Articles
- Page Path
- HOME > Endocrinol Metab > Volume 39(1); 2024 > Article
-
Review ArticleThyroid A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
 Keypoint
Keypoint
The "2023 Korean Thyroid Association (KTA) Management Guideline for Thyroid Nodules" is an update of the "2016 KTA Management Guidelines for Thyroid Nodules and Cancers," in which the nodule portion is separated. This review provides a summary of the 2023 KTA guideline for the management of thyroid nodules released in May 2023 and gives balanced insight through a comparison with recent guidelines from other societies. -
Eun Kyung Lee1
 , Young Joo Park2
, Young Joo Park2 , Chan Kwon Jung3, Dong Gyu Na4
, Chan Kwon Jung3, Dong Gyu Na4 -
Endocrinology and Metabolism 2024;39(1):61-72.
DOI: https://doi.org/10.3803/EnM.2024.1938
Published online: February 14, 2024
1Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, Goyang, Korea
2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
3Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
4Department of Radiology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- Corresponding author: Young Joo Park. Department of Internal Medicine, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-4183, Fax: +82-2-764-2199, E-mail: yjparkmd@snu.ac.kr
- This guideline has been originally written in Korean and published in the International Journal of Thyroidology 2023;16:1-31.
Copyright © 2024 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,512 Views
- 98 Download
- 1 Crossref
- ABSTRACT
- INTRODUCTION
- DEVELOPMENT PROCESS OF THE 2023 GUIDELINE
- SECTION 1. DEFINITION OF HIGH-RISK GROUPS FOR THYROID CANCER SCREENING
- SECTION 2. DIAGNOSTIC EVALUATION OF INCIDENTAL OR CLINICALLY DETECTED THYROID NODULES
- SECTION 3. THE PATHOLOGICAL DIAGNOSIS OF THYROID NODULES
- SECTION 4. LONG-TERM FOLLOW-UP OF THYROID NODULES
- SECTION 5. TREATMENT OF THYROID NODULES
- SECTION 6. THYROID NODULES IN PREGNANT WOMEN
- CONCLUSIONS
- Article information
- References
ABSTRACT
- The 2023 Korean Thyroid Association (KTA) Management Guideline for Patients with Thyroid Nodules constitute an update of the 2016 KTA guideline for thyroid nodules and cancers that focuses specifically on nodules. The 2023 guideline aim to offer updated guidance based on new evidence that reflects the changes in clinical practice since the 2016 KTA guideline. To update the 2023 guideline, a comprehensive literature search was conducted from January 2022 to May 2022. The literature search included studies, reviews, and other evidence involving human subjects that were published in English in MEDLINE (PubMed), Embase, and other relevant databases. Additional significant clinical trials and research studies published up to April 2023 were also reviewed. The limitations of the current evidence are discussed, and suggestions for areas in need of further research are identified. The purpose of this review is to provide a summary of the 2023 KTA guideline for the management of thyroid nodules released in May 2023 and to give a balanced insight with comparison of recent guidelines from other societies.
- Thyroid nodules are a common endocrine disorder, typically with a favorable prognosis. The diagnostic approach of ultrasonography (US) followed by fine-needle aspiration (FNA) is well-established as the gold standard [1]. The incorporation of molecular pathology tests based on high-throughput sequencing techniques has improved the accuracy of preoperative diagnosis. Furthermore, a novel clinical strategy termed active surveillance (AS) involves postponing treatment from the time of diagnosis until disease progression.
- These changes have led to a paradigm shift in the diagnosis and management of thyroid nodules, marked by the emergence of more surgical and/or non-surgical options for thyroid cancer and new drugs for advanced thyroid cancer cases. In response, the members of the Guideline Development Committee of the Korean Thyroid Association (KTA) agreed on the need to update the guideline and decided to develop separate guideline for thyroid nodules instead of a single guideline covering both thyroid nodules and cancers.
- The 2023 guideline updated the research base for each topic covered by the 2016 guideline and added new topics, including the role of pathological and molecular marker tests in diagnosis, the noninvasive treatment of benign thyroid nodules, and special considerations for pregnant women. In this update, the major changes are (1) the definition of high-risk groups for thyroid cancer screening; (2) the application of the revised Korean Thyroid Imaging Reporting and Data System (K-TIRADS) and 2023 World Health Organization classification of thyroid neoplasms; (3) the addition of the role of core needle biopsy (CNB) and molecular marker tests; (4) the application of AS in low-risk papillary thyroid microcarcinoma (PTMC); and (5) updated indications for the non-surgical treatment of benign thyroid nodules. This review provides an overview of the 2023 KTA Management Guideline for Patients with Thyroid Nodules, with an emphasis on updates.
INTRODUCTION
- A comprehensive literature search was conducted between January 2022 and May 2022, which included studies, reviews, and other forms of evidence related to human subjects and published in English. The databases searched were MEDLINE (PubMed), Embase, and other pertinent sources. Additionally, relevant clinical trials and research studies published up to April 2023 were considered. The manuscript and its specific recommendations were crafted by integrating the best available research evidence with the panelists’ knowledge and clinical experience. This process was grounded in a systematic review, assessment of related evidence, consideration of principles of care, and reasoned inferences.
- The recommendations were graded using a modified version of the American College of Physicians guideline methodology [2], which has also been adopted in previous KTA guidelines, to facilitate comparison with the previous version and to maintain consistency among the guidelines (Table 1) [3]. The customary KTA classifications of levels 1, 2, and 3 are used in Table 2 to summarize both the evidence and expert opinion and to provide final recommendations for patient assessment and therapy. Given the inherent challenges in generating evidence for certain aspects of thyroid nodule or cancer management, some level 1 recommendations are based on the judgment that there is a significant net benefit from the recommended behavior, even when the evidence is low quality or scarce [4].
- The draft of the manuscript was revised after receiving comments from KTA members at a public hearing, and the revised draft was sent to the Advisory Board for additional feedback and then posted on the KTA website for 4 weeks for critical evaluation by the KTA members. Six related societies have endorsed the clinical practice guideline: the Korean Endocrine Society, the Korean Association of Endocrine Surgeons, the Korean Society of Head and Neck Surgery, the Korean Society of Thyroid Radiology, the Korean Society of Nuclear Medicine, and the Endocrine Pathology Study Group of the Korean Society of Pathologists. All proposed changes and comments were considered by the guideline task force, and the resulting changes were incorporated into the final document, which was published in the International Journal of Thyroidology in May 2023, after approval by the KTA Board of Directors [5]. The recommendations for the management of thyroid nodules are summarized in Table 2.
DEVELOPMENT PROCESS OF THE 2023 GUIDELINE
- When considering candidates for thyroid cancer screening within the context of hereditary syndromes, it is important to consider factors such as the benefit of early detection. Conditions such as multiple endocrine neoplasia syndrome and hereditary medullary thyroid cancer (MTC) syndrome are examples where oncologic outcomes may be improved through screening [6,7]. While individuals with Graves’ disease [8,9], Hashimoto’s thyroiditis [10], and primary hyperparathyroidism [11-13] have a relatively similar or even higher risk of thyroid cancer compared to individuals without those diseases, their prognosis may not significantly differ. Familial non-MTC, defined as three or more affected thyroid cancer patients in a family, has not demonstrated a poorer prognosis compared to a control group [14-16]. Consequently, routine thyroid cancer screening is not indicated for this particular group in the 2023 guideline.
- However, specific populations, such as childhood cancer survivors, require careful consideration for thyroid cancer screening. For these individuals, screening should begin after the successful treatment of the primary cancer. It is recommended that they undergo annual or biannual physical examinations, accompanied by thyroid US every 5 years [7,17,18].
- Staying up to date with the latest medical guidelines is crucial, and consulting with a multidisciplinary team of healthcare professionals—including endocrinologists, surgeons, radiologists, nuclear medicine physicians, and pathologists—will help determine the most appropriate and evidence-based screening strategies for individuals with hereditary syndromes and related conditions.
SECTION 1. DEFINITION OF HIGH-RISK GROUPS FOR THYROID CANCER SCREENING
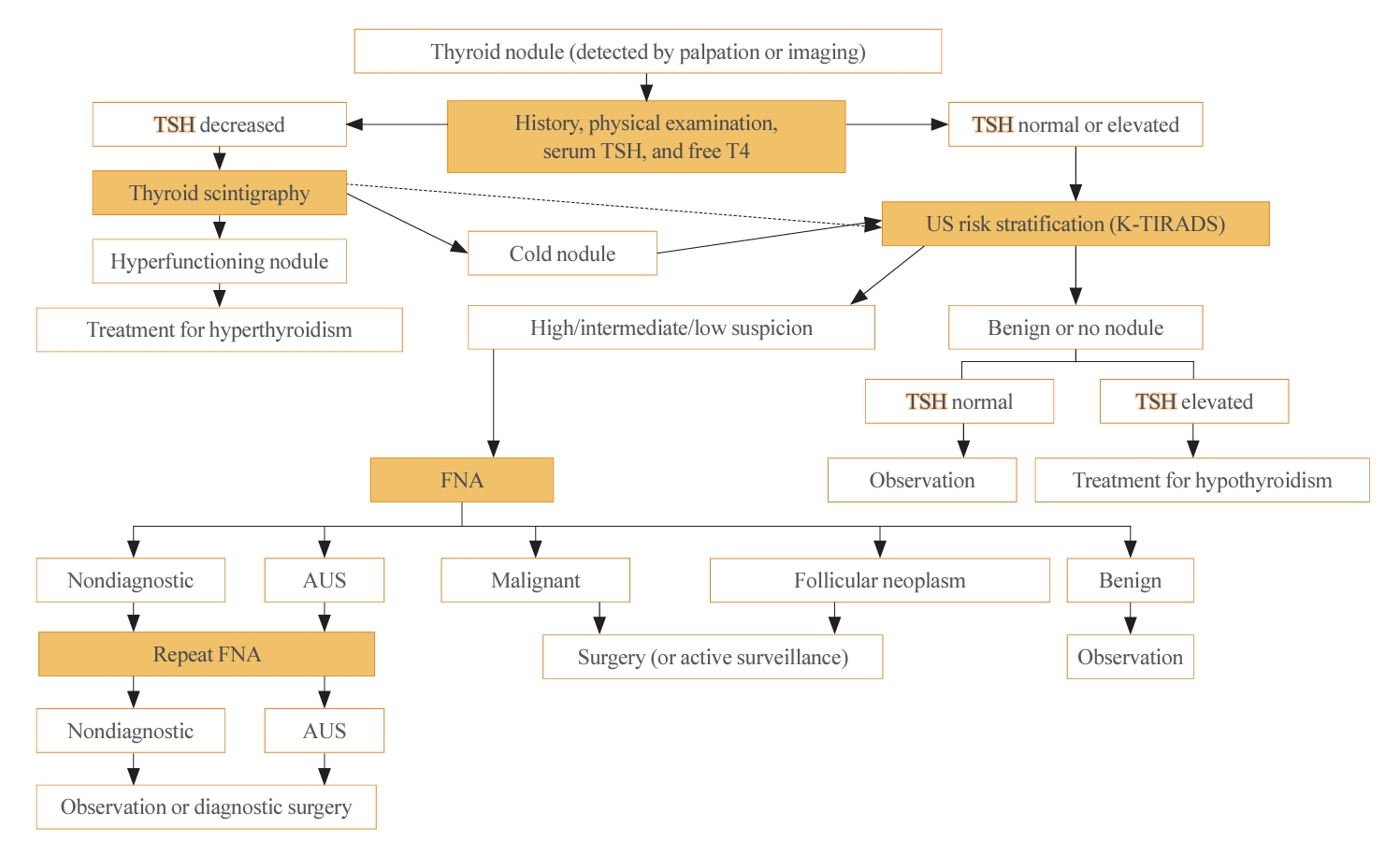
- The initial evaluation of thyroid nodules includes thyroid function test (TFT) (thyroid stimulating hormone [TSH] and free thyroxine). Routine measurements of serum thyroglobulin or calcitonin are not recommended because of their low specificity [7,19]. However, calcitonin testing is recommended in certain situations—specifically, for thyroid nodules suspicious for MTC, patients with a family history of MTC, and before surgery [7]. The algorithm for the diagnostic evaluation of thyroid nodules is presented in Fig. 1. For incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography, a pathological evaluation should be considered, guided by a US-based risk stratification [20].
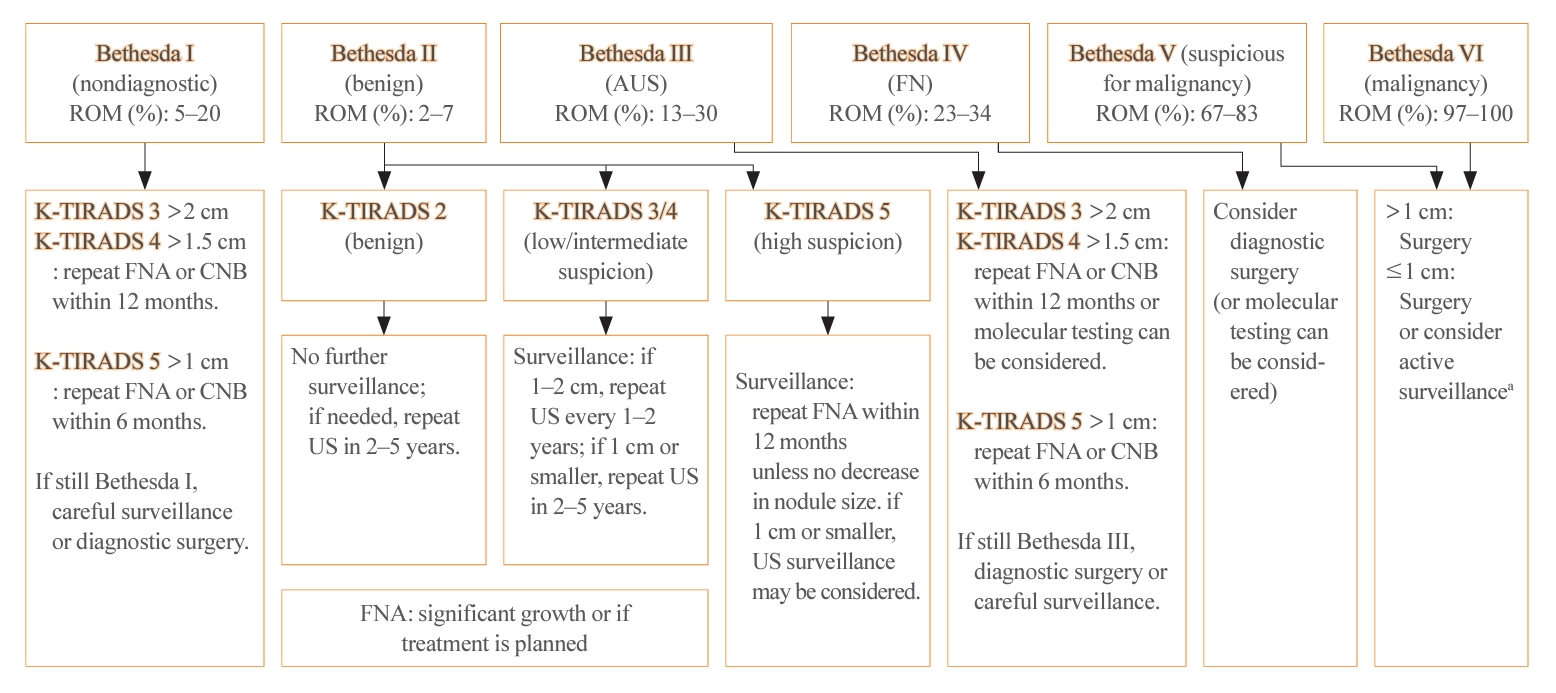
- Thyroid US is a noninvasive imaging test that is both safe and highly sensitive and accurate. Some groups have proposed USbased risk stratification, and the KTA has adopted the K-TIRADS. The 2021 K-TIRADS has been incorporated into the 2023 guideline [1]. K-TIRADS classifies US findings into five categories (high, intermediate, low suspicion, benign, and no nodule) (Table 3). Compared to the 2016 guideline, the 2023 guideline suggest a larger cut-off size for recommending a pathological diagnosis of thyroid nodules and extend the intervals between surveillance to minimize unnecessary procedures or operations (Fig. 2).
SECTION 2. DIAGNOSTIC EVALUATION OF INCIDENTAL OR CLINICALLY DETECTED THYROID NODULES
