Articles
- Page Path
- HOME > Endocrinol Metab > Volume 38(4); 2023 > Article
-
Original ArticleDiabetes, obesity and metabolism Greater Severity of Steatosis Is Associated with a Higher Risk of Incident Diabetes: A Retrospective Longitudinal Study
 Keypoint
Keypoint
Fatty liver has been linked to a heightened risk of type 2 diabetes. A longitudinal study involving 1,798 participants used abdominal CT scans to determine the association between hepatic steatosis severity and the onset of diabetes. Over a 5-year median follow-up, participants with moderate to severe hepatic steatosis were over three times more likely to develop diabetes than those without steatosis. Thus, the severity of hepatic steatosis positively correlates with an increased risk of diabetes onset. -
Ji Min Han1*
 , Jung Hwan Cho1*
, Jung Hwan Cho1* , Hye In Kim1, Sunghwan Suh1, Yu-Ji Lee2, Jung Won Lee3, Kwang Min Kim3, Ji Cheol Bae1
, Hye In Kim1, Sunghwan Suh1, Yu-Ji Lee2, Jung Won Lee3, Kwang Min Kim3, Ji Cheol Bae1
-
Endocrinology and Metabolism 2023;38(4):418-425.
DOI: https://doi.org/10.3803/EnM.2023.1729
Published online: July 12, 2023
1Division of Endocrinology and Metabolism, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
2Division of Nephrology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
3Division of Gastroenterology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- Corresponding author: Ji Cheol Bae. Division of Endocrinology and Metabolism, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, 158 Paryong-ro, Masanhoewon-gu, Changwon 51353, Korea Tel: +82-55-233-5831, Fax: +82-55-233-5109, E-mail: drkuri10@gmail.com
- *These authors contributed equally to this work.
Copyright © 2023 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,065 Views
- 77 Download
ABSTRACT
-
Background
- Fatty liver is associated with increased risk of developing type 2 diabetes. We aimed to evaluate whether the severity of hepatic steatosis is associated with incident diabetes.
-
Methods
- We conducted a longitudinal analysis using data from 1,798 participants who underwent a comprehensive health checkup and abdominal computed tomography (CT). We assessed the association between baseline liver attenuation value on non-contrast CT images and risk of incident diabetes. All the participants were categorized into three groups based on the baseline liver attenuation value on non-contrast CT images: without hepatic steatosis (>57 Hounsfield unit [HU]), mild hepatic steatosis (41–57 HU), and moderate to severe hepatic steatosis (≤40 HU).
-
Results
- During a median follow-up period of 5 years, 6.0% of the study participants progressed to diabetes. The incidence of diabetes was 17.3% in the moderate to severe hepatic steatosis group, 9.0% in the mild steatosis group, and 2.9% in those without hepatic steatosis. In a multivariate adjustment model, as compared with participants without hepatic steatosis, those with moderate to severe steatosis had a hazard ratio (HR) of 3.24 (95% confidence interval [CI], 1.64 to 4.2) for the development of diabetes, and those in the mild steatosis group had a HR of 2.33 (95% CI, 1.42 to 3.80). One standard deviation decrease in mean CT attenuation values of the liver was associated with a 40% increase in the development of diabetes (multivariate adjusted HR, 1.40; 95% CI, 1.2 to 1.63).
-
Conclusion
- We found a positive association between severity of hepatic steatosis and risk of incident diabetes. Greater severity of steatosis was associated with a higher risk of incident diabetes.
- Ectopic fat is defined as excess fat deposition in non-adipose tissues, such as the liver, skeletal muscle, heart, and pancreas, which normally contain only small amounts of fat [1]. It is well established that accumulation of ectopic fat is a major contributor to metabolic risk, with some depots having systemic effects [1,2]. As a key organ in systemic metabolism, the liver contributes substantially to the development of insulin resistance and type 2 diabetes [3]. Ectopic fat accumulation in the liver causes nonalcoholic fatty liver disease (NAFLD), encompassing simple steatosis, nonalcoholic steatohepatitis, fibrosis, and cirrhosis [2,4]. Accumulating evidence shows that NAFLD is associated with increased risk of incident diabetes, and this risk is independent of adiposity measures and other common metabolic risk factors [5].
- There are still few large-scale studies on the quantitative relationship between NAFLD and type 2 diabetes, in terms of the severity of steatosis or NAFLD [5]. Liver biopsy is the gold standard for differentiating simple steatosis from steatohepatitis and evaluating the severity of hepatic steatosis and fibrosis. However, liver biopsy is invasive, expensive, and infeasible for population-based studies [6]. Therefore, studies based on histological findings were mostly small-scale and included patients in liver clinics [5,7,8]. Noninvasive modalities such as magnetic resonance imaging (MRI)-estimated proton density fat fraction (PDFF) and magnetic resonance elastography (MRE) are highly sensitive and specific for measuring liver fat contents; however, they are costly, time-consuming, and limited by technical feasibility [9]. Ultrasound is relatively limited in quantitatively measuring hepatic steatosis and distinguishing the severity of fatty liver. In most large-scale studies that examined the association between NAFLD and risk of developing diabetes, participants were recruited from general populations, and the diagnosis of NAFLD was based on ultrasonography [5].
- In an observational cohort study of Korean adults, we carried out an analysis that examines the quantitative association between hepatic steatosis and risk of new-onset diabetes. In this study, we assessed the severity of hepatis steatosis using unenhanced hepatic computed tomography (CT). Although not as sensitive or specific as MRI-PDFF or MRE, unenhanced hepatic CT imaging correlates well with those measure by biopsy [10]. We aimed to investigate the association of hepatic steatosis with incident diabetes, and to determine if there are differences in these associations according to the severity of hepatic steatosis. The risk of diabetes may increase with alteration in glucose metabolism and insulin sensitivity that occurs as hepatic fat contents increase [11,12]. We hypothesized that the severity of hepatic steatosis is associated with incident diabetes.
INTRODUCTION
- Study population
- This study included 2,785 participants aged ≥20 years who underwent a comprehensive health check-up and abdominal CT between January 2010 and December 2013 at the Samsung Changwon Hospital Healthcare Center. In the present study, a visit between 2010 and 2013 was termed a baseline visit. Among these participants, 996 were excluded based on the following criteria: (1) self-reported diabetes or undiagnosed diabetes (fasting glucose concentration ≥126 mg/dL or hemoglobin A1c [HbA1c] ≥6.5%) (n=209); (2) those who never underwent a follow-up examination after baseline visit (n=731); (3) those whose CT showed evidence of liver cirrhosis (n=5); (4) those with a history of malignant disease (n=3); and (5) missing data, including HbA1c and fasting plasma glucose (FPG) (n=45). After applying the above exclusion criteria, 1,798 participants were eligible for the analysis.
- Study design and statistical analysis
- This study was a retrospective, longitudinal, observational study of Korean adults without diabetes at baseline. We assessed hepatic steatosis and investigated associations with incident diabetes. We examined longitudinal associations between baseline liver attenuation value on non-contrast CT images and the risk of incident diabetes. All the participants were categorized into three groups using the baseline liver attenuation value on non-contrast CT images (>57, 41–57, and ≤40 Hounsfield unit [HU]). The incidence of diabetes was calculated in these groups, and the hazard ratio (HR) for the association of hepatic steatosis with incident diabetes was estimated using Cox proportional hazard regression analysis. The probability of developing diabetes, according to the baseline liver attenuation value, was estimated in multivariate logistic regression models using the margin command in Stata software. The median duration of follow-up for our study was 5 years (interquartile range, 4.2 to 5.3). All the models were adjusted for age, sex, body mass index (BMI), FPG, alcohol, triglyceride, and hepatitis B and C viral infections. A P value of <0.05 was considered statistically significant. All analyses were performed using Stata program version 15.1 (Stata Corp., College Station, TX, USA).
