients with chronic kidney disease. Kidney Int 2019;95:447–54.ArticlePubMed
- 64. Shevroja E, Lamy O, Hans D. Review on the utility of trabecular bone score, a surrogate of bone micro-architecture, in the chronic kidney disease spectrum and in kidney transplant recipients. Front Endocrinol (Lausanne) 2018;9:561.ArticlePubMedPMC
- 65. Evenepoel P, Cavalier E, D’Haese PC. Biomarkers predicting bone turnover in the setting of CKD. Curr Osteoporos Rep 2017;15:178–86.ArticlePubMedPDF
- 66. Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis 2016;67:559–66.ArticlePubMed
- 67. Evenepoel P, Behets GJ, Laurent MR, D’Haese PC. Update on the role of bone biopsy in the management of patients with CKD-MBD. J Nephrol 2017;30:645–52.ArticlePubMedPDF
- 68. Vangala C, Pan J, Cotton RT, Ramanathan V. Mineral and bone disorders after kidney transplantation. Front Med (Lausanne) 2018;5:211.ArticlePubMedPMC
- 69. Segaud N, Legroux I, Hazzan M, Noel C, Cortet B. Changes in bone mineral density after kidney transplantation: 2-year assessment of a French cohort. Osteoporos Int 2018;29:1165–75.ArticlePubMedPDF
- 70. Torregrosa JV, Ferreira AC, Cucchiari D, Ferreira A. Bone mineral disease after kidney transplantation. Calcif Tissue Int 2021;108:551–60.ArticlePubMedPDF
- 71. Perez-Saez MJ, Herrera S, Prieto-Alhambra D, Nogues X, Vera M, Redondo-Pachon D, et al. Bone density, microarchitecture, and tissue quality long-term after kidney transplant. Transplantation 2017;101:1290–4.ArticlePubMed
- 72. Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol 2000;11:1093–9.ArticlePubMed
- 73. Versele EB, Van Laecke S, Dhondt AW, Verbeke F, Vanholder R, Van Biesen W, et al. Bisphosphonates for preventing bone disease in kidney transplant recipients: a meta-analysis of randomized controlled trials. Transpl Int 2016;29:153–64.ArticlePubMed
- 74. Nanmoku K, Shinzato T, Kubo T, Shimizu T, Yagisawa T. Effects of denosumab on hypercalcemia and bone mineral density loss in kidney transplant recipients. Clin Nephrol 2019;92:1–8.ArticlePubMed
- 75. Marques ID, Araujo MJ, Graciolli FG, Dos Reis LM, Pereira RM, Alvarenga JC, et al. A randomized trial of zoledronic acid to prevent bone loss in the first year after kidney transplantation. J Am Soc Nephrol 2019;30:355–65.ArticlePubMedPMC
- 76. El-Agroudy AE, El-Husseini AA, El-Sayed M, Mohsen T, Ghoneim MA. A prospective randomized study for prevention of postrenal transplantation bone loss. Kidney Int 2005;67:2039–45.ArticlePubMed
- 77. Bonani M, Frey D, Brockmann J, Fehr T, Mueller TF, Saleh L, et al. Effect of twice-yearly denosumab on prevention of bone mineral density loss in de novo kidney transplant recipients: a randomized controlled trial. Am J Transplant 2016;16:1882–91.ArticlePubMed
- 78. Park W, Lee SH, Park KR, Rho SH, Chung WY, Kim HJ. Characteristics of bisphosphonate-related osteonecrosis of the jaw after kidney transplantation. J Craniofac Surg 2012;23:e510–4.ArticlePubMed
- 79. Rodriguez-Aguilar EF, Perez-Escobar J, Sanchez Herrera D, Garcia-Alanis M, Toapanta-Yanchapaxi L, GonzalezFlores E, et al. Bone disease and liver transplantation: a review. Transplant Proc 2021;53:2346–53.ArticlePubMed
- 80. Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl 2006;12:1390–402.ArticlePubMed
- 81. Monegal A, Navasa M, Guanabens N, Peris P, Pons F, Martinez de Osaba MJ, et al. Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int 2001;12:484–92.ArticlePubMedPDF
- 82. Baccaro LF, Boin IF, Pedro AO, Costa-Paiva L, Leal AL, Ramos CD, et al. Decrease in bone mass in women after liver transplantation: associated factors. Transplant Proc 2011;43:1351–6.ArticlePubMed
- 83. Collier J. Bone disorders in chronic liver disease. Hepatology 2007;46:1271–8.ArticlePubMed
- 84. Guanabens N, Pares A. Liver and bone. Arch Biochem Biophys 2010;503:84–94.ArticlePubMed
- 85. van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000;39:1383–9.ArticlePubMed
- 86. Han A, Park Y, Lee YK, Park SY, Park CY. Position statement: vitamin D intake to prevent osteoporosis and fracture in adults. J Bone Metab 2022;29:205–15.ArticlePubMedPMCPDF
- 87. Kim KM, Choi HS, Choi MJ, Chung HY. Calcium and vitamin D supplementations: 2015 position statement of the Korean Society for Bone and Mineral Research. J Bone Metab 2015;22:143–9.ArticlePubMedPMC
- 88. Jeong HM, Kim DJ. Bone diseases in patients with chronic liver disease. Int J Mol Sci 2019;20:4270.ArticlePubMedPMC
- 89. Atamaz F, Hepguler S, Akyildiz M, Karasu Z, Kilic M. Effects of alendronate on bone mineral density and bone metabolic markers in patients with liver transplantation. Osteoporos Int 2006;17:942–9.ArticlePubMedPDF
- 90. Kaemmerer D, Schmidt B, Lehmann G, Wolf G, Hommann M, Settmacher U. Monthly ibandronate for the prevention of bone loss in patients after liver transplantation. Transplant Proc 2012;44:1362–7.ArticlePubMed
- 91. Misof BM, Bodingbauer M, Roschger P, Wekerle T, Pakrah B, Haas M, et al. Short-term effects of high-dose zoledronic acid treatment on bone mineralization density distribution after orthotopic liver transplantation. Calcif Tissue Int 2008;83:167–75.ArticlePubMedPDF
- 92. Levy C, Harnois DM, Angulo P, Jorgensen R, Lindor KD. Raloxifene improves bone mass in osteopenic women with primary biliary cirrhosis: results of a pilot study. Liver Int 2005;25:117–21.ArticlePubMedPDF
- 93. Brunova J, Kratochvilova S, Stepankova J. Osteoporosis therapy with denosumab in organ transplant recipients. Front Endocrinol (Lausanne) 2018;9:162.ArticlePubMedPMC
- 94. Zavatta G, Vandi G, Di Dalmazi G, Repaci A, Ravaioli M, Cescon M, et al. Denosumab in post-liver transplantation osteoporosis: preliminary data on the effects on bone mineral density and turnover markers. Endocr Abstr 2019;63:GP110.Article
- 95. Stein E, Ebeling P, Shane E. Post-transplantation osteoporosis. Endocrinol Metab Clin North Am 2007;36:937–63.ArticlePubMed
- 96. Forien M, Coralli R, Verdonk C, Ottaviani S, Ebstein E, Demaria L, et al. Osteoporosis and risk of fracture in heart transplant patients. Front Endocrinol (Lausanne) 2023;14:1252966.ArticlePubMedPMC
- 97. Dalle Carbonare L, Zanatta M, Braga V, Sella S, Vilei MT, Feltrin G, et al. Densitometric threshold and vertebral fractures in heart transplant patients. Transplantation 2011;92:106–11.ArticlePubMed
- 98. Shane E, Mancini D, Aaronson K, Silverberg SJ, Seibel MJ, Addesso V, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med 1997;103:197–207.ArticlePubMed
- 99. Lofdahl E, Radegran G. Osteoporosis following heart transplantation and immunosuppressive therapy. Transplant Rev (Orlando) 2017;31:232–9.ArticlePubMed
- 100. Schnoll-Sussman F, Katz PO. Clinical implications of emerging data on the safety of proton pump inhibitors. Curr Treat Options Gastroenterol 2017;15:1–9.ArticlePubMedPDF
- 101. Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report-2012. J Heart Lung Transplant 2012;31:1052–64.ArticlePubMed
- 102. Wang TK, O’Sullivan S, Gamble GD, Ruygrok PN. Bone density in heart or lung transplant recipients: a longitudinal study. Transplant Proc 2013;45:2357–65.ArticlePubMed
- 103. Van Cleemput J, Daenen W, Nijs J, Geusens P, Dequeker J, Vanhaecke J. Timing and quantification of bone loss in cardiac transplant recipients. Transpl Int 1995;8:196–200.ArticlePubMed
- 104. Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914–56.PubMed
- 105. Kourek C, Karatzanos E, Nanas S, Karabinis A, Dimopoulos S. Exercise training in heart transplantation. World J Transplant 2021;11:466–79.ArticlePubMedPMC
- 106. Cremer J, Struber M, Wagenbreth I, Nischelsky J, Demertzis S, Graeter T, et al. Progression of steroid-associated osteoporosis after heart transplantation. Ann Thorac Surg 1999;67:130–3.ArticlePubMed
- 107. Sambrook P, Henderson NK, Keogh A, MacDonald P, Glanville A, Spratt P, et al. Effect of calcitriol on bone loss after cardiac or lung transplantation. J Bone Miner Res 2000;15:1818–24.ArticlePubMedPDF
- 108. Shane E, Addesso V, Namerow PB, McMahon DJ, Lo SH, Staron RB, et al. Alendronate versus calcitriol for the prevention of bone loss after cardiac transplantation. N Engl J Med 2004;350:767–76.ArticlePubMed
- 109. Fahrleitner-Pammer A, Piswanger-Soelkner JC, Pieber TR, Obermayer-Pietsch BM, Pilz S, Dimai HP, et al. Ibandronate prevents bone loss and reduces vertebral fracture risk in male cardiac transplant patients: a randomized doubleblind, placebo-controlled trial. J Bone Miner Res 2009;24:1335–44.ArticlePubMedPDF
- 110. Shane E, Cohen A, Stein EM, McMahon DJ, Zhang C, Young P, et al. Zoledronic acid versus alendronate for the prevention of bone loss after heart or liver transplantation. J Clin Endocrinol Metab 2012;97:4481–90.ArticlePubMedPMC
- 111. Uzquiano JC, Mendez AA, Bielsa AJ, Carmena MD, Jimenez JF, Sanz-Ayan P. Denosumab treatment for osteopenia or osteoporosis in heart transplant recipients: effects and safety. Transplant Rep 2022;7:100103.Article
- 112. Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 2017;377:1417–27.ArticlePubMed
- 113. Fahrleitner-Pammer A, Wagner D, Krisper P, Amrein K, Dimai H. Teriparatide treatment in a heart transplant patient with a chronic kidney disease and a low-turnover bone disease: a case report. Osteoporos Int 2017;28:1149–52.ArticlePubMedPDF
- 114. Vrieze A, de Greef MH, Wijkstra PJ, Wempe JB. Low bone mineral density in COPD patients related to worse lung function, low weight and decreased fat-free mass. Osteoporos Int 2007;18:1197–202.ArticlePubMedPDF
- 115. Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J 2009;34:209–18.ArticlePubMed
- 116. Maggi S, Siviero P, Gonnelli S, Schiraldi C, Malavolta N, Nuti R, et al. Osteoporosis risk in patients with chronic obstructive pulmonary disease: the EOLO study. J Clin Densitom 2009;12:345–52.ArticlePubMed
- 117. Ferrari SL, Nicod LP, Hamacher J, Spiliopoulos A, Slosman DO, Rochat T, et al. Osteoporosis in patients undergoing lung transplantation. Eur Respir J 1996;9:2378–82.ArticlePubMed
- 118. Hariman A, Alex C, Heroux A, Camacho P. Incidence of fractures after cardiac and lung transplantation: a single center experience. J Osteoporos 2014;2014:573041.ArticlePubMedPMCPDF
- 119. Caffarelli C, Tomai Pitinca MD, Alessandri M, Cameli P, Bargagli E, Bennett D, et al. Timing of osteoporotic vertebral fractures in lung and heart transplantation: a longitudinal study. J Clin Med 2020;9:2941.ArticlePubMedPMC
- 120. Venado A, Kolaitis NA, Huang CY, Gao Y, Glidden DV, Soong A, et al. Frailty after lung transplantation is associated with impaired health-related quality of life and mortality. Thorax 2020;75:669–78.ArticlePubMed
- 121. Lyu DM, Zamora MR. Medical complications of lung transplantation. Proc Am Thorac Soc 2009;6:101–7.ArticlePubMed
- 122. Cahill BC, O’Rourke MK, Parker S, Stringham JC, Karwande SV, Knecht TP. Prevention of bone loss and fracture after lung transplantation: a pilot study. Transplantation 2001;72:1251–5.ArticlePubMed
- 123. Shane E, Papadopoulos A, Staron RB, Addesso V, Donovan D, McGregor C, et al. Bone loss and fracture after lung transplantation. Transplantation 1999;68:220–7.ArticlePubMed
- 124. Trombetti A, Gerbase MW, Spiliopoulos A, Slosman DO, Nicod LP, Rizzoli R. Bone mineral density in lung-transplant recipients before and after graft: prevention of lumbar spine post-transplantation-accelerated bone loss by pamidronate. J Heart Lung Transplant 2000;19:736–43.ArticlePubMed
- 125. Braith RW, Conner JA, Fulton MN, Lisor CF, Casey DP, Howe KS, et al. Comparison of alendronate vs alendronate plus mechanical loading as prophylaxis for osteoporosis in lung transplant recipients: a pilot study. J Heart Lung Transplant 2007;26:132–7.ArticlePubMed
- 126. Ng E, Topliss DJ, Paraskeva M, Paul E, Sztal-Mazer S. The utility of prophylactic zoledronic acid in patients undergoing lung transplantation. J Clin Densitom 2021;24:581–90.ArticlePubMed
- 127. Shrosbree JE, Elder GJ, Eisman JA, Center JR. Acute hypocalcaemia following denosumab in heart and lung transplant patients with osteoporosis. Intern Med J 2018;48:681–7.ArticlePubMedPDF
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite