Search
- Page Path
- HOME > Search
- Miscellaneous
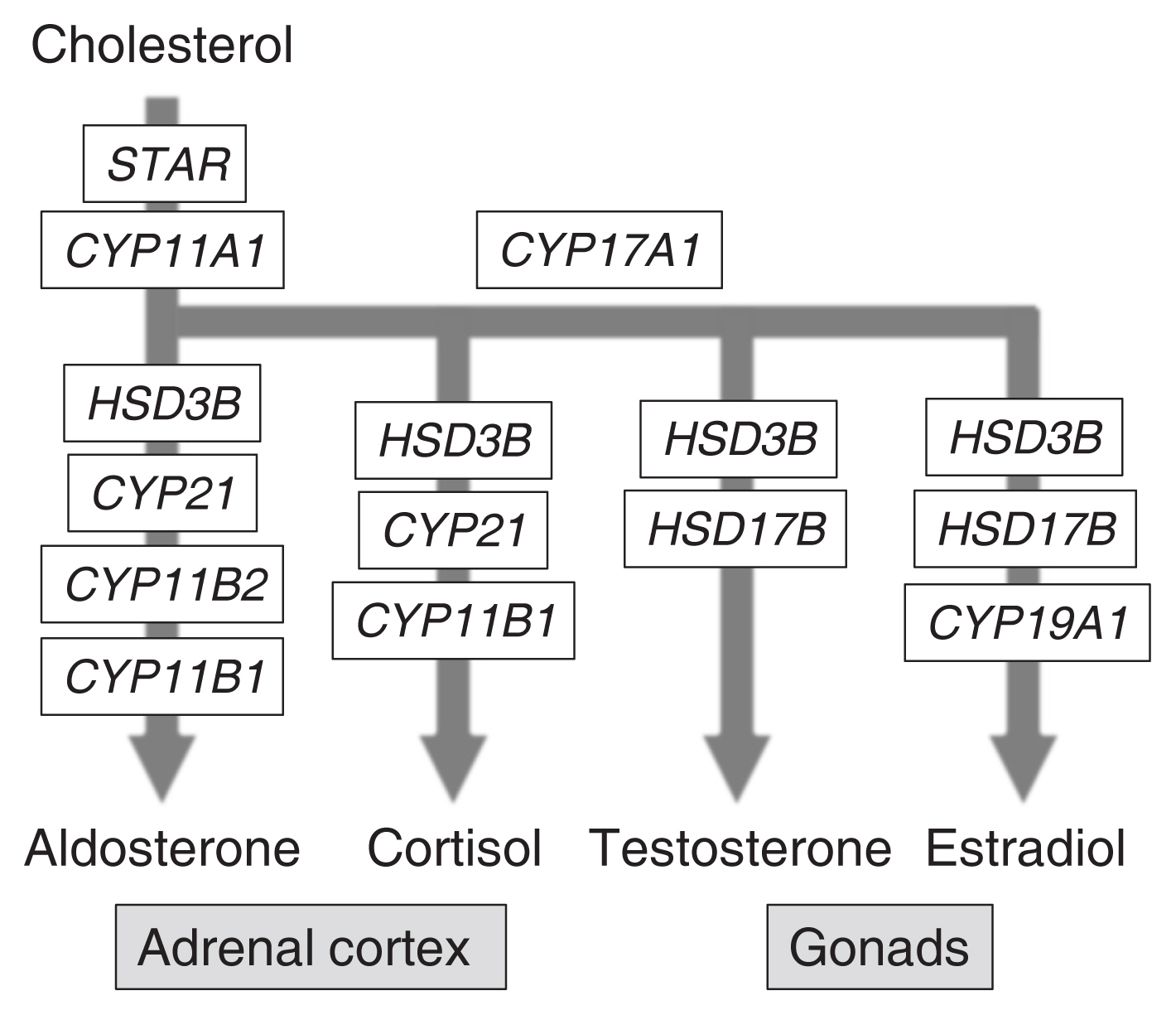
- Coordination of Multiple Cellular Processes by NR5A1/Nr5a1
- Ken-ichirou Morohashi, Miki Inoue, Takashi Baba
- Endocrinol Metab. 2020;35(4):756-764. Published online December 23, 2020
- DOI: https://doi.org/10.3803/EnM.2020.402
- 4,982 View
- 159 Download
- 7 Web of Science
- 7 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The agenesis of the gonads and adrenal gland in revealed by knockout mouse studies strongly suggested a crucial role for Nr5a1 (SF-1 or Ad4BP) in organ development. In relation to these striking phenotypes, NR5A1/Nr5a1 has the potential to reprogram cells to steroidogenic cells, endow pluripotency, and regulate cell proliferation. However, due to limited knowledge regarding NR5A1 target genes, the mechanism by which NR5A1/Nr5a1 regulates these fundamental processes has remained unknown. Recently, newlyestablished technologies have enabled the identification of NR5A1 target genes related to multiple metabolic processes, as well as the aforementioned biological processes. Considering that active cellular processes are expected to be accompanied by active metabolism, NR5A1 may act as a key factor for processes such as cell differentiation, proliferation, and survival by coordinating these processes with cellular metabolism. A complete and definite picture of the cellular processes coordinated by NR5A1/Nr5a1 could be depicted by accumulating evidence of the potential target genes through whole genome studies.
-
Citations
Citations to this article as recorded by- Development of sexual dimorphism of skeletal muscles through the adrenal cortex, caused by androgen-induced global gene suppression
Fumiya Takahashi, Takashi Baba, Antonius Christianto, Shogo Yanai, Hyeon-Cheol Lee-Okada, Keisuke Ishiwata, Kazuhiko Nakabayashi, Kenichiro Hata, Tomohiro Ishii, Tomonobu Hasegawa, Takehiko Yokomizo, Man Ho Choi, Ken-ichirou Morohashi
Cell Reports.2024; 43(2): 113715. CrossRef - A novel heterozygous SF1/NR5A1 gene variant causes 46,XY DSD-gonadal dysgenesis with hypergonadotropic hypogonadism without adrenal insufficiency
Luis Ramos
Genes & Diseases.2024; 11(4): 101160. CrossRef - A conserved NR5A1-responsive enhancer regulates SRY in testis-determination
Denis Houzelstein, Caroline Eozenou, Carlos F. Lagos, Maëva Elzaiat, Joelle Bignon-Topalovic, Inma Gonzalez, Vincent Laville, Laurène Schlick, Somboon Wankanit, Prochi Madon, Jyotsna Kirtane, Arundhati Athalye, Federica Buonocore, Stéphanie Bigou, Gerard
Nature Communications.2024;[Epub] CrossRef - Nuclear Receptor Gene Variants Underlying Disorders/Differences of Sex Development through Abnormal Testicular Development
Atsushi Hattori, Maki Fukami
Biomolecules.2023; 13(4): 691. CrossRef - Identification of a novel class of cortisol biosynthesis inhibitors and its implications in a therapeutic strategy for hypercortisolism
Soo Hyun Kim, Gi Hoon Son, Joo Young Seok, Sung Kook Chun, Hwayoung Yun, Jaebong Jang, Young-Ger Suh, Kyungjin Kim, Jong-Wha Jung, Sooyoung Chung
Life Sciences.2023; 325: 121744. CrossRef - Induced pluripotent stem cell line generated from a patient with differences in sex development (DSD) and multiple genetic variants including a large deletion in NR5A1
Aisha L. Siebert, Grace B. Schwartz, Hana Kubo, Monica M. Laronda
Stem Cell Research.2023; 71: 103154. CrossRef - Loss of NR5A1 in mouse Sertoli cells after sex determination changes cellular identity and induces cell death by anoikis
Sirine Souali-Crespo, Diana Condrea, Nadège Vernet, Betty Féret, Muriel Klopfenstein, Erwan Grandgirard, Violaine Alunni, Marie Cerciat, Matthieu Jung, Chloé Mayere, Serge Nef, Manuel Mark, Frédéric Chalmel, Norbert B. Ghyselinck
Development.2023;[Epub] CrossRef
- Development of sexual dimorphism of skeletal muscles through the adrenal cortex, caused by androgen-induced global gene suppression

- Endocrine Research
- Effects of Oxytocin on Cell Proliferation in a Corticotroph Adenoma Cell Line
- Jung Soo Lim, Young Woo Eom, Eun Soo Lee, Hyeong Ju Kwon, Ja-Young Kwon, Junjeong Choi, Choon Hee Chung, Young Suk Jo, Eun Jig Lee
- Endocrinol Metab. 2019;34(3):302-313. Published online September 26, 2019
- DOI: https://doi.org/10.3803/EnM.2019.34.3.302
- 4,999 View
- 74 Download
- 3 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Oxytocin (OXT) has been reported to act as a growth regulator in various tumor cells. However, there is a paucity of data on the influence of OXT on cell proliferation of corticotroph adenomas. This study aimed to examine whether OXT affects cell growth in pituitary tumor cell lines (AtT20 and GH3 cells) with a focus on corticotroph adenoma cells.
Methods Reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay were conducted with AtT20 cells to confirm the effects of OXT on hormonal activity; flow cytometry was used to assess changes in the cell cycle after OXT treatment. Moreover, the impact of OXT on proliferating cell nuclear antigen (PCNA), nuclear factor κB, and mitogen-activated protein kinase signaling pathway was analyzed by Western blot.
Results OXT treatment of 50 nM changed the gene expression of OXT receptor and pro-opiomelanocortin within a short time. In addition, OXT significantly reduced adrenocorticotropic hormone secretion within 1 hour. S and G2/M populations of AtT20 cells treated with OXT for 24 hours were significantly decreased compared to the control. Furthermore, OXT treatment decreased the protein levels of PCNA and phosphorylated extracellular-signal-regulated kinase (P-ERK) in AtT20 cells.
Conclusion Although the cytotoxic effect of OXT in AtT20 cells was not definite, OXT may blunt cell proliferation of corticotroph adenomas by altering the cell cycle or reducing PCNA and P-ERK levels. Further research is required to investigate the role of OXT as a potential therapeutic target in corticotroph adenomas.
-
Citations
Citations to this article as recorded by- Increased proliferation and neuronal fate in prairie vole brain progenitor cells cultured in vitro: effects by social exposure and sexual dimorphism
Daniela Ávila-González, Italo Romero-Morales, Lizette Caro, Alejandro Martínez-Juárez, Larry J. Young, Francisco Camacho-Barrios, Omar Martínez-Alarcón, Analía E. Castro, Raúl G. Paredes, Néstor F. Díaz, Wendy Portillo
Biology of Sex Differences.2023;[Epub] CrossRef - Anterior pituitary gland synthesises dopamine from l‐3,4‐dihydroxyphenylalanine (l‐dopa)
Santiago Jordi Orrillo, Nataly de Dios, Antonela Sofía Asad, Fernanda De Fino, Mercedes Imsen, Ana Clara Romero, Sandra Zárate, Jimena Ferraris, Daniel Pisera
Journal of Neuroendocrinology.2020;[Epub] CrossRef
- Increased proliferation and neuronal fate in prairie vole brain progenitor cells cultured in vitro: effects by social exposure and sexual dimorphism

- The Effects of Iodide on the Cellular Functions and Expression of Thyroid Autoantigens in Thyroid Cells.
- Young Joo Park, Eun Shin Park, Tae Yong Kim, Hye Seung Jung, Hyeong Kyu Park, Do Joon Park, Won Bae Kim, Chan Soo Shin, Kyoung Soo Park, Seong Yeon Kim, Hong Kyu Lee, Bo Youn Cho
- J Korean Endocr Soc. 2002;17(1):69-78. Published online February 1, 2002
- 1,191 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Iodide has been known to control the function and the growth of the thyroid gland, and to be used as a substrate of thyroid hormone. Moreover, it has been suggested that excessive iodide stimulates the thyroid autoimmune responses. To evaluate the effects of iodide on thyrocytes, we investigated cell function and proliferation, or thyroid autoantigen expression after administering iodide to rats or FRTL-5 cells. MEHTODS AND RESULTS: Ten-weeks-old Sprague-Dawley rats were sacrificed after 7 days of NaI treatment. The expressions of thyroid autoantigens were examined by northern blot analysis. Chronic administration of iodide resulted in no effect on TSH receptor (TSHR) and thyroperoxidase (TPO) mRNA expression, while it increased thyroglobulin (TG) and diminished sodium-iodide symporter (NIS) mRNA expression. FRTL-5 cells were also treated with various concentrations of NaI. The generation of cAMP or iodide uptake was decreased, and the cellular growth was also inhibited by iodide. However, the expressions of all thyroid autoantigens (TSHR, TG, TPO, MHC class I and class II) except NIS were unchanged for 72 hours after iodide administration. The expression of NIS was mildly increased after 24 hours. CONCLUSION: Iodide resulted in decreased cell proliferation and cellular function of cAMP generation and iodide uptake. Chronic administration of iodide increased TG and diminished NIS mRNA expression in vivo but not in vitro


 KES
KES

 First
First Prev
Prev