- This study was approved by the Institutional Review Board (IRB) of the Samsung Changwon Hospital (IRB number: SMC201908003). Informed consent for this study was waived by the IRB because the researchers only accessed the database to obtain clinical data for the analyses, and personal information was not accessed.
- Definition
- The development of diabetes was assessed from the records of participants at each follow-up visit through December 2018, and diabetes was diagnosed in participants who had FPG of ≥ 126 mg/dL or HbA1c ≥6.5% [13]. Additionally, participants who currently reported taking glucose-lowering medications based on a self-report questionnaire were defined as having diabetes. We used a mean liver attenuation cut-off value of 57 HU on unenhanced CT for mild hepatic steatosis (corresponding to hepatic fat contents ≥5%) and 40 HU for moderate to severe hepatic steatosis (corresponding to hepatic fat contents ≥30%) [10,14-16]. Significant alcohol consumption was defined as >21 standard drinks per week in men and >14 standard drinks per week in women. A standard alcoholic drink was any drink that contains 14 g of pure alcohol [17].
- Measurement
- Information on demographic characteristics and health-related history was obtained using self-reported questionnaires. The BMI was calculated as weight in kilogram divided by height in meters squared and then rounded to one decimal place. Blood samples were collected after an overnight fast. FPG concentration was determined on an automated chemistry analyzer (Hitachi Modular DPP, Roche Diagnostics, Tokyo, Japan) using a hexokinase method. HbA1c was measured using a non-porous ion-exchange liquid chromatography with a HLC-723 Tosoh G8 automatic analyzer (Tosoh Corporation, Tokyo, Japan).
- Computed tomography measurement of hepatic steatosis
- Hepatic steatosis was assessed by measuring the liver attenuation value on non-contrast CT images expressed as HU. The unenhanced images were reviewed on a picture archiving and communication system (Marosis m-view, Marotech, Seoul, Korea) by researchers (J.C.B., J.M.H., H.I.K., J.W.L., K.M.K., and Y.J.L.) blinded to patient data. Details of the method have been described previously [18]. For each case, hepatic attenuation was measured by means of 12 circular regions of interests (ROIs) within three different transverse sections of the liver, with each section containing the umbilical portion of the left portal vein, confluence of the right hepatic vein, and posterior branch of the right portal vein. At each representative level, the liver was divided into four sectors (right anterior, right posterior, left medial, and left lateral sectors), and one ROI randomly drawn inside each sector, avoiding the large vessels, biliary structure, and any focal lesions, was considered representative of the sector [10]. The size of each ROI ranged between 1.0 and 1.1 cm2. The abdominal CTs were performed using a 64-detector row helical scanner (SOMATOM Definition AS 64, Siemens Healthcare, Forchheim, Germany).
METHODS
- Cohort characteristics
- The characteristics of the study participants are presented in Table 1. Overall, the participants had a mean BMI of 24.0±3.1 kg/m2. The mean age was 44.3±8.8 years, and 69.2% of the study participants were men. Of the total participants, 43.0% (773 of 1,798 participants) had at least mild hepatic steatosis with a mean liver attenuation of ≤57 HU [14,16], and 5.8% (104 of 1,798 participants) had a mean liver attenuation of ≤40 HU, which is consistent with moderate to severe hepatic steatosis [10,15,16]. As the severity of hepatic steatosis increased, the values of metabolic parameters, including FPG, HbA1c, BMI, and triglyceride, increased, while high-density lipoprotein cholesterol decreased. A sex difference was also shown with more men being affected by hepatic steatosis than women.
- Relationship between steatosis and incident diabetes
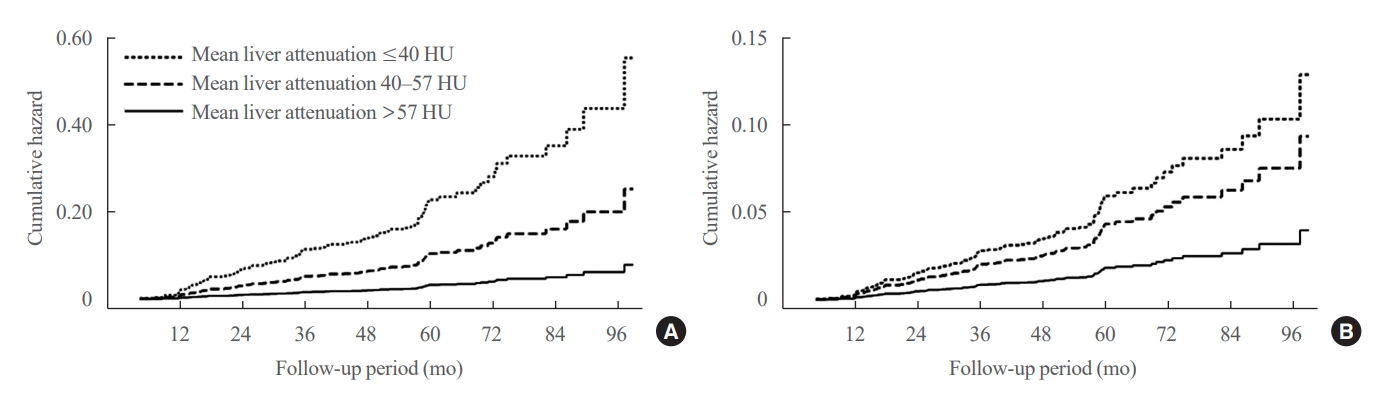
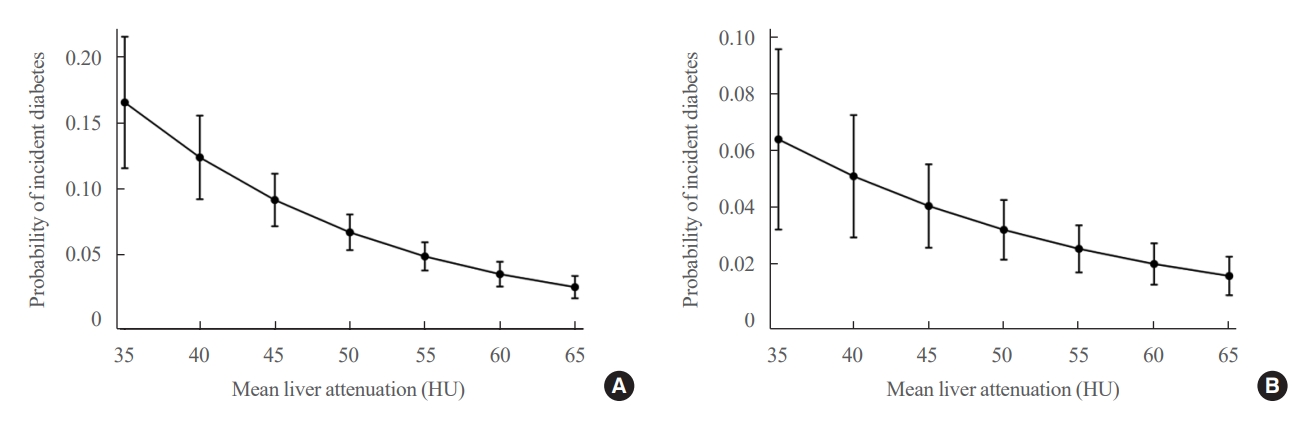
- Table 2, Fig. 1 show the risk of incident diabetes according to the categorized mean liver attenuation. During the median follow-up period of nearly 5 years (interquartile range, 4.2 to 5.3), 108 of 1,798 participants (6.0%) progressed to diabetes. The incidence of diabetes was 17.3% in the moderate to severe hepatic steatosis group (a mean liver attenuation of ≤40 HU) and 9.0% in the mild steatosis group (a mean liver attenuation of 40 to 57 HU), while it was 2.9% in participants without hepatic steatosis (a mean liver attenuation of >57 HU). In a multivariate adjustment model, participants in the moderate to severe steatosis group had a HR of 3.24 (95% confidence interval [CI], 1.64 to 6.42) for the development of diabetes, and those in the mild steatosis group had a HR of 2.33 (95% CI, 1.42 to 3.80) when compared with participants without hepatic steatosis. One standard deviation (SD) decrease in mean CT attenuation values of the liver was associated with a 40% increase in the development of diabetes (multivariate adjusted HR per SD decrement, 1.40; 95% CI, 1.2 to 1.63). The probability of incident diabetes increased with decreasing mean CT liver attenuation (Fig. 2).
RESULTS
- In this asymptomatic cohort, we found that 43.0% of 1,798 Korean adults were affected by hepatic steatosis, and 5.8% (104 individuals) had moderate or greater hepatic steatosis. The risk of incident diabetes increased among individuals with hepatic steatosis, and most importantly, greater severity of steatosis was associated with a higher risk of incident diabetes.
- In the present study, we used a liver attenuation value of 57 HU measured by unenhanced CT as a threshold indicative of at least mild hepatic steatosis [14], and the prevalence of hepatic steatosis was 43.0%. This value exceeds the global prevalence of NAFLD based on ultrasound, which has previously been reported to be between 20% and 35% of the adult population [19]. However, direct comparison is difficult, given the different sensitivities of CT and ultrasound in identifying steatosis [20]. Moreover, the prevalence of fatty liver depended on the specific diagnostic CT attenuation criteria, and these criteria also varied in their sensitivity for detecting mild hepatic steatosis [21]. Therefore, for other CT attenuation criteria that include milder severity of steatosis [21], using a liver attenuation of ≤ (spleen attenuation+5 HU) and liver-to-spleen ratio of ≤1.1 lowered the prevalence to 30.1% and 29.3%, respectively. Meanwhile, in our study, the prevalence of moderate to severe steatosis was 5.8% when 40-HU threshold was applied. This was similar to the prevalence reported in other studies that used same criterion of 40-HU threshold [21,22], although the study population had different background characteristics. A liver attenuation value of ≤40 HU represents the most accurate criterion for moderate to severe disease [10,21].
- We previously demonstrated that sustained presence of hepatic steatosis detected by ultrasound had an independent effect on incident diabetes [23]. The presence of fatty liver had a differential association with incident diabetes based on its duration. In the present study, fatty liver had a graded association with the risk of incident diabetes based on the severity of steatosis. The risk of incident diabetes increased with increasing severity of hepatic steatosis. Our results suggest that not only the persistence of fatty liver but also the severity of steatosis was independently associated with an increased risk of diabetes. Recently, hepatic steatosis has also been reported to have a quantitative association with liver fibrosis, showing higher probability of significant liver fibrosis with increasing severity of hepatic steatosis [24]. Evidence from liver biopsy cohort studies revealed that in patients with fatty liver disease, the risk of incident diabetes was higher when significant liver fibrosis was present [5]. Identifying the severity of hepatic steatosis could play a more important role in assessing the risk of developing diabetes than just detection of fatty liver.
- Insulin resistance is not independent of hepatic steatosis and can be exacerbated by bidirectional communication through metabolic inflammation [25]. Intrahepatic fat leads to activation of immune response represented by hepatic c-Jun N-terminal kinase and nuclear factor κB signaling cascades [25,26]. Chronic metabolic inflammation induced by intrahepatic fat promotes skeletal muscle, adipose tissue, and pancreatic dysfunction through liver-derived inflammatory mediators, including cytokines, acute-phase protein, and hepatokines [25,27,28]. All of these contribute to the development of type 2 diabetes by causing hepatic and peripheral insulin resistance, leading to derangements in glucose and lipid metabolism [4,25]. Peripheral insulin resistance also exacerbates hepatic steatosis by increasing lipolysis of adipocytes and circulating free fatty acids [27]. These pathophysiological mechanisms helped to interpret our findings that hepatic steatosis is a preferential risk factor for the development of type 2 diabetes.
- Greater severity of steatosis was reported to be associated with higher risk of significant fibrosis [24,29]. In our study, more severe hepatic steatosis was associated with an increased risk of developing diabetes. Individuals with clinically significant fibrosis, especially those with type 2 diabetes, have a greater risk of cirrhosis, cardiovascular disease, and all-cause mortality [30]. These findings suggest that patients with moderate to severe hepatic steatosis are at higher risk for hepatic and extrahepatic outcomes. The goal of screening is not to identify steatosis itself, but rather to identify patients in these high-risk groups [30]. Unenhanced CT is highly specific for identifying patients with moderate to severe hepatic steatosis [16,21,31]. CT images provide an objective measurement of liver fat content, and this noninvasive imaging modality has been increasingly used for the evaluation of fatty liver disease in many studies over the past two decades [16]. In our study, we used an abdominal CT to quantify the severity of hepatic steatosis.
- Our study has several strengths and limitations. We categorized all the participants into three groups (non-steatosis, mild, and moderate to severe steatosis groups) using the baseline liver attenuation value on non-contrast CT images. Although it is a very accurate test for detecting moderate to severe hepatic steatosis, unenhanced CT is less accurate in the assessment of mild steatosis [16,21]. We did not exclude participants with significant alcohol consumption or positive serologic markers for hepatitis B or C infection. Excessive alcohol consumption and chronic infection with viral hepatitis can cause hepatic steatosis [32]. Given the definition of NAFLD [13], this may be a limitation of our study. However, we do not believe that this biased our results because we found similar results even after adjusting for alcohol consumption and the presence of viral hepatitis B or C infection. Recently, a new nomenclature, metabolic-associated fatty liver disease, was proposed to replace the term NAFLD, thereby highlighting the association between fatty liver disease and metabolic dysregulation, regardless of alcohol consumption or the presence of other liver diseases [33]. We did not consider physical activity and dietary data in our analysis, which might affect the results. Lastly, the lack of 2-hour post-load glucose tests might have underestimated the incidence of diabetes. This study is significant in that it uses longitudinal data to verify the association between the severity of hepatic steatosis and incident diabetes. Although observational studies do not allow for proving of causality, longitudinal studies better establish the correct sequence of events, identify changes over time, and provide insight into cause-and-effect relationships.
- In conclusion, we found a positive association between the severity of hepatic steatosis and risk of incident diabetes. Greater severity of steatosis was associated with a higher risk of incident diabetes. Our findings suggest that quantitative measurement of hepatic steatosis is clinically important for assessing the risk of developing diabetes in patients with fatty liver.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.M.H., J.C.B. Acquisition, analysis, or interpretation of data: J.H.C., H.I.K., S.S., Y.J.L., J.W.L., K.M.K., J.C.B. Drafting the work or revising: J.M.H., J.H.C., J.C.B. Final approval of the manuscript: J.C.B.
Article information
-
Acknowledgements
- We thank the staff of Samsung Changwon Hospital Healthcare Center who have contributed to this data. We also thank Mi Hyeon Jin, Department of Research Support, Samsung Changwon Hospital, South Korea, for providing advice regarding statistical methodology.
| Characteristic | All (n=1,798) |
Mean liver attenuation on CT, Hua |
||
|---|---|---|---|---|
| >57 (n=1,025) | 57–40 (n=669) | ≤40 (n=104) | ||
| Age, yr | 44.3±8.8 | 44.3±8.9 | 44.4±8.7 | 42.8±8.8 |
| Male sex | 1,245 (69.2) | 625 (61.0) | 524 (78.3) | 96 (92.3) |
| BMI, kg/m2 | 24.0±3.1 | 22.8±2.5 | 25.2±2.9 | 27.4±2.9 |
| FPG, mg/dL | 89.0±8.9 | 88.2±8.3 | 89.6±9.5 | 93.1±10.2 |
| HbA1c, % | 5.5±0.3 | 5.4±0.3 | 5.5±0.3 | 5.6±0.3 |
| Triglyceride, mg/dL | 100 (70–144) | 88 (65–122) | 113 (77–168) | 148 (108–208) |
| HDL-C, mg/dL | 57.4±14.2 | 60.7±14.1 | 53.5±13.3 | 49.8±9.8 |
| AST, IU/L | 22 (18, 28) | 20 (17, 25) | 23 (19, 30) | 31 (26, 42) |
| ALT, IU/L | 20 (14, 29) | 16 (13, 23) | 25 (19, 35) | 49 (33, 66) |
| Alcohol | 197 (11.0) | 102 (9.9) | 85 (12.7) | 10 (9.6) |
| HBsAg positive | 114 (6.3) | 70 (6.8) | 38 (5.7) | 6 (5.8) |
| HCV Ab positive | 5 (0.2) | 3 (0.3) | 2 (0.3) | 0 |
| Mean liver attenuation, HUa | 55.2±8.0 | 60.2±2.8 | 51.1±4.2 | 32.3±7.2 |
Values are expressed as mean±standard deviation, number (%), or median (interquartile range).
CT, computed tomography; HU, Hounsfield unit; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; Ab, antibody.
a Measured in unenhanced CT.
| Variable |
Liver attenuation, HU |
Continuous liver attenuation, /SD decrease | |||
|---|---|---|---|---|---|
| >57 | 57–40 | ≤40 | |||
| No. of participants | 1,025 | 669 | 104 | ||
| No. of participants who developed DM (%) | 30 (2.9) | 60 (9.0) | 18 (17.3) | ||
| Person-years of follow-up | 4,793 | 3,027 | 425 | ||
| Incident cases of DM per 1,000 person-years | 6.3 | 19.8 | 42.4 | ||
| Adjusted HR (95% CI)a | |||||
| Unadjusted | 1 | 3.21 (2.07–4.99) | 7.04 (3.92–12.64) | 1.67 (1.49–1.88) | |
| Age and sex | 1 | 2.98 (1.91–4.65) | 6.26 (3.44–11.40) | 1.66 (1.46–1.88) | |
| Age, sex, BMI, FPG, TG, and HDL-C | 1 | 2.30 (1.42–3.73) | 3.19 (1.63–6.23) | 1.39 (1.19–1.62) | |
| Multivariateb | 1 | 2.33 (1.42–3.80) | 3.24 (1.64–6.42) | 1.40 (1.20–1.63) | |
HU, Hounsfield unit; SD, standard deviation; DM, diabetes mellitus; HR, hazard ratio; CI, confidence interval; BMI, body mass index; FPG, fasting plasma glucose; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol.
a Estimated by Cox proportional hazard analysis;
b Adjusted for age, sex, BMI, FPG, triglyceride, HDL-C, alcohol consumption, presence of hepatitis B surface antigen and hepatitis C virus antibody.
- 1. Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation 2011;124:e837–41.ArticlePubMed
- 2. Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715–25.ArticlePubMed
- 3. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017;14:32–42.ArticlePubMedPDF
- 4. Rhee EJ. Nonalcoholic fatty liver disease and diabetes: an epidemiological perspective. Endocrinol Metab (Seoul) 2019;34:226–33.ArticlePubMedPMCPDF
- 5. Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut 2021;70:962–9.ArticlePubMed
- 6. Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc 2015;90:1233–46.ArticlePubMedPMC
- 7. Nasr P, Fredrikson M, Ekstedt M, Kechagias S. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int 2020;40:1069–78.ArticlePubMedPDF
- 8. Bjorkstrom K, Stal P, Hultcrantz R, Hagstrom H. Histologic scores for fat and fibrosis associate with development of type 2 diabetes in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2017;15:1461–8.ArticlePubMed
- 9. Lee DH. Noninvasive evaluation of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul) 2020;35:243–59.ArticlePubMedPMCPDF
- 10. Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol 2007;188:1307–12.ArticlePubMed
- 11. Xia MF, Bian H, Yan HM, Lin HD, Chang XX, Li XM, et al. Assessment of liver fat content using quantitative ultrasonography to evaluate risks for metabolic diseases. Obesity (Silver Spring) 2015;23:1929–37.ArticlePubMedPDF
- 12. Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology 2017;65:1132–44.ArticlePubMedPDF
- 13. American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S17–38.ArticlePubMedPDF
- 14. Graffy PM, Sandfort V, Summers RM, Pickhardt PJ. Automated liver fat quantification at nonenhanced abdominal CT for population-based steatosis assessment. Radiology 2019;293:334–42.ArticlePubMedPMC
- 15. Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology 2006;239:105–12.ArticlePubMed
- 16. Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of liver fat content with CT and MRI: state of the art. Radiology 2021;301:250–62.ArticlePubMedPMC
- 17. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–57.ArticlePubMedPDF
- 18. Han JM, Kim HI, Lee YJ, Lee JW, Kim KM, Bae JC. Differing associations between fatty liver and dyslipidemia according to the degree of hepatic steatosis in Korea. J Lipid Atheroscler 2019;8:258–66.ArticlePubMedPMCPDF
- 19. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84.ArticlePubMed
- 20. Zhang YN, Fowler KJ, Hamilton G, Cui JY, Sy EZ, Balanay M, et al. Liver fat imaging: a clinical overview of ultrasound, CT, and MR imaging. Br J Radiol 2018;91:20170959.ArticlePubMedPMC
- 21. Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol 2010;194:623–8.ArticlePubMed
- 22. Garg T, Chu LC, Zimmerman SL, Weiss CR, Fishman EK, Azadi JR. Prevalence of hepatic steatosis in adults presenting to the emergency department identified by unenhanced chest CT. Curr Probl Diagn Radiol 2023;52:35–40.ArticlePubMed
- 23. Bae JC, Han JM, Cho JH, Kwon H, Park SE, Park CY, et al. The persistence of fatty liver has a differential impact on the development of diabetes: the Kangbuk Samsung Health Study. Diabetes Res Clin Pract 2018;135:1–6.ArticlePubMed
- 24. Bae JC, Beste LA, Utzschneider KM. The impact of insulin resistance on hepatic fibrosis among United States adults with non-alcoholic fatty liver disease: NHANES 2017 to 2018. Endocrinol Metab (Seoul) 2022;37:455–65.ArticlePubMedPMCPDF
- 25. Gehrke N, Schattenberg JM. Metabolic inflammation: a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 2020;158:1929–47.ArticlePubMed
- 26. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177–85.ArticlePubMedPDF
- 27. Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol 2017;13:509–20.ArticlePubMedPDF
- 28. Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008;103:1372–9.ArticlePubMed
- 29. Mak LY, Hui RW, Fung J, Liu F, Wong DK, Cheung KS, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol 2020;73:800–6.ArticlePubMed
- 30. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes. 2023. Diabetes Care 2023;46(Suppl 1):S49–67.ArticlePubMedPMCPDF
- 31. Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES. Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol 2012;22:1075–82.ArticlePubMedPDF
- 32. Zakhari S. Bermuda Triangle for the liver: alcohol, obesity, and viral hepatitis. J Gastroenterol Hepatol 2013;28 Suppl 1:18–25.ArticlePubMedPDF
- 33. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9.ArticlePubMed
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite