Clinical Characteristics of Patients with Adrenal Insufficiency in a General Hospital
Article information
Abstract
Background
Adrenal insufficiency (AI) is a life-threatening disorder caused by the deficiency of adrenal steroid hormones. This retrospective cross-sectional study investigated the characteristics of patients with AI in Korea.
Methods
All consecutive patients with suspected AI who received care at a tertiary referral center in Korea in 2014 and underwent adrenocorticotropic hormone stimulation or insulin-tolerance testing were identified through a review of medical charts. Patients diagnosed with AI were enrolled. Their demographic, clinical, and treatment details were extracted.
Results
Of 771 patients with suspected AI, 183 (23.7%) received a definitive diagnosis. The most common reason for testing was the presence of suspicious AI-related symptoms (30.0%), followed by a history of steroid medications (23.5%). Their mean age was 66.7 years, and females predominated (67.8%). The most common symptoms were general weakness, anorexia, arthralgia, and fever. Approximately half (53.6%) had a history of steroid use. Hydrocortisone was the most common treatment (71.6%), with most patients taking a 30 mg dose (44.2%). The most common dose frequency was twice a day (78.6%). Fourteen patients were treated for adrenal crisis (n=10, 5.5%) or an intercurrent illness (n=4, 2.2%).
Conclusion
AI may have been caused by steroid medication use in many of the patients included in this study. The detection of AI can be improved by careful history-taking and being alert to the possibility that a patient has used steroids.
INTRODUCTION
Adrenal insufficiency (AI) is a life-threatening disease caused by the insufficient production of steroid hormones in the adrenal glands. It can be described as primary, secondary, or tertiary depending on the cause. Primary AI results from disease intrinsic to the adrenal cortex. Central AI, the collective name for secondary and tertiary AI, is caused by impaired production or action of corticotropin due to disorder of the hypothalamic-pituitary axis [1234]. It was first described by Thomas Addison in 1855, and is characterized by symptoms such as general weakness, fatigue, anorexia, abdominal pain, weight loss, orthostatic hypotension, and salt cravings due to adrenal destruction [145]. Until 1949, the survival rate was very low; more than 80% of patients died within 2 years of diagnosis [14678]. However, in 1949, cortisone was synthesized for the first time. Today, cortisone treatment allows patients with AI to carry on their normal life activities. However, because the main symptoms of AI are nonspecific and usually arise gradually, the diagnosis and treatment of AI are often delayed [1491011]. Although the diagnostic process is not complicated, caution is needed because multiple points must be considered when implementing and interpreting the diagnostic tests, and many other diagnostic possibilities must be excluded [149]. Information about the characteristics of AI in Korea is lacking. Therefore, we performed this retrospective cross-sectional study to characterize the clinical features of patients with AI in Korea.
METHODS
Study design
The study included all consecutive patients with suspected AI who visited Keimyung University Dongsan Medical Center (a tertiary referral center) between January 2014 and December 2014. Patients were included in the study if they had undergone a rapid adrenocorticotropic hormone (ACTH) stimulation test or an insulin tolerance test due to suspected AI symptoms, with the test leading to a diagnosis of AI. The rapid ACTH stimulation test was performed by administering one ampule (250 µg) of corticotropin intravenously and measuring serum cortisol levels 30 to 60 minutes later. AI was defined as a peak cortisol concentration of <500 nmol/L (18 µg/dL) [9]. The insulin tolerance test was performed by administering insulin (0.10 to 0.15 U/kg) intravenously to induce hypoglycemia, after which serum cortisol levels were measured every 30 minutes for at least 120 minutes [412]. Depending on the underlying mechanism, AI was diagnosed as primary or central. The following data were collected retrospectively from patients' medical records: age, gender, laboratory test results, and the presence of underlying diseases such as hypertension, hyperlipidemia, diabetes, thyroid disease, rheumatic diseases, respiratory diseases, or osteoporosis. The clinical symptoms present at the time that the patient underwent the test were also recorded. These symptoms included general weakness, loss of appetite, weight loss, nausea, vomiting, abdominal pain, arthralgia, dizziness, salt cravings, dry skin, itching, decreased libido, excessive skin pigmentation, fever, dehydration, and alopecia. Steroid medication history before and after the diagnosis, the type of drug used for treatment, the drug concentrations, and the daily dose were documented. The frequency and causes of adrenal crisis and intercurrent illnesses were recorded. Adrenal crisis was defined as a life-threatening emergency that acutely impaired general health and required treatment with parenteral hydrocortisone and saline infusion [413]. An intercurrent illness was defined as a disease state that required the glucocorticoid dose to be significantly but temporarily increased [13]. This study was conducted with the approval of the Keimyung University Dongsan Medical Center Institutional Review Board (IRB 2015-01-010).
Statistics
Continuous and categorical data are presented as mean±standard deviation (SD) or number and percentage, respectively. Groups were compared using the two-tailed t test. P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS version 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline characteristics of the patients
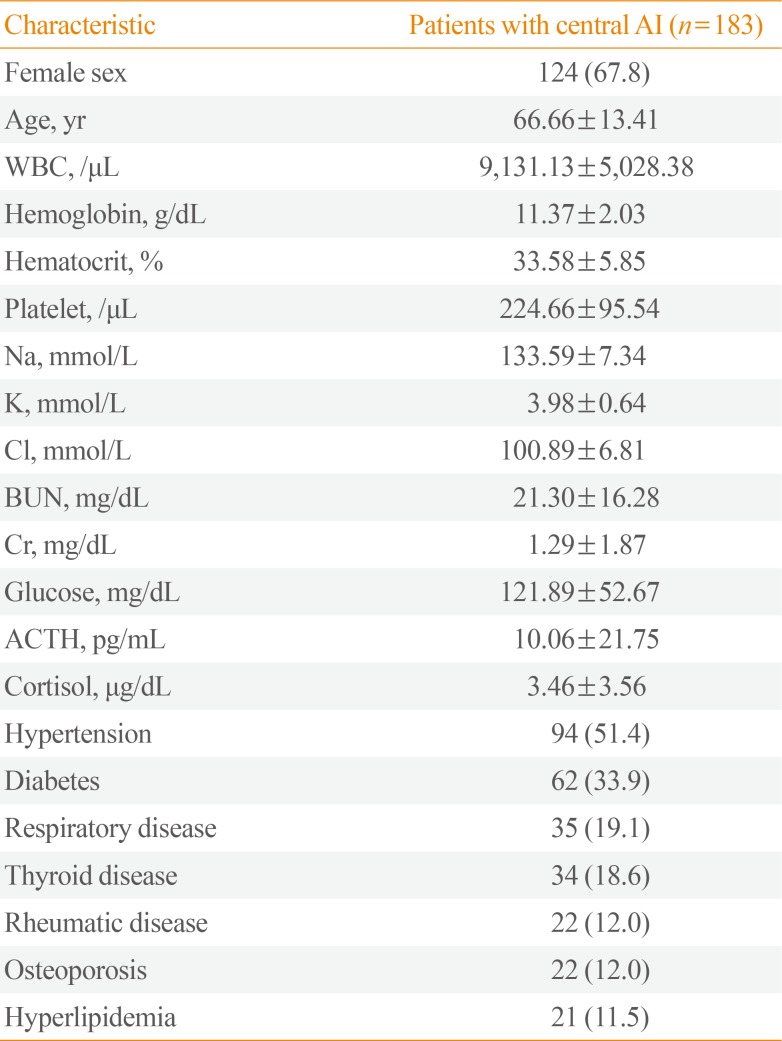
Of the 771 patients who presented with suspected AI in 2014, 183 (23.7%) were diagnosed with AI on the basis of a rapid ACTH stimulation test or an insulin tolerance test. These 183 patients formed the study cohort. All of these patients had central AI and none had primary AI (Fig. 1). The mean age of the cohort was 66.66±13.41 years. Of the total study cohort, 59 were male (32.2%) and 124 were female (67.8%). The mean ACTH and cortisol levels were 10.06±21.75 pg/mL and 3.46±3.56 µg/dL, respectively. The mean Na+, K+, and glucose levels were 133.59±7.34 mmol/L, 3.98±0.64 mmol/L, and 121.89±52.67 mg/dL, respectively. The results of the other laboratory blood tests are shown in Table 1. Hypertension (n=94, 51.4%) was the most common comorbidity, followed by diabetes (n=62, 33.9%) and respiratory disease (n=35, 19.1%) (Table 1).
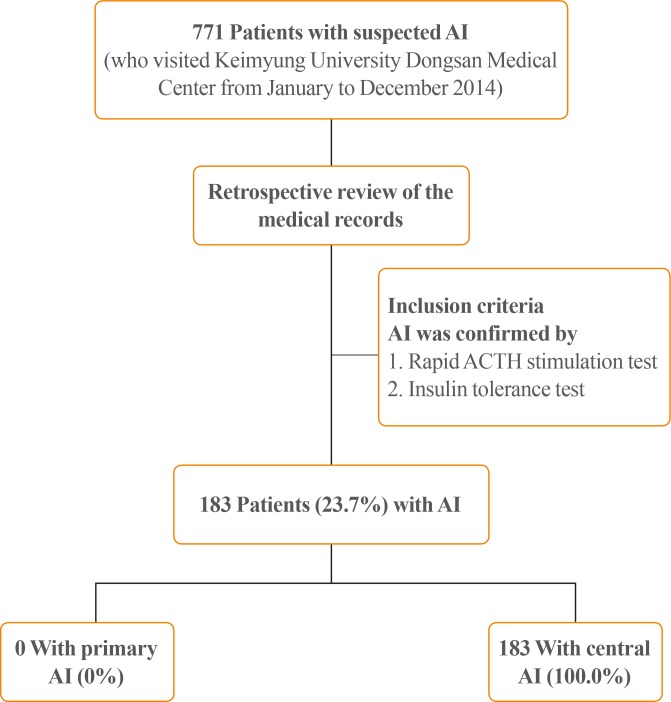
Flow chart showing the disposition of patients with adrenal insufficiency (AI) in the study. ACTH, adrenocorticotropic hormone.
Reasons for the blood test and the symptoms present at the time the blood test was conducted
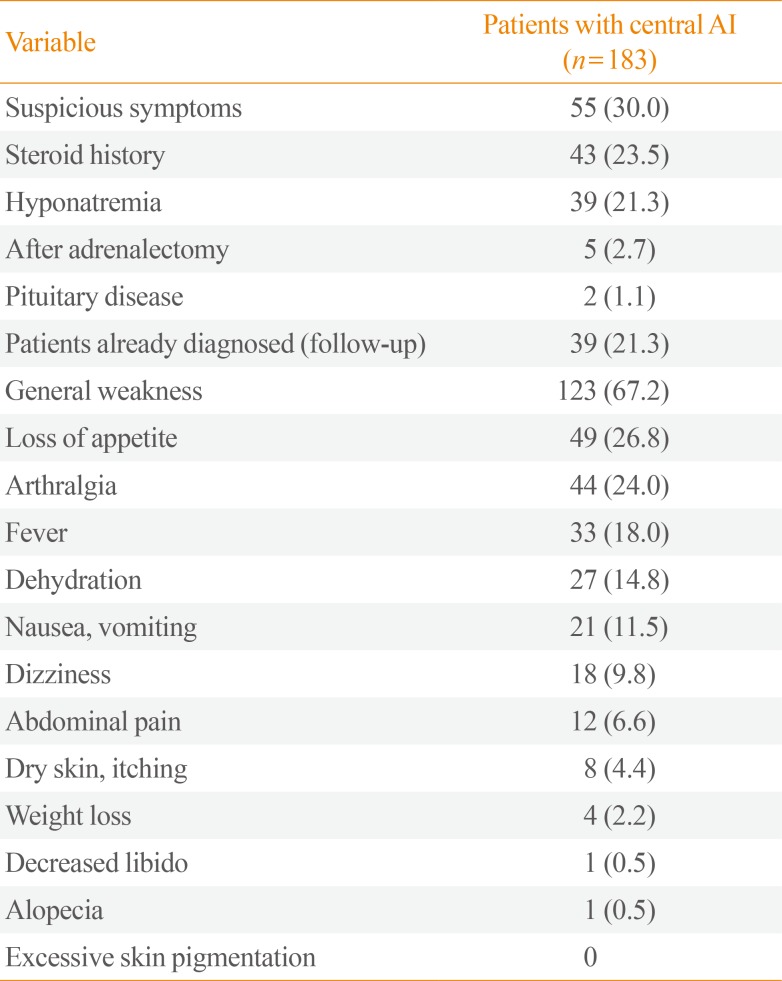
The most common reason for conducting the blood test was the presence of suspicious symptoms that were suggestive of AI (n=55, 30.0%), followed by a history of steroid medication use (n=43, 23.5%). The next most common reasons were the need to diagnose the cause of hyponatremia and a previous diagnosis of AI (both n=39, 21.3%).
The most common symptom that led to the decision to conduct the test was general weakness (n=123, 67.2%), followed by anorexia (n=49, 26.8%), arthralgia (n=44, 24.0%), and fever (n=33, 18.0%) (Table 2).
Medication history
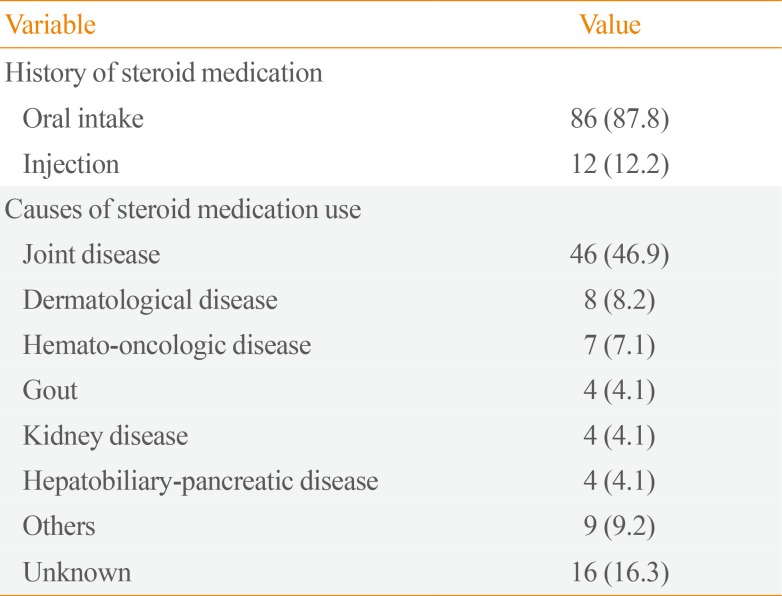
We evaluated how many patients had a relevant medication history. In our cohort, 98 of the 183 patients with AI (53.6%) had a history of steroid medication use. Of these, 86 (87.8%) took steroids orally and 12 (12.2%) took steroids by injection. The most common reason for taking steroid medications was joint disease (n=46, 46.9%), followed by dermatological disease (n=8, 8.2%) (Table 3).
Hormone replacement therapy for AI
In terms of the type of steroid used for daily replacement therapy after the diagnosis of AI in the overall cohort, 131 patients (71.6%) received hydrocortisone, 50 (27.3%) received prednisolone, one (0.5%) received both, and one (0.5%) did not receive treatment. The most common daily dosage of a drug was 30 mg of hydrocortisone (n=58, 44.2%). Most patients took hydrocortisone twice a day (n=103, 78.6%) (Table 4). The next most commonly used steroid was prednisolone. The most common daily prednisolone dose was 5 mg (n=17, 34.0%), followed by 7.5 mg (n=13, 26.0%). Most patients who took prednisolone used it once a day (n=31, 62.0%) (Table 4).
Frequency of adrenal crisis and intercurrent illness
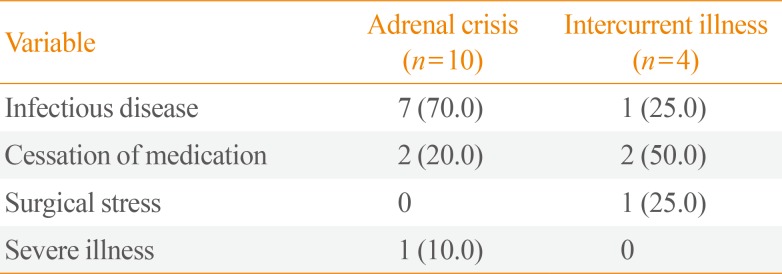
Adrenal crisis is life-threatening. To evaluate how many patients in our cohort experienced adrenal crisis or intercurrent illness, we reviewed their medical records. In total, 14 patients (7.7%) were treated for adrenal crisis (n=10, 5.5%) or intercurrent illness (n=4, 2.2%) during the study period. The reasons for adrenal crisis were infection (n=7, 70.0%), cessation of medication (n=2, 20.0%), and severe illness (n=1, 10.0%) (Table 5). Of the 10 patients who experienced an adrenal crisis, four (40.0%) died during treatment. Of these, three had an infectious disease and one had stopped taking medication. The causes of intercurrent illness were infection (n=1, 25.0%), cessation of medication (n=2, 50.0%), and surgical stress (n=1, 25.0%) (Table 5).
DISCUSSION
This retrospective cross-sectional study found that 183 of 771 patients suspected of having AI were actually diagnosed with the condition. All patients had central AI and women predominated. The most common symptom was general weakness. Half of the patients had a history of steroid medication use. Most patients underwent replacement therapy with hydrocortisone. Approximately 7.7% of the patients were treated for adrenal crisis or intercurrent illness.
In Europe, the prevalence and incidence of chronic primary AI are 93 to 144 cases per million and 4.4 to 6.0 new cases per million population per year, respectively [141415]. Central AI is more common than primary AI. Secondary AI has an estimated prevalence of 150 to 280 cases per million [141617181920]. Tertiary AI is believed to be the most common form of AI [1421]. Among the patients who underwent a rapid ACTH stimulation test or an insulin tolerance test to rule out AI in our study, approximately one-fourth were actually diagnosed with AI, and all these patients had central AI. No cases of primary AI were found in our cohort. The prevalence and incidence of primary AI in Korea have not yet been reported [22]. Thus, the lack of cases of primary AI in our study either reflects a lower prevalence of primary AI in Korea than in other countries, or is the result of limitations placed on determining which patients were eligible for an ACTH stimulation or insulin tolerance test. Studies of secondary AI in Europe have shown that it was most commonly diagnosed in the sixth decade of life, and that women were more frequently affected than men [141718]. In our study, the mean age of the patients was 66.7 years and women were over-represented. Patients with AI have been reported to have a higher risk of cardiovascular disease [2023]. This association may reflect the effects of cortisol on metabolism; for example, Rosmond and Bjorntorp [24] showed that pathological changes in the hypothalamic-pituitary-adrenal axis were associated with cardiovascular disease, type 2 diabetes, and stroke, and might be a risk factor for these diseases. In our study, comorbidities were hypertension, diabetes, respiratory disease, thyroid disease, rheumatic disease, and osteoporosis in order of frequency. The prevalence of hypertension was similar to that in the general population, while the prevalence of diabetes was higher than in the general population [25]. This shows that our patients with AI can have a higher risk of cardiovascular disease, as previously reported. In addition, respiratory disease and rheumatic disease were relatively common comorbidities. Since steroids can be used to treat these diseases, this may reflect the fact that glucocorticoids used to treat a variety of diseases are a common cause of central AI. The symptoms of AI are nonspecific and include general weakness, fatigue, anorexia, abdominal pain, weight loss, and orthostatic hypotension [14]. As a result, the diagnosis of AI is often delayed [1491011]. Therefore, when patients complain of these symptoms, AI must be suspected [26]. In our study, the most common symptoms at diagnosis were general weakness and loss of appetite. These are also the most common symptoms of central AI and are the result of a glucocorticoid deficiency [14]. Drugs can cause a glucocorticoid deficiency at the hypothalamic-pituitary-adrenal axis [221]. Indeed, it is suggested that any patient who has received the equivalent of 20 to 30 mg/day of prednisone for longer than 1 week may be at risk of AI [2728]. In our study, half of the patients (53.6%) had a history of steroid medication. The most common reason for taking steroid medication was joint disease. However the number of patients who underwent an AI diagnostic test based on their history of steroid use was smaller (23.5%) than the number of patients who took steroids. Our observations suggest that if a patient taking a steroid medication presents with AI-related symptoms, or if the patient has recently stopped taking the steroid medication, the adrenal function of the patient must be evaluated.
Hydrocortisone is the preferred choice for glucocorticoid replacement in AI because hydrocortisone tablets taken twice or thrice a day result in circulating glucocorticoid levels similar to the physiological circadian-based serum cortisol exposure-time profile [29]. Thus, the recommended total daily dose of hydrocortisone for replacement therapy in AI is 15 to 25 mg, and the recommended dose frequency is twice a day [2129]. In our study, hydrocortisone was the most frequently used glucocorticoid replacement therapy, and the most common dose frequency was twice a day. However, among patients receiving hydrocortisone, only 35.9% received daily doses of 15 to 20 mg, while 44.2% received daily doses of 30 mg. This means that a considerable number of patients may have received unnecessarily high replacement doses. Glucocorticoid under-replacement carries the risk of adrenal crisis and impaired well-being. However, chronic over-replacement may lead to substantial morbidity, including impaired glucose tolerance, obesity, osteoporosis, and cardiovascular disease [4]. Since an objective metric for assessing the adequacy of replacement therapy has yet to be identified, physicians must rely primarily on the patient's symptoms and signs and titrate the glucocorticoid replacement dose appropriately.
Since AI can be lethal if it is not managed properly, it is important that medical staff are trained to recognize the signs of AI and to institute appropriate and rapid treatment. A European retrospective study on a large cohort of patients with AI showed that adrenal crises are relatively common, with an overall frequency of 6.3 adrenal crises per 100 patient-years. The most important precipitating factors of adrenal crisis were infectious diseases, especially gastrointestinal infections [30]. Similarly, in our study, the frequency of adrenal crisis was 5.5 adrenal crises per 100 patient-years, and infectious disease was the most common precipitating factor of both adrenal crisis and intercurrent illness. Notably, four patients exhibited intercurrent illness in our study, none of whom developed adrenal crisis. Thus, if patients who are at an early stage of an intercurrent illness and could proceed to adrenal crisis are detected early and managed well, the incidence of adrenal crisis can be reduced significantly.
Our study has several limitations. First, it is a retrospective study that relied on medical chart review. Thus, it is prone to information bias. Second, the study was only performed at a single center. Therefore, generalizing its findings to other patient populations in Korea requires caution. Nevertheless, this is the first study to report the clinical characteristics and treatment of AI in real-world practice in Korea. Therefore, these results are likely to make a significant contribution to research into AI and its treatment in Korea.
AI is not highly prevalent, but inappropriate treatment affects the quality of life of patients. In our study, many patients had AI related to steroid medication, whereas the number of patients who actually underwent an AI diagnostic test based on their history of steroid use was much smaller. Currently, chronic glucocorticoid therapy is used to treat a variety of disorders. Therefore, patients who are receiving steroid therapy should always be carefully monitored to detect AI at an early stage and avoid adrenal crisis. Moreover, it is important to titrate the glucocorticoid replacement dose appropriately in order to prevent significant morbidity. To improve the treatment of patients, further study is needed to monitor the effect of medication and biomarkers, with the goal of determining how to adjust the dose appropriately.
ACKNOWLEDGMENTS
This study was supported by the research fund of the Daegu and Gyeongbuk Branch of the Korean Endocrine Society (2016).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.