Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(1); 2024 > Article
-
Review ArticleDiabetes, obesity and metabolism Glucagon: Physiological and Pharmacological Functions and Pathophysiological Significance in Type 2 Diabetes
 Keypoint
Keypoint
Glucagon has many functions, including the promotion of hepatic glucose production, fatty acid oxidation, thermogenesis, energy consumption, lipolysis, and myocardial contraction, as well as the suppression of lipogenesis, appetite, and gastrointestinal motility. A recently developed new glucagon sandwich ELISA is a more sensitive and specific method for detecting glucagon, and its application to clinical examinations is expected to contribute to personalized medicine for diabetes by facilitating the diagnosis of the pathophysiological condition of individual diabetes patients and informing the choice of treatment strategy. -
Tadahiro Kitamura

-
Endocrinology and Metabolism 2024;39(1):33-39.
DOI: https://doi.org/10.3803/EnM.2024.1911
Published online: February 22, 2024
Metabolic Signal Research Center, Institute for Molecular and Cellular Regulation, Gunma University, Maebashi, Japan
- Corresponding author: Tadahiro Kitamura. Metabolic Signal Research Center, Institute for Molecular and Cellular Regulation, Gunma University, 3-39-15 Showa-machi, Maebashi, Gunma 371-8512, Japan Tal: +81-27-220-8845, Fax: +81-27-220-8849, E-mail: kitamura@gunma-u.ac.jp
• Received: December 25, 2023 • Revised: January 15, 2024 • Accepted: January 20, 2024
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,473 Views
- 127 Download
- ABSTRACT
- INTRODUCTION
- PHYSIOLOGICAL FUNCTIONS OF GLUCAGON
- DIFFERENCE BETWEEN THE PHYSIOLOGICAL AND PHARMACOLOGICAL FUNCTIONS OF GLUCAGON
- PROBLEMS IN GLUCAGON MEASUREMENT AND A COMPARISON OF THE ACCURACY OF CONVENTIONAL COMPETITIVE RADIOIMMUNOASSAY AND MERCODIA SANDWICH ELISA
- EVALUATION OF PLASMA GLUCAGON LEVELS IN TYPE 2 DIABETES PATIENTS USING MERCODIA SANDWICH ELISA
- DEVELOPMENT OF A NEW GLUCAGON SANDWICH ELISA WITH HIGHER SPECIFICITY AND SENSITIVITY THAN MERCODIA SANDWICH ELISA
- CONCLUSIONS
- Article information
- References
ABSTRACT
- Glucagon has many functions, including the promotion of hepatic glucose production, fatty acid oxidation, thermogenesis, energy consumption, lipolysis, and myocardial contraction, as well as the suppression of lipogenesis, appetite, and gastrointestinal motility. However, it remains unclear which of these functions are physiological and which are pharmacological. Research on glucagon has lagged behind research on insulin because cross-reactivity with glucagon-related peptides in plasma has hindered the development of an accurate measurement system for glucagon. We recently developed a new glucagon sandwich enzyme-linked immunosorbent assay (ELISA) that is more specific and more sensitive to glucagon than the currently used measurement systems. The new sandwich ELISA is expected to contribute to personalized medicine for diabetes through its use in clinical examinations, the diagnosis of the pathophysiological condition of individual diabetes patients, and the choice of a treatment strategy. Efforts are continuing to develop glucagon/glucagon-like peptide-1 receptor dual agonists to improve obesity and fatty liver by enhancing glucagon’s appetite-suppressing and lipolysis- and thermogenesis-promoting effects. Thus, glucagon is expected to be applied to new diagnostic and therapeutic strategies based on a more accurate understanding of its functions.
- Two years after the discovery of insulin, glucagon was identified as a hormone that elevates blood glucose levels, and 2023 marked the 100th anniversary of its discovery. To date, glucagon has played a relatively minor role in the field of diabetes as an antagonistic hormone to insulin. However, with the clinical application of antidiabetic drugs that have glucagon secretionsuppressing effects in addition to insulin secretion-enhancing effects, such as dipeptidyl peptidase 4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, attention to glucagon has gradually increased. In addition, the importance of glucagon in regulating blood glucose levels was further underscored by the finding that, even when pancreatic β cells are destroyed by streptozotocin, blood glucose levels hardly increase in pancreatic α cell-deficient mice and glucagon receptor-deficient mice. Moreover, glucagon research has not substantially progressed for many years because of the lack of an accurate method for measuring plasma glucagon concentrations. Therefore, no consensus has been reached on glucagon abnormalities in the pathophysiology of diabetes, and plasma glucagon levels have infrequently been measured in diabetes patients. In this article, we discuss the limitations of traditional glucagon measurement techniques and summarize the clinical trials of the Mercodia glucagon sandwich enzyme-linked immunosorbent assay (ELISA), which is currently used in clinical examinations. We also introduce the new, highly accurate sandwich ELISA that we recently developed. We hope that this newly developed sandwich ELISA will contribute to the pathophysiological diagnosis of individual diabetes patients and future personalized medicine for diabetes.
INTRODUCTION
- Glucagon is a peptide hormone consisting of 29 amino acids. It has traditionally been considered an antagonist to insulin, with insulin reducing blood glucose levels and glucagon increasing them. However, glucagon’s physiological functions extend beyond its antagonism to insulin. The liver is the most important target organ of glucagon, as glucagon receptors are predominantly expressed in hepatocytes. Glucagon produces glucose through the promotion of glycogenolysis and gluconeogenesis in the liver and supplies glucose to various organs throughout the body, including the brain. The receptor is also abundantly expressed in the kidney, but the extent to which glucagon is involved in renal gluconeogenesis is unknown. Furthermore, in the liver, glucagon strongly suppresses fat accumulation in hepatocytes by promoting fatty acid oxidation and suppressing fat synthesis. Additionally, glucagon has been found to suppress appetite and gastrointestinal motility, as well as to increase heat production in brown adipose tissue, all of which contribute to a reduction in body weight. It is also commonly thought that glucagon increases myocardial contraction and the heart rate and stimulates lipolysis in white adipose tissue; however, the physiological relevance of these effects is debatable (see below). Glucagon secreted from pancreatic α cells also stimulates GLP-1 receptors in neighboring β cells to promote insulin secretion. These functions of glucagon are summarized in Fig. 1.
PHYSIOLOGICAL FUNCTIONS OF GLUCAGON
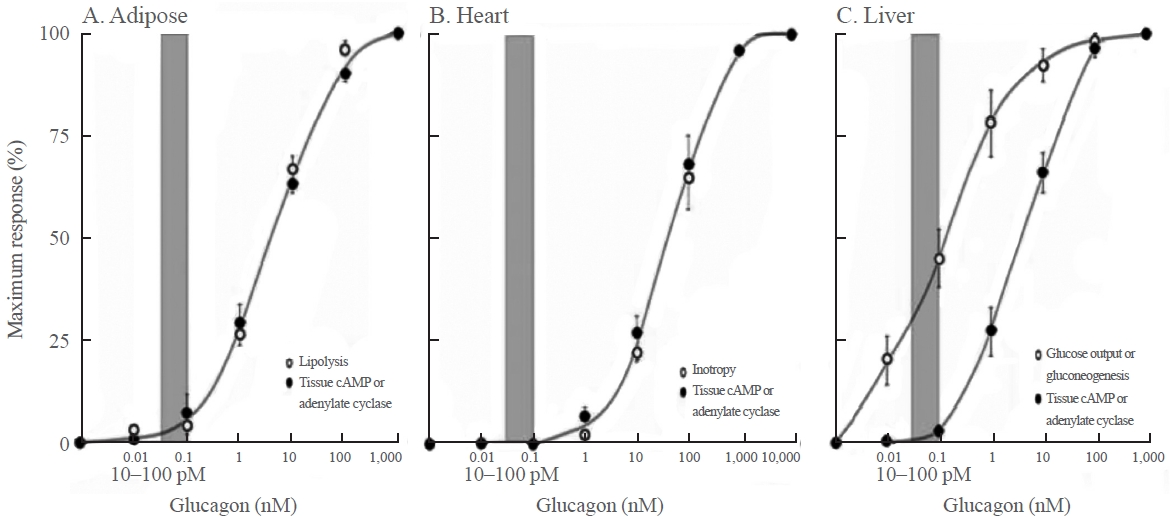
- Recently, glucose-dependent insulinotropic polypeptide (GIP)/ GLP-1 receptor dual agonists have been applied clinically, and the difference between the physiological and pharmacological functions of GIP has been attracting attention. This issue has long been recognized in the context of glucagon. As shown in Fig. 2, in 2012, Rodgers [1] cited a figure from a paper published in 1970. At physiological concentrations, glucagon promoted gluconeogenesis in the liver, but lipolysis in adipose tissue and myocardial contraction were not observed. In addition, a glucagon-induced increase in cyclic adenosine monophosphate (cAMP) did not occur in the physiological range of glucagon, suggesting that the promotion of gluconeogenesis by glucagon in the liver is probably mediated by Ca+2 signals, not by cAMP signaling. Furthermore, these findings indicate that cAMP-mediated lipolysis and myocardial contractility are pharmacological functions of glucagon. In that paper, the authors used the expression “the elephant in the room” to claim that we have been turning a blind eye to these important facts regarding glucagon [1]. As discussed below, when glucagon receptor agonists are applied clinically in the future, it will be necessary to keep in mind the difference between the physiological and pharmacological functions of glucagon.
DIFFERENCE BETWEEN THE PHYSIOLOGICAL AND PHARMACOLOGICAL FUNCTIONS OF GLUCAGON
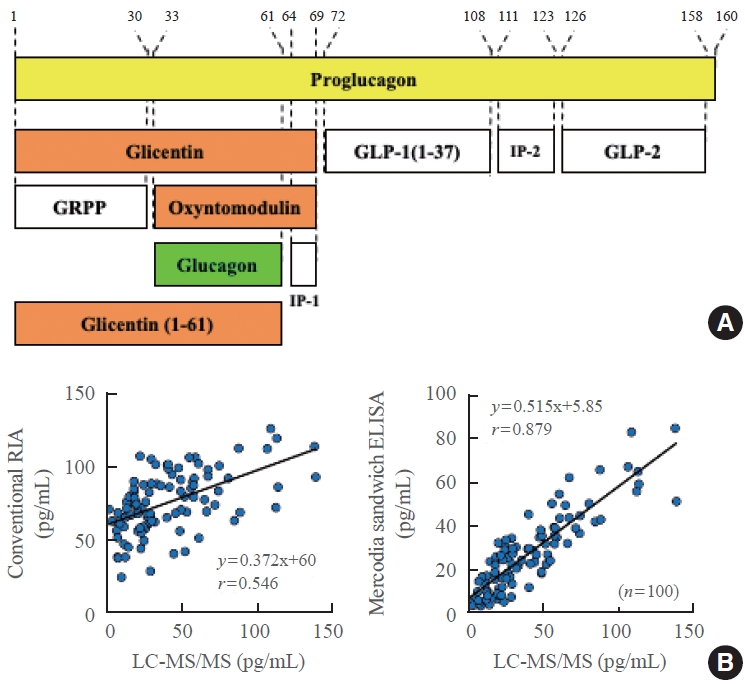
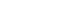
- Glucagon measurement using the competitive radioimmunoassay (RIA), which is a conventional measurement system, was established in the 1960s, around the same time as the established insulin measurement technique. However, a problem with this method is cross-reactivity with other peptides containing a similar structure to that of glucagon. As shown in the upper panel of Fig. 3, proglucagon, which is a precursor of glucagon, undergoes processing mainly in the stomach and intestines, and numerous peptides having the same amino acid sequence as glucagon are secreted. This is in contrast to proinsulin, which produces only two hormones, insulin and C-peptide, without any reciprocal overlap. Therefore, conventional glucagon measurement methods use an antibody that specifically recognizes the C-terminal end of glucagon, as most proglucagon-derived peptides have a C-terminal structure that is distinct from glucagon. However, a recent, more precise analysis revealed the existence of glicentin (1-61), which shares a common C-terminal structure with glucagon, in human plasma; thus, cross-reactivity may occur even with an antibody recognizing the C-terminal end of glucagon. To overcome these problems, a sandwich ELISA using both N-terminal and C-terminal glucagon antibodies was developed. This method can theoretically avoid cross-reactivity with peptides containing the same C-terminal structure as glucagon. Bak et al. [2] and Wewer Albrechtsen et al. [3] reported that Mercodia sandwich ELISA (glucagon ELISA #10-1271-01) is currently the most accurate method for measuring plasma glucagon levels. However, several cross-reactivity tests conducted by various groups, including our laboratory, revealed that Mercodia sandwich ELISA still exhibits cross-reactivity with multiple glucagon-related peptides [4]. The measurement principle of sandwich ELISA is an immunoassay, and it is difficult to completely exclude nonspecific antibody cross-reactivity. To overcome the problems inherent in immunoassays using antibodies, we developed a glucagon measurement system using mass spectrometry (liquid chromatography-mass spectrometry/mass spectrometry [LC-MS/MS]) instead of antibodies as a new method based on a different measurement principle [5]. Because glucagon-related peptides that cause cross-reactivity in immunoassays have different molecular weights from glucagon, they are not detected by this method. However, glucagon measurement using LC-MS/MS cannot be applied to clinical examinations for two critical reasons: it is both time-consuming and expensive. Therefore, we carried out validation tests to adopt the measurement method that provides the closest values to those obtained by LC-MS/MS in clinical examinations (Fig. 3). In contrast to the conventional competitive RIA, which showed a poor correlation with LC-MS/MS, Mercodia sandwich ELISA showed a good correlation with LC-MS/MS, though it did not completely match, indicating that the Mercodia sandwich ELISA is superior to conventional competitive RIA [5].
PROBLEMS IN GLUCAGON MEASUREMENT AND A COMPARISON OF THE ACCURACY OF CONVENTIONAL COMPETITIVE RADIOIMMUNOASSAY AND MERCODIA SANDWICH ELISA
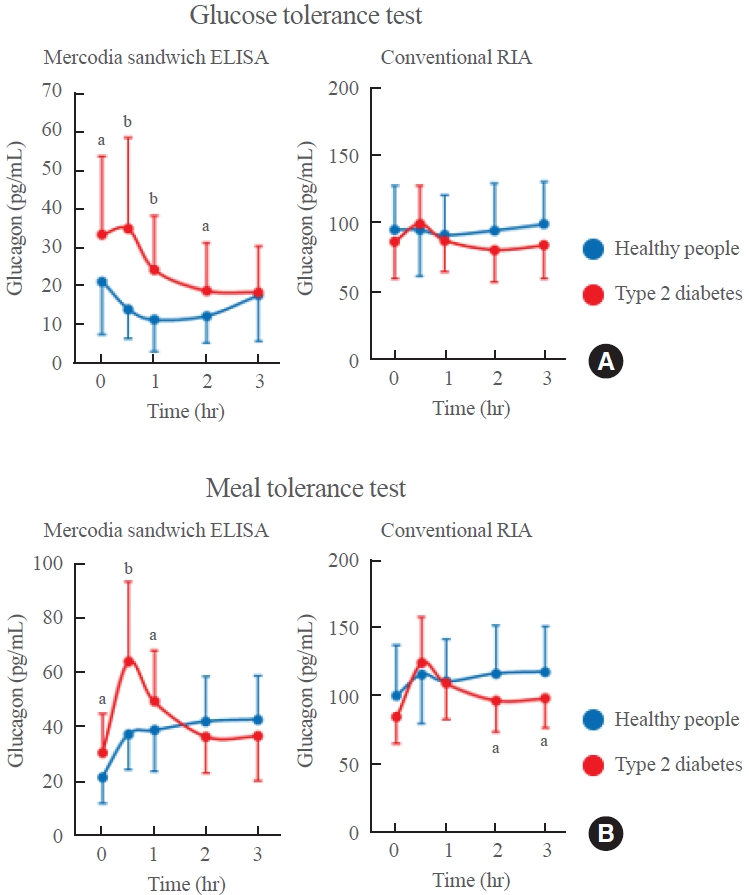
- Although dysregulation of glucagon secretion is known to be associated with the pathophysiology of type 2 diabetes, the mechanism underlying this relationship remains unclear. Furthermore, most previous conclusions are not reliable because they were derived from plasma glucagon levels measured using conventional RIA systems. Therefore, we attempted to evaluate plasma glucagon levels in type 2 diabetes patients using Mercodia sandwich ELISA. Both the glucose tolerance test (Fig. 4A) and meal tolerance test (Fig. 4B) were conducted in the same type 2 diabetes patients and the same healthy people. The type 2 diabetes patients had significantly higher fasting plasma glucagon levels than their healthy counterparts. In addition, plasma glucagon levels decreased in healthy people 30 minutes after glucose load but did not decrease in type 2 diabetes patients and even increased in some patients. Plasma glucagon levels 30 minutes after meal load increased even in healthy people, probably due to the protein content of the meal stimulating glucagon secretion, but they increased significantly more in type 2 diabetes patients [6]. In contrast, when the same plasma sample was measured by the conventional competitive RIA, these important differences were not observed. Furthermore, the difference in plasma glucagon levels before and 30 minutes after meal load measured by the Mercodia sandwich ELISA correlated well with the levels of blood glucose and glucose tolerance in type 2 diabetes patients [6]. The abnormalities in glucagon secretion in type 2 diabetes patients were independent of the abnormalities in either insulin or incretin secretion, suggesting that plasma glucagon values can be used as a new pathophysiological diagnostic marker in type 2 diabetes.
EVALUATION OF PLASMA GLUCAGON LEVELS IN TYPE 2 DIABETES PATIENTS USING MERCODIA SANDWICH ELISA
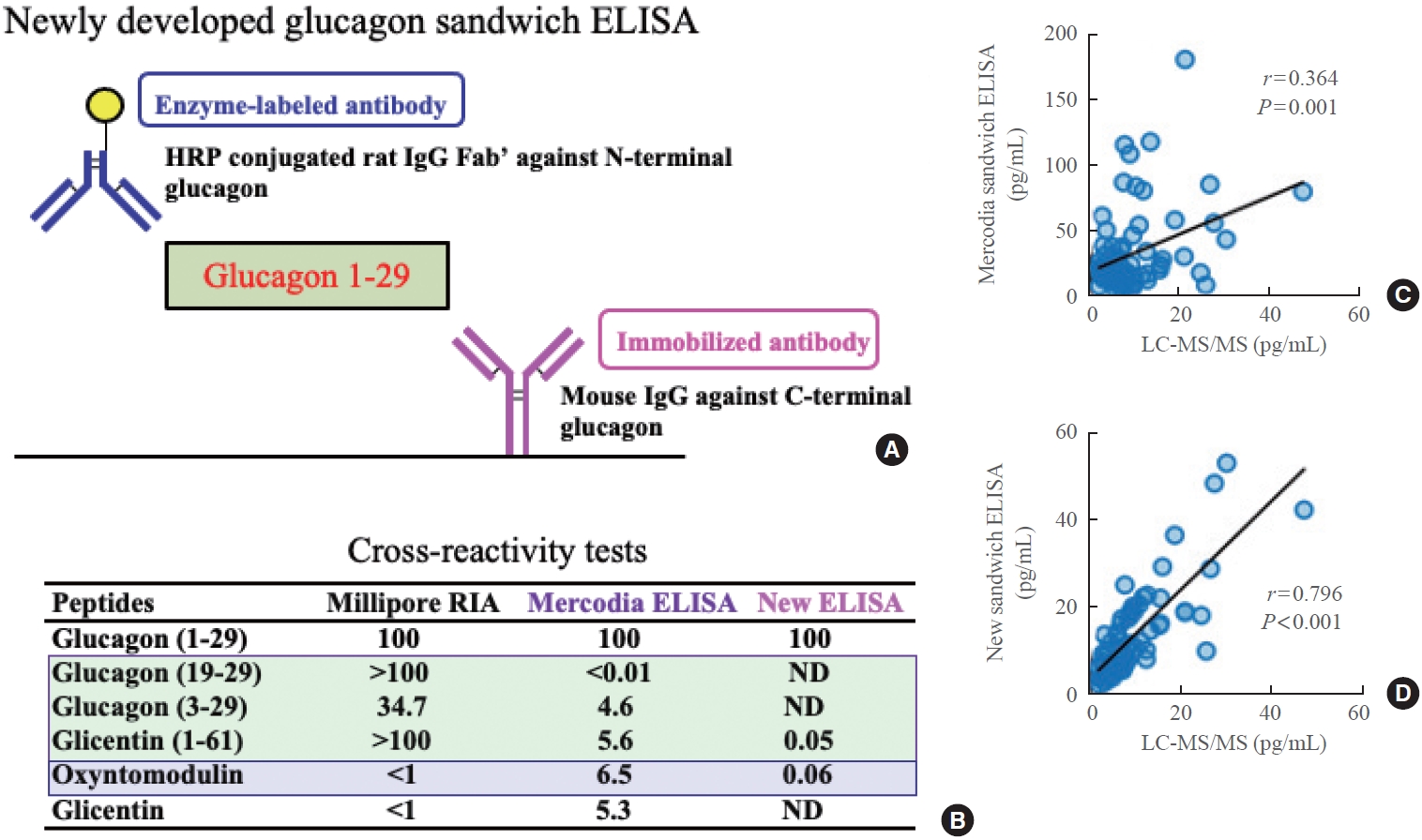
- As discussed above, the Mercodia sandwich ELISA exhibits cross-reactivity with various glucagon-related peptides [4]. While some may consider cross-reactivity within a few percentage points to reflect an acceptable error margin, this issue cannot be overlooked when cross-reacting peptides are present at high concentrations in plasma. We have previously reported that the Mercodia sandwich ELISA yields falsely elevated plasma glucagon levels post-pancreatectomy due to the increased presence of glicentin in patients’ plasma [7]. A similar phenomenon was observed in patients who underwent bariatric surgery (gastric sleeve resection) [8]. Therefore, although Mercodia sandwich ELISA is more accurate than the conventional competitive RIA, it may provide incorrect glucagon values after resection of the pancreas or stomach. Therefore, there was a need to develop a more specific glucagon sandwich ELISA that would exhibit reduced cross-reactivity with other glucagon-related peptides, such as glicentin, compared to the Mercodia assay. For this purpose, we generated highly specific and highly sensitive monoclonal antibodies against the N-terminal or C-terminal end of glucagon and constructed a sandwich ELISA system (Fig. 5A, C). As shown in the bottom panel of Fig. 5, cross-reactivity tests revealed almost no cross-reactivity with glucagon-related peptides, including glicentin, in the new sandwich ELISA, a striking contrast to the Mercodia sandwich ELISA, which showed cross-reactivity (at several percentage points) with other peptides [8]. The newly developed sandwich ELISA also showed a close correlation with LC-MS/MS (Fig. 5C, D), even after bariatric surgery, whereas the Mercodia sandwich ELISA showed a poor correlation with LC-MS/MS measurements [8].
DEVELOPMENT OF A NEW GLUCAGON SANDWICH ELISA WITH HIGHER SPECIFICITY AND SENSITIVITY THAN MERCODIA SANDWICH ELISA
- Accurate plasma glucagon values measured by the new sandwich ELISA could become a new marker for pathophysiological diagnosis of diabetes, in addition to the conventional markers of blood glucose and plasma insulin. In the future, it will be important to clarify the relationship between impaired glucagon secretion and the clinical background or the pathological factors in diabetes patients.
- Among diabetes drugs, DPP-4 inhibitors and GLP-1 receptor agonists suppress glucagon secretion, biguanides (metformin) suppress glucagon action in the liver, and sodium glucose cotransporter 2 inhibitors promote glucagon secretion [9]. The development of glucagon receptor antagonists that improve blood glucose levels by inhibiting glucagon’s gluconeogenesis-promoting effects has been discontinued due to several side effects, including liver dysfunction and deterioration of lipid markers. However, there are ongoing efforts to develop glucagon receptor agonists that aim to improve obesity and fatty liver by enhancing glucagon’s appetite-suppressing and lipolysis- and thermogenesis-promoting effects [10-13]. Notably, in the latter case, a dual agonist with the GLP-1 receptor is being considered in order to offset the increase in blood glucose caused by glucagon receptor agonism. Applying these glucagon-targeting drugs in the treatment of diabetes, obesity, and fatty liver will open up more opportunities to evaluate plasma glucagon levels in patients with these diseases, thereby increasing the importance of accurate glucagon measurement using the new sandwich ELISA. We expect to be able to understand the pathophysiological condition of individual diabetes patients from the viewpoint of both glucagon and insulin, which could contribute to future personalized medicine for diabetes.
CONCLUSIONS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
-
Acknowledgements
- I thank all the members of the Metabolic Signal Research Center at the Institute for Molecular and Cellular Regulation, Gunma University, for their sincere and enthusiastic attitude toward glucagon research.
Fig. 2.The concentration-dependent effects of glucagon on lipolysis, myocardial contraction, hepatic gluconeogenesis, and the cyclic adenosine monophosphate (cAMP) concentration in each tissue. The gray bar in each graph indicates the physiological range of the plasma glucagon concentration. Modified from Rodgers [1], with permission from Bentham Science Publishers.


Fig. 3.(A) Various glucagon-related peptides produced from proglucagon. (B) Comparison of the plasma glucagon values obtained by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) to those obtained by conventional competitive radioimmunoassay (RIA) or Mercodia sandwich enzyme-linked immunosorbent assay (ELISA). Modified from Miyachi et al. [5], with permission from Springer Nature. GLP, glucagon-like peptide; IP, intervening peptide; GRPP, glicentin-related pancreatic polypeptide.
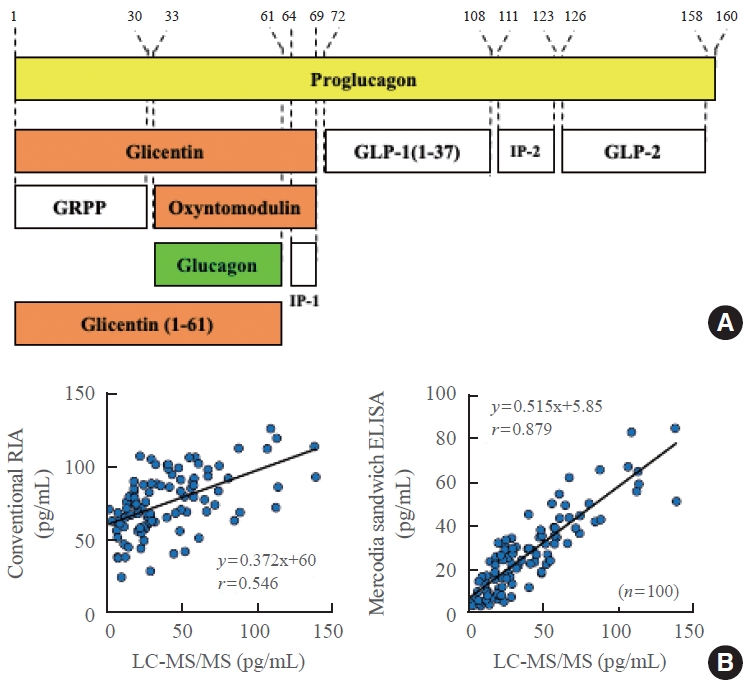

Fig. 4.Plasma glucagon levels measured by the Mercodia sandwich enzyme-linked immunosorbent assay (ELISA; left) or conventional competitive radioimmunoassay (RIA; right) during the (A) glucose tolerance test or (B) meal tolerance test in type 2 diabetes patients (red) and healthy people (blue). Modified from Kobayashi et al. [6]. aP<0.05; bP<0.001.
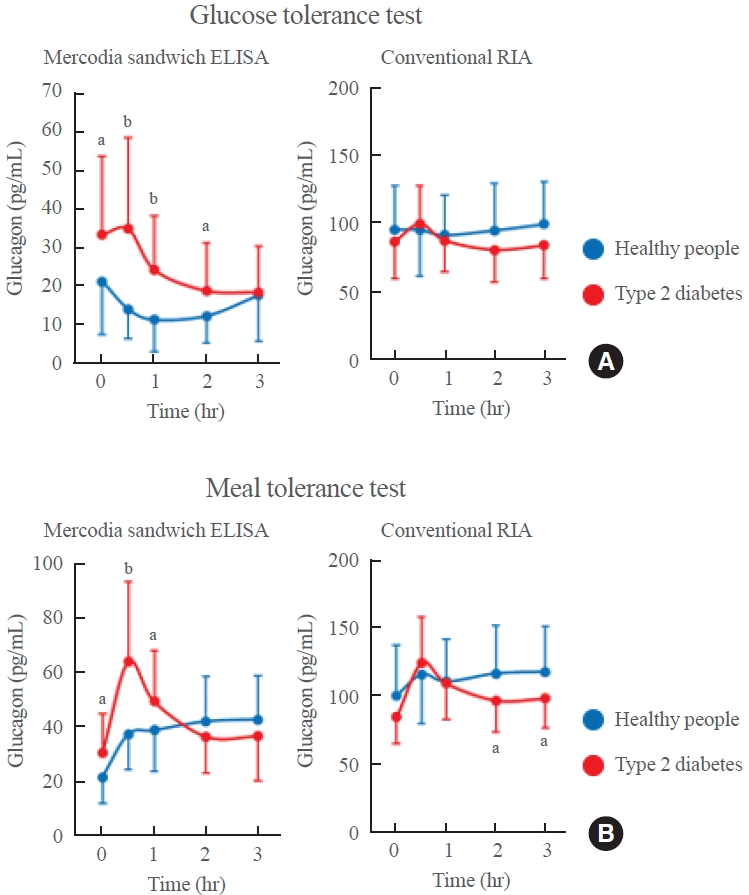

Fig. 5.Schematic diagram showing (A) the newly developed glucagon sandwich enzyme-linked immunosorbent assay (ELISA) and (B) the results of cross-reactivity tests in the Mercodia sandwich ELISA and new sandwich ELISA. Plasma glucagon values after bariatric surgery measured by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) and compared to the values obtained by (C) the Mercodia sandwich ELISA or (D) the new sandwich ELISA. Modified from Kobayashi et al. [8]. HRP, horseradish peroxidase; IgG, immunoglobulin G; RIA, radioimmunoassay; ND, not detected.


- 1. Rodgers RL. Glucagon and cyclic AMP: time to turn the page? Curr Diabetes Rev 2012;8:362–81.ArticlePubMed
- 2. Bak MJ, Albrechtsen NW, Pedersen J, Hartmann B, Christensen M, Vilsboll T, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol 2014;170:529–38.ArticlePubMed
- 3. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, Windelov JA, Plamboeck A, Bojsen-Moller KN, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia 2014;57:1919–26.ArticlePubMedPDF
- 4. Matsuo T, Miyagawa J, Kusunoki Y, Miuchi M, Ikawa T, Akagami T, et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzymelinked immunosorbent assay. J Diabetes Investig 2016;7:324–31.ArticlePubMedPMCPDF
- 5. Miyachi A, Kobayashi M, Mieno E, Goto M, Furusawa K, Inagaki T, et al. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem 2017;409:5911–8.ArticlePubMedPDF
- 6. Kobayashi M, Satoh H, Matsuo T, Kusunoki Y, Tokushima M, Watada H, et al. Plasma glucagon levels measured by sandwich ELISA are correlated with impaired glucose tolerance in type 2 diabetes. Endocr J 2020;67:903–22.ArticlePubMed
- 7. Kobayashi M, Waki H, Nakayama H, Miyachi A, Mieno E, Hamajima H, et al. Pseudo-hyperglucagonemia was observed in pancreatectomized patients when measured by glucagon sandwich enzyme-linked immunosorbent assay. J Diabetes Investig 2021;12:286–9.ArticlePubMedPMCPDF
- 8. Kobayashi M, Maruyama N, Yamamoto Y, Togawa T, Ida T, Yoshida M, et al. A newly developed glucagon sandwich ELISA is useful for more accurate glucagon evaluation than the currently used sandwich ELISA in subjects with elevated plasma proglucagon-derived peptide levels. J Diabetes Investig 2023;14:648–58.PubMedPMC
- 9. Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, et al. SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels. Mol Metab 2019;19:1–12.ArticlePubMedPMC
- 10. Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 2009;5:749–57.ArticlePubMedPDF
- 11. Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 2018;391:2607–18.ArticlePubMed
- 12. Alba M, Yee J, Frustaci ME, Samtani MN, Fleck P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor coagonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose-ranging study. Clin Obes 2021;11:e12432.PubMed
- 13. Romero-Gomez M, Lawitz E, Shankar RR, Chaudhri E, Liu J, Lam RLH, et al. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J Hepatol 2023;79:888–97.PubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 


 KES
KES

 PubReader
PubReader ePub Link
ePub Link Cite
Cite