Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(1); 2024 > Article
-
Original ArticleHypothalamus and pituitary gland Preoperative Serum Copeptin Can Predict Delayed Hyponatremia after Pituitary Surgery in the Absence of Arginine Vasopressin Deficiency
 Keypoint
Keypoint
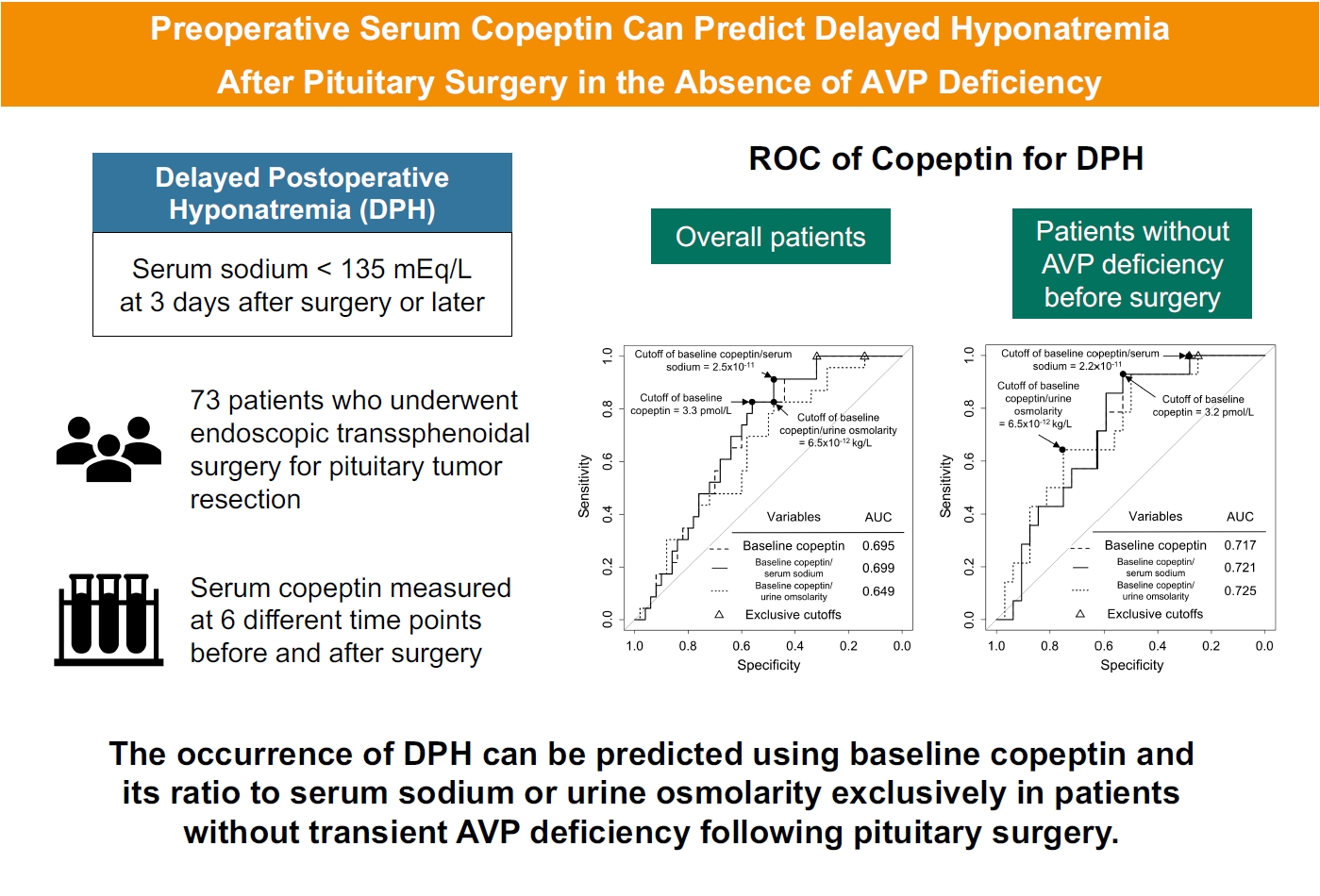
This study measured serum copeptin concentrations at 6 different time points before and after pituitary surgery and assessed the ability of copeptin to predict delayed postoperative hyponatremia. For patients without transient arginine vasopressin deficiency, the preoperative copeptin-to-urine osmolarity ratio demonstrated the highest predictive performance, with an AUROC of 0.725. -
Ho Kang1*
 , Seung Shin Park2*
, Seung Shin Park2* , Yoo Hyung Kim2, Hwan Sub Lim3, Mi-Kyeong Lee3, Kyoung-Ryul Lee3, Jung Hee Kim2,4
, Yoo Hyung Kim2, Hwan Sub Lim3, Mi-Kyeong Lee3, Kyoung-Ryul Lee3, Jung Hee Kim2,4 , Yong Hwy Kim4,5
, Yong Hwy Kim4,5
-
Endocrinology and Metabolism 2024;39(1):164-175.
DOI: https://doi.org/10.3803/EnM.2023.1792
Published online: January 3, 2024
1Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
3Department of Laboratory Medicine, Seoul Clinical Laboratories, Yongin, Korea
4Pituitary Center, Seoul National University Hospital, Seoul, Korea
5Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- Corresponding authors: Jung Hee Kim. Department of Internal Medicine and Pituitary Center, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-4839, Fax: +82-2-2072-2987, E-mail: jhee1@snu.ac.kr
- Yong Hwy Kim. Department of Neurosurgery and Pituitary Center, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-4062, Fax: +82-2-2072-2987, E-mail: kimyh96@snu.ac.kr
- *These authors contributed equally to this work.
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,072 Views
- 49 Download
ABSTRACT
-
Background
- Delayed postoperative hyponatremia (DPH) is the most common cause of readmission after pituitary surgery. In this study, we aimed to evaluate the cutoff values of serum copeptin and determine the optimal timing for copeptin measurement for the prediction of the occurrence of DPH in patients who undergo endoscopic transsphenoidal approach (eTSA) surgery and tumor resection.
-
Methods
- This was a prospective observational study of 73 patients who underwent eTSA surgery for pituitary or stalk lesions. Copeptin levels were measured before surgery, 1 hour after extubation, and on postoperative days 1, 2, 7, and 90.
-
Results
- Among 73 patients, 23 patients (31.5%) developed DPH. The baseline ratio of copeptin to serum sodium level showed the highest predictive performance (area under the curve [AUROC], 0.699), and its optimal cutoff to maximize Youden’s index was 2.5×10–11, with a sensitivity of 91.3% and negative predictive value of 92.0%. No significant predictors were identified for patients with transient arginine vasopressin (AVP) deficiency. However, for patients without transient AVP deficiency, the copeptin-to-urine osmolarity ratio at baseline demonstrated the highest predictive performance (AUROC, 0.725). An optimal cutoff of 6.5×10–12 maximized Youden’s index, with a sensitivity of 92.9% and a negative predictive value of 94.1%.
-
Conclusion
- The occurrence of DPH can be predicted using baseline copeptin and its ratio with serum sodium or urine osmolarity only in patients without transient AVP deficiency after pituitary surgery.
- Delayed postoperative hyponatremia (DPH) is a complication that occurs after pituitary surgery and is the most common cause of readmission after pituitary surgery [1-5]. The pathophysiology of DPH is not fully understood, but several mechanisms have been proposed, including hypocortisolism, hypothyroidism, syndrome of inappropriate diuretic hormone release (SIADH), primary polydipsia related to dry mouth, and sodium depletion due to postoperative nausea and anorexia [5-8]. DPH typically occurs between postoperative days (PODs) 4 and 10, with the highest incidence at approximately POD 7, with an incidence of 2% to 35% [1,3,4,6-9]. Since DPH can lead to sudden neurological deterioration, such as confusion or seizures, and delayed treatment may result in severe complications, early diagnosis is crucial. Fluid restriction may be prescribed during the peak period of DPH, or serum sodium levels may be checked at an outpatient clinic around POD 7 [1,8,10-12]. However, predicting the occurrence of DPH remains challenging, and only the prediction of SIADH as a second phase in the triphasic response is helpful in clinical practice for patients with severe intraoperative damage to the pituitary stalk [13,14]. Several factors have been suggested as potential predictors of DPH, such as older age, larger tumor size, Cushing disease, rapid decrease in serum sodium levels, low sodium levels during POD 1 to 2, and longer operation times. However, these factors are still controversial and not universally agreed upon as reliable predictors [5,7,15].
- Copeptin is a C-terminal polypeptide of pre-pro-vasopressin stimulated by high plasma osmolarity or volume depletion and secreted with arginine vasopressin (AVP). Unlike AVP, copeptin is stable for over 7 days at room temperature, making it a useful surrogate marker for AVP measurement through sandwich immunoassay [16,17]. Copeptin has been reported to help discriminate between different pathophysiological causes in cases of hypernatremia or hyponatremia. It has also been used to evaluate the integrity of the hypothalamus-pituitary axis in patients after pituitary surgery, mainly regarding AVP deficiency [18-20]. Studies have shown that the absolute copeptin value and the ratio of copeptin to baseline copeptin on POD 2 can predict the occurrence of permanent AVP deficiency [21]. However, the feasibility of using copeptin as a diagnostic tool for DPH remains controversial. While copeptin may help discriminate primary polydipsia, its usefulness in differentiating other causes of DPH is still uncertain.
- We aimed to evaluate the cutoff values of serum copeptin levels with maximal predictive power and to identify the optimal timing for copeptin measurement to predict the occurrence of DPH in patients who undergo endoscopic transsphenoidal approach (eTSA) surgery and tumor resection.
INTRODUCTION
- Study patients
- We consecutively enrolled 83 patients (age ≥18 years) who underwent eTSA surgery for resection of neoplasms on the pituitary stalk or gland between August 2020 and June 2021. This prospective cohort was described previously [21]. In the final analysis, we collected clinical data and blood samples from 73 patients at six different time points: before surgery (baseline), 1 hour after extubation, and on PODs 1, 2, 7, and 90.
- This study was approved by the Institutional Review Board of Seoul National University (No. 2006-169-1136), conducted in compliance with the Declaration of Helsinki and registered at the Korean Clinical Research Information Service (KCT0006488). All patients provided written informed consent.
- Data collection
- Definitions and methods of collecting parameters were described previously [21]. The following parameters were evaluated: age, sex, height, weight, blood urea nitrogen, creatinine, estimated glomerular filtration rate (using Chronic Kidney Disease Epidemiology Collaboration equations), preoperative comorbidities such as diabetes mellitus, hypertension and chronic renal disease, previous surgery for sellar lesions, and pathological diagnosis. DPH was defined as a serum sodium concentration <135 mEq/L on or after POD 3.
- For pituitary adenomas, maximal diameter, tumor height, and Knosp grade were assessed using preoperative magnetic resonance imaging (MRI) [22]. Gross total resection (GTR) was defined as complete tumor resection according to the surgeon’s opinion, as confirmed by postoperative MRI within 48 hours after surgery. Postoperative complications were assessed through a review of the medical records and images.
- Anterior pituitary function was evaluated in all patients before and 3 months after surgery. The assessment method followed the protocol described in our previous study [19,21,23]. Adrenocorticotropic hormone (ACTH) deficiency was diagnosed when the peak cortisol level was <18 µg/dL with a low to normal serum ACTH concentration in a short Synacthen test. Thyroid-stimulating hormone (TSH) deficiency was diagnosed when the serum free thyroxine concentration was <0.7 ng/dL with a low to normal serum TSH level. The number of impaired anterior pituitary hormonal axes at POD 90 was compared with the number of short hormonal axes assessed before surgery; the anterior pituitary hormonal outcomes were classified as “improved,” “stationary,” or “aggravated” according to their respective fluctuations.
- Surgery
- All patients underwent eTSA surgery, the operative technique of which has been described previously [23,24]. Microdissection was performed to remove all tumors without blunt dissection. Extracapsular dissection for pituitary adenomas and dissection of the tumor from the pia or arachnoid membrane on the pituitary stalk and hypothalamus were conducted.
- Preoperative management and anesthesia
- Before surgery, patients with ACTH or TSH deficiency were replenished with hydrocortisone or levothyroxine. In particular, in patients with ACTH deficiency, 100 mg of hydrocortisone was administered intravenously immediately before surgery. Single injection/continuous infusion of propofol and continuous infusion of remifentanil were used for induction of anesthesia, and continuous infusion of propofol and remifentanil or sevoflurane inhalation with continuous infusion of remifentanil was used for maintenance of anesthesia. A detailed description of anesthesia was previously reported [5,21].
- Diagnosis and management of postoperative AVP deficiency
- Postoperative AVP deficiency was diagnosed when new onset hypotonic polyuria (>50 mL/kg/day with inappropriately low urine osmolality for serum osmolality) was accompanied by hypernatremia (>145 mmol/L) or serum osmolality>300 mOsm/kg. When AVP deficiency was diagnosed, 1 µg of intravenous desmopressin was administered. After 2 hours, follow-up serum electrolytes, serum osmolality, urine electrolytes, and urine osmolality were reassessed to confirm the pharmacologic response. If AVP deficiency was maintained until discharge, oral desmopressin was administered only when urine output increased to >50 mL/kg/day. However, if the stalk was sacrificed during surgery, 0.1 mg oral desmopressin was administered every 12 hours after discharge; if urine output decreased (<50 mL/kg/day), it was temporarily waived. Transient central diabetes insipidus was defined if desmopressin was no longer needed without hypotonic polyuria after postoperative 3 months. Permanent AVP deficiency was diagnosed if there was recurrence of hypotonic polyuria after desmopressin withdrawal 3 months postoperatively [18,21].
- Management of hypocortisolism and DPH
- During hospitalization, serum sodium and osmolarity were measured daily. If the patient was discharged earlier than POD 7, strict water restriction was not applied, and serum sodium level was measured at an outpatient clinic at POD 7. If the serum sodium level was <130 mEq/L or if the related symptoms, such as fatigue, nausea, and syncope, were severe, even with sodium levels ≥130 mEq/L, the patient was rehospitalized. When hypocortisolism was present before surgery or apparent injury of the pituitary stalk occurred during surgery, 100 mg of hydrocortisone per day was intravenously administered on POD 0, and 50 mg in divided dose twice a day was administered as a stress dose on POD 1 with the supplement of synthyroid. From POD 2, a physiologic dose of 15 mg divided into twice a day (10 mg at 7:00 AM and 5 mg at 5:00 PM) was administered, but in case of fever or sepsis, the dose was temporarily increased as needed as a stress dose. For patients without preoperative hypocortisolism and nonevident intraoperative injury of the pituitary stalk, morning serum cortisol was measured at 7:00 AM on PODs 1 and 2 in the state of fasting since midnight, and if it was less than 8 μg/dL, cortisol replacement was performed in the same way as for patients with preoperative hypocortisolism or suspicious stalk injury during surgery [25-27]. Hydrocortisone was discontinued if possible, and the decision to permanently discontinue was made based on the result of a short Synacthen test on POD 90.
- When DPH was diagnosed in patients who had not previously received hydrocortisone, 50 to 100 mg of hydrocortisone was intravenously or orally administered for 1 or 2 days and then administered at a dose of 15 mg/day (10 mg at 7:00 AM, 5 mg at 7:00 PM). If a patient who had been taking desmopressin due to persistent AVP deficiency was diagnosed with DPH, desmopressin was immediately discontinued after the diagnosis of DPH but resumed when the serum sodium level returned to normal and AVP deficiency features were seen again as in the triphasic response [14]. For patients with serum sodium levels less than 130 mEq/L or symptomatic DPH, water restriction (<1,000 mL intake per day) was applied, and 3% hypertonic saline was used if necessary.
- Copeptin measurement
- Blood samples were collected before surgery, 1 hour after extubation, and on PODs 1 (18±4 hours), 2 (42±4 hours), 7, and 90. Except for water, patients were instructed not to consume food for more than 12 hours. Even when desmopressin was regularly administered, morning desmopressin was not administered before collection of a blood sample. Blood samples were collected in a serum-separating tube and centrifuged at 4,000 rpm for 5 minutes. The obtained serum aliquot was immediately frozen and stored at −80°C. The stored samples were thawed and subsequently analyzed according to the manufacturer’s recommendations.
- Copeptin levels were measured using a KRYPTOR Compact Plus device and a commercially available chemiluminescence sandwich immunoassay copeptin ProAVP (BRAHMS Copeptin ProAVP KRYPTOR, Thermo Fisher Scientific, Dreieich, Germany) as previously described [28,29]. The assay measuring range was 0.7 to 500 pmol/L. The lower limit of detection of the assay was 0.69 pmol/L, and its intra- and interassay coefficients of variation were below 6.0% and 2.2%, respectively. The technician in charge of these assays was blinded to the patient’s features. There was no cross-reactivity between copeptin and AVP due to differences in the amino acid sequence.
- Statistical analysis
- This prospective cohort was originally designed to obtain the predictive power of copeptin for postoperative AVP deficiency [21]. For continuous variables, normality was tested using the Shapiro-Wilk test and presented as the mean±standard deviation or median (interquartile range) according to their P values. For categorical variables, a comparative analysis was performed using the chi-square test. Comparative analysis of continuous variables between two groups according to the occurrence of DPH was conducted by Student’s t test or the Mann-Whitney test according to normality. Additionally, we subclassified patients according to the presence or absence of transient AVP deficiency, which is expected to be one of the most important confounding factors, and performed subgroup analyses.
- Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive abilities of the copeptin measurement for DPH. The optimal cutoff for the prediction of DPH was set as the point that maximized Youden’s index (sensitivity+ specificity−1). After excluding patients who had already developed DPH before POD 7 (n=71), we performed ROC curve analysis and further analysis to evaluate the diagnostic power of copeptin level or ratios of copeptin on POD 7 for DPH. The predictive performance of each variable was compared using DeLong’s test and presented as an odds ratio (OR) in logistic regression models; all statistical analyses were performed using R version 4.2.2 (Foundation for Statistical Computing, Vienna, Austria).
METHODS
- Baseline characteristics of patients
- Seventy-three patients underwent eTSA surgery. Among them, 23 (32%) developed DPH, and there was no patient with postoperative hyponatremia (<135 mEq/L) before POD 3. Table 1 summarizes the baseline characteristics according to the presence of DPH. There was no difference in variables except preoperative copeptin and meningitis according to the presence of DPH.
- DPH was diagnosed with a median serum sodium level of 133 mEq/L on the median day 7 postoperatively (Supplemental Fig. S1). GTR was achieved in 61 patients (83.6%), with no significant difference in the GTR rate according to the presence of DPH. Nine patients (12.3%) and 27 patients (40.0%) had postoperative deterioration of anterior pituitary hormones and transient AVP deficiency, respectively, with no significant difference with or without DPH. Among 66 patients without preoperative hypocortisolism, 23 patients (34.8%) received preemptive hydrocortisone supplementation after surgery, including 10 patients with pituitary adenoma and all 13 patients with craniopharyngioma. Four (17.4%) of the patients who received preemptive steroid supplements developed DPH, and all four had craniopharyngioma.
- Differences in copeptin concentration according to the presence of DPH
- According to the occurrence of DPH, copeptin concentrations at each time point are shown in Table 2, Fig. 1, and the time trend in each AVP deficiency group is shown in Supplemental Fig. S2. The level of copeptin 1 hour after extubation showed a trend that increased from baseline and decreased from POD 1 regardless of the presence of DPH. The absolute value of preoperative copeptin and ratios of preoperative copeptin to serum sodium level (CSR) and urine osmolarity (CUR) were significantly lower in patients with DPH. Copeptin levels and ratios of copeptin from 1 hour after extubation to POD 2 were not different by the occurrence of DPH. The serum sodium level and CUR on POD 7 were significantly lower in patients with DPH.
- Predictive and diagnostic performance of copeptin for DPH
- The ROC curves of the absolute copeptin value, CSR, and CUR at baseline are shown in Fig. 2A. Although baseline CSR showed the highest area under the curve (AUROC) of 0.699, it was not statistically superior to the absolute copeptin value or CUR at baseline (Fig. 2A). No combination using absolute copeptin values at baseline achieved ROC curves with AUROCs higher than 0.699, the AUROC of baseline CSR.
- The cutoffs that maximized Youden’s index and negative predictive value (NPV) at baseline are listed in Table 3. The cutoff value to maximize Youden’s index was 3.3 pmol/L for the baseline copeptin level, with a sensitivity of 82.6% and NPV of 87.1%. The cutoff value of the baseline copeptin level to exclude the possibility of DPH was 4.5 pmol/L. For the baseline CSR, the cutoff value to maximize Youden’s index was 2.5×10–11, with a sensitivity of 91.3% and NPV of 92.0%. The cutoff of baseline CSR that could exclude the possibility of DPH was 3.2×10–11. The cutoff of baseline CUR that maximized Youden’s index was 6.5×10–12, with a sensitivity of 82.6% and NPV of 85.7%. The cutoff of baseline CUR to exclude the possibility of DPH was 11.1×10–12.
- The diagnostic performance of serum sodium level and CUR on POD 7 for the occurrence of DPH on or after POD 7 are presented in Supplemental Table S1. When DPH did not develop until POD 6, the cutoff value to maximize Youden’s index of CUR on POD 7 for the diagnosis of DPH was 7.8×10–12.
- Prediction models were presented along with the variables that showed significant differences according to the presence of DPH (Table 4). Patients with an absolute copeptin value, CSR, and CUR at baseline below cutoffs had a 5.6 (95% confidence interval [CI], 1.8 to 21.4), 8.9 (95% CI, 2.3 to 59.7), and 4.4-fold (95% CI, 1.4 to 16.8) higher risk of DPH, respectively.
- Predictive performance of copeptin for DPH according to the presence of transient AVP deficiency
- In patients without AVP deficiency (n=46), 14 (30.4%) developed DPH; these patients with DPH showed lower values of absolute copeptin, CSR, and CUR at baseline, CSR on POD 2, and serum sodium level, absolute copeptin value, and CUR on POD 7 than those without DPH (Supplemental Table S2). The ROC curves of the absolute copeptin value, CSR, and CUR at baseline in patients without AVP deficiency are shown in Fig. 2B. Baseline CUR showed the highest AUROC of 0.725, but it was not substantially superior to other variables at baseline. The cutoff that maximized Youden’s index of baseline CUR was 6.5×10–12, with a sensitivity of 92.9% and NPV of 94.1% (Supplemental Table S3). The cutoffs of the absolute copeptin value and the CSR at baseline to maximize Youden’s index were 3.2 pmol/L and 2.2×10–11, respectively, and the cutoff for the CSR on POD 2 was 2.1×10–11. The cutoffs showed significant ORs (Supplemental Table S4).
- In patients with transient AVP deficiency (n=27), there was no difference in the absolute copeptin value or copeptin ratios according to the presence of DPH (Supplemental Table S5). The cutoff of serum sodium level on POD 7 that could exclude the possibility of DPH after POD 7 was 147 mEq/L (Supplemental Table S6).
RESULTS
- In this prospective study, the absolute copeptin value and copeptin ratios at baseline and POD 7 were significantly related to DPH occurrence. Despite the absence of statistical superiority, the baseline CSR exhibited the highest AUROC of 0.695 in the ROC analysis. Significant ORs for DPH were observed for the baseline absolute copeptin value, CSR, and CUR. This was particularly notable in the group excluding those with transient AVP deficiency, compared to the entire cohort analysis. However, in patients with transient AVP deficiency, baseline copeptin and its ratios did not show significant predictive ability for DPH. Furthermore, no differences were observed in DPH incidence based on the pathological diagnosis or the presence of AVP deficiency.
- In our study, we aimed to mitigate postoperative ACTH or TSH deficiencies through the preemptive administration of hydrocortisone and levothyroxine. Additionally, we explored the predictive capabilities of copeptin for DPH arising from causes like SIADH. We hypothesize that this preemptive hormone therapy might explain why traditionally recognized DPH risk factors—such as older age, larger tumor size, low sodium levels in the early PODs, and tumor cephalocaudal diameter—did not significantly correlate with DPH occurrence in our study.
- In routine practice, fluid restriction after discharge was advised in patients who underwent eTSA. However, we aimed to assess copeptin’s predictive value for DPH without interfering with the natural physiological responses following hypothalamus-pituitary axis injury during surgery. This consideration led us to avoid routine fluid restriction. Specifically, in this research, we aimed to assess the applicability of copeptin in guiding clinical decision-making in actual practice. Given that DPH predominantly occurs on POD 7 to 8, we investigated whether DPH could be predicted using clinical information obtainable before POD 7, such as the presence of transient AVP deficiency and copeptin levels or their ratios prior to or on POD 2.
- The AUROC of 0.725 for the baseline CUR, specifically in patients without transient AVP deficiency, may not be definitive but can be informative in actual clinical decision-making. Earlier studies designed to explore the relationship between copeptin and hyponatremia were primarily focused on investigating its usefulness to elucidate the causes of hyponatremia in emergency department setting. The results of these studies of copeptin demonstrated its strong diagnostic value in distinguishing primary polydipsia but lacked robust diagnostic power for other causes [29-31]. The efficacy of copeptin in predicting and differentiating hyponatremia following pituitary surgery remains contentious, making the predictive ability of baseline copeptin for DPH presented in this study important [29-32]. In this study, the exclusion of postoperative diuretic use and the preemptive administration of hydrocortisone in patients suspected of having hypocortisolism allows us to eliminate diuretics and hypocortisolism as potential causes of DPH observed in this research. Therefore, we should consider several other mechanisms, such SIADH, primary polydipsia, and sodium depletion due to postoperative nausea. These mechanisms often occur simultaneously after surgery, which may explain the difficulty we encountered in predicting DPH using postoperative copeptin levels in our study. Nevertheless, considering the baseline copeptin level, it can be inferred that copeptin partly reflects the basal capacity of AVP secretion. The significantly lower copeptin levels on POD 90 in patients with DPH further support this notion (Supplemental Table S7). Thus, we hypothesize that preoperative subclinical damage to the hypothalamus-pituitary axis caused by a tumor exists, the extent of which is mirrored in preoperative copeptin levels, and that damage to this axis during surgery manifests as DPH.
- Baseline copeptin and its ratios demonstrated significant predictive power for DPH only in patients without transient AVP deficiency. In our prior study conducted with the same cohort, preoperative copeptin levels were higher in patients with AVP deficiency than in those without AVP deficiency, although the difference was not statistically significant, regardless of the tumor’s pathological diagnosis [21]. Thus, although the mechanism remains unclear, preoperative copeptin levels were higher in patients with AVP deficiency before surgery, and it is inferred that copeptin lacks predictive power for DPH even before surgery in patients with AVP deficiency. Additionally, when transient AVP deficiency occurred, we administered desmopressin, which might also contribute to lowering copeptin levels through a decrease in serum osmolarity and an increase in volume. Even if the predictive power of copeptin is limited to patients without transient AVP deficiency, the finding remains sufficiently significant. In cases of craniopharyngioma, even if stalk injury is incomplete during surgery, the possibility of DPH is considered, and close monitoring ensues [14,24]. Rather, it is pituitary adenoma where the likelihood of DPH is often underestimated. Among the 53 patients with pituitary adenoma in this series, 41 did not have transient AVP deficiency, and 14 of them developed DPH. This study’s results are important as they provide evidence for identifying which patients should be closely monitored for hyponatremia when patients with pituitary adenoma do not exhibit postoperative transient AVP deficiency.
- There are limitations to the current study as well. Since there were lack of a detailed analysis of patients’ volume status and differential diagnosis of DPH such as SIADH, primary polydipsia, and sodium depletion, the predictive power of copeptin for each differential diagnosis could not be assessed. Furthermore, since this cohort was initially designed to evaluate copeptin’s predictive power for postoperative AVP deficiency, a larger number of patients should have been included, given that DPH is typically reported at a lower incidence than postoperative AVP deficiency. Other factors, such as operation time, extubation time, or drugs used for anesthesia, might affect the occurrence of DPH. However, we did not incorporate it in this study due to a lack of information.
- The occurrence of DPH can be predicted using baseline copeptin and its ratio to serum sodium or urine osmolarity exclusively in patients without transient AVP deficiency following pituitary surgery.
DISCUSSION
Supplementary Material
Supplemental Table S1.
Supplemental Table S2.
Supplemental Table S3.
Supplemental Table S4.
Supplemental Table S5.
Supplemental Table S6.
Supplemental Table S7.
Supplemental Fig. S1.
Supplemental Fig. S2.
-
CONFLICTS OF INTEREST
Jung Hee Kim is a deputy editor of the journal. But she was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.H.K., Y.H.K. Acquisition, analysis, or interpretation of data: H.K., S.S.P., Y.H.K., H.S.L., M.K.L., K. R.L., J.H.K., Y.H.K. Drafting the work or revising: H.K., S.S.P., Y.H.K., H.S.L., M.K.L., K.R.L., J.H.K., Y.H.K. Final approval of the manuscript: H.K., S.S.P., Y.H.K., H.S.L., M.K.L., K.R.L., J.H.K., Y.H.K.
Article information
-
Acknowledgements
- This work was supported by the EnM Research Award from the Korean Endocrinology Society to Jung Hee Kim in 2021 and the New Faculty Startup Fund from Seoul National University to Yong Hwy Kim. We would like to thank the Seoul Clinical Laboratories. The biospecimens used in this study were provided by the Biobank of Seoul National University Hospital, a member of the Korea Biobank Network. We also thank Serena Park for data collection.


| Characteristic | No DPH (n=50) | DPH (n=23) | P value |
|---|---|---|---|
| Age, yr | 46.5 (36.0–57.0) | 40.0 (35.0–60.0) | 0.896 |
| Male sex | 29 (58.0) | 9 (39.1) | 0.212 |
| BMI, kg/m2 | 25.1 (23.1–27.8) | 23.8 (22.3–26.6) | 0.174 |
| Diabetes mellitus | 5 (10.0) | 2 (8.7) | 1.000 |
| Hypertension | 10 (20.0) | 6 (26.1) | 0.780 |
| CKD | 0 | 0 | |
| BUN, mg/dL | 14.0 (11.0–16.0) | 14.0 (11.5–16.5) | 0.721 |
| Creatinine, mg/dL | 0.8±0.2 | 0.8±0.2 | 0.491 |
| eGFR, mL/min/1.73 m2 | 103.9 (96.1–113.9) | 102.4 (93.2–112.5) | 0.821 |
| Revised | 12 (24.0) | 9 (39.1) | 0.294 |
| Pathology | 0.279 | ||
| Pituitary adenoma | 35 (70.0) | 18 (78.3) | 0.651 |
| Somatotroph | 20 (40.0) | 14 (60.9) | 0.159 |
| Corticotroph | 3 (6.0) | 1 (4.3) | 0.791 |
| Gonadotroph | 7 (14.0) | 2 (8.7) | 0.797 |
| Mammotroph | 3 (6.0) | 1 (4.3) | 0.791 |
| Null cell | 2 (4.0) | 0 | 0.841 |
| Craniopharyngioma | 9 (18.0) | 4 (17.4) | 1.000 |
| Rathke’s cleft cyst | 4 (8.0) | 0 | 0.400 |
| Meningioma | 2 (4.0) | 0 | 0.841 |
| Others | 0 | 1 (4.3) | 0.689 |
| Characteristics of pituitary adenomas (n=53) | |||
| Knosp grade | 0.787 | ||
| 0 | 6 (17.1) | 6 (33.3) | 0.324 |
| 1 | 9 (25.7) | 5 (27.8) | 1.000 |
| 2 | 12 (34.3) | 5 (27.8) | 0.865 |
| 3 | 5 (14.3) | 2 (11.1) | 1.000 |
| 4 | 3 (8.6) | 0 | 0.515 |
| Maximal diameter, mm | 27±11 | 24±8 | 0.411 |
| Tumor height from planum sphenoidale, mm | 6.2 (–3.0 to 8.5) | 6.7 (0.0 to 8.4) | 0.888 |
| Cephalocaudal diameter, mm | 22.6±11.0 | 20.4±7.8 | 0.445 |
| Preoperative endocrinological characteristics | |||
| ACTH deficiency | 4 (8.0) | 3 (13.0) | 0.801 |
| TSH deficiency | 6 (12.0) | 4 (17.4) | 0.798 |
| FSH/LH deficiency | 1 (2.0) | 1 (4.3) | 1.000 |
| Panhypopituitarism | 0 | 0 | |
| Preoperative DI | 0 | 0 | |
| Preoperative copeptin, pmol/L | 3.4 (2.5–5.0) | 2.5 (2.2–3.0) | 0.008a |
| Surgical outcome | |||
| Gross total resection | 39 (78.0) | 22 (95.7) | 0.121 |
| Anterior pituitary function | 0.587 | ||
| Improved | 5 (10.0) | 4 (17.4) | 0.611 |
| Stationary | 38 (76.0) | 17 (73.9) | 1.000 |
| Aggravated | 7 (14.0) | 2 (8.7) | 0.797 |
| DI | 18 (36.0) | 9 (39.1) | 0.997 |
| Only transient DI | 7 (14.0) | 6 (26.1) | 0.355 |
| Permanent DI | 11 (22.0) | 3 (13.0) | 0.560 |
| Pre-emptive hydrocortisone supplement among patients without preoperative ACTH deficiency | 19 (41.3) | 4 (20.0) | 0.165 |
| Time interval to the detection of DPH, postoperative day | - | 8 (8–8) | |
| Complications | |||
| Meningitis | 0 | 2 (8.7) | 0.034a |
| CSF leakage | 0 | 1 (4.3) | 0.689 |
| Intracranial hemorrhage | 0 | 0 | |
| Hydrocephalus | 1 (2.0) | 0 | 0.688 |
| Uncontrollable epistaxis | 0 | 1 (4.3) | 0.689 |
| Others | 1 (2.0) | 0 | 0.688 |
| Revision due to complication | 1 (2.0) | 0 | 0.688 |
Values are expressed as median (interquartile range), number (%), or mean±standard deviation.
DPH, delayed postoperative hyponatremia; BMI, body mass index; CKD, chronic kidney disease; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; ACTH, adrenocorticotrophic hormone; TSH, thyroid-stimulating hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; DI, diabetes insipidus; CSF, cerebrospinal fluid.
a Significant P values are indicated.
| Variable | No DPH (n=50) | DPH (n=23) | P value |
|---|---|---|---|
| Baseline | |||
| Serum Na, mEq/L | 142±2 | 141±2 | 0.536 |
| Serum copeptin, pmol/L | 3.4 (2.5–5.0) | 2.5 (2.2–3.0) | 0.008a |
| Copeptin/serum sodium ratio, 10–11 | 2.4 (1.7–3.6) | 1.8 (1.5–2.1) | 0.007a |
| Copeptin/urine osmolarity ratio, 10–12 kg/L | 6.2 (3.9–8.9) | 4.6 (3.3–6.2) | 0.043a |
| 1 hr after extubation | |||
| Serum Na, mEq/L | 142 (141–143) | 142 (141–143) | 0.718 |
| Serum copeptin, pmol/L | 5.0 (3.9–10.3) | 5.1 (3.2–11.2) | 0.495 |
| Copeptin/baseline copeptin ratio | 1.61 (1.03–3.20) | 1.88 (1.12–5.27) | 0.402 |
| Copeptin/serum sodium ratio, 10–11 | 3.5 (2.7–7.3) | 3.6 (2.3–8.0) | 0.487 |
| Copeptin/urine osmolarity ratio, 10–12 kg/L | 12.6 (8.0–27.2) | 15.6 (9.9–27.1) | 0.708 |
| POD 1 | |||
| Serum Na, mEq/L | 142 (140–144) | 141 (139–143) | 0.412 |
| Serum copeptin, pmol/L | 3.5 (2.5–5.1) | 2.9 (2.0–4.1) | 0.099 |
| Copeptin/baseline copeptin ratio | 1.04 (0.74–1.38) | 1.13 (0.71–1.57) | 0.502 |
| Copeptin/serum sodium ratio, 10–11 | 2.5 (1.8–3.7) | 2.1 (1.4–2.9) | 0.116 |
| Copeptin/urine osmolarity ratio, 10–12 kg/L | 6.7 (4.1–13.1) | 5.3 (3.6–10.0) | 0.130 |
| POD 2 | |||
| Serum Na, mEq/L | 143 (142–145) | 144 (142–146) | 0.494 |
| Serum copeptin, pmol/L | 3.2 (2.7–4.1) | 2.6 (2.0–3.3) | 0.073 |
| Copeptin/baseline copeptin ratio | 0.96 (0.67–1.34) | 1.15 (0.72–1.46) | 0.263 |
| Copeptin/serum sodium ratio, 10–11 | 2.2 (1.8–2.9) | 1.8 (1.4–2.4) | 0.078 |
| Copeptin/urine osmolarity ratio, 10–12 kg/L | 8.5 (4.3–11.0) | 7.5 (6.3–12.5) | 0.421 |
| POD 7 | |||
| Serum Na, mEq/L | 141±3 | 134±6 | <0.001a |
| Serum copeptin, pmol/L | 3.3 (2.5–4.8) | 2.7 (2.4–3.5) | 0.138 |
| Copeptin/baseline copeptin ratio | 1.08 (0.75–1.44) | 1.19 (1.09–1.32) | 0.494 |
| Copeptin/serum sodium ratio, 10–11 | 2.3 (1.7–3.4) | 2.1 (1.8–2.5) | 0.307 |
| Copeptin/urine osmolarity ratio, 10–12 kg/L | 5.6 (4.2–10.2) | 4.7 (3.5–6.3) | 0.038a |
| Cutoff value | No. (%) | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % |
|---|---|---|---|---|---|---|
| Copeptin level | ||||||
| <3.3 pmol/La | 42 (57.5) | 82.6 | 54.0 | 45.2 | 87.1 | 63.0 |
| <4.5 pmol/Lb | 57 (78.1) | 100.0 | 32.0 | 40.4 | 100.0 | 53.4 |
| Copeptin/serum sodium ratio | ||||||
| <2.5×10–11a | 48 (65.8) | 91.3 | 46.0 | 43.8 | 92.0 | 60.3 |
| <3.2×10–11b | 57 (78.1) | 100.0 | 32.0 | 40.4 | 100.0 | 53.4 |
| Copeptin/urine osmolarity ratio | ||||||
| <6.5×10–12 kg/La | 45 (61.6) | 82.6 | 48.0 | 42.2 | 85.7 | 58.9 |
| <11.1×10–12 kg/Lb | 66 (90.4) | 100.0 | 14.0 | 34.8 | 100.0 | 41.1 |
| Variable | OR (95% CI) | P value |
|---|---|---|
| Copeptin at baseline <3.3 pmol/L | 5.6 (1.8–21.4) | 0.005a |
| Copeptin/serum sodium at baseline <2.5×10–11 | 8.9 (2.3–59.7) | 0.006a |
| Copeptin/urine osmolarity at baseline <6.5×10–12 kg/L | 4.4 (1.4–16.8) | 0.017a |
- 1. Bohl MA, Ahmad S, Jahnke H, Shepherd D, Knecht L, White WL, et al. Delayed hyponatremia is the most common cause of 30-day unplanned readmission after transsphenoidal surgery for pituitary tumors. Neurosurgery 2016;78:84–90.ArticlePubMedPDF
- 2. Bohl MA, Ahmad S, White WL, Little AS. Implementation of a postoperative outpatient care pathway for delayed hyponatremia following transsphenoidal surgery. Neurosurgery 2018;82:110–7.ArticlePubMedPDF
- 3. Hong YG, Kim SH, Kim EH. Delayed hyponatremia after transsphenoidal surgery for pituitary adenomas: a single institutional experience. Brain Tumor Res Treat 2021;9:16–20.ArticlePubMedPMCPDF
- 4. Hussain NS, Piper M, Ludlam WG, Ludlam WH, Fuller CJ, Mayberg MR. Delayed postoperative hyponatremia after transsphenoidal surgery: prevalence and associated factors. J Neurosurg 2013;119:1453–60.ArticlePubMed
- 5. Yoon HK, Lee HC, Kim YH, Lim YJ, Park HP. Predictive factors for delayed hyponatremia after endoscopic transsphenoidal surgery in patients with nonfunctioning pituitary tumors: a retrospective observational study. World Neurosurg 2019;122:e1457–64.ArticlePubMed
- 6. Burke WT, Cote DJ, Iuliano SI, Zaidi HA, Laws ER. A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary 2018;21:25–31.ArticlePubMedPDF
- 7. Cote DJ, Alzarea A, Acosta MA, Hulou MM, Huang KT, Almutairi H, et al. Predictors and rates of delayed symptomatic hyponatremia after transsphenoidal surgery: a systematic review [corrected]. World Neurosurg 2016;88:1–6.PubMed
- 8. Lee CC, Wang YC, Liu YT, Huang YC, Hsu PW, Wei KC, et al. Incidence and factors associated with postoperative delayed hyponatremia after transsphenoidal pituitary surgery: a meta-analysis and systematic review. Int J Endocrinol 2021;2021:6659152.ArticlePubMedPMCPDF
- 9. Tomita Y, Kurozumi K, Inagaki K, Kameda M, Ishida J, Yasuhara T, et al. Delayed postoperative hyponatremia after endoscopic transsphenoidal surgery for pituitary adenoma. Acta Neurochir (Wien) 2019;161:707–15.ArticlePubMedPDF
- 10. Deaver KE, Catel CP, Lillehei KO, Wierman ME, Kerr JM. Strategies to reduce readmissions for hyponatremia after transsphenoidal surgery for pituitary adenomas. Endocrine 2018;62:333–9.ArticlePubMedPDF
- 11. Patel KS, Shu Chen J, Yuan F, Attiah M, Wilson B, Wang MB, et al. Prediction of post-operative delayed hyponatremia after endoscopic transsphenoidal surgery. Clin Neurol Neurosurg 2019;182:87–91.ArticlePubMed
- 12. Winograd D, Staggers KA, Sebastian S, Takashima M, Yoshor D, Samson SL. An effective and practical fluid restriction protocol to decrease the risk of hyponatremia and readmissions after transsphenoidal surgery. Neurosurgery 2020;87:761–9.ArticlePubMedPDF
- 13. Brooks EK, Inder WJ. Disorders of salt and water balance after pituitary surgery. J Clin Endocrinol Metab 2022;108:198–208.ArticlePubMedPMCPDF
- 14. Lopez DC, Almeida JP, Momin AA, Andrade EJ, Soni P, Yogi-Morren D, et al. Triphasic response after endoscopic craniopharyngioma resection and its dependency on infundibular preservation or sacrifice. J Neurosurg 2023;139:790–7.ArticlePubMed
- 15. Makino R, Fujio S, Hanada T, Yonenaga M, Kawade S, Hashiguchi H, et al. Delayed postoperative hyponatremia in patients with acromegaly: incidence and predictive factors. Pituitary 2023;26:42–50.ArticlePubMedPDF
- 16. Barat C, Simpson L, Breslow E. Properties of human vasopressin precursor constructs: inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry 2004;43:8191–203.ArticlePubMed
- 17. Levy B, Chauvet MT, Chauvet J, Acher R. Ontogeny of bovine neurohypophysial hormone precursors. II. Foetal copeptin, the third domain of the vasopressin precursor. Int J Pept Protein Res 1986;27:320–4.PubMed
- 18. Berton AM, Gatti F, Penner F, Varaldo E, Prencipe N, Rumbolo F, et al. Early copeptin determination allows prompt diagnosis of post-neurosurgical central diabetes insipidus. Neuroendocrinology 2020;110:525–34.ArticlePubMedPDF
- 19. Kim YH, Kim YH, Je YS, Lee KR, Lim HS, Kim JH. Changes in copeptin levels before and 3 months after transsphenoidal surgery according to the presence of postoperative central diabetes insipidus. Sci Rep 2021;11:17240.ArticlePubMedPMCPDF
- 20. Rostom H, Noronha S, Jafar-Mohammadi B, May C, Borg A, Halliday J, et al. Post-pituitary surgery copeptin analysis as a ‘rule-out’ test for post-operative diabetes insipidus. Endocrine 2023;79:358–64.ArticlePubMedPMCPDF
- 21. Jang HN, Kang H, Kim YH, Lim HS, Lee MK, Lee KR, et al. Serum copeptin levels at day two after pituitary surgery and ratio to baseline predict postoperative central diabetes insipidus. Pituitary 2022;25:1004–14.ArticlePubMedPDF
- 22. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993;33:610–8.ArticlePubMed
- 23. Kim JH, Lee JH, Lee JH, Hong AR, Kim YJ, Kim YH. Endoscopic transsphenoidal surgery outcomes in 331 nonfunctioning pituitary adenoma cases after a single surgeon learning curve. World Neurosurg 2018;109:e409–16.ArticlePubMed
- 24. Dho YS, Kim YH, Se YB, Han DH, Kim JH, Park CK, et al. Endoscopic endonasal approach for craniopharyngioma: the importance of the relationship between pituitary stalk and tumor. J Neurosurg 2018;129:611–9.ArticlePubMed
- 25. Loriaux DL. Diagnosis and differential diagnosis of Cushing’s syndrome. N Engl J Med 2017;376:1451–9.ArticlePubMed
- 26. Wang F, Catalino MP, Bi WL, Dunn IF, Smith TR, Guo Y, et al. Postoperative day 1 morning cortisol value as a biomarker to predict long-term remission of Cushing disease. J Clin Endocrinol Metab 2021;106:e94–102.ArticlePubMedPDF
- 27. Cote DJ, Iuliano SL, Catalino MP, Laws ER. Optimizing pre-, intra-, and postoperative management of patients with sellar pathology undergoing transsphenoidal surgery. Neurosurg Focus 2020;48:E2.Article
- 28. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112–9.ArticlePubMedPDF
- 29. Fenske W, Stork S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab 2009;94:123–9.ArticlePubMedPDF
- 30. Christ-Crain M, Morgenthaler NG, Fenske W. Copeptin as a biomarker and a diagnostic tool in the evaluation of patients with polyuria-polydipsia and hyponatremia. Best Pract Res Clin Endocrinol Metab 2016;30:235–47.ArticlePubMed
- 31. Nigro N, Winzeler B, Suter-Widmer I, Schuetz P, Arici B, Bally M, et al. Evaluation of copeptin and commonly used laboratory parameters for the differential diagnosis of profound hyponatraemia in hospitalized patients: ‘The Co-MED Study’. Clin Endocrinol (Oxf) 2017;86:456–62.ArticlePubMedPDF
- 32. Baldrighi M, Castello LM, Bartoli E. Copeptin in hyponatremia: is there a role for this biomarker in the diagnostic workup? Endocrine 2018;60:384–5.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations


 KES
KES

 PubReader
PubReader ePub Link
ePub Link Cite
Cite