Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(2); 2024 > Article
-
Review ArticleDiabetes, obesity and metabolism Scaling Insulin-Producing Cells by Multiple Strategies
 Keypoint
Keypoint
· Human-induced pluripotent stem cells hold promise as treatments for insulin-dependent diabetes mellitus, but scalable production remains a challenge.
· Recent advancements in expanding pancreatic progenitors and beta cells show potential, yet practical reproducibility and cost-efficiency remain significant hurdles.
· This manuscript reviews these innovative techniques, analyzing their current limitations and providing a roadmap for optimizing and scaling up these cellular therapies for insulin-dependent diabetes mellitus. -
Jinhyuk Choi1*
 , Fritz Cayabyab1*
, Fritz Cayabyab1* , Harvey Perez1
, Harvey Perez1 , Eiji Yoshihara1,2
, Eiji Yoshihara1,2
-
Endocrinology and Metabolism 2024;39(2):191-205.
DOI: https://doi.org/10.3803/EnM.2023.1910
Published online: April 4, 2024
1The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, CA, USA
2David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA, USA
- Corresponding author: Eiji Yoshihara The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, 1124 W Carson St, Torrance, CA 90502, USA Tel: +1-310-781-1480, E-mail: eiji.yoshihara@lundquist.org
- *These authors contributed equally to this work.
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,344 Views
- 74 Download
ABSTRACT
- In the quest to combat insulin-dependent diabetes mellitus (IDDM), allogenic pancreatic islet cell therapy sourced from deceased donors represents a significant therapeutic advance. However, the applicability of this approach is hampered by donor scarcity and the demand for sustained immunosuppression. Human induced pluripotent stem cells are a game-changing resource for generating synthetic functional insulin-producing β cells. In addition, novel methodologies allow the direct expansion of pancreatic progenitors and mature β cells, thereby circumventing prolonged differentiation. Nevertheless, achieving practical reproducibility and scalability presents a substantial challenge for this technology. As these innovative approaches become more prominent, it is crucial to thoroughly evaluate existing expansion techniques with an emphasis on their optimization and scalability. This manuscript delineates these cutting-edge advancements, offers a critical analysis of the prevailing strategies, and underscores pivotal challenges, including cost-efficiency and logistical issues. Our insights provide a roadmap, elucidating both the promises and the imperatives in harnessing the potential of these cellular therapies for IDDM.
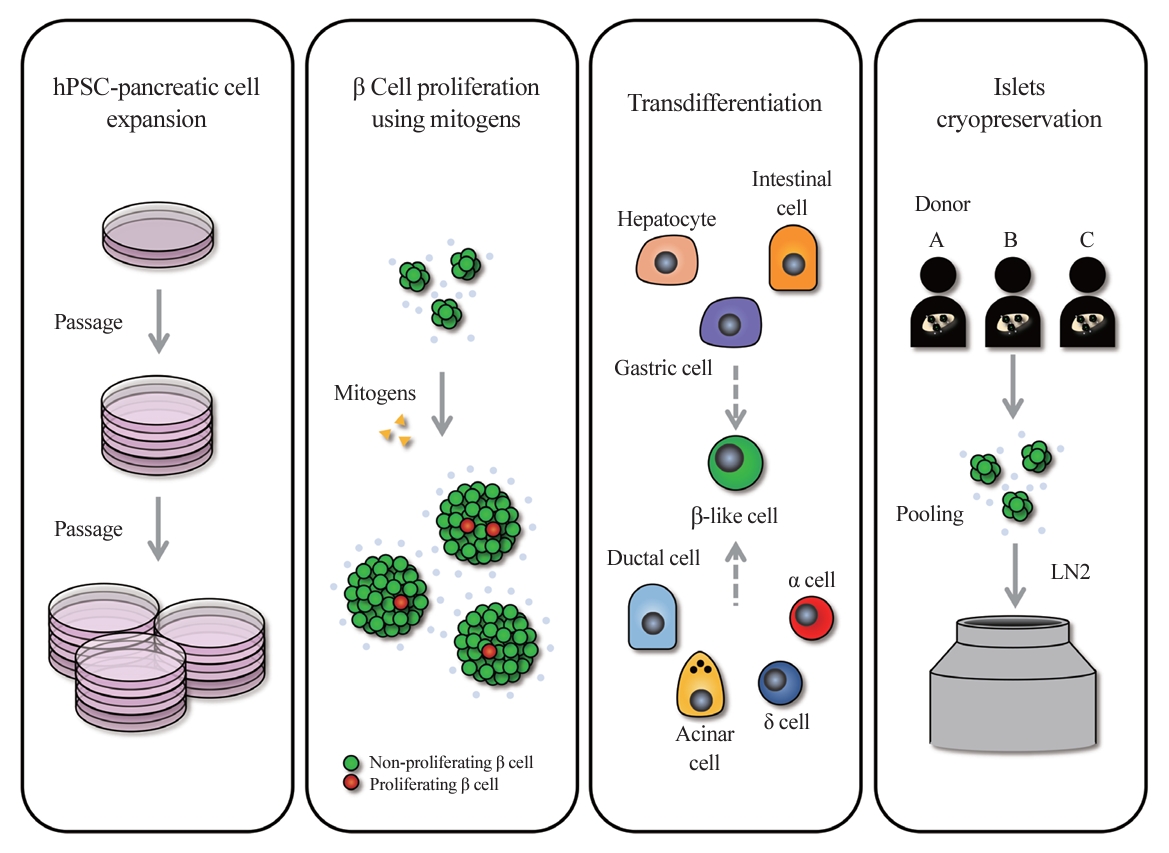
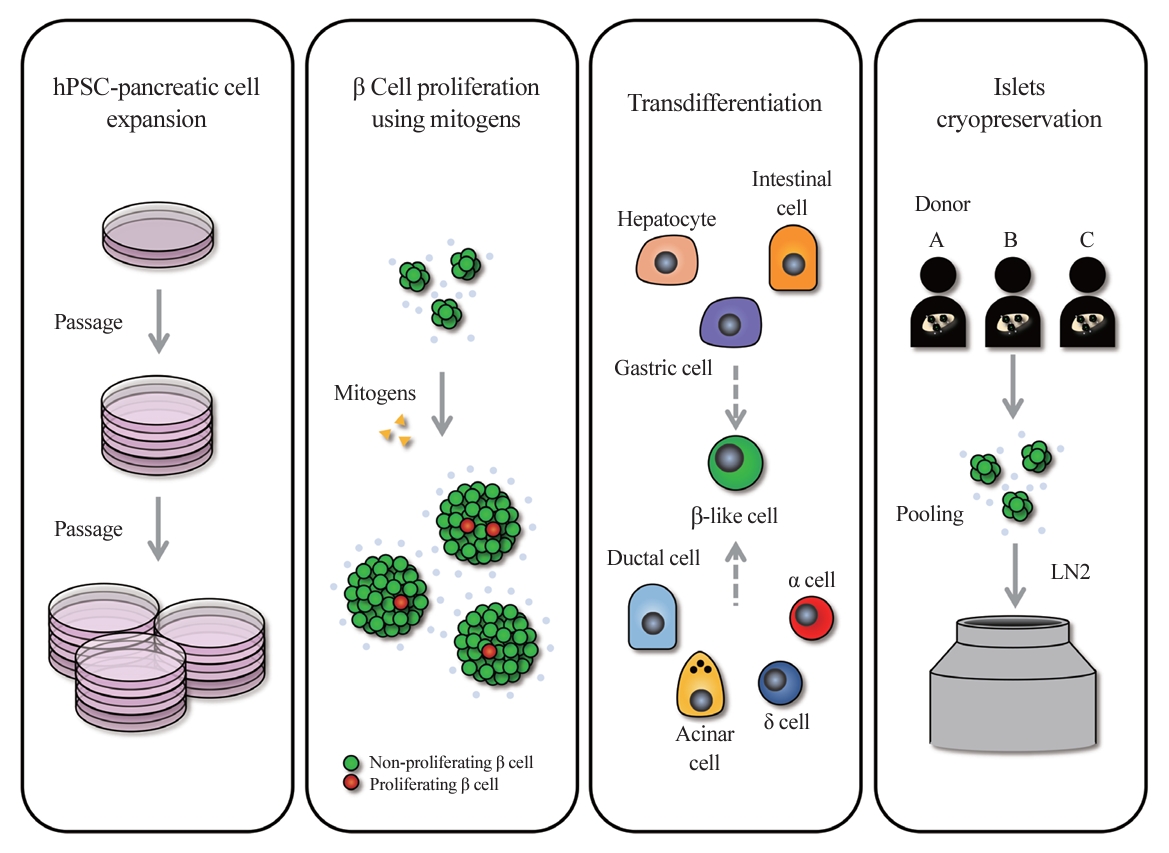
- Diabetes is a heterogeneous metabolic disorder characterized by chronic hyperglycemia, principally due to the loss of β cell mass or a decline in β cell function [1-3]. Although current therapeutic strategies mitigate symptoms and temporarily improve glycemic control, they do not prevent or significantly forestall the progression to associated comorbidities. True remediation of diabetes necessitates the replenishment of β cells capable of precise blood glucose regulation. This can be accomplished primarily through two approaches: increasing the number of endogenous β cells or transplanting β cells from external sources. Stem cell-based islet replacement therapy has emerged as a curative approach for insulin-dependent diabetes mellitus (IDDM) across several preclinical animal studies [4-12] and human trials [13-15]. In 2000, the capabilities of islet replacement therapy were demonstrated by a hallmark islet transplantation study, wherein all seven patients with type 1 diabetes (T1D) who underwent transplantation with islets from cadaveric donors became insulin-independent for a minimum duration of 1 year [16]. Advancements in the islet isolation protocol, along with improvements in immunosuppressive regimen, known as the Edmonton Protocol, have set a new benchmark in diabetes treatment. However, the limited availability of cadaveric donor islets in comparison to the demand by T1D patients presents a substantial challenge. Over the past decades, efforts have been made to establish a reliable islet supply chain through the use of advanced islet storage and pooling of islets from multiple donors. Another renewable source for the generation of β cells using human pluripotent stem cells (hPSCs) has been attracting attention as an alternative strategy to overcome the shortage of healthy donor pancreases [17,18]. Clinical trials typically employ hPSC-derived pancreatic and endocrine progenitor cells for transplantation. Viacyte’s 2014 phase 1/2 clinical trial (NCT02239354) used pancreatic progenitor cells with a macroencapsulation device to treat T1D, followed by a 2016 study (NCT02939118) to assess adverse effects. Although minor adverse effects were reported, graft longevity requires further study. Recently, a phase 1/2 clinical trial (NCT04786262) by Vertex Pharmaceuticals demonstrated that all six T1D patients receiving transplants of hPSC-derived islets (VX-880) showed improved glycemic control through endogenous insulin production. Moving beyond the scope of islet transplantation from external sources, there is a growing focus on therapeutic strategies aimed at increasing the body’s own production of insulin-producing cells, a paradigm shift that offers the potential for more integrated and sustainable diabetes management. To increase the number of endogenous β cells, one strategy is to stimulate the proliferation of existing β cells, while another involves inducing the transdifferentiation of non-insulin-producing cells into β cells (Fig. 1). β cell proliferation has shown promise in preclinical animal models, as well as in select human cases, such as during pregnancy, offering potential therapeutic avenues for IDDM [19,20]. However, human β cells exhibit a lower proliferative capacity than those in preclinical animal models, achieving an average proliferation rate of less than 1% in human adults and diminishing further with age [21]. Current research efforts are directed toward identifying small molecules, biologics, and pathways that can enhance the proliferative capacity of human β cells. Compounds targeting glucagon-like peptide 1 receptor (GLP-1R) [22] and dual-specific tyrosine-phosphorylation regulated kinase 1A (DYRK1A) [23] are emerging as front-runners, with evidence from in vivo diabetic mouse models transplanted with human β cells, which have shown that even a 1% to 2% proliferation increase can lead to significant glycemic control improvements, reaching a level of control similar to that in normal mice [24-26]. Nevertheless, the clinical viability of these findings for enhancing adult human β cell proliferation remains uncertain. Alternative β cell replication strategies include the transdifferentiation of liver cells [27,28], stomach and intestinal cells [29], and other pancreatic origin cells such as ductal cells, yet no Food and Drug Administration-approved strategy for this purpose has been applied clinically [30,31]. Despite these advances, current production methods for creating homogeneous, high-quality hPSC-derived insulin-producing β-like cells (hPSC-derived insulin-producing cells hereinafter referred to as “β-like” cells) face challenges. Large-scale clinical application is impeded by complex differentiation processes, batch variability, cost inefficiency, and underdeveloped cryostorage and delivery methods (Fig. 1). This review discusses progressive strategies to surmount hurdles in islet transplantation, including islet availability and the critical aspects of islet preservation.
INTRODUCTION
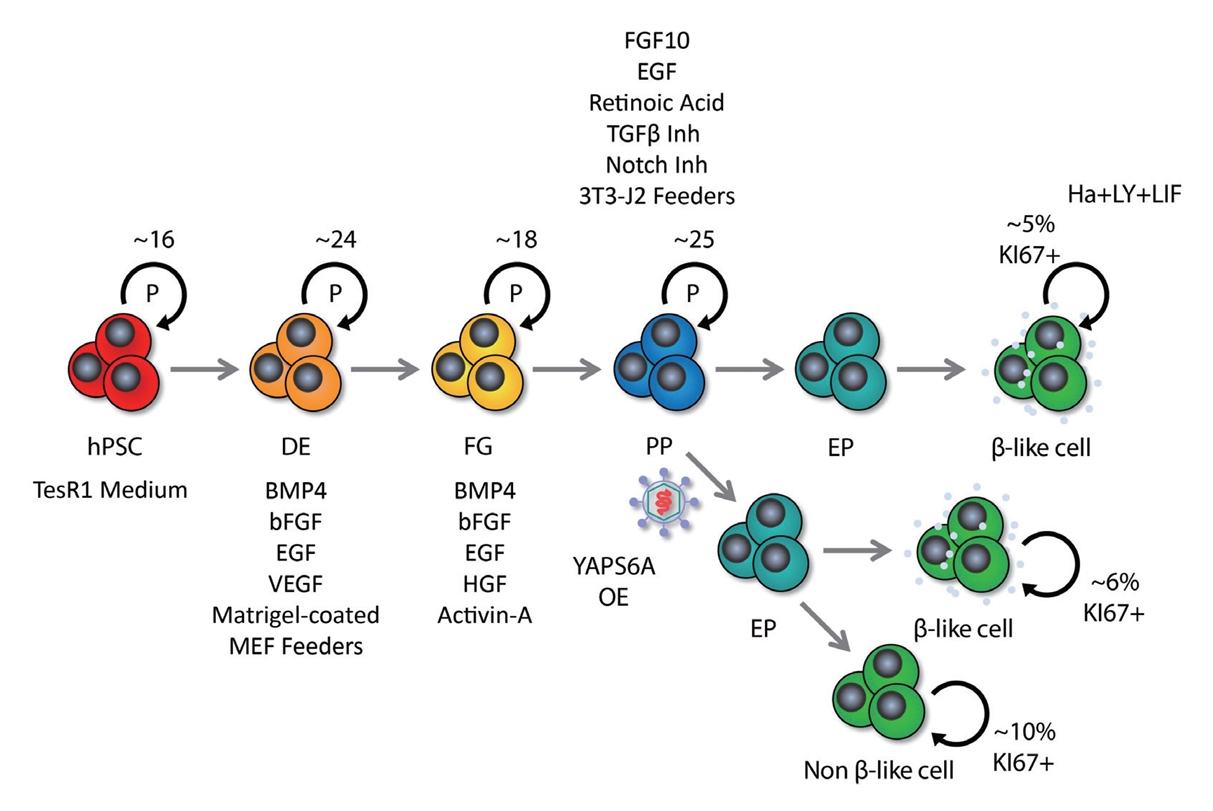
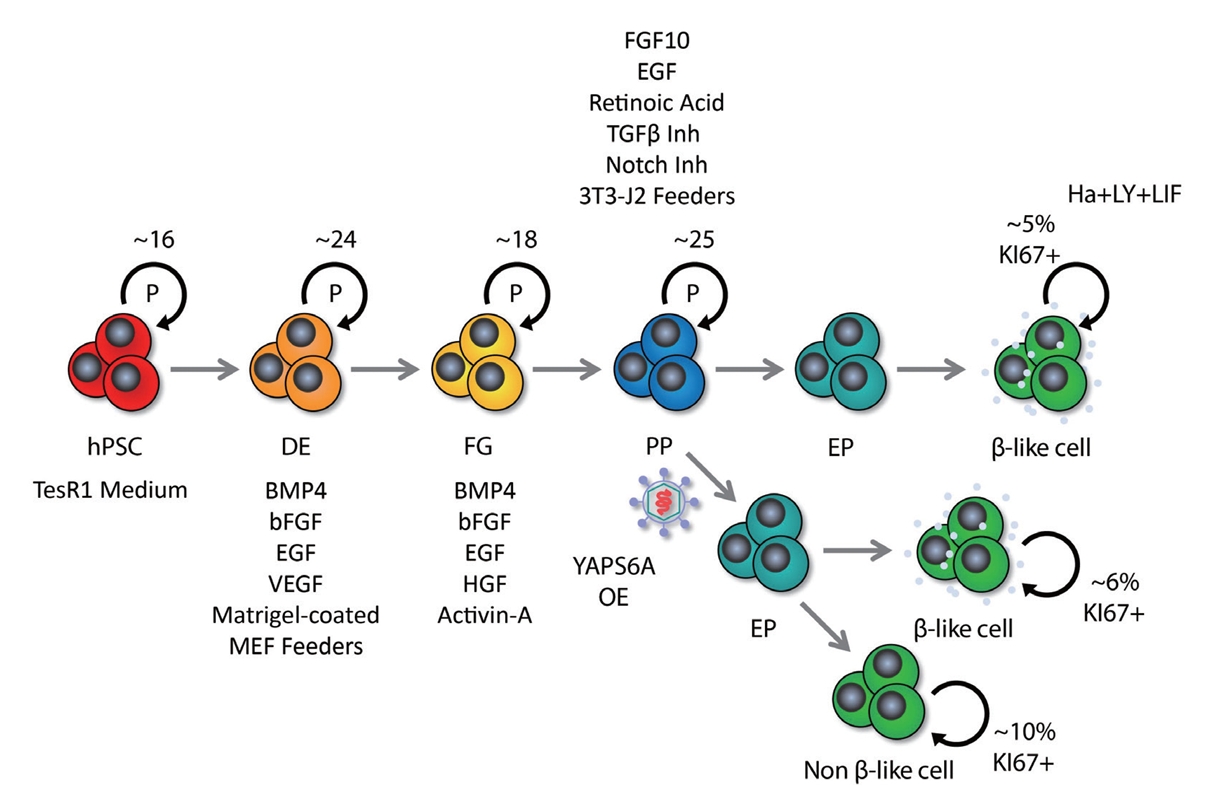
- While hPSC technology holds promise for addressing various challenges in cell replacement therapies, including the generation of β cells, there are significant scalability issues that must be addressed. The indefinite replication potential of PSCs and their ability to differentiate into any cell type, including β cells, underpins the technology’s promise. hPSCs come from two main sources: embryonic pluripotent stem cells (ESC), and induced pluripotent stem cells (iPSC). The same differentiation process can be applied to both ESCs and iPSCs; however iPSCs offer an ethical advantage over ESCs. The generation of iPSCs involves reprogramming adult cells by targeting transcription factors like octamer-binding transcription factor 4 (OCT4), SRY-box transcription factor 2 (SOX2), Krüppel-like factor 4 (KLF4), and cellular myelocytomatosis oncogene (c-MYC) [32,33], which can lead to inconsistent differentiation efficiencies due to residual epigenetic memory [34]. This variability can severely limit the large-scale production of functionally homogeneous β cells. Additionally, the differentiation stage of the β cells may influence their propensity to form teratomas, a type of tumor. Less differentiated cells carry a higher tumorigenic risk, raising concerns about the safety of cell therapies [35]. Ensuring the success, purity, and homogeneity of differentiated β cells is critical and remains a significant hurdle for scaling up this technology for widespread clinical application. The differentiation of β cells from hPSCs follows a meticulous, stepwise protocol that guides cells through sequential lineage commitments: starting with induction into definitive endoderm, followed by commitment to foregut and pancreatic progenitor stages, further specializing into endocrine progenitors, and culminating towards mono-hormonal endocrine cells, including insulin-secreting β cells, glucagon-secreting α cells and somatostatin-secreting δ cells. This approach to β cell differentiation has been successfully implemented in both monolayer cultures and three-dimensional (3D) cell aggregates [36]. hPSC-derived β cells express the hallmark markers, such as pancreatic and duodenal homeobox1 (PDX1), NK6 homeobox 1 (NKX6-1), urocortin 3 (UCN3), MAF bZIP transcription factor A (MAFA), insulin (INS), and neurogenic differentiation 1 (NEUROD1), and have the functionality of glucose-sensing and insulin-secretion at varying levels [4,5]. Despite these successes, hPSC-derived β cells still face major issues that hinder their therapeutic application.
- Identity and purity
- Protocols for in vitro differentiation to generate β-like cells from iPSCs commonly emulate the developmental progression of pancreatic islets. Consequently, the resulting cultures typically comprise not only β-like cells, but also α-like cells and δ-like cells. The complex and labor-intensive nature of these protocols, along with the dynamics of morphogen signaling gradients and the susceptibility of different precursor cells to develop into different lineages, occasionally leads to the emergence of cell types atypical to cadaveric islets. Such anomalies include enterochromaffin cells, which are closer to intestinal than pancreatic endocrine lineages, as well as polyhormonal cells that exhibit a less developed state than monohormonal β, α, or δ cells [37-39]. Moreover, the differentiation process is not universally efficient throughout the culture process, with some cells stalling at progenitor stages, others deviating to alternative lineages, and yet others displaying traits of both intestinal and pancreatic types. These undesired cells can be considered contaminants that can potentially impair the functionality of the β-like cells or alter the differentiation trajectory of neighboring cells via direct contact or the secretion of soluble factors. These cells can also persist even after transplantation despite allowing the in vivo maturation of derived β-like cells [39,40]. Sorting and reaggregation of β-like cells with the removal of the undesired cell types has been shown to enhance the functional maturation of β-like cells clusters [41]. Variability in the differentiation efficiency of β-like cells across different protocols and the disparate responsiveness of various iPSC lines further complicate the process. Determining the optimal ratio of β-like cells to other pancreatic endocrine cells for clinical applications remains a topic of debate. Nonetheless, controlling this ratio and reducing contaminant cell populations are critical research objectives. An emerging aspect of β-like cell differentiation that is coming into focus is the importance of epigenetics and chromatin states. Although single cell transcriptomic analyses have provided useful insights into the cellular identities of cells generated by various differentiation protocols, they fall short in explaining the emergence of non-β-like cells and their interrelations. The prevailing theory suggests a divergence from a common progenitor lineage into distinct end-branches, which precludes the possibility of transdifferentiation or a continuum of cellular identities. Thus, in the past few years, strategies to improve β-like cell differentiation efficiency have involved employing cell surface markers such as CD49a [37], CD9 [36,42], glycoprotein 2 [43-45], CD142 [45], CD24 [46], and CD63 [47], followed by purification and/or reaggregation [41]. However, these methods might be challenging to implement on a larger scale for the mass production of β-like cells. In contrast, single cell transcriptomic analysis complemented by single cell transposase-accessible chromatin sequencing on β-like cells, revealed that the presence of enterochromaffin cells may represent an intermediary transitory state of pancreatic endocrine cells and intestinal cells [48]. This finding suggests the possibility of transdifferentiating enterochromaffin cells into a β cell identity by modulating the chromatin states, timing, and expression of key transcription factors and signals, similar to the process of transdifferentiating stomach cells into β-like cells [49,50]. Nonetheless, it is essential to evaluate the purity and identity of the differentiated cells for the presence of β cell markers and the absence of stem cell and other cell type markers. Utilizing single cell multiomic assays can deepen our understanding of β cell differentiation in vitro. This approach can help analyze the different resulting cells and may pave the way to optimize the in vitro differentiation protocols to achieve a desirable proportion of mature and functional β cells, along with other pancreatic endocrine cells [46].
- Transplantation site and immune reaction
- The selection of a transplantation site for PSC-derived β-like cells is critical in mitigating graft rejection and immune responses [51]. To achieve functional efficacy, the loss of transplanted insulin-producing cells should be minimized. For this purpose, a current focus of interest is increasing graft survival by rapid vascularization to deliver nutrition and oxygen, as well as protecting from harsh allogenic and autoimmune responses. Certain transplantation sites may provide an immune-privileged or immune-tolerant environment, potentially reducing graft rejection risk. Sites including the anterior chamber of the eye and the omentum are under preclinical investigation for their efficacy in this regard [52,53]. Immune evasion via genetic engineering of β cells is also being tested. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 deletion of the human leukocyte antigen-A/B/C (HLA-A/B/C) and class II transactivator (CIITA) genes, and the introduction of the programmed death-ligand 1 (PD-L1), HLA-G, and CD47 genes allow cells to be less immunogenic [10,54-58]. The use of encapsulation devices is another innovative transplantation strategy. These devices are designed to protect the cells from immune attacks; closed-type devices prevent immune interactions, while open-type devices facilitate vascularization and nutrient exchange [59]. These approaches are in a developmental stage and have not yet achieved full protection of transplanted cells, presenting a trade-off. Previous reviews discuss these strategies in depth [51,60].
- Maturity
- Maturity in hPSC-derived β-like cells is marked by the expression of key β cell markers, including MAFA, UCN3, islet amyloid polypeptide (IAPP), SIX homeobox 2 (SIX2), and Wnt family member 4 (WNT4), proper glycolysis and mitochondrial metabolic activity, and some functional capacity for glucose sensing and insulin secretion [7,10,18]. Although it is widely acknowledged that these differentiated cells do not yet exhibit definitive β cell metabolic maturity and functionality comparable to human islets, they can attain in vivo maturity and reduce hyperglycemia when transplanted in diabetic mice. Advancement toward defining signals and pathways that can further mature hPSC-derived monohormonal β cells, mimicking in vivo maturity, is a current subject of intense research. Furthermore, it is being increasingly recognized that there is heterogeneity of mature β cells with putatively different functions. Determining whether current protocols are able to replicate these heterogeneous subtypes and how they influence β cell functionality for transplantation and clinical applications remain to be thoroughly investigated. The signal pathways involved in β cell maturation have recently been reviewed in detail [18,51].
- Expansion and scalability
- Typically, around 3,000 islet equivalents (IEQ) per mouse and approximately 100,000 IEQ per kilogram of body weight in humans are required to observe a beneficial effect on glucose homeostasis in diabetes (Table 1) [4,5,10-12,61-75]. Consequently, a strategic expansion of fully mature and functionally homogeneous β cells is necessary to standardize these therapeutic interventions. The self-renewal capacity of hPSCs is expected to provide an infinite resource for newly synthetic β cells. However, it is known that the passage number of hPSCs influences their differentiation function. In general, hPSCs with a higher passage number show more variability and increased genomic instability, and lose the function of proper differentiation [76]. In addition, different sources of human iPSCs or human embryonic stem cells show distinct patterns of epigenetic inheritance [34], which can result in variability in their efficacy for generating β cells. The cell count tends to decrease during the lengthy differentiation process, highlighting the limitations of scalability with hPSCs. Besides relying on the self-renewal function of hPSCs for expansion, the regulation of proliferation at the advanced stages of differentiation of hPSCs to the pancreatic lineage has been explored. Distinct stages of the β cell development have different proliferative capacities; thus, in vitro culture of these cells is under investigation for intensive proliferation before differentiation to increase the scalability of β cells. We discuss these approaches in the next section.
SOURCES OF FUNCTIONAL HUMAN β CELLS
- The expansion of residual β cells is considered a promising therapeutic approach for T1D and type 2 diabetes. In fact, 2% to 3% of human β cells are observed to divide during the infancy-childhood β cell expansion period, a rate that gradually declines to less than 0.5% in adulthood [77]. While mitogens that induce β cell proliferation in rodents have been identified, the majority of those mitogens have not been as effective in human β cells. This discrepancy may stem from differences in cell cycle regulation mechanisms between species. For example, human β cells have high expression of cyclin-dependent kinase 6 (CDK6), which is important for cell division in these cells, but not in rodent β cells [78-80]. Additionally, rodent β cells express all three D-cyclins, and the genetic deletion of cyclin D2 leads to β cell hypoplasia and diabetes [81]. In contrast, human β cells express very little or no cyclin D2 [80,82].
- Inhibition of DYRK1A activity is known as a representative pathway that induces human β cell division; therefore, DYRK1A inhibitors such as harmine, INDY, leuketine-41, GNF4877, 5-iodotubericidin, TG003, AZ191, and CC-401 have been used to promote human β cell proliferation [24,83]. DYRK1A phosphorylates nuclear factor of activated T-cell transcription factors (NFATs) and prevents their translocation to the nucleus, thereby preventing their activation. Activation of NFATs through inhibition of DYRK1A induces cell cycle regulator expression and increases human β cell proliferation and mass [24]. Additionally, DYRK1A inhibition induces human β cell proliferation through reduced expression of the cell cycle inhibitor p27kip1 and conversion of the repressive DREAM (DP, RB-like, E2F, and MuvB) complex to the pro-proliferative MMB (MYB, MuvB, and FOXM1) conformation [84,85]. Harmine, a prominent DYRK1A inhibitor, can enhance adult human β cell proliferation by up to approximately 3% in vitro and in vivo [24]. In a study, streptozotocin (STZ)-induced diabetic nonobese diabetic/severe combined immunodeficiency (NOD-SCID) mice that received transplanted human islets and were treated with harmine exhibited a significant reduction in blood glucose levels—measuring approximately 200 mg/dL within 21 days, compared to around 300 mg/dL in the control group. Interestingly, harmine treatment not only induced human β cell proliferation, but also increased the expression of important β cell transcription factors, such as PDX1, NKX6.1, and MAFA [24]. While transforming growth factor β (TGFβ) signaling has been shown to suppress β cell proliferation by increasing the expression of CDK inhibitors, including P15, P16, P21, and P57 [86,87], its suppression through inhibitors like LY364947, ALK5, and GW788388 did not markedly increase human β cell proliferation [25]. However, it has recently been reported that leukemia inhibitory factor (LIF) signaling stimulated the expression of cyclins and CDKs via the signal transducer and activator of transcription 3 and CCAAT Enhancer Binding Protein Delta (CEBPD) pathways, facilitating human β cell cycle progression [26]. Treatment with recombinant LIF (rLIF) led to a modest 1.5% rise in human β cell proliferation in vitro [26]. rLIF treatment for 14 days improved glycemic regulation by up to approximately 200 mg/dL (phosphate-buffered saline treatment group: approximately 350 mg/dL) before a single nephrectomy in STZ-induced diabetic NOD-SCID mice with human islet transplantation. In particular, in an in vivo glucose-stimulated insulin secretion test, the rLIF-treated group showed a twofold increase in insulin secretion compared to controls [26]. Additionally, it has been reported that γ-aminobutyric acid (GABA) signaling, an inhibitory neurotransmitter, induces protein kinase B (PKB or AKT) and cAMP-response element binding protein (CREB) pathway activity to increase β cell proliferation, and glycogen synthase kinase 3β (GSK3β) inhibitors such as 1-azakenpaullone, CHIR99021, or 6-bromoindirubin-30-oxime (BIO) also induces rat β cell survival and proliferation [88,89]. Contrary to previous findings, it was recently reported that GABA did not restore islet capacity and function in diet-induced obese mice, and GSK3β inhibitors alone were unable to increase human β cell proliferation [83,90]. Synergistic effects—where two or more drugs, when used in combination, produce an amplified effect—have been harnessed in recent studies to enhance human β cell proliferation. Research has demonstrated that combined treatment of harmine (DYRK1A inhibitor)+LY364947 (TGFβ inhibitor) or CC-401 (DYRK1A inhibitor)+ALK5 inhibitor II (TGFβ inhibitor) can increase human β cell proliferation by 4% to 8% in vitro [25,91]. Similarly, the combined treatment of harmine and GW788388 (TGFβ inhibitor) showed a synergistic effect, increasing human β cell proliferation by 1.5% (harmine treatment group: approximately 1.2%) in NOD-SCID mice [25]. In addition, harmine also synergized with GLP-1 agonists to induce human β cell proliferation in vitro and in vivo. The combination treatment improved normoglycemic levels in STZ-induced diabetic NOD-SCID gamma (NSG) mice transplanted with 500 IEQ of human islets, and it increased human β cell proliferation approximately twofold over the harmine treatment group. However, a single treatment with harmine failed to reduce blood glucose levels [92]. Moreover, in hPSC-β cells exhibiting a 1% cell division rate, a triple combination of LIF+harmine+LY364947 was able to boost cell division to approximately 5% in vitro [26]. Moreover, efforts to increase the mass of insulin-producing cells have targeted not only human β cell proliferation, but also the expansion of pancreatic progenitors [93-95]. The recent success in cultivating expandable protein C receptor positive pancreatic progenitors that can generate functional islet organoids in a mouse model has further encouraged these approaches [96]. The synergistic effect of several drugs based on the new understanding of the mechanism of pancreatic β cell replication has significantly increased human β cell proliferation (Table 2, Fig. 2) [24-26,83,89,92,93,97-102]. These findings hold promise for the potential clinical application of these drug combinations in the future.
REGULATION OF HUMAN β CELL PROLIFERATION
- Cryopreservation of islets is a crucial component in scaling the delivery of insulin-producing cells. This method has been extensively studied, as it offers a solution to challenges in the islet supply chain by enabling high-quality storage and the pooling of islets from multiple donors. However, islets are highly susceptible to cellular stress and damage during the freeze-thaw cycle, which may lead to impaired function or cell death. Over the past decades, various conditions have been studied for islet cryopreservation in order to improve islet survival and functional recovery after thawing. In particular, major factors affecting successful islet cryopreservation include the use of cryoprotective agents (CPAs), the management of cellular stress, and the maintenance of the islets’ 3D structure.
- Rapid freezing can cause the formation of intracellular and extracellular ice crystals, which are detrimental to cell viability [103]. In 1949, the discovery of glycerol’s cryoprotective properties paved the way for the use of CPAs such as dimethyl sulfoxide (DMSO), glycerol, ethylene glycol, and propylene, which are introduced prior to freezing to prevent ice crystallization [104,105]. Effective cryopreservation thus requires sufficient time to equilibrate with CPAs within and around the cells. Furthermore, it is important to set the optimal temperature and concentration of CPAs, as different CPAs have varying rates of diffusion and levels of cytotoxicity [106].
- In 1977, cryopreserved rat islets were transplanted into the livers of diabetic rats through the portal vein [107]. The diabetic rats transplanted with cryopreserved rat islets showed hyperglycemia for 6 weeks after transplantation but thereafter maintained normal blood glucose levels until 13 weeks. These findings have spurred the development of various islet cryopreservation protocols [108]. Despite success in small rodents, only 20% of pigs with transplanted cryopreserved porcine islets achieved normal glucose regulation, highlighting the need for improved cryopreservation methods for larger mammals, including humans [109]. In 2001, it was shown that islet survival improved with a protocol incorporating slow cooling at a rate of 0.25°C per minute and rapid thawing, facilitated by the addition of 2 moles (M) DMSO to the University of Wisconsin organ preservation solution or to a hypothermosol preservation solution [110].
- Although CPAs are effective in preventing ice crystal formation, they do not alleviate the cellular stress associated with freezing and thawing. In particular, the oxidative stress that occurs during this process poses a threat to islet survival due to the inherently low antioxidant defense of islet cells [111]. Over past decades, research has focused on various CPA additives to reduce oxidative stress in islets, including taurine (an antioxidant), metformin (an antidiabetic drug), GABA, eicosapentaenoic acid (a polyunsaturated fatty acid), or docosahexanoic acid (a polyunsaturated fatty acid). When combined with CPAs, these additives have been shown to significantly lower reactive oxygen species levels in islets, thereby enhancing their function and survival after cryopreservation [112,113] .
- Human islets are aggregates of approximately 1,500 to 2,000 cells with an average diameter of 100 to 150 μm [114]. The 3D structure of islets prevents CPAs from spreading uniformly as the temperature of each cell within the islets changes [115]. The differential temperature pattern formed in this way can generate intracellular ice crystals and eventually lead to cell death. To overcome this problem, a method was proposed to separate islets into single cells, freeze them, and reassemble them into their original spheroid form after thawing. In fact, islets reconstituted after cryopreservation showed higher cell survival and functional recovery than native islets, both in vitro and in vivo [116].
- In addition, improved islet survival rate and functional recovery after cryopreservation have been achieved by reducing the amount of CPAs using hollow fiber vitrification, encapsulating islets with 1.75% alginate, or combining CPAs with ethylene glycol and DMSO [117,118]. In particular, a recent islet cryopreservation protocol using cryomesh has achieved survival rates exceeding 89% and an islet recovery rate of more than 95% in 2,500 islets after thawing. In addition, it was suggested that clinically meaningful throughput could be achieved if a larger-sized cryomesh and cryomesh overlapping method were used [119]. In the near future, high-quality islet banking through the establishment of successful islet cryopreservation methods holds the potential to substantially reduce the geographical and temporal barriers between donors and recipients. This advancement is anticipated to markedly improve the success rates of islet translation by increasing the opportunity for high-dose transplantation and more precise HLA matching through the pooling of islet resources.
ISLET CRYOPRESERVATION
- Taken together, this review highlights recent findings on novel methodologies that provide game-changing resources for generating synthetic functional insulin-producing β cells and directly expanding human β cells using small molecules or pooling via islet cryopreservation. Although these developments are promising, significant hurdles remain. For instance, the production of uniform hPSC-derived β-like cells in quantities sufficient for clinical applications is still a challenge. Current differentiation protocols are labor-intensive and struggle with the heterogeneity and nonuniformity of the resulting β cells, which are critical issues to resolve for industrial-scale production. Despite these challenges, the various cutting-edge methods for obtaining human β cells are paving the way toward making islet transplantation a clinically viable and more successful treatment in the foreseeable future.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
-
Acknowledgements
- This work was supported by grants from Beatson Foundation (2022-006), Tobacco-Related Disease Research Program (TRDRP) research award (T33IR6551) and National Institute of Diabetes And Digestive And Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under Award Number R01DK136888. Eiji Yoshihara is supported by the Juvenile Diabetes Research Foundation (JDRF) Career Development Award (5-CDA-2022-1178-A-N). Jinhyuk Choi and Harvey Perez are supported by postdoctoral fellowships from the California Institute for Regenerative Medicine (CIRM)-training grant (EDUC4-12837).
|
Species |
Types | Sites | Islet quantity, IEQ | Reference | |
|---|---|---|---|---|---|
| Donor | Recipient | ||||
| Mouse | Mouse | Primary islets | Portal vein | 350 | [61] |
| Kidney capsule | Approximately 1,000 | [10] | |||
| Dog | Dog | Primary islets | Kidney | 3,000–5,000 | [62] |
| Portal vein | |||||
| Porcine | Mouse | Primary islets | Portal vein | 2,000 | [61] |
| NHPs | Approximately 50,000/kg (approximately 6.2×106 β cells/kg) | [65] | |||
| Approximately 25,000/kg (approximately 7.9±4.8×106 β cells/kg) | [66] | ||||
| 85,000–100,000/kg of BW | [67] | ||||
| Human | Mouse | Primary islets | Portal vein | Approximately 2,000 | [61] |
| Kidney capsule | Approximately 2,000 | [10] | |||
| Approximately 3,000 | [68] | ||||
| Human | Primary islets | Portal vein | 782,550 | [69] | |
| <10,000/kg of BW | [16] | ||||
| <9,000/kg of BW | [71] | ||||
| Human | Mouse | hPSC-β | Kidney capsule | 3×106–7×106 cells | [68] |
| 3×106–5×106 cells | [5] | ||||
| 3×106 cells | [75] | ||||
| Approximately 1.25×106 cells | [4] | ||||
| Approximately 1.6×106 cells | [72] | ||||
| 250–750 (diameter 100–200 μm) | [11] | ||||
| 3.2×106–4.9×106 cells | [73] | ||||
| 3×106 cells | [74] | ||||
| NHPs | Omentum | Approximately 17,000 | [12] | ||
| Portal vein | 30,000–40,000/kg of BW | [75] | |||
| Treatment | Target | Molecules | Experiment | Cell types |
Proliferation index (vs. CON) |
Mechanism of action | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| KI67 | BrdU | EdU | P-HH3 | |||||||
| Single | DYRK1A inhibitor | Harmine | In vitro | Human β cells | 1%–3% | –2% | –0.3% | NFAT signaling pathway ↑ | [24] | |
| In vitro | Human β cells | –3% | [83] | |||||||
| In vitro | Human β cells | –2% | –2% | –0.4% | [25] | |||||
| In vivo | Human β cells | –1% | ||||||||
| In vitro | hPSC-β cells | –2.5% | [26] | |||||||
| INDY | In vitro | Human β cells | 1.5% | –0.2% | [24] | |||||
| In vitro | Human β cells | –3% | [83] | |||||||
| In vitro | Human β cells | –2% | [25] | |||||||
| Leucettine-41 | In vitro | Human β cells | 4% | [83] | ||||||
| In vitro | Human β cells | –2% | [25] | |||||||
| 5-IT | In vitro | Human β cells | 3% | [83] | ||||||
| TG003 | In vitro | Human β cells | 2% | |||||||
| CC-401 | In vitro | Human β cells | 1% | |||||||
| DYRK1A inhibitor+GSK3β inhibitor | GNF7156 | In vitro | Human β cells | 3%–6% | [97] | |||||
| GNF4877 | In vitro | Human β cells | 3%–6% | |||||||
| In vivo | Human β cells | 3% | ||||||||
| TGFβ inhibitor | SB431542 | In vitro | Human β cells | –2.5% | CDKIs (P15, P16, P21,P57) ↓ | [98] | ||||
| In vivo | Human β cells | –1% | ||||||||
| LY364947 | In vitro | Human β cells | –1% | [25] | ||||||
| ALK5 | In vitro | Human β cells | –1% | |||||||
| GW788388 | In vitro | Human β cells | –1% | |||||||
| In vivo | Human β cells | –1% | ||||||||
| A83-01 | In vitro | Human β cells | –1% | |||||||
| K02288 | In vitro | Human β cells | –1% | |||||||
| LDN193189 | In vitro | Human β cells | –1% | |||||||
| LIF | Recombinant LIF | In vitro | Human β cells | –2% | STAT3 & CEBPD singaling pathway↑ | [26] | ||||
| In vivo | Human β cells | –1.5% | ||||||||
| In vitro | hPSC-β cells | –1.5% | ||||||||
| GABA | Recombinant GABA | In vitro | Human β cells | –2% | –0.3% | PKA-CREB signaling pathway↑ | [89] | |||
| In vivo | Human β cells | –2% | ||||||||
| GSK3β inhibitor | Tideglusib | In vitro | Human β cells | NS | [83] | |||||
| CHIR99021 | In vitro | Human β cells | NS | |||||||
| GLP-1 | recombinant GLP-1 | In vitro | NS | NS | [92] | |||||
| Expendin-4 | In vivo | Human β cells | NS | |||||||
| Combination | DYRK1A inhibitor+TGFβ inhibitor | Harmine+SB431542 | In vitro | Human β cells | –4% | [25] | ||||
| Harmine+LY364947 | In vitro | Human β cells | –7% | |||||||
| Harmine+ALK5 | In vitro | Human β cells | –7% | |||||||
| Harmine+GW788388 | In vitro | Human β cells | –5% | |||||||
| Harmine+A83-01 | In vitro | Human β cells | –6% | |||||||
| Harmine+K02288 | In vitro | Human β cells | –5% | |||||||
| Harmine+LDN193189 | In vitro | Human β cells | –4% | |||||||
| DYRK1A inhibitor+GSK3β inhibitor | Harmine+Tidglusib | In vitro | Human β cells | –4% | [83] | |||||
| Harmine+CHIR99021 | In vitro | Human β cells | –4% | |||||||
| DYRK1A inhibitor+TGFβ inhibitor+LIF | Harmine+LY364947+LIF | In vitro | hPSC-β cells | –5% | [26] | |||||
| DYRK1A inhibitor+GLP-1 | Harmine+GLP-1 | In vitro | Human β cells | –5% | –3.5% | –1.5% | [92] | |||
| Harmine+Expendin-4 | In vivo | Human β cells | –1% | |||||||
CON, control; KI67, antigen Kiel 67; BrdU, 5-bromo-2’-deoxyuridine; EdU, 5-ethynyl-2’-deoxyuridine; P-HH3, phospho-histone H3; DYRK1A, dual-specific tyrosine-phosphorylation regulated kinase 1A; NFAT, nuclear factor of activated T-cell transcription factor; hPSC, human pluripotent stem cell; INDY, 1Z-(3-ethyl-5-hydroxy-2(3H)-benzothiazolylidene)-2-propanone; 5-IT, 5-iodotubericidin; GSK3β, glycogen synthase kinase 3β; GNF, Genomics Institute of the Novartis Research Foundation; TGFβ, transforming growth factor β; CDKI, cyclin-dependent kinase inhibitors; P, protein; ALK5, activin like kinase 5; LIF, leukemia inhibitory factor; hPSC-β, human pluripotent stem cell-derived β cell; STAT3, signal transducer and activator of transcription 3; CEBPD, CCAAT/enhancer-binding protein delta; GABA, γ-aminobutyric acid; PKA-CREB, protein kinase A-cAMP response element-binding protein; NS, not significant; GLP-1, glucagon-like peptide-1.
- 1. Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell 2012;148:1160–71.ArticlePubMedPMC
- 2. Rutter GA, Georgiadou E, Martinez-Sanchez A, Pullen TJ. Metabolic and functional specialisations of the pancreatic beta cell: gene disallowance, mitochondrial metabolism and intercellular connectivity. Diabetologia 2020;63:1990–8.ArticlePubMedPMCPDF
- 3. Ikegami H, Babaya N, Noso S. β-Cell failure in diabetes: common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J Diabetes Investig 2021;12:1526–39.ArticlePubMedPMCPDF
- 4. Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014;32:1121–33.ArticlePubMedPDF
- 5. Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic β cells in vitro. Cell 2014;159:428–39.ArticlePubMedPMC
- 6. Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 2015;34:1759–72.ArticlePubMedPMCPDF
- 7. Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, et al. ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells. Cell Metab 2016;23:622–34.ArticlePubMedPMC
- 8. Vegas AJ, Veiseh O, Gurtler M, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016;22:306–11.PubMedPMC
- 9. Hogrebe NJ, Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat Biotechnol 2020;38:460–70.ArticlePubMedPMCPDF
- 10. Yoshihara E, O’Connor C, Gasser E, Wei Z, Oh TG, Tseng TW, et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 2020;586:606–11.ArticlePubMedPMCPDF
- 11. Balboa D, Barsby T, Lithovius V, Saarimaki-Vire J, Omar-Hmeadi M, Dyachok O, et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat Biotechnol 2022;40:1042–55.PubMedPMC
- 12. Deng H, Zhang A, Pang DR, Xi Y, Yang Z, Matheson R, et al. Bioengineered omental transplant site promotes pancreatic islet allografts survival in non-human primates. Cell Rep Med 2023;4:100959.ArticlePubMedPMC
- 13. Ramzy A, Thompson DM, Ward-Hartstonge KA, Ivison S, Cook L, Garcia RV, et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021;28:2047–61.ArticlePubMed
- 14. Shapiro AM, Thompson D, Donner TW, Bellin MD, Hsueh W, Pettus J, et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med 2021;2:100466.ArticlePubMedPMC
- 15. Keymeulen B, De Groot K, Jacobs-Tulleneers-Thevissen D, Thompson DM, Bellin MD, Kroon EJ, et al. Encapsulated stem cell-derived β cells exert glucose control in patients with type 1 diabetes. Nat Biotechnol 2023 Nov 27 [Epub]. https://doi.org/10.1038/s41587-023-02055-5.Article
- 16. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–8.ArticlePubMed
- 17. Salib A, Cayabyab F, Yoshihara E. Stem cell-derived islets for type 2 diabetes. Int J Mol Sci 2022;23:5099.ArticlePubMedPMC
- 18. Yoshihara E. Adapting physiology in functional human islet organogenesis. Front Cell Dev Biol 2022;10:854604.ArticlePubMedPMC
- 19. Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 1992;130:1459–66.ArticlePubMed
- 20. Toselli C, Hyslop CM, Hughes M, Natale DR, Santamaria P, Huang CT. Contribution of a non-β-cell source to β-cell mass during pregnancy. PLoS One 2014;9:e100398.ArticlePubMedPMC
- 21. Lam CJ, Cox AR, Jacobson DR, Rankin MM, Kushner JA. Highly proliferative α-cell-related islet endocrine cells in human pancreata. Diabetes 2018;67:674–86.ArticlePubMedPMCPDF
- 22. Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J Clin Invest 2017;127:3835–44.ArticlePubMedPMC
- 23. Aamodt KI, Aramandla R, Brown JJ, Fiaschi-Taesch N, Wang P, Stewart AF, et al. Development of a reliable automated screening system to identify small molecules and biologics that promote human β-cell regeneration. Am J Physiol Endocrinol Metab 2016;311:E859–68.ArticlePubMedPMC
- 24. Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 2015;21:383–8.ArticlePubMedPMCPDF
- 25. Wang P, Karakose E, Liu H, Swartz E, Ackeifi C, Zlatanic V, et al. Combined inhibition of DYRK1A, SMAD, and trithorax pathways synergizes to induce robust replication in adult human beta cells. Cell Metab 2019;29:638–52.ArticlePubMed
- 26. Rosado-Olivieri EA, Aigha II, Kenty JH, Melton DA. Identification of a LIF-responsive, replication-competent subpopulation of human β cells. Cell Metab 2020;31:327–38.ArticlePubMed
- 27. Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 2000;6:568–72.ArticlePubMedPDF
- 28. Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol 2000;2:879–87.ArticlePubMedPDF
- 29. Eicher AK, Kechele DO, Sundaram N, Berns HM, Poling HM, Haines LE, et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell 2022;29:36–51.ArticlePubMedPMC
- 30. Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife 2013;2:e00940.ArticlePubMedPMCPDF
- 31. Rhee M, Lee SH, Kim JW, Ham DS, Park HS, Yang HK, et al. Preadipocyte factor 1 induces pancreatic ductal cell differentiation into insulin-producing cells. Sci Rep 2016;6:23960.ArticlePubMedPMCPDF
- 32. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–72.ArticlePubMed
- 33. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76.ArticlePubMed
- 34. Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010;467:285–90.PubMedPMC
- 35. Bulic-Jakus F, Katusic Bojanac A, Juric-Lekic G, Vlahovic M, Sincic N. Teratoma: from spontaneous tumors to the pluripotency/malignancy assay. Wiley Interdiscip Rev Dev Biol 2016;5:186–209.ArticlePubMedPDF
- 36. Li X, Yang KY, Chan VW, Leung KT, Zhang XB, Wong AS, et al. Single-cell RNA-seq reveals that CD9 is a negative marker of glucose-responsive pancreatic β-like cells derived from human pluripotent stem cells. Stem Cell Reports 2020;15:1111–26.ArticlePubMedPMC
- 37. Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JH, Harb G, et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 2019;569:368–73.ArticlePubMedPMCPDF
- 38. Davis JC, Alves TC, Helman A, Chen JC, Kenty JH, Cardone RL, et al. Glucose response by stem cell-derived β cells in vitro is inhibited by a bottleneck in glycolysis. Cell Rep 2020;31:107623.ArticlePubMedPMC
- 39. Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR. Single-cell transcriptome profiling reveals β cell maturation in stem cell-derived islets after transplantation. Cell Rep 2020;32:108067.ArticlePubMedPMC
- 40. Velazco-Cruz L, Song J, Maxwell KG, Goedegebuure MM, Augsornworawat P, Hogrebe NJ, et al. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Reports 2019;12:351–65.ArticlePubMedPMC
- 41. Nair GG, Liu JS, Russ HA, Tran S, Saxton MS, Chen R, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat Cell Biol 2019;21:263–74.ArticlePubMedPMCPDF
- 42. Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, et al. Human islets contain four distinct subtypes of β cells. Nat Commun 2016;7:11756.ArticlePubMedPMCPDF
- 43. Ameri J, Borup R, Prawiro C, Ramond C, Schachter KA, Scharfmann R, et al. Efficient generation of glucose-responsive beta cells from isolated GP2+ human pancreatic progenitors. Cell Rep 2017;19:36–49.ArticlePubMed
- 44. Cogger KF, Sinha A, Sarangi F, McGaugh EC, Saunders D, Dorrell C, et al. Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat Commun 2017;8:331.ArticlePubMedPMCPDF
- 45. Ramond C, Glaser N, Berthault C, Ameri J, Kirkegaard JS, Hansson M, et al. Reconstructing human pancreatic differentiation by mapping specific cell populations during development. Elife 2017;6:e27564.ArticlePubMedPMCPDF
- 46. Jiang W, Sui X, Zhang D, Liu M, Ding M, Shi Y, et al. CD24: a novel surface marker for PDX1-positive pancreatic progenitors derived from human embryonic stem cells. Stem Cells 2011;29:609–17.ArticlePubMedPDF
- 47. Rubio-Navarro A, Gomez-Banoy N, Stoll L, Dundar F, Mawla AM, Ma L, et al. A beta cell subset with enhanced insulin secretion and glucose metabolism is reduced in type 2 diabetes. Nat Cell Biol 2023;25:565–78.ArticlePubMedPMCPDF
- 48. Augsornworawat P, Hogrebe NJ, Ishahak M, Schmidt MD, Marquez E, Maestas MM, et al. Single-nucleus multi-omics of human stem cell-derived islets identifies deficiencies in lineage specification. Nat Cell Biol 2023;25:904–16.ArticlePubMedPMCPDF
- 49. Huang X, Gu W, Zhang J, Lan Y, Colarusso JL, Li S, et al. Stomach-derived human insulin-secreting organoids restore glucose homeostasis. Nat Cell Biol 2023;25:778–86.ArticlePubMedPMCPDF
- 50. Choi J, Cayabyab F, Yoshihara E. A guide from the stomach to β cells. Nat Cell Biol 2023;25:637–8.ArticlePubMedPDF
- 51. Tahbaz M, Yoshihara E. Immune protection of stem cell-derived islet cell therapy for treating diabetes. Front Endocrinol (Lausanne) 2021;12:716625.ArticlePubMedPMC
- 52. Perez VL, Caicedo A, Berman DM, Arrieta E, Abdulreda MH, Rodriguez-Diaz R, et al. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: a study in a baboon model of diabetes. Diabetologia 2011;54:1121–6.ArticlePubMedPMCPDF
- 53. Bartholomeus K, Jacobs-Tulleneers-Thevissen D, Shouyue S, Suenens K, In’t Veld PA, Pipeleers-Marichal M, et al. Omentum is better site than kidney capsule for growth, differentiation, and vascularization of immature porcine β-cell implants in immunodeficient rats. Transplantation 2013;96:1026–33.ArticlePubMedPMC
- 54. Mandal PK, Ferreira LM, Collins R, Meissner TB, Boutwell CL, Friesen M, et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 2014;15:643–52.ArticlePubMedPMC
- 55. Han X, Wang M, Duan S, Franco PJ, Kenty JH, Hedrick P, et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc Natl Acad Sci U S A 2019;116:10441–6.ArticlePubMedPMC
- 56. Ye Q, Sung TC, Yang JM, Ling QD, He Y, Higuchi A. Generation of universal and hypoimmunogenic human pluripotent stem cells. Cell Prolif 2020;53:e12946.ArticlePubMedPMCPDF
- 57. Shi L, Li W, Liu Y, Chen Z, Hui Y, Hao P, et al. Generation of hypoimmunogenic human pluripotent stem cells via expression of membrane-bound and secreted β2m-HLA-G fusion proteins. Stem Cells 2020;38:1423–37.ArticlePubMedPDF
- 58. Cefalu WT, Andersen DK, Arreaza-Rubin G, Pin CL, Sato S, Verchere CB, et al. Heterogeneity of diabetes: β-cells, phenotypes, and precision medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes 2022;71:1–22.ArticlePubMedPMCPDF
- 59. Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov 2017;16:338–50.ArticlePubMedPDF
- 60. Cayabyab F, Nih LR, Yoshihara E. Advances in pancreatic islet transplantation sites for the treatment of diabetes. Front Endocrinol (Lausanne) 2021;12:732431.ArticlePubMedPMC
- 61. Khatri R, Hussmann B, Rawat D, Gurol AO, Linn T. Intraportal transplantation of pancreatic islets in mouse model. J Vis Exp 2018;135:57559.ArticlePubMedPMC
- 62. Warnock GL, Rajotte RV. Critical mass of purified islets that induce normoglycemia after implantation into dogs. Diabetes 1988;37:467–70.ArticlePubMed
- 63. Lingwal N, Padmasekar M, Samikannu B, Bretzel RG, Preissner KT, Linn T. Inhibition of gelatinase B (matrix metalloprotease-9) activity reduces cellular inflammation and restores function of transplanted pancreatic islets. Diabetes 2012;61:2045–53.ArticlePubMedPMCPDF
- 64. Chen C, Moreno R, Samikannu B, Bretzel RG, Schmitz ML, Linn T. Improved intraportal islet transplantation outcome by systemic IKK-beta inhibition: NF-κB activity in pancreatic islets depends on oxygen availability. Am J Transplant 2011;11:215–24.ArticlePubMed
- 65. Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 2006;12:304–6.ArticlePubMedPDF
- 66. Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006;12:301–3.ArticlePubMedPDF
- 67. van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant 2009;9:2716–26.ArticlePubMed
- 68. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–52.PubMed
- 69. Scharp DW, Lacy PE, Santiago JV, McCullough CS, Weide LG, Falqui L, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes 1990;39:515–8.ArticlePubMed
- 70. Miller CA, Brooks EF, DeFriese GH, Gilbert B, Jain SC, Kavaler F. A survey of local public health departments and their directors. Am J Public Health 1977;67:931–9.ArticlePubMedPMC
- 71. Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes 2002;51:2148–57.PubMed
- 72. Liu H, Li R, Liao HK, Min Z, Wang C, Yu Y, et al. Chemical combinations potentiate human pluripotent stem cell-derived 3D pancreatic progenitor clusters toward functional β cells. Nat Commun 2021;12:3330.ArticlePubMedPMCPDF
- 73. Braam MJ, Zhao J, Liang S, Ida S, Kloostra NK, Iworima DG, et al. Protocol development to further differentiate and transition stem cell-derived pancreatic progenitors from a monolayer into endocrine cells in suspension culture. Sci Rep 2023;13:8877.ArticlePubMedPMCPDF
- 74. Ma Q, Xiao Y, Xu W, Wang M, Li S, Yang Z, et al. ZnT8 loss-of-function accelerates functional maturation of hESC-derived β cells and resists metabolic stress in diabetes. Nat Commun 2022;13:4142.ArticlePubMedPMCPDF
- 75. Du Y, Liang Z, Wang S, Sun D, Wang X, Liew SY, et al. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat Med 2022;28:272–82.ArticlePubMedPDF
- 76. Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic instability of iPSCs: challenges towards their clinical applications. Stem Cell Rev Rep 2017;13:7–16.ArticlePubMedPMCPDF
- 77. Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, Garcia-Ocana A, Stewart AF. Diabetes mellitus: advances and challenges in human β-cell proliferation. Nat Rev Endocrinol 2015;11:201–12.ArticlePubMedPDF
- 78. Cozar-Castellano I, Weinstock M, Haught M, VelazquezGarcia S, Sipula D, Stewart AF. Evaluation of beta-cell replication in mice transgenic for hepatocyte growth factor and placental lactogen: comprehensive characterization of the G1/S regulatory proteins reveals unique involvement of p21cip. Diabetes 2006;55:70–7.ArticlePubMed
- 79. Fiaschi-Taesch N, Bigatel TA, Sicari B, Takane KK, Salim F, Velazquez-Garcia S, et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes 2009;58:882–93.PubMedPMC
- 80. Fiaschi-Taesch NM, Salim F, Kleinberger J, Troxell R, Cozar-Castellano I, Selk K, et al. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes 2010;59:1926–36.PubMedPMC
- 81. Georgia S, Hinault C, Kawamori D, Hu J, Meyer J, Kanji M, et al. Cyclin D2 is essential for the compensatory beta-cell hyperplastic response to insulin resistance in rodents. Diabetes 2010;59:987–96.PubMedPMC
- 82. Stamateris RE, Sharma RB, Kong Y, Ebrahimpour P, Panday D, Ranganath P, et al. Glucose induces mouse β-cell proliferation via IRS2, MTOR, and cyclin D2 but not the insulin receptor. Diabetes 2016;65:981–95.ArticlePubMedPMCPDF
- 83. Ackeifi C, Swartz E, Kumar K, Liu H, Chalada S, Karakose E, et al. Pharmacologic and genetic approaches define human pancreatic β cell mitogenic targets of DYRK1A inhibitors. JCI Insight 2020;5:e132594.ArticlePubMedPMC
- 84. Dirice E, Walpita D, Vetere A, Meier BC, Kahraman S, Hu J, et al. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes 2016;65:1660–71.ArticlePubMedPMCPDF
- 85. Wang P, Karakose E, Argmann C, Wang H, Balev M, Brody RI, et al. Disrupting the DREAM complex enables proliferation of adult human pancreatic β cells. J Clin Invest 2022;132:e157086.ArticlePubMedPMC
- 86. Xiao X, Wiersch J, El-Gohary Y, Guo P, Prasadan K, Paredes J, et al. TGFβ receptor signaling is essential for inflammation-induced but not β-cell workload-induced β-cell proliferation. Diabetes 2013;62:1217–26.ArticlePubMedPMCPDF
- 87. Lin HM, Lee JH, Yadav H, Kamaraju AK, Liu E, Zhigang D, et al. Transforming growth factor-beta/Smad3 signaling regulates insulin gene transcription and pancreatic islet beta-cell function. J Biol Chem 2009;284:12246–57.PubMedPMC
- 88. Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, et al. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem 2007;282:12030–7.ArticlePubMed
- 89. Purwana I, Zheng J, Li X, Deurloo M, Son DO, Zhang Z, et al. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 2014;63:4197–205.ArticlePubMedPDF
- 90. Untereiner A, Abdo S, Bhattacharjee A, Gohil H, Pourasgari F, Ibeh N, et al. GABA promotes β-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB J 2019;33:3968–84.ArticlePubMedPDF
- 91. Abdolazimi Y, Zhao Z, Lee S, Xu H, Allegretti P, Horton TM, et al. CC-401 promotes β-cell replication via pleiotropic consequences of DYRK1A/B inhibition. Endocrinology 2018;159:3143–57.ArticlePubMedPMC
- 92. Ackeifi C, Wang P, Karakose E, Manning Fox JE, Gonzalez BJ, Liu H, et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human β cell regeneration. Sci Transl Med 2020;12:eaaw9996.ArticlePubMedPMC
- 93. Trott J, Tan EK, Ong S, Titmarsh DM, Denil SL, Giam M, et al. Long-term culture of self-renewing pancreatic progenitors derived from human pluripotent stem cells. Stem Cell Reports 2017;8:1675–88.ArticlePubMedPMC
- 94. Kimura A, Toyoda T, Nishi Y, Nasu M, Ohta A, Osafune K. Small molecule AT7867 proliferates PDX1-expressing pancreatic progenitor cells derived from human pluripotent stem cells. Stem Cell Res 2017;24:61–8.ArticlePubMed
- 95. Ma X, Lu Y, Zhou Z, Li Q, Chen X, Wang W, et al. Human expandable pancreatic progenitor-derived β cells ameliorate diabetes. Sci Adv 2022;8:eabk1826.ArticlePubMedPMC
- 96. Wang D, Wang J, Bai L, Pan H, Feng H, Clevers H, et al. Long-term expansion of pancreatic islet organoids from resident Procr+ progenitors. Cell 2020;180:1198–211.ArticlePubMed
- 97. Shen W, Taylor B, Jin Q, Nguyen-Tran V, Meeusen S, Zhang YQ, et al. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat Commun 2015;6:8372.ArticlePubMedPMCPDF
- 98. Dhawan S, Dirice E, Kulkarni RN, Bhushan A. Inhibition of TGF-β signaling promotes human pancreatic β-cell replication. Diabetes 2016;65:1208–18.ArticlePubMedPMCPDF
- 99. Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 2006;24:185–7.ArticlePubMedPDF
- 100. Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014;14:810–23.ArticlePubMedPMC
- 101. Hannan NR, Fordham RP, Syed YA, Moignard V, Berry A, Bautista R, et al. Generation of multipotent foregut stem cells from human pluripotent stem cells. Stem Cell Reports 2013;1:293–306.ArticlePubMedPMC
- 102. Rosado-Olivieri EA, Anderson K, Kenty JH, Melton DA. YAP inhibition enhances the differentiation of functional stem cell-derived insulin-producing β cells. Nat Commun 2019;10:1464.ArticlePubMedPMCPDF
- 103. Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol 1984;247(3 Pt 1):C125–42.ArticlePubMed
- 104. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949;164:666.ArticlePubMedPDF
- 105. Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology 1984;21:407–26.ArticlePubMed
- 106. Best BP. Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res 2015;18:422–36.ArticlePubMedPMC
- 107. Rajotte RV, Stewart HL, Voss WA, Shnitka TK. Viability studies on frozen: thawed rat islets of Langerhans. Cryobiology 1977;14:116–20.ArticlePubMed
- 108. Bank HL. Cryobiology of isolated islets of Langerhans circa 1982. Cryobiology 1983;20:119–28.ArticlePubMed
- 109. Wise MH, Gordon C, Johnson RW. Intraportal autotransplantation of cryopreserved porcine islets of Langerhans. Cryobiology 1985;22:359–66.ArticlePubMed
- 110. Lakey JR, Rajotte RV, Fedorow CA, Taylor MJ. Islet cryopreservation using intracellular preservation solutions. Cell Transplant 2001;10:583–9.ArticlePubMedPDF
- 111. Modak MA, Parab PB, Ghaskadbi SS. Pancreatic islets are very poor in rectifying oxidative DNA damage. Pancreas 2009;38:23–9.ArticlePubMed
- 112. Hardikar AA, Risbud MV, Remacle C, Reusens B, Hoet JJ, Bhonde RR. Islet cryopreservation: improved recovery following taurine pretreatment. Cell Transplant 2001;10:247–53.ArticlePubMedPDF
- 113. Chandravanshi B, Dhanushkodi A, Bhonde R. High recovery of functional islets stored at low and ultralow temperatures. Rev Diabet Stud 2014;11:267–78.ArticlePubMedPMC
- 114. Pisania A, Weir GC, O’Neil JJ, Omer A, Tchipashvili V, Lei J, et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest 2010;90:1661–75.ArticlePubMedPMCPDF
- 115. Dolezalova N, Gruszczyk A, Barkan K, Gamble JA, Galvin S, Moreth T, et al. Accelerating cryoprotectant diffusion kinetics improves cryopreservation of pancreatic islets. Sci Rep 2021;11:10418.ArticlePubMedPMCPDF
- 116. Rawal S, Harrington S, Williams SJ, Ramachandran K, Stehno-Bittel L. Long-term cryopreservation of reaggregated pancreatic islets resulting in successful transplantation in rats. Cryobiology 2017;76:41–50.ArticlePubMed
- 117. Nagaya M, Matsunari H, Kanai T, Maehara M, Nakano K, Umeki I, et al. An effective new cryopreservation procedure for pancreatic islets using hollow fiber vitrification. Horm Metab Res 2016;48:540–9.ArticlePubMed
- 118. Kojayan GG, Flores A, Li S, Alexander M, Lakey JR. Cryopreserved alginate-encapsulated islets can restore euglycemia in a diabetic animal model better than cryopreserved non-encapsulated islets. Cell Med 2019;11:2155179019876641.ArticlePubMedPMCPDF
- 119. Zhan L, Rao JS, Sethia N, Slama MQ, Han Z, Tobolt D, et al. Pancreatic islet cryopreservation by vitrification achieves high viability, function, recovery and clinical scalability for transplantation. Nat Med 2022;28:798–808.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite