Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(1); 2024 > Article
-
Original ArticleDiabetes, obesity and metabolism Efficacy and Safety of Omarigliptin, a Novel Once-Weekly Dipeptidyl Peptidase-4 Inhibitor, in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
 Keypoint
Keypoint
Omarigliptin is a once-weekly DPP4i approved for use in patients with type 2 diabetes mellitus in Japan and other countries. Omarigliptin has shown good glucose-lowering capacity with an additional 0.58% HbA1c reduction compared to the placebo. The safety profile of omarigliptin is also reassuring, evidenced by comparable adverse events, serious adverse events, hypoglycemia, and severe hypoglycemia in the control group. -
A.B.M. Kamrul-Hasan1
 , Muhammad Shah Alam2, Samir Kumar Talukder3, Deep Dutta4, Shahjada Selim5
, Muhammad Shah Alam2, Samir Kumar Talukder3, Deep Dutta4, Shahjada Selim5 -
Endocrinology and Metabolism 2024;39(1):109-126.
DOI: https://doi.org/10.3803/EnM.2023.1839
Published online: January 23, 2024
1Department of Endocrinology, Mymensingh Medical College, Mymensingh, Bangladesh
2Department of Medicine, Army Medical College Cumilla, Cumilla, Bangladesh
3Department of Endocrinology, Rangpur Medical College, Rangpur, Bangladesh
4Department of Endocrinology, Center for Endocrinology, Diabetes, Arthritis and Rheumatism (CEDAR) Superspeciality Healthcare, New Delhi, India
5Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
- Corresponding author: A.B.M. Kamrul-Hasan. Department of Endocrinology, Mymensingh Medical College, Char Para, Medical Rd, Mymensingh 2200, Bangladesh Tel: +880-9166063, Fax: +880-9166064, E-mail: rangassmc@gmail.com
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,190 Views
- 40 Download
ABSTRACT
-
Background
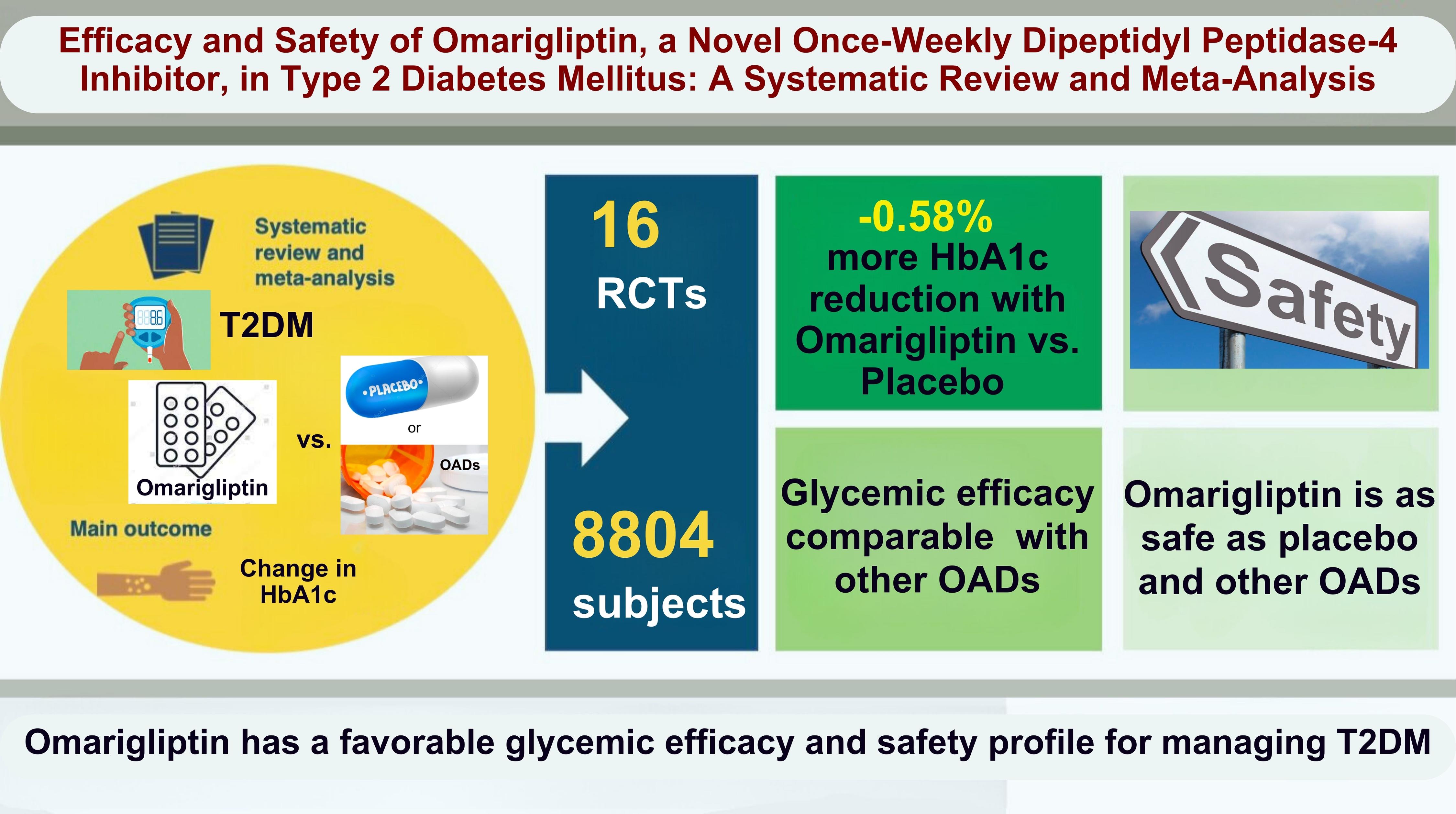
- No recent meta-analysis has holistically analyzed and summarized the efficacy and safety of omarigliptin in type 2 diabetes mellitus (T2DM). We conducted a meta-analysis to address this knowledge gap.
-
Methods
- Electronic databases were searched to identify randomized controlled trials (RCTs) that included patients with T2DM who received omarigliptin in the intervention arm. The control arm consisted of either a placebo (passive control group [PCG]) or an active comparator (active control group [ACG]). The primary outcome assessed was changes in hemoglobin A1c (HbA1c), while secondary outcomes included variations in glucose levels, achievement of glycemic targets, adverse events (AEs), and hypoglycemic events.
-
Results
- From 332 initially screened articles, data from 16 RCTs involving 8,804 subjects were analyzed. Omarigliptin demonstrated superiority over placebo in reducing HbA1c levels (mean difference, –0.58%; 95% confidence interval, –0.75 to –0.40; P<0.00001; I2=91%). Additionally, omarigliptin outperformed placebo in lowering fasting plasma glucose, 2-hour postprandial glucose, and in the percentage of participants achieving HbA1c levels below 7.0% and 6.5%. The glycemic efficacy of omarigliptin was similar to that of the ACG across all measures. Although the omarigliptin group experienced a higher incidence of hypoglycemic events compared to the PCG, the overall AEs, serious AEs, hypoglycemia, and severe hypoglycemia were comparable between the omarigliptin and control groups (PCG and ACG).
-
Conclusion
- Omarigliptin has a favorable glycemic efficacy and safety profile for managing T2DM.
- Dipeptidyl peptidase-4 (DPP-4) inhibitors, or DPP-4is, are a class of oral antihyperglycemic agents (AHAs) that work by extending the half-life of endogenous incretins [1]. These incretins, which are peptides originating in the gut, enhance insulin secretion and suppress glucagon release in response to glucose levels [1]. DDP-4is are well tolerated and have a low risk of causing hypoglycemia, making them a mainstay in the treatment of type 2 diabetes mellitus (T2DM) [2]. Individuals with chronic conditions such as hypertension and T2DM often require long-term medication, spanning years, or even decades. The higher the number of pills a patient must take, the greater the likelihood of missed doses, which can lead to suboptimal glycemic control and an increased risk of both microvascular and macrovascular complications [3]. Research indicates that once-weekly medication regimens are linked to improved adherence and better longterm health outcomes [3]. In this context, omarigliptin, a potent and selective once-weekly DPP-4i, was first introduced in Japan in November 2015 and has since been approved for use in several other countries, including some in Asia [3]. On August 1, 2023, this product was introduced to the Bangladesh market. Omarigliptin is minimally metabolized in the liver, does not accumulate in any specific tissues, and is distributed widely in the body, resulting in a low rate of kidney filtration [4,5]. When it is filtered in the renal glomeruli, approximately 60% of it is reabsorbed in the renal tubules in its unchanged form. These distinctive properties enable omarigliptin to maintain stable DPP-4 inhibition for a full week following oral administration [4,5]. The international treatment landscape for T2DM has been thoroughly investigated in numerous randomized controlled trials (RCTs) and observational studies that have focused on omarigliptin. These studies, originating from various countries and covering a spectrum of clinical situations, have consistently demonstrated the drug’s efficacy in glycemic control and a safety profile comparable to that of daily DPP-4i and other oral AHAs, including sulfonylureas [6-22].
- In 2018, a meta-analysis that included 11 RCTs on the efficacy and safety of omarigliptin was published [23]. However, the research landscape has since evolved, with at least five more RCTs published since then. Therefore, this updated meta-analysis seeks to evaluate the effectiveness and safety of omarigliptin in the management of T2DM, taking into account all the most recent RCTs available.
INTRODUCTION
- Ethical considerations
- The meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO), bearing the registration number CRD42023451785. It adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, which promote transparency and methodological rigor in reporting. The completed PRISMA checklist is available as Supplemental Table S1 [24]. Separate ethical approval was not required for this meta-analysis, as the individual studies included had their own existing approvals.
- Search method for identifying studies
- A comprehensive search was conducted across several databases, including MEDLINE (via PubMed), Scopus, Google Scholar, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov, from their inception to August 15, 2023. The search strategy utilized a Boolean approach with the terms (omarigliptin) AND (diabetes). Additionally, a thorough manual search of references within previous meta-analyses, the RCTs included in this study, and relevant journals was carried out to identify any recently published or unpublished studies.
- Study selection
- We employed the Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria to screen and select studies for this meta-analysis. The patient population (P) consisted of individuals living with T2DM. The intervention (I) was the administration of omarigliptin for T2DM management. The control (C) included patients receiving either a placebo or any other approved oral AHA. The outcomes (O) assessed were the effects on glycemic parameters, including hemoglobin A1c (HbA1c), and adverse events (AEs) such as hypoglycemia. The RCTs included in this analysis met specific inclusion criteria: a minimum trial duration of 12 weeks; participants aged ≥18 years, regardless of ethnic background or sex, with a diagnosis of T2DM; the presence of at least two treatment arms/groups, with one involving subjects with T2DM receiving omarigliptin either as monotherapy or as part of a standard diabetes treatment regimen, and the other receiving either a placebo or an alternative AHA, either alone or in combination with other treatments; and the measurement of HbA1c change from baseline as one of the outcomes, with or without the concurrent evaluation of secondary outcomes and AEs. The exclusion criteria ruled out trials involving animal or healthy human subjects, nonrandomized trials, letters to editors, case reports, articles with insufficient information of interest or primary data, and trials with a duration of <12 weeks.
- The primary outcome of this meta-analysis was the change in HbA1c levels from baseline to the end of the trial. Secondary outcomes included changes in fasting plasma glucose (FPG), 2-hour postprandial plasma glucose (2-hour PPG), the percentage of participants achieving HbA1c levels <7.0% and <6.5% at the end of the trial, changes in body weight, the incidence of AEs, serious AEs, hypoglycemia, severe hypoglycemia, and changes in serum amylase and serum lipase levels from baseline. The analysis of outcomes was stratified based on whether the control group received an active comparator (any AHA), which constituted the active control group (ACG), or a placebo, which formed the passive control group (PCG).
- Data extraction
- Data extraction was conducted independently by three review authors using standardized data extraction forms. When multiple publications from a single study group were identified, the results were consolidated, and pertinent data from each article were included in the analyses. From each RCT, we collected the following information: first author, year of publication, sample size, mean age, percentage of male participants, duration of diabetes, baseline HbA1c levels, medications used in the treatment and control groups, and the length of the interventions. Additionally, as stated above, data on primary and secondary outcomes were extracted. Any disagreements were resolved by the fourth and fifth authors.
- Assessment of risk of bias
- Four authors independently assessed the risk of bias using the risk assessment tool provided in Review Manager Web (RevMan Web) version 6.0.0 software (The Cochrane Collaboration, August 2023; https://revman.cochrane.org). They considered various factors, including proper sequence generation (to address selection bias), adequate allocation concealment (to address selection bias), prevention of foreknowledge of allocated interventions throughout the study, appropriate blinding of participants and personnel (to address performance bias), blinding of outcome assessors (to address detection bias), proper management of incomplete outcome data (to address attrition bias), absence of selective outcome reporting (to address reporting bias), and the mitigation of other potential sources of bias. In cases of disagreement, a fifth author served as an arbitrator to reach a consensus.
- Measures of treatment effect
- The outcomes were reported as mean differences (MDs) for continuous variables. HbA1c levels were presented as percentages for analysis, while other outcomes, including plasma glucose, were reported in International System (SI) units. Results from studies that used different units were converted to SI units for consistency, using appropriate conversion factors. For dichotomous outcomes, such as treatment success and AEs, we presented the results as odds ratios (ORs) with 95% confidence intervals (CIs). The 2023 version of RevMan Web, developed by Cochrane in Oxford, UK, was employed to compare the MDs for both primary and secondary outcomes between the omarigliptin and control groups in the included studies.
- Dealing with missing data
- The necessary supplementary details from the original authors were obtained via written e-mail correspondence. All relevant information gathered in this way was carefully integrated into the meta-analysis. A detailed review of key numerical data, including the number of individuals screened and randomized, as well as a rigorous examination of intention-to-treat, as-treated, and per-protocol populations, was conducted with diligence. Furthermore, attrition rates, including drop-outs, losses to follow-up, and withdrawals, were thoroughly investigated.
- Assessment of heterogeneity
- An initial assessment of heterogeneity was conducted by reviewing the forest plot generated for the primary and secondary outcomes of this study. Subsequently, the chi-square test was employed with N-1 degrees of freedom to analyze heterogeneity, using an alpha of 0.05, and the I2 test was applied. The interpretation of I2 values is as follows:
(1) 0% to 25% might not be important.
(2) 25% to 50% may represent low heterogeneity.
(3) 50% to 75% may represent moderate heterogeneity.
(4) 75% to 100% may represent high heterogeneity [25].
- The importance of the observed value of I2 depends on (1) the magnitude and direction of the effect and (2) the strength of evidence for heterogeneity (e.g., the P value from the chi-square test or the CI for I2 : uncertainty in the value of I2 is substantial when the number of studies is small) [25].
- Data synthesis
- Data were pooled using random-effects models to analyze primary and secondary outcomes. These outcomes were expressed as 95% CIs. Forest plots were generated, with the left side of the graph indicating a favor towards omarigliptin and the right side favoring the control, utilizing RevMan Web software. Only forest plots that incorporated data from at least two RCTs were included in the results. A P value of less than 0.05 was considered to indicate statistical significance.
- Grading of the results
- An overall assessment of the evidence quality related to the primary outcome and the major secondary outcomes of the meta-analysis was conducted using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach [26]. The GRADE approach assesses the quality of a body of evidence by determining how confident one can be that an effect or association estimate is close to the actual value of interest. This quality assessment includes considerations of withintrial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and the risk of publication bias [26]. To evaluate publication bias, we generated a funnel plot (Supplemental Fig. S1). The presence of one or more smaller studies outside the inverted funnel plot was interpreted as evidence of significant publication bias [27]. We used the GRADEpro Guideline Development Tool (GDT) software (McMaster University and Evidence Prime Inc., Hamilton, ON, Canada; 2015) to create the summary of findings (SoF) table for this meta-analysis. The certainty of the evidence was categorized into four levels: high certainty (indicating strong confidence that the true effect is close to the estimated effect), moderate certainty (suggesting moderate confidence in the effect estimate, with the true effect likely close to the estimate but possibly substantially different), low certainty (implying limited confidence in the effect estimate, with the true effect potentially being substantially different from the estimate), and very low certainty (meaning there is very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate) [26].
- Registration
- The meta-analysis was registered in PROSPERO, having Registration number CRD42023451785. The review protocol summary can be accessed at the PROSPERO website.
- Availability of data and materials
- All data generated or analyzed during this study are available in this published article.
METHODS
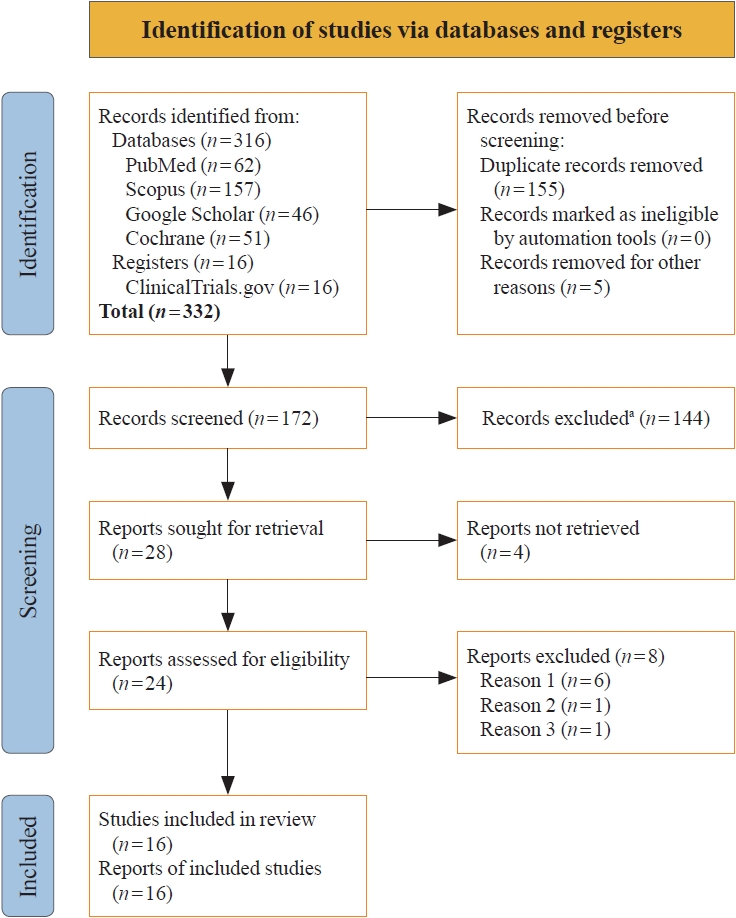
- Search results
- The study selection process is illustrated in Fig. 1. Initially, 332 articles were identified. Following the screening of titles and abstracts, and subsequent full-text reviews, the number of studies considered for this meta-analysis was narrowed down to 24. Detailed evaluation led to the inclusion of 16 RCTs involving 8,804 subjects with T2DM, which met all the inclusion criteria [6-21]. Eight studies were excluded because they either assessed the pharmacokinetic and pharmacodynamic properties of omarigliptin [4,5,28-31], were observational [32], or were retrospective studies [22].
- Study characteristics
- In this meta-analysis, which included 16 RCTs, subgroup analyses were conducted based on the nature of the control group, either ACG or PCG. The study by Gantz et al. [8] (Gantz 2017b) featured two control groups: one receiving a placebo and the other receiving sitagliptin 50 mg once-daily. Consequently, in the forest plot, the results of this study are presented separately. The comparison of omarigliptin with placebo is labeled as “Gantz 2017b(p),” while the comparison with sitagliptin is labeled as “Gantz 2017b(s).” Nine studies (Chacra 2017 [6], Gantz 2017a [7], Gantz 2017c [9], Gantz 2017d [10], Home 2018 [14], Kadowaki 2021 [16], Lee 2017 [17], Shankar 2017 [19], and Sheu 2015 [20]) included only a placebo in the control group. Together with the placebo group from the study by Gantz et al. [8] (Gantz 2017b1), a total of 10 studies were analyzed in the PCG. Six studies (Goldenberg 2017 [11], Handelsman 2017 [12], Hattori 2020 [13], Ishii 2023 [15], Ohara 2021 [18], and Yoshizawa 2021 [21]) included only an oral AHA in the control group. Along with the AHA group from the study by Gantz et al. [8] (Gantz 2017b2), a total of seven studies were analyzed in the ACG. The details of the studies included in this meta-analysis are provided in Table 1.
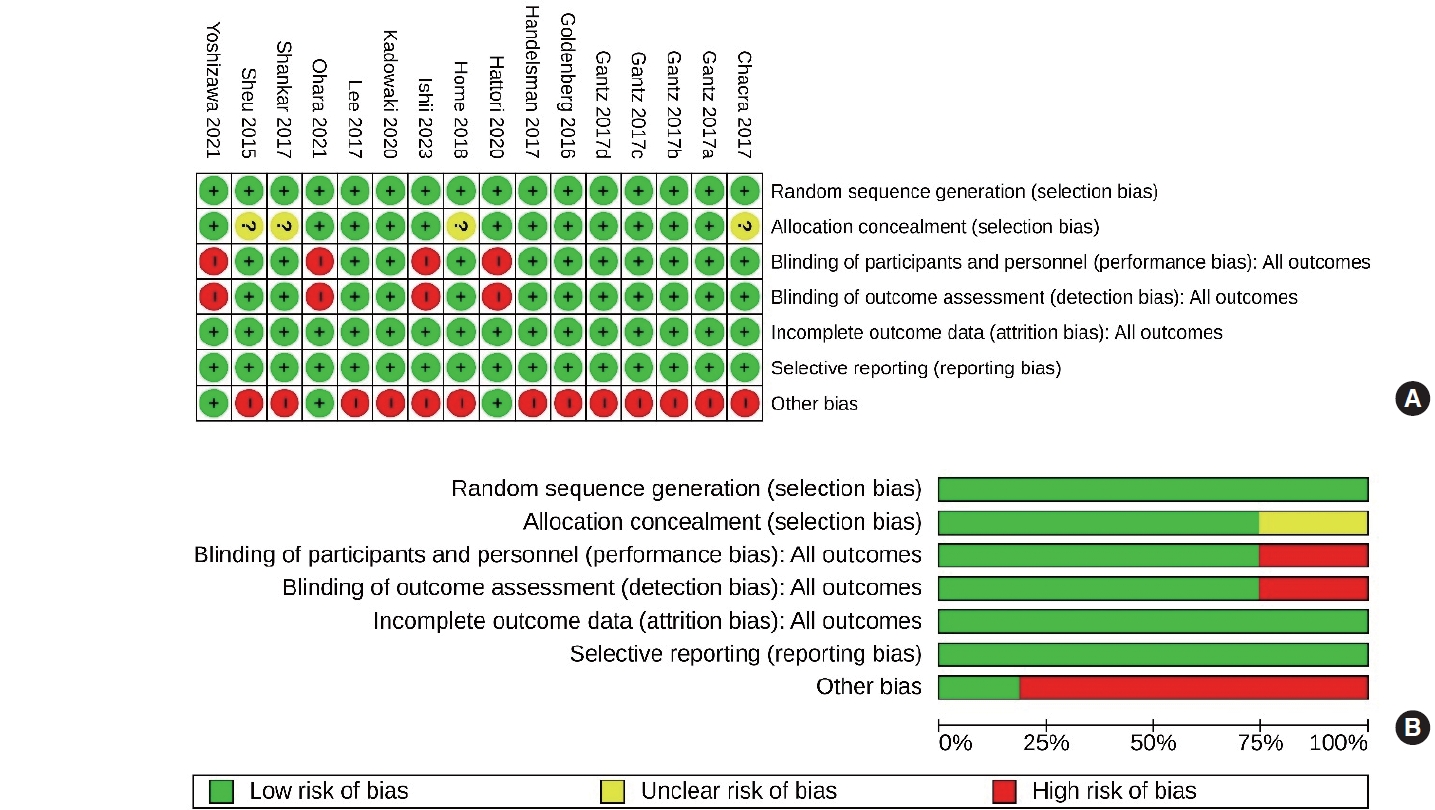
- Risk of bias in the included studies
- The risk of bias across the 16 studies included in the meta-analysis is illustrated in Fig. 2. All 16 studies (100%) were assessed as having a low risk of bias in terms of random sequence generation, attrition bias, and reporting bias. When it came to allocation concealment bias (selection bias), 12 of the studies (75%) were determined to have a low risk. The assessment of performance bias (blinding of participants and investigators) and detection bias (blinding of outcome assessors) also indicated a low risk for 12 studies (75%). The “other bias” category included an examination of funding sources, with a particular focus on pharmaceutical companies, affiliations with pharmaceutical organizations, and potential conflicts of interest. Only three of the 16 studies (18.75%) were considered to have a low risk of other biases. The comprehensive bias risk assessment process is provided as a supplemental file (Supplemental Table S2).
- Grading of the results
- The grades for the certainty of evidence of the results are given in the SoF table (Table 2).
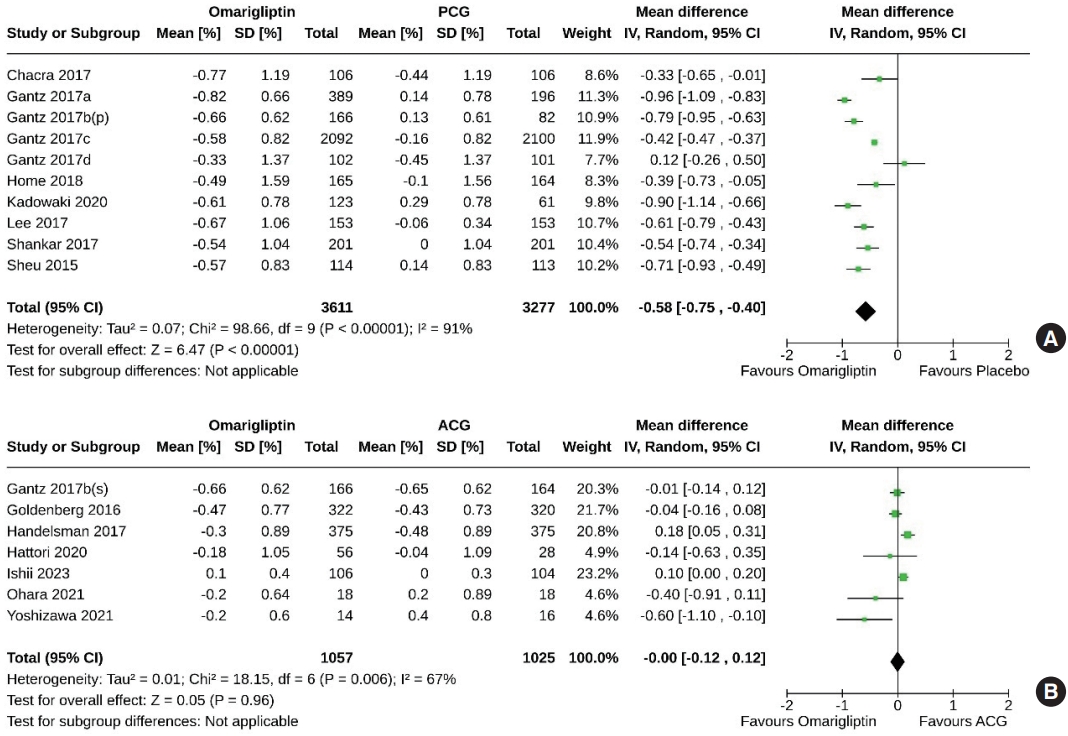
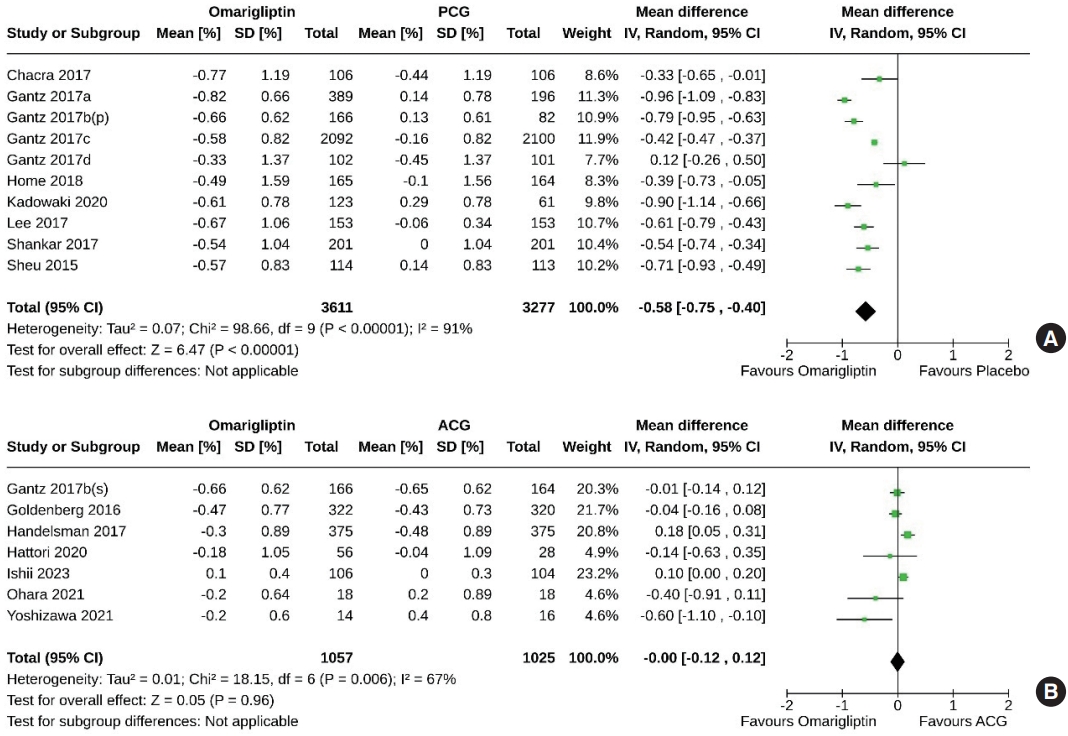
- Effect of omarigliptin on the primary outcome (change in HbA1c)
- Ten studies (6,888 subjects: 3,611 in the omarigliptin group, 3,277 in the PCG) compared the HbA1c-lowering effect of omarigliptin to placebo. Omarigliptin was superior to placebo in HbA1c reduction (MD, –0.58%; 95% CI, –0.75 to –0.40; P<0.00001; I2=91% [high heterogeneity], very low certainty of evidence) (Fig. 3A). Seven studies (2,082 subjects: 1,057 in the omarigliptin group, 1,025 in the ACG) compared the HbA1c-lowering effect of omarigliptin to active comparators. Reductions in HbA1c were similar in omarigliptin group and ACG (MD, –0.00%; 95% CI, –0.12 to 0.12; P=0.96; I2=67% [moderate heterogeneity], low certainty of evidence) (Fig. 3B).
- Effect of omarigliptin on secondary outcomes
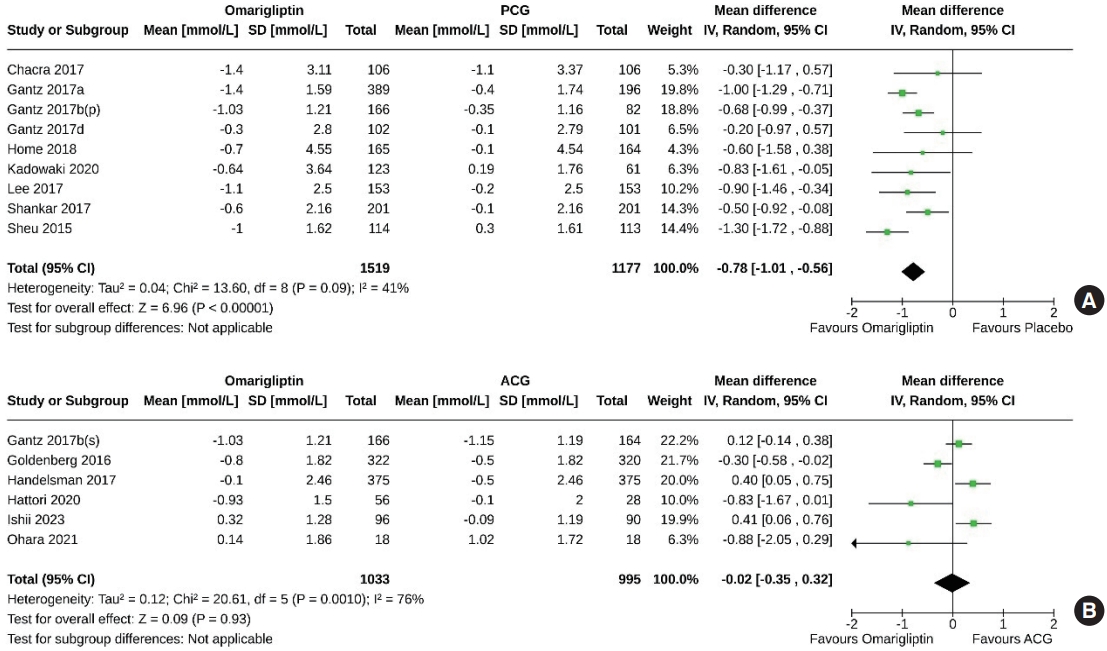
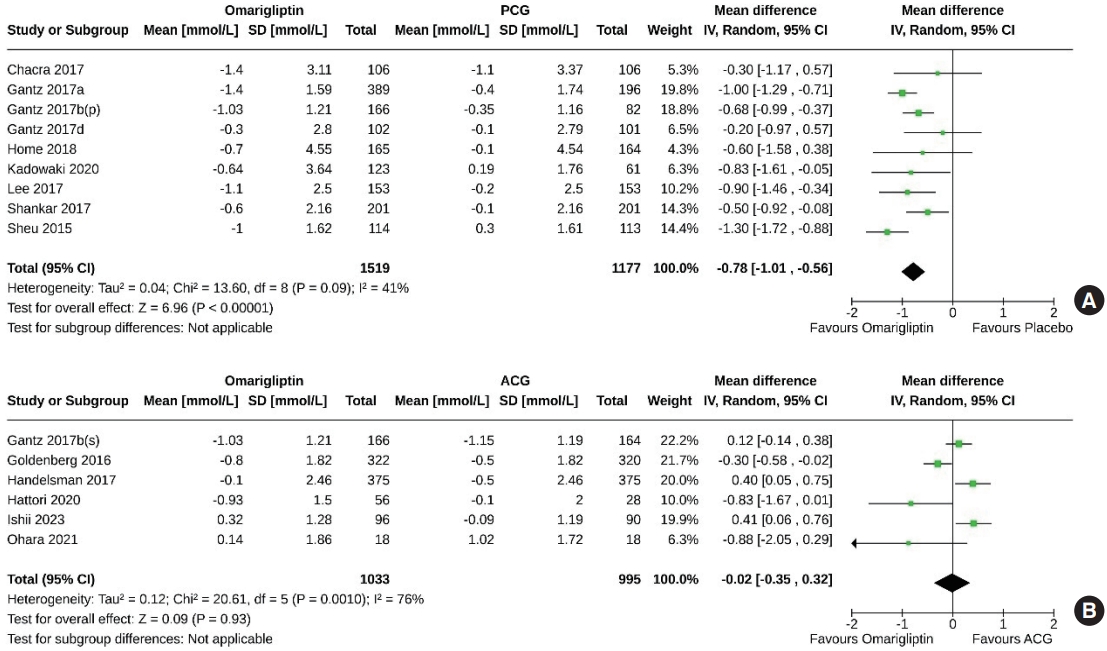
- Nine studies (2,696 subjects: 1,519 in the omarigliptin group, 1,177 in the PCG) compared the FPG-lowering effect of omarigliptin to placebo. Omarigliptin was superior to placebo in FPG reduction (MD, –0.78 mmol/L; 95% CI, –1.01 to –0.56; P<0.00001; I2=41% [mild heterogeneity]) (Fig. 4A). Six studies (2,028 subjects: 1,033 in the omarigliptin group, 995 in the ACG) compared the FPG-lowering effect of omarigliptin to active comparators; the reductions in FPG were similar in the omarigliptin group and ACG (MD, –0.02 mmol/L; 95% CI, –0.35 to 0.32; P=0.93; I2=76% [high heterogeneity]) (Fig. 4B).
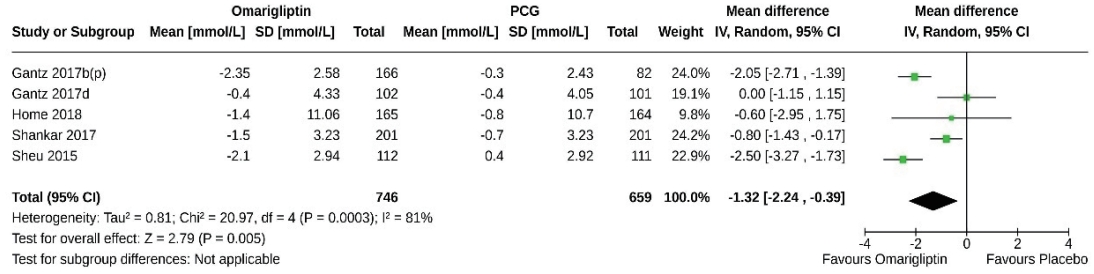
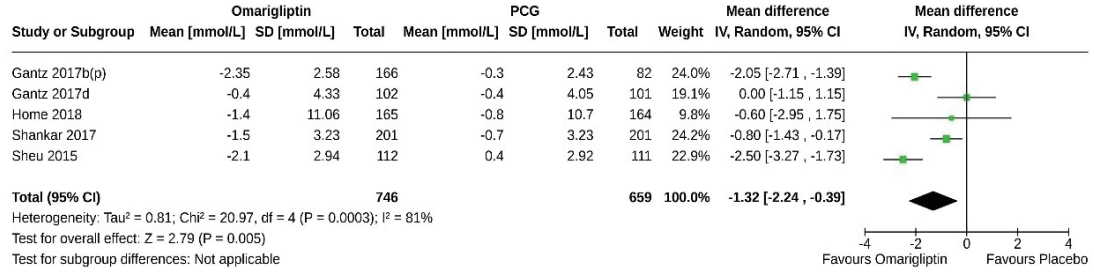
- Five studies (1,405 subjects: 746 in the omarigliptin group, 659 in the PCG) compared the 2-hour PPG-lowering effect of omarigliptin to placebo. Omarigliptin was superior to placebo in 2-hour PPG reduction (MD, –1.32 mmol/L; 95% CI, –2.24 to –0.39; P=0.0005; I2=81% [high heterogeneity]) (Fig. 5). Only one study compared the 2-hour PPG-lowering effect of omarigliptin to active comparators and found a comparable effect [8].
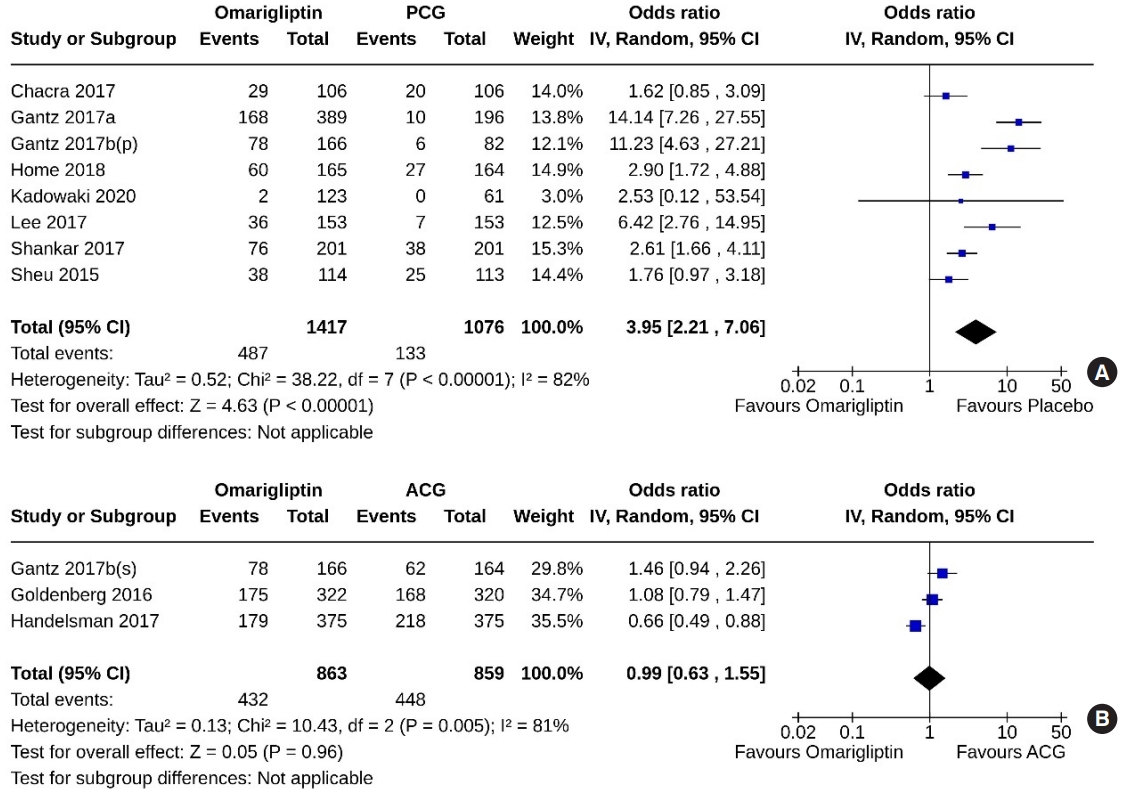
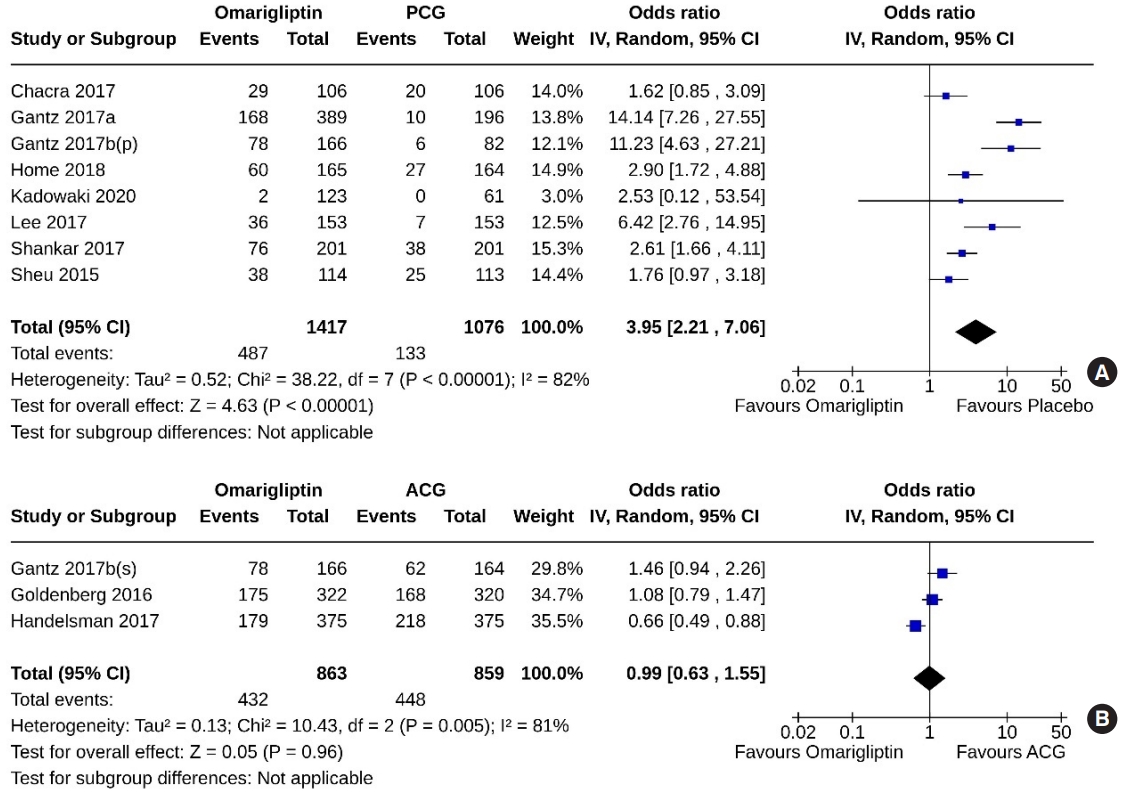
- Eight studies (2,493 subjects: 1,417 in the omarigliptin group, 1,076 in the PCG) compared omarigliptin to placebo, and three studies (1,722 subjects: 863 in the omarigliptin group, 859 in the ACG) compared omarigliptin to active comparators to determine the percentage of subjects achieving HbA1c <7% at the end of the trial. Omarigliptin was superior to placebo (OR, 3.95; 95% CI, 2.21 to 7.06; P<0.00001; I2=82% [high heterogeneity], very low certainty of evidence) (Fig. 6A), and non-inferior to the ACG (OR, 0.99; 95% CI, 0.63 to 1.55; P=0.96; I2=81% [high heterogeneity]) (Fig. 6B) in this regard.
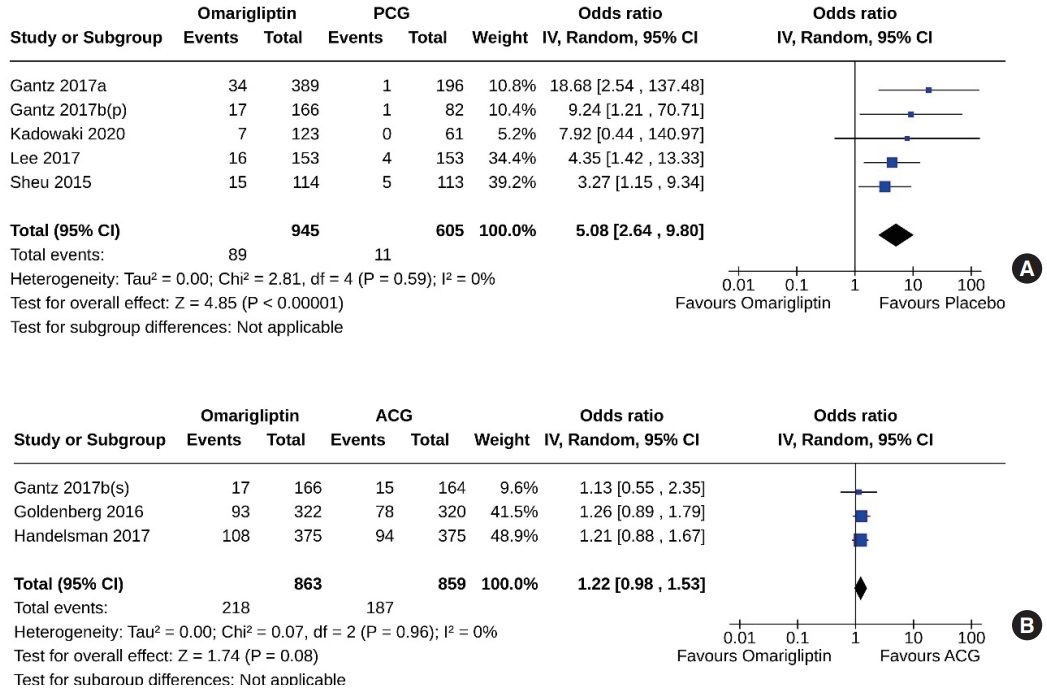
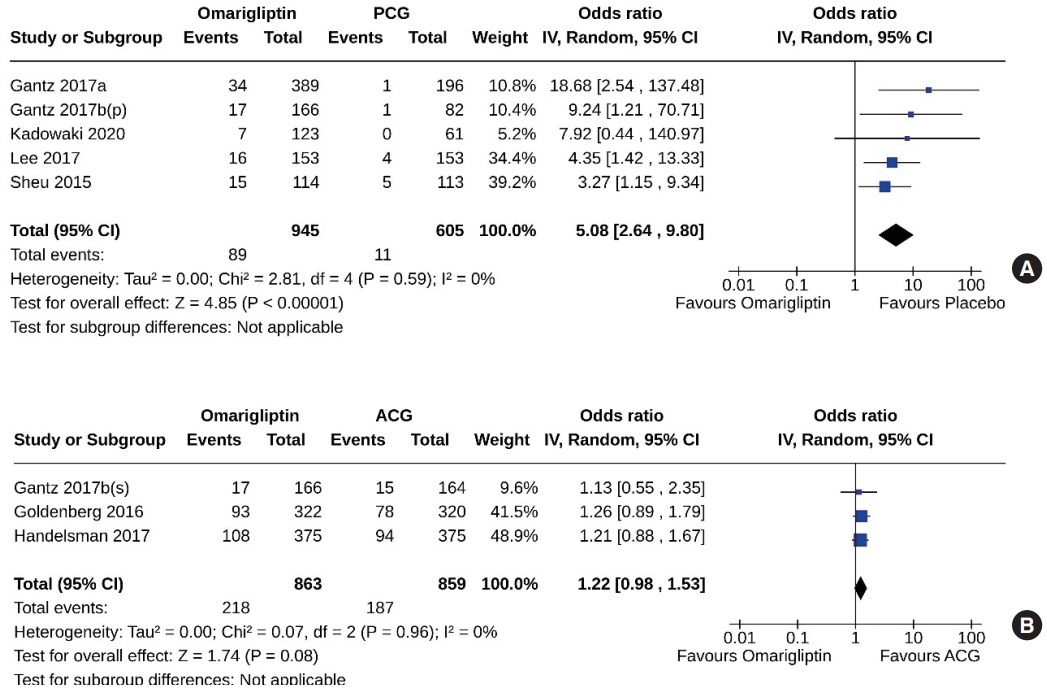
- Five studies (1,550 subjects: 945 in the omarigliptin group, 605 in the PCG) compared omarigliptin to placebo, and three studies (1,722 subjects: 863 in the omarigliptin group, 859 in the ACG) compared omarigliptin to active comparators to determine the percentage of subjects achieving HbA1c <6.5% at the end of the trial. Omarigliptin was superior to placebo (OR, 5.08; 95% CI, 2.64 to 9.80; P<0.00001; I2=0% [not important heterogeneity], moderate certainty of evidence) (Fig. 7A), and non-inferior to the ACG (OR, 1.22; 95% CI, 0.98 to 1.53; P=0.08; I2=0% [not important heterogeneity]) (Fig. 7B) in this regard.
- AEs and serious AEs
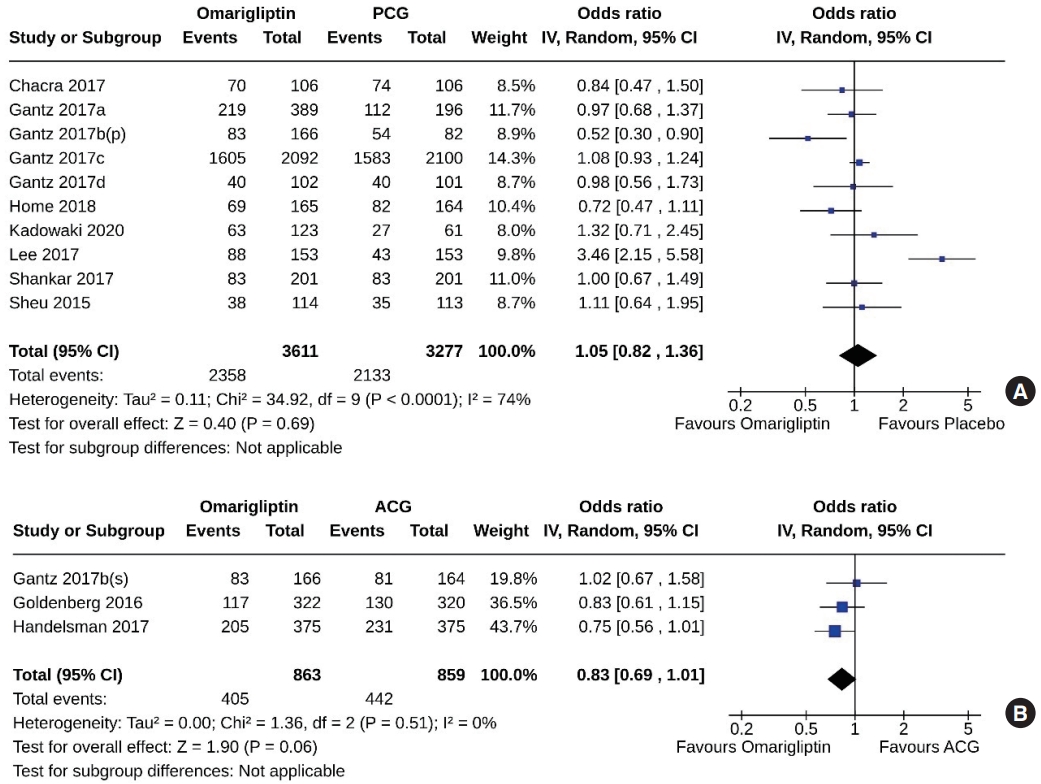
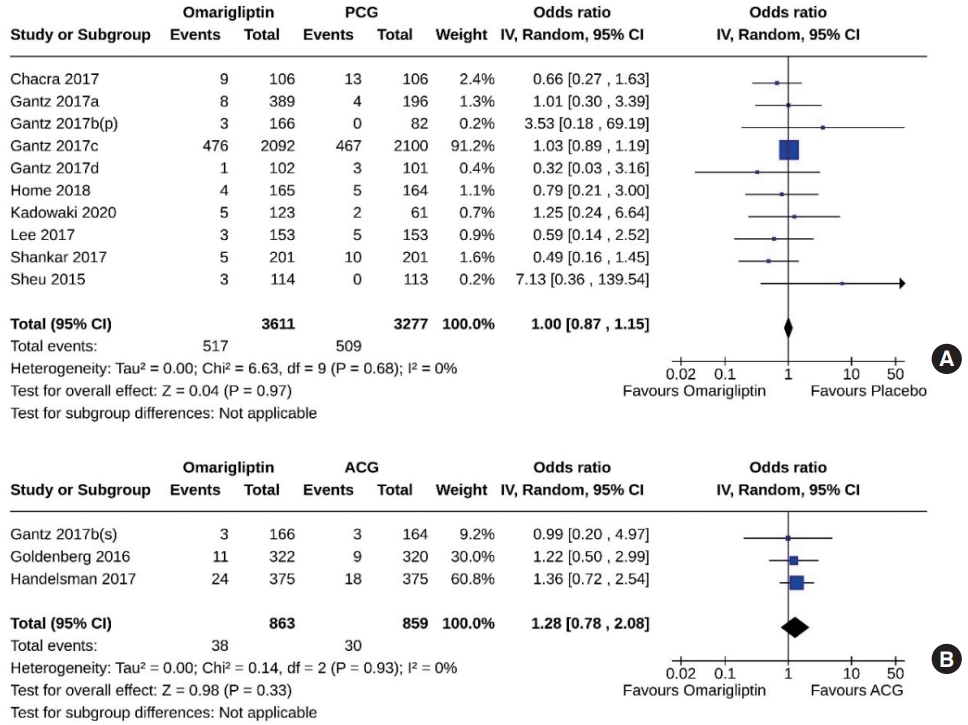
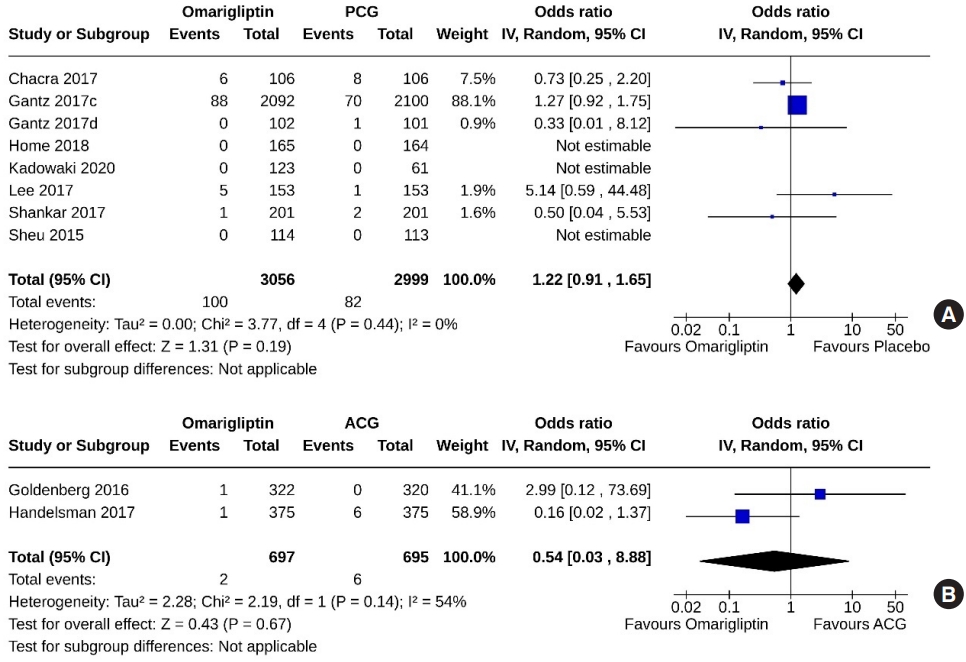
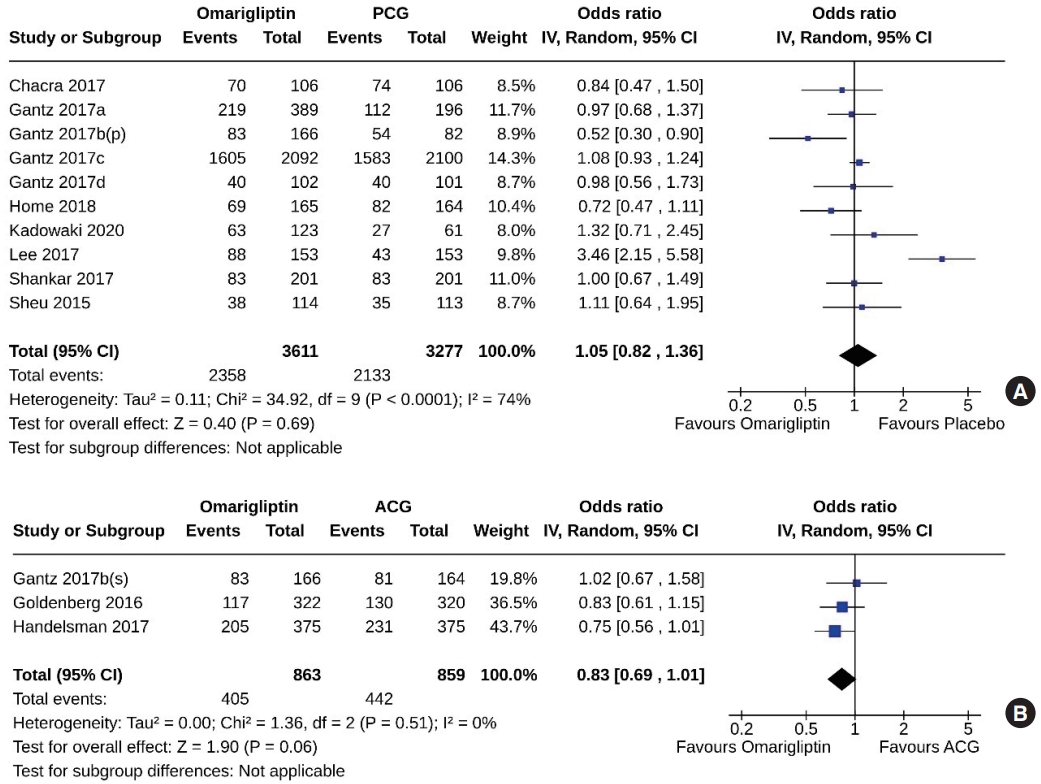
- Ten studies (6,888 subjects: 3,611 in the omarigliptin group, 3,277 in the PCG) compared omarigliptin to placebo, and three studies (1,722 subjects: 863 in the omarigliptin group, 859 in the ACG) compared omarigliptin to active comparators for the AEs (total AEs and serious AEs). Total AEs were similar in the omarigliptin group and PCG (OR, 1.05; 95% CI, 0.82 to 1.36; P=0.69; I2=74% [moderate heterogeneity]) (Fig. 8A), and in the omarigliptin group and ACG (OR, 0.83; 95% CI, 0.69 to 1.01; P=0.06; I2=0% [not important heterogeneity]) (Fig. 8B). The serious AEs were also similar in the omarigliptin group and PCG (OR, 1.00; 95% CI, 0.87 to 1.15; P=0.97; I2=0% [not important heterogeneity]) (Fig. 9A), and in the omarigliptin group and ACG (OR, 1.28; 95% CI, 0.78 to 2.08; P=0.33; I2=0% [not important heterogeneity]) (Fig. 9B).
- Hypoglycemia and severe hypoglycemia
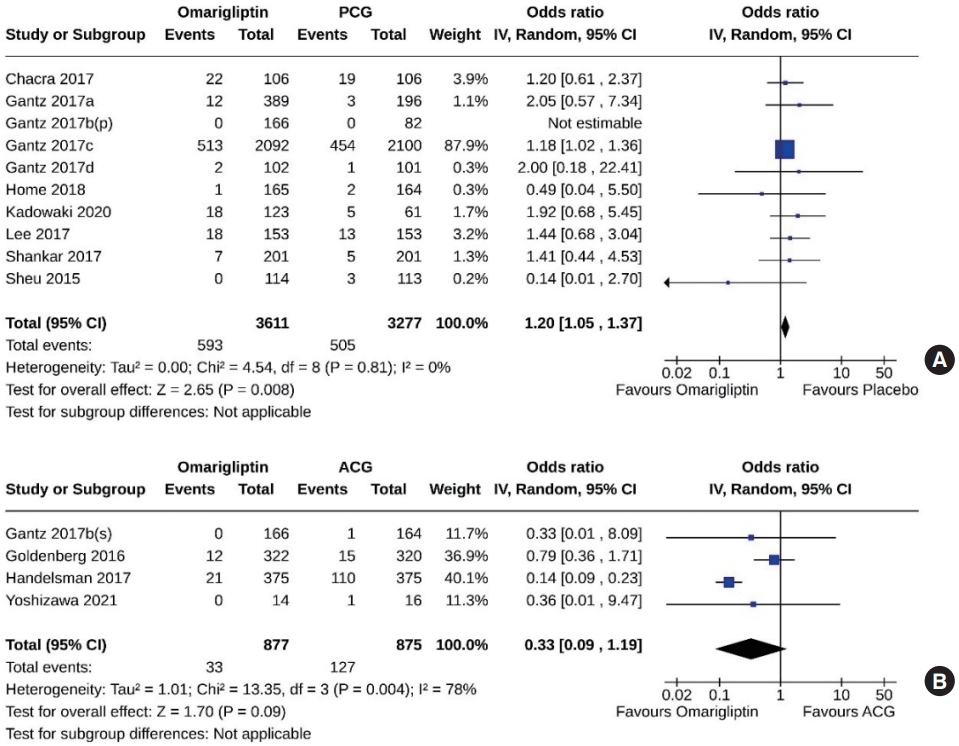
- Ten studies (6,888 subjects: 3,611 in the omarigliptin group, 3,277 in the PCG) compared omarigliptin to placebo, and four studies (1,752 subjects: 877 in the omarigliptin group, 875 in the ACG) compared omarigliptin to active comparators for hypoglycemic events. Hypoglycemic events were higher in the omarigliptin group than in the PCG (OR, 1.20; 95% CI, 1.05 to 1.37; P=0.008; I2=0% [not important heterogeneity]) (Fig. 10A) and similar in the omarigliptin group and ACG (OR, 0.33; 95% CI, 0.09 to 1.19; P=0.09; I2=78% [high heterogeneity]) (Fig. 10B). An additional sensitivity analysis (Supplemental Fig. S2) revealed that one study (Gantz 2017c) [9], which included the majority of the subjects, showed a significantly increased OR for hypoglycemia. However, in the remaining studies, the OR did not appear to be significantly high.
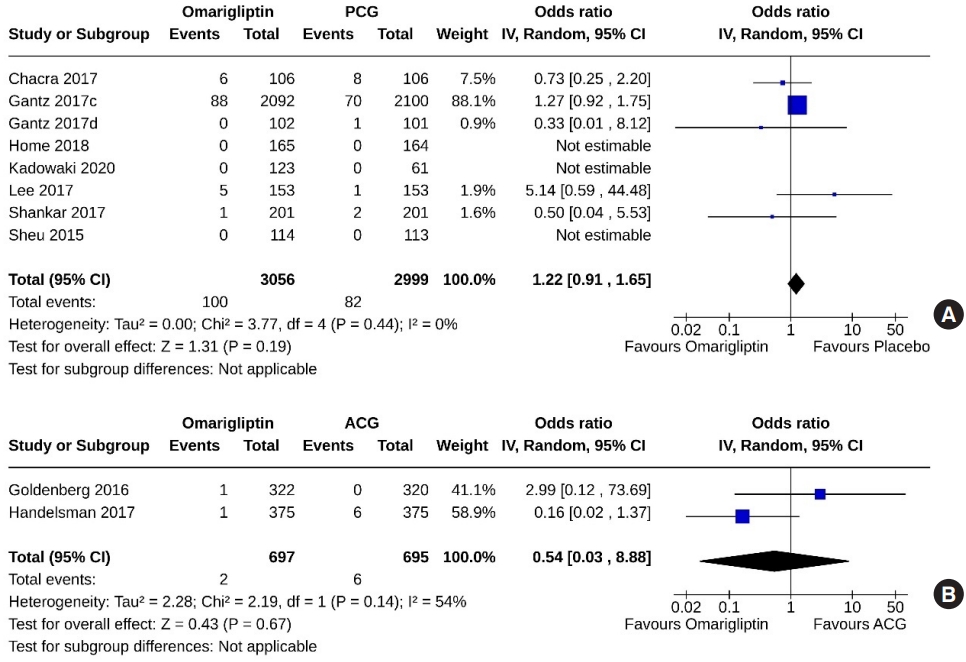
- Eight studies (6,055 subjects: 3,056 in the omarigliptin group, 2,999 in the PCG) compared omarigliptin to placebo, and two studies (1,392 subjects: 697 in the omarigliptin group, 695 in the ACG) compared omarigliptin to active comparators for severe hypoglycemic events. Severe hypoglycemic events were similar in the omarigliptin group and PCG (OR, 1.22; 95% CI, 0.91 to 1.65; P=0.19; I2=0% [not important heterogeneity]) (Fig. 11A), and in the omarigliptin group and ACG (OR, 0.54; 95% CI, 0.03 to 8.88; P=0.67; I2=54% [moderate heterogeneity]) (Fig. 11B).
- Body weight
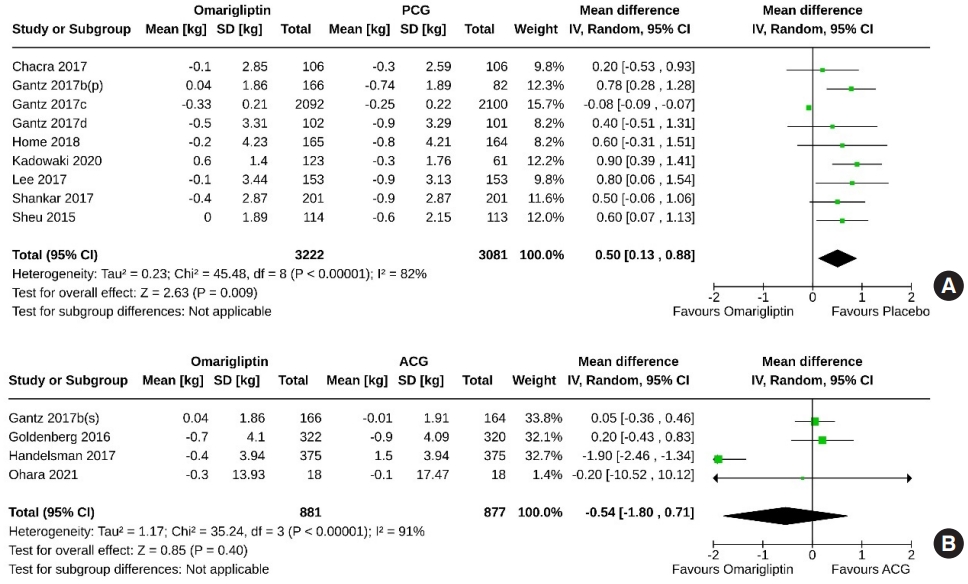
- Nine studies (6,303 subjects: 3,222 in the omarigliptin group, 3,081 in the PCG) compared omarigliptin to placebo, and four studies (1,758 subjects: 881 in the omarigliptin group, 877 in the ACG) compared omarigliptin to active comparators for changes in body weight. Placebo was superior to omarigliptin in terms of body weight reduction (MD, 0.50 kg; 95% CI, 0.13 to 0.88; P=0.009; I2=82% [high heterogenicity]) (Fig. 12A). However, changes in body weight were similar in the omarigliptin group and ACG (MD, –0.54 kg; 95% CI, –1.80 to 0.71; P=0.40; I2=91% [high heterogeneity]) (Fig. 12B).
- Estimated glomerular filtration rate
- Two studies (4,404 subjects: 2,198 in the omarigliptin group; 2,206 in the PCG) compared omarigliptin to placebo, and two studies (120 subjects: 74 in the omarigliptin group; 46 in the ACG) compared omarigliptin to active comparators for changes in estimated glomerular filtration rate (eGFR). A greater decline in eGFR (MD, –1.84 mL/min/1.73 m2; 95% CI, –3.57 to –0.12; P=0.04; I2=60% [moderate heterogeneity]) was observed in the omarigliptin group than in the PCG (Fig. 13A). However, changes in eGFR were similar in the omarigliptin group and ACG (MD, –0.86 mL/min/1.73 m2; 95% CI, –9.65 to 7.92; P=0.85; I2=0% [not important heterogenicity]) (Fig. 13B).
- Serum amylase and lipase
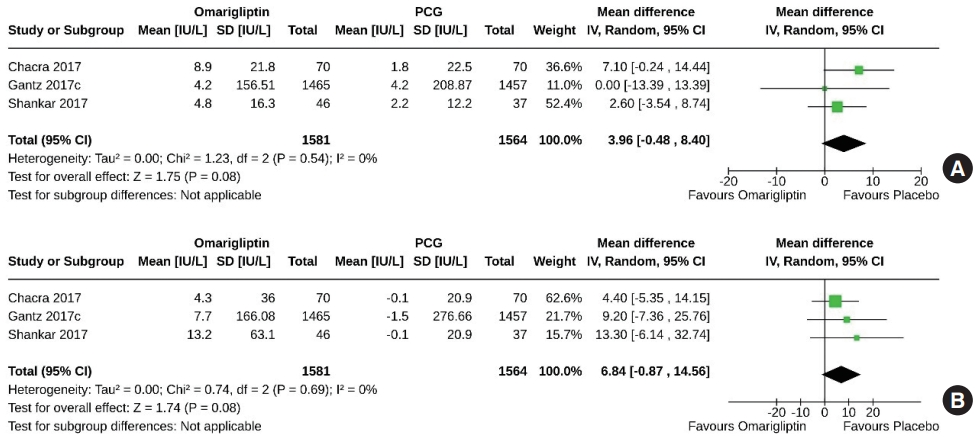
- Two studies (3,145 subjects: 1,581 in the omarigliptin group, 1,564 in the PCG) compared omarigliptin to placebo for changes in serum amylase and lipase. Changes in serum amylase (MD, 3.96 IU/L; 95% CI, –0.48 to 8.40; P=0.08; I2=0% [not important heterogenicity]) (Fig. 14A), and serum lipase (MD, 6.84 IU/ L; 95% CI, –0.87 to 14.56; P=0.08; I2=0% [not important heterogenicity]) (Fig. 14B) were similar in the two groups. Only one study compared the changes in serum amylase and lipase with omarigliptin to an active comparator and found comparable effects [12].
RESULTS
Fasting plasma glucose
2-Hour postprandial glucose
Percentage of subjects achieving HbA1c <7%
Percentage of subjects achieving HbA1c <6.5%
- The current meta-analysis synthesized the results of RCTs of omarigliptin in T2DM published to date. It emphasizes the drug’s efficacy in glycemic control, its safety profile in terms of AEs, and its potential for causing hypoglycemia when compared to controls, which include placebo and other AHAs. Omarigliptin has been found to be more effective in lowering HbA1c levels than controls, while exhibiting a similar incidence of adverse and hypoglycemic events.
- This meta-analysis included RCTs that evaluated the efficacy of omarigliptin, either as monotherapy or in combination with other AHAs, in comparison to placebo or other AHAs. The primary comparators were once-daily DPP-4i and glimepiride, over durations ranging from 12 to 142 weeks. Omarigliptin demonstrated a clear superiority over placebo in improving HbA1c levels. Additionally, omarigliptin showed good and comparable glycemic efficacy to established once-daily DDP4i and glimepiride. The advantage of omarigliptin over placebo was also evident in the reduction of FPG and 2-hour PPG; however, the reduction in FPG was similar to that achieved with other once-daily DDP4i and glimepiride. Furthermore, compared to placebo, omarigliptin exhibited greater glycemic efficacy than trelagliptin, another once-weekly DPP-4i, with reductions in HbA1c (–0.58% vs. –0.54%) and FPG (–0.78 mmol/L vs. –0.34 mmol/L). The reductions in PPG were comparable (–1.32 mmol/L vs. –1.33 mmol/L), as reported in a recent meta-analysis [33]. Additionally, one study in this meta-analysis demonstrated omarigliptin’s superiority over placebo in maintaining time-in-range [18]. Our study provides reassuring data on the efficacy of omarigliptin. When compared to other DDP-4is used in various RCTs, once-weekly omarigliptin was equally effective in reducing HbA1c and FPG, as well as in the percentage of patients achieving HbA1c targets of <7% and <6.5%, respectively.
- The meta-analysis also provided reassuring safety data for omarigliptin. Given the broad tissue distribution of the DPP-4 enzyme, DPP-4is could potentially be linked to a diverse array of AEs. The AEs commonly associated with this class of drugs include gastrointestinal intolerance, which manifests as nausea, vomiting, diarrhea, and dyspepsia, as well as acute pancreatitis and pancreatic carcinoma. There is also an increased incidence of infections, such as upper respiratory tract infections, nasopharyngitis, and urinary tract infections, along with arthralgia and myalgia. Hypersensitivity reactions have been reported as well, including anaphylaxis, rash, and angioedema [34,35]. Omarigliptin has been well tolerated in all studies included in this meta-analysis, with no increased risks of AEs or serious AEs reported for this once-weekly DPP-4i. Relative to the PCG and ACG, there was a small, non-significant increase in serum amylase and lipase levels from baseline in the omarigliptin group. However, the study results indicated that these increases were minor, and in nearly all cases, the mean serum amylase and lipase levels remained within normal laboratory ranges throughout the treatment period. Only a few studies noted mildly elevated serum lipase levels at certain points during the trial, but these were not linked to any clinical consequences [6,9,17]. Moreover, the majority of studies reported no instances of acute or chronic pancreatitis [6,8,10-12,16,17,19,20]. Only one study (Gantz 2017c) documented a small number of acute pancreatitis cases (six out of 2,092 in the omarigliptin group and three out of 2,100 in the placebo group); there was one case of chronic pancreatitis in the placebo group and none in the omarigliptin group [9]. This study also noted three cases of pancreatic carcinoma in the omarigliptin group compared to one in the placebo group [9]. It is important to recognize that diabetes itself is associated with elevated levels of amylase and lipase and is a risk factor for pancreatitis. Therefore, the observed increase in amylase and lipase levels with omarigliptin use warrants further investigation.
- DPP-4is promote insulin secretion and suppress glucagon secretion in a glucose-dependent manner, which prevents them from causing hypoglycemia [36]. In this meta-analysis, omarigliptin demonstrated a greater potential for hypoglycemia compared to placebo. This effect was primarily attributed to one study (Gantz 2017c) [9], which accounted for the majority of subjects analyzed and revealed a significantly increased OR for hypoglycemia. However, the OR for hypoglycemia in the other studies did not appear to be significantly elevated. The high proportion of participants (approximately 75%) in the aforementioned study (Gantz 2017c) [9] who were concurrently using insulin or sulphonylureas may account for the variability in hypoglycemic outcomes. Nonetheless, the risk of hypoglycemia associated with omarigliptin was comparable to that of active comparators.
- Moreover, severe hypoglycemic events were comparable between the omarigliptin group and both the PCG and ACG. This meta-analysis found that omarigliptin is weight-neutral, consistent with other DPP-4is [36]. Omarigliptin use was associated with a greater reduction in eGFR than that observed in the PCG in this meta-analysis. However, when compared to other DPP4i, omarigliptin did not result in any additional decline in glomerular filtration rate (GFR). The reductions in eGFR were minor and not associated with any measurable clinical outcomes in the studies [6,9,13,18]. The cause of this mild decrease in GFR with omarigliptin remains unclear and warrants further investigation in future studies. Furthermore, omarigliptin did not increase the risk of major adverse cardiovascular events or hospitalization for heart failure, as reported in a trial by Gantz et al. [9] (Gantz 2017c). There were no clinically meaningful changes from baseline in vital signs, laboratory safety parameters (including liver function tests), lipid profiles, or electrocardiogram parameters (including corrected QT interval) in most of the studies [6,8,9,11,12,14,17,19,20].
- The once-weekly dosage of omarigliptin could significantly decrease the monthly pill count, potentially reducing medication burden and enhancing adherence. Ishii et al. [15] found that transitioning from a daily DPP-4i to once-weekly omarigliptin may lessen the medication burden for patients. These characteristics render omarigliptin an appealing option for sustained clinical use in individuals with T2DM, leading to improved longterm compliance and glycemic outcomes.
- Strengths and limitations
- The main strength of this meta-analysis lies in its inclusion of a large population drawn from a substantial number of studies. The overall quality of the trials included is high; all were RCTs, with the exception of four, which were not double-blind. Our analysis examined changes in body weight, eGFR, serum amylase, and lipase levels between the omarigliptin and control groups—data that were absent from previous meta-analyses. Additionally, this meta-analysis included all available RCTs published up to the present. However, there are several limitations to consider. The study by Gantz et al. [9] (Gantz 2017c) represents approximately 47% of the subjects in the meta-analysis, which significantly influenced the outcomes. There was notable heterogeneity observed for the primary outcome and many secondary outcomes. The certainty of the evidence produced ranged from very low to moderate, with only a few exceptions. Lastly, due to the inclusion of only published data, the meta-analysis may be susceptible to reporting bias, potentially leading to an overestimation of the effects of omarigliptin.
- Conclusions
- To conclude, this meta-analysis examining the efficacy and safety of once-weekly omarigliptin offers reassuring evidence of its good glycemic control, tolerability, and safety across a prolonged period of clinical use in a varied population of subjects with T2DM. The use of omarigliptin was linked to a mild, asymptomatic decline in the eGFR and a slight, asymptomatic rise in amylase and lipase levels, the clinical significance of which requires further investigation in future studies.
DISCUSSION
Supplementary Material
Supplemental Fig. S1.
Supplemental Fig. S2.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: A.B.M.K., M.S.A., S.S. Acquisition, analysis, or interpretation of data: A.B.M.K., M.S.A., S.K.T., D.D. Drafting the work or revising: A.B.M.K., D.D. Final approval of the manuscript: A.B.M.K., M.S.A., S.K.T., D.D., S.S.
Article information





| RCT ID | Study place | Characteristics of study subjects | Study arms | No. | Age, yr | Male, % | Duration of T2DM, yr | Baseline HbA1c, % | Study duration, wk |
|---|---|---|---|---|---|---|---|---|---|
| Chacra et al. (2017) [6] | Multi-country, multicenter | eGFR <60 mL/min/1.73 m2 or ESRD | Omarigliptin 12.5/25 mg | 106 | 65.9±9.4 | 63.6 | 14.9±8.2 | 8.3±0.8 | Phase A: 24a |
| On no, single, or combination AHA or stable dose of insulin with HbA1c ≥ 6.5%–≤10.0% | Placebo | 106 | 64.5±9.7 | 59.4 | 15.1±8.7 | 8.3±0.8 | Phase B: 30 | ||
| Pre-randomization FPG <7.22–<14.43 mmol/L | |||||||||
| Gantz et al. (2017a) [7] | Multicenter in Japan | On stable dose of an SU, GL, BG, TZD, or AGI | Omarigliptin 25 mg+Background OADs | 389 | 61±10 | 69.7 | 9.3±5.8 | 8.0±0.7 | Phase A: 24a |
| Pre-randomization HbA1c 7.0%–10.0% | Placebo+Background OADs | 196 | 61±11 | 74.0 | 9.7±5.7 | 8.0±0.7 | Phase B: 28 | ||
| Gantz et al. (2017b) [8] | Multicenter in Japan | Treatment-naïve or on AHA | Omarigliptin 25 mg | 166 | 60±11 | 62.7 | 7.4±5.5 | 7.9±0.7 | Phase A: 24a |
| Pre-randomization HbA1c 7.0%–10.0% | Sitagliptin 50 mg | 164 | 60±9 | 69.7 | 7.4±5.3 | 8.0±0.8 | Phase B: 28 | ||
| Placebo | 82 | 61±9 | 68.7 | 8.6±5.1 | 8.1±0.7 | ||||
| Gantz et al. (2017c) [9] | Multi-country, multicenter | Established CVD | Omarigliptin 25 mg | 2,092 | 63.7±8.5 | 69.6 | 12.0±7.6 | 8.0±0.9 | 142 (with an 18-week period of unadjusted background medicationa) |
| Stable diabetes treatment regimens for at least 12 weeks | |||||||||
| Pre-randomization HbA1c 6.5%–10.0% | |||||||||
| Placebo | 2,100 | 63.6±8.5 | 70.7 | 12.1±8.0 | 8.0±0.9 | ||||
| Gantz et al. (2017d) [10] | Multi-country, multicenter | Drug-naïve or not on an AHA for ≥12 weeks | Omarigliptin 25 mg | 102 | 38.8±4.7 | 65.7 | 2.9±2.2 | 7.9±0.8 | 24 |
| HbA1c 7.0%–10.0% at screening and FPG >7.2–<14.4 mmol/L at randomization | Placebo | 101 | 39.5±4.5 | 59.4 | 3.3±3.0 | 8.1±0.9 | |||
| Goldenberg et al. (2017) [11] | Multi-country, multicenter | On a stable dose of metformin (≥1.5 g) for ≥12 weeks | Omarigliptin 25 mg+Metformin | 322 | 57±10 | 46.9 | 7.0±4.5 | 7.5±0.8 | 24 |
| HbA1c ≥6.5%–≤9.0% at screening and FPG >7.2–<14.4 mmol/L at randomization | Sitagliptin 100 mg+ Metformin | 320 | 58±10 | 54.7 | 7.5±5.6 | 7.5±0.7 | |||
| Handelsman et al. (2017) [12] | Multi-country, multicenter | On a stable dose of metformin (≥1.5 g) for ≥12 weeks | Omarigliptin 25 mg+ Metformin | 375 | 58±10 | 54.0 | 7.6±5.1 | 7.5±0.8 | 54 |
| HbA1c ≥6.5%–≤9.0% at screening and FPG >7.0–<14.4 mmol/L at randomization | Glimepiride+Metformin | 375 | 58±9 | 56.3 | 7.7±4.9 | 7.4±0.7 | |||
| Hattori (2020) [13] | Single-center in Japan | Attended the study center for at least 12 months | Omarigliptin 25 mg | 56 | 59.00±7.33 | 71 | Not available | 6.91±0.77 | 52 |
| HbA1c >6.0% regardless of diet, exercise, and daily medications with the DDP-4 inhibitors sitagliptin (50 mg) or linagliptin (5 mg) | Sitagliptin 50 mg or linagliptin 5 mg | 28 | 59.17±7.85 | 75 | Not available | 6.85±0.75 | |||
| Home et al. (2018) [14] | Multi-country, multicenter | At screening were either not on an OGLD for at least 12 weeks and had a screening visit HbA1c ≥7.0% and ≤10.0% on diet and exercise alone, or had HbA1c ≥6.5% and ≤9.0% on OGLD monotherapy or low-dose (50% of maximum label dose of each agent) dual oral therapy | Omarigliptin 25 mg | 165 | 57.4±9.2 | 57.6 | 5.4±3.8 | 8.0±0.9 | Phase A: 24a |
| Placebo | 164 | 57.0±9.7 | 59.1 | 5.7±4.7 | 8.1±1.0 | Phase B: 30 | |||
| Ishii et al. (2023) [15] | Multicenter in Japan | Used once- or twice-daily DPP-4 inhibitors | Omarigliptin 25 mg | 106 | 65.3±11.8 | 50.9 | Not available | 6.8±0.6 | 12 |
| Did not change the anti-diabetic agents (dose, usage, or type) within 8 weeks before giving their consent | Once or twice-daily DPP-4 inhibitors | 106 | 65.1±11.7 | 48.1 | Not available | 6.9±0.7 | |||
| HbA1c <10.0% upon giving consent | |||||||||
| Omarigliptin 25 mg | 123 | 61.1±11.0 | 69.9 | 12.6±9.0 | 8.8±0.7 | Phase A: 16a | |||
| Kadowaki et al (2021) [16] | Multicenter in Japan | At screening, either on a stable regimen of insulin ± a single OHA with an HbA1c of ≥7.0% and ≤10.0% | Placebo | 61 | 60.9±11.7 | 77.0 | 13.8±7.8 | 8.8±0.8 | Phase B: 36 |
| 2 weeks before randomization, HbA1c of ≥7.5% and ≤10.0%, as well as FPG of ≥7.0 and ≤12.8 mmol/L | |||||||||
| Lee et al. (2017) [17] | Multi-country, multicenter | On dual combination therapy with metformin ≥1.5 g/day for ≥12 weeks and either glimepiride or another sulfonylurea | Omarigliptin 25 mg+Metformin+Sus | 153 | 57.2±8.4 | 47.4 | 9.8±5.3 | 8.5±0.8 | 24 |
| HbA1c ≥7.5% and ≤10.5% | Placebo+Metformin+SUs | 153 | 58.4±9.4 | 48.4 | 10.4±5.5 | 8.6±0.8 | |||
| Ohara et al. (2021) [18] | Multicenter in Japan | Treatment with daily DPP-4 inhibitors for ≥12 weeks | Omarigliptin 25 mg | 18 | 66.8±6.6 | 72.2 | 11.9±7.6 | 7.2±0.4 | 24 |
| HbA1c ≥6.5% | Daily DPP-4 inhibitorsb | 18 | 69.0±9.2 | 66.7 | 14.5±6.5 | 7.2±0.4 | |||
| Shankar et al. (2017) [19] | Multi-country, multicenter | On a stable dose of metformin monotherapy (≥1.5 g/day) for at least 12 weeks | Omarigliptin 25 mg+Metformin | 201 | 57.5±8.1 | 50.2 | 8.2±5.2 | 8.1±0.9 | Phase A: 24a |
| HbA1c of 7.0%–10.5% at screening | Placebo+Metformin | 201 | 56.8±9.1 | 50.7 | 7.4±5.6 | 8.0±0.9 | Phase B: 80 | ||
| Sheu et al. (2015) [20] | Multi-country, multicenter | Not on an oral AHA (off AHA medication for ≥14 weeks) | Omarigliptin 25 mg | 114 | 55.1±8.8 | 60.5 | 5.9±5.2 | 8.1±1.0 | Phase A: 12a |
| HbA1c ≥7.0% and ≤10.0% | Placebo | 113 | 55.9±8.4 | 57.0 | 5.8±4.6 | 8.1±0.9 | Phase B: 66 | ||
| Yoshizawa et al. (2021) [21] | Multicenter in Japan | On maintenance hemodialysis for >6 months | Omarigliptin 12.5 mg | 14 | 67.7±8.9 | 85.7 | 16.0±8.7 | 6.2±0.9 | 24 |
| Using DPP-4 inhibitors for more than 3 months | Linagliptin 5 mg | 16 | 67.5±9.0 | 75.0 | 20.8±11.3 | 6.5±1.0 | |||
| HbA1c <9.0% |
RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; AHA, antihyperglycemic agent; FPG, fasting plasma glucose; SU, sulphonylureas; GL, glinides; BG, biguanides; TZD, thiazolidinediones; AGI, alpha-glucosidase inhibitors; OAD, oral anti-diabetic drug; CVD, cardiovascular disease; DDP-4, dipeptidyl peptidase-4; OGLD, oral glucose-lowering drug; OHA, oral hypoglycemic agent.
a Included in the meta-analysis;
b Sitagliptin 50 mg/day, linagliptin 5 mg/day, alogliptin 25 mg/day, vildagliptin 100 mg/day, saxagliptin 5 mg/day, teneligliptin 20 mg/day.
| Outcomes |
Anticipated absolute effectse (95% CI) |
Relative effect, OR (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with control | Risk with omarigliptin | ||||
| Change in HbA1c-PCG | The mean change in HbA1c in PCG was 0.12% | MD 0.58% lower (0.75 lower to 0.4 lower) | - | 6,888 (10 RCTs) | ⨁◯◯◯ |
| Very lowb,c | |||||
| Proportion of subjects achieved HbA1c <7.0%-PCG | 124 per 1,000 | 358 per 1,000 (238–499) | 3.95 (2.21–7.06) | 2,493 (8 RCTs) | ⨁◯◯◯ |
| Very lowb,c | |||||
| Proportion of subjects achieved HbA1c <6.5%-PCG | 18 per 1,000 | 86 per 1,000 (47–155) | 5.08 (2.64–9.89) | 1,550 (5 RCTs) | ⨁⨁⨁◯ |
| Moderateb | |||||
| AEs-PCG | 651 per 1,000 | 662 per 1,000 (605–717) | 1.05 (0.82–1.36) | 6,888 (10 RCTs) | ⨁⨁◯◯ |
| Lowa,d | |||||
| Serious AEs-PCG | 155 per 1,000 | 155 per 1,000 (138–175) | 1.00 (0.87–1.15) | 6,888 (10 RCTs) | ⨁⨁⨁⨁ |
| High | |||||
| Hypoglycemia-PCG | 154 per 1,000 | 179 per 1,000 (161–200) | 1.20 (1.05–1.37) | 6,888 (10 RCTs) | ⨁⨁⨁◯ |
| Moderateb | |||||
| Severe hypoglycemia-PCG | 27 per 1,000 | 33 per 1,000 (25–44) | 1.22 (0.91–1.65) | 6,055 (8 RCTs) | ⨁⨁⨁◯ |
| Moderated | |||||
CI, confidence interval; OR, odds ratio; HbA1c, hemoglobin A1c; PCG, passive control group; MD, mean difference; RCT, randomized controlled trial; AE, adverse event.
a Moderate heterogeneity among the studies present;
b The funnel plot is suggestive of an asymmetrical presence of research on each side of the central line and the presence of most of the studies outside the plot; hence, it is likely that significant publication bias is present;
c High heterogeneity among the studies present;
d The funnel plot is suggestive of an asymmetrical presence of research on each side of the central line; hence, it is likely that significant publication bias is present;
e The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
- 1. Vella A. Mechanism of action of DPP-4 inhibitors: new insights. J Clin Endocrinol Metab 2012;97:2626–8.ArticlePubMedPMC
- 2. Godinho R, Mega C, Teixeira-de-Lemos E, Carvalho E, Teixeira F, Fernandes R, et al. The place of dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapeutics: a “me too” or “the special one” antidiabetic class? J Diabetes Res 2015;2015:806979.ArticlePubMedPMCPDF
- 3. Burness CB. Omarigliptin: first global approval. Drugs 2015;75:1947–52.ArticlePubMedPDF
- 4. Krishna R, Addy C, Tatosian D, Glasgow XS, Gendrano Iii IN, Robberechts M, et al. Pharmacokinetics and pharmacodynamics of omarigliptin, a once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, after single and multiple doses in healthy subjects. J Clin Pharmacol 2016;56:1528–37.ArticlePubMedPMCPDF
- 5. Tsuchiya S, Friedman E, Addy C, Wakana A, Tatosian D, Matsumoto Y, et al. Single and multiple dose pharmacokinetics and pharmacodynamics of omarigliptin, a novel, once-weekly dipeptidyl peptidase-4 inhibitor, in healthy Japanese men. J Diabetes Investig 2017;8:84–92.ArticlePubMedPMCPDF
- 6. Chacra A, Gantz I, Mendizabal G, Durlach L, O’Neill EA, Zimmer Z, et al. A randomised, double-blind, trial of the safety and efficacy of omarigliptin (a once-weekly DPP-4 inhibitor) in subjects with type 2 diabetes and renal impairment. Int J Clin Pract 2017;71:e12955.ArticlePubMedPMCPDF
- 7. Gantz I, Okamoto T, Ito Y, Sato A, Okuyama K, O’Neill EA, et al. A randomized, placebo-controlled trial evaluating the safety and efficacy of adding omarigliptin to antihyperglycemic therapies in Japanese patients with type 2 diabetes and inadequate glycemic control. Diabetes Ther 2017;8:793–810.ArticlePubMedPMCPDF
- 8. Gantz I, Okamoto T, Ito Y, Okuyama K, O’Neill EA, Kaufman KD, et al. A randomized, placebo- and sitagliptin-controlled trial of the safety and efficacy of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2017;19:1602–9.PubMedPMC
- 9. Gantz I, Chen M, Suryawanshi S, Ntabadde C, Shah S, O’Neill EA, et al. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2017;16:112.ArticlePubMedPMCPDF
- 10. Gantz I, Sokolova L, Jain L, Iredale C, O’Neill EA, Wei Z, et al. Use of prohibited medication, a potentially overlooked confounder in clinical trials: omarigliptin (once-weekly DPP4 inhibitor) monotherapy trial in 18- to 45-year-olds. Clin Ther 2017;39:2024–37.ArticlePubMed
- 11. Goldenberg R, Gantz I, Andryuk PJ, O’Neill EA, Kaufman KD, Lai E, et al. Randomized clinical trial comparing the efficacy and safety of treatment with the once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor omarigliptin or the oncedaily DPP-4 inhibitor sitagliptin in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Diabetes Obes Metab 2017;19:394–400.PubMedPMC
- 12. Handelsman Y, Lauring B, Gantz I, Iredale C, O’Neill EA, Wei Z, et al. A randomized, double-blind, non-inferiority trial evaluating the efficacy and safety of omarigliptin, a onceweekly DPP-4 inhibitor, or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin 2017;33:1861–8.ArticlePubMed
- 13. Hattori S. Omarigliptin decreases inflammation and insulin resistance in a pleiotropic manner in patients with type 2 diabetes. Diabetol Metab Syndr 2020;12:24.ArticlePubMedPMCPDF
- 14. Home P, Shankar RR, Gantz I, Iredale C, O’Neill EA, Jain L, et al. A randomized, double-blind trial evaluating the efficacy and safety of monotherapy with the once-weekly dipeptidyl peptidase-4 inhibitor omarigliptin in people with type 2 diabetes. Diabetes Res Clin Pract 2018;138:253–61.ArticlePubMed
- 15. Ishii H, Kamei N, Shimono D, Niiya T, Tosaki T, Kitazawa T, et al. Treatment burden on once-weekly omarigliptin versus daily dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: randomized controlled trial (ONWARD-DPP4 Study). Diabetes Ther 2023;14:1639–58.ArticlePubMedPMCPDF
- 16. Kadowaki T, Seino Y, Kaku K, Okamoto T, Kameya M, Sato A, et al. A randomized, placebo-controlled study to evaluate the efficacy and safety of adding omarigliptin to insulin therapy in Japanese patients with type 2 diabetes and inadequate glycaemic control. Diabetes Obes Metab 2021;23:1242–51.PubMedPMC
- 17. Lee SH, Gantz I, Round E, Latham M, O’Neill EA, Ceesay P, et al. A randomized, placebo-controlled clinical trial evaluating the safety and efficacy of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus inadequately controlled by glimepiride and metformin. BMC Endocr Disord 2017;17:70.ArticlePubMedPMCPDF
- 18. Ohara M, Nagaike H, Fujikawa T, Kohata Y, Ogawa M, Omachi T, et al. Effects of omarigliptin on glucose variability and oxidative stress in type 2 diabetes patients: a prospective study. Diabetes Res Clin Pract 2021;179:108999.ArticlePubMed
- 19. Shankar RR, Inzucchi SE, Scarabello V, Gantz I, Kaufman KD, Lai E, et al. A randomized clinical trial evaluating the efficacy and safety of the once-weekly dipeptidyl peptidase-4 inhibitor omarigliptin in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin 2017;33:1853–60.ArticlePubMed
- 20. Sheu WH, Gantz I, Chen M, Suryawanshi S, Mirza A, Goldstein BJ, et al. Safety and efficacy of omarigliptin (MK-3102), a novel once-weekly DPP-4 inhibitor for the treatment of patients with type 2 diabetes. Diabetes Care 2015;38:2106–14.ArticlePubMedPDF
- 21. Yoshizawa Y, Hosojima M, Kabasawa H, Tanabe N, Ugamura D, Koda Y, et al. Effects of the once-weekly DPP4 inhibitor omarigliptin on glycemic control in patients with type 2 diabetes mellitus on maintenance hemodialysis: a 24-week open-label, multicenter randomized controlled study. Diabetes Ther 2021;12:655–67.ArticlePubMedPMCPDF
- 22. Kawasaki E, Nakano Y, Fukuyama T, Uchida A, Sagara Y, Tamai H, et al. Efficacy of omarigliptin, once-weekly dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes. World J Diabetes 2021;12:2087–95.ArticlePubMedPMC
- 23. Wang X, Li X, Qie S, Zheng Y, Liu Y, Liu G. The efficacy and safety of once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus: a systemic review and meta-analysis. Medicine (Baltimore) 2018;97:e11946.PubMedPMC
- 24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.ArticlePubMedPMC
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.ArticlePubMedPMC
- 26. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94.ArticlePubMed
- 27. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. (updated August 2023) Chichester: John Wiley & Sons; 2023 Chapter 13, Assessing risk of bias due to missing results in a synthesis [cited 2023 Dec 19]. Available from: www.training.cochrane.org/handbook.
- 28. Addy C, Tatosian D, Glasgow XS, Gendrano IN 3rd, Kauh E, Martucci A, et al. Pharmacokinetic and pharmacodynamic effects of multiple-dose administration of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in obese participants with and without type 2 diabetes mellitus. Clin Ther 2016;38:516–30.ArticlePubMed
- 29. Addy C, Tatosian DA, Glasgow XS, Gendrano IN III, Sisk CM, Kauh EA, et al. Effects of age, sex, and obesity on the single-dose pharmacokinetics of omarigliptin in healthy subjects. Clin Pharmacol Drug Dev 2016;5:374–82.ArticlePubMedPDF
- 30. Xu S, Tatosian D, Mcintosh I, Caceres M, Matthews C, Samuel K, et al. Absorption, metabolism and excretion of [14C] omarigliptin, a once-weekly DPP-4 inhibitor, in humans. Xenobiotica 2018;48:584–91.ArticlePubMed
- 31. Jain L, Chain AS, Tatosian DA, Hing J, Passarell JA, Kauh EA, et al. Pharmacokinetic-pharmacodynamic (dipeptidyl peptidase-4 inhibition) model to support dose rationale in diabetes patients, including those with renal impairment, for once-weekly administered omarigliptin. Br J Clin Pharmacol 2019;85:2759–71.ArticlePubMedPMCPDF
- 32. Tosaki T, Kamiya H, Yamamoto Y, Himeno T, Kato Y, Kondo M, et al. Efficacy and patient satisfaction of the weekly DPP-4 inhibitors trelagliptin and omarigliptin in 80 Japanese patients with type 2 diabetes. Intern Med 2017;56:2563–9.ArticlePubMedPMC
- 33. Dutta D, Mohindra R, Surana V, Sharma M. Safety and efficacy of once weekly dipeptidyl-peptidase-4 inhibitor trelagliptin in type-2 diabetes: a meta-analysis. Diabetes Metab Syndr 2022;16:102469.ArticlePubMed
- 34. Huang J, Jia Y, Sun S, Meng L. Adverse event profiles of dipeptidyl peptidase-4 inhibitors: data mining of the public version of the FDA adverse event reporting system. BMC Pharmacol Toxicol 2020;21:68.ArticlePubMedPMCPDF
- 35. Ling J, Cheng P, Ge L, Zhang DH, Shi AC, Tian JH, et al. The efficacy and safety of dipeptidyl peptidase-4 inhibitors for type 2 diabetes: a Bayesian network meta-analysis of 58 randomized controlled trials. Acta Diabetol 2019;56:249–72.ArticlePubMedPDF
- 36. Florentin M, Kostapanos MS, Papazafiropoulou AK. Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment. World J Diabetes 2022;13:85–96.ArticlePubMedPMC
References
Figure & Data
References
Citations


 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite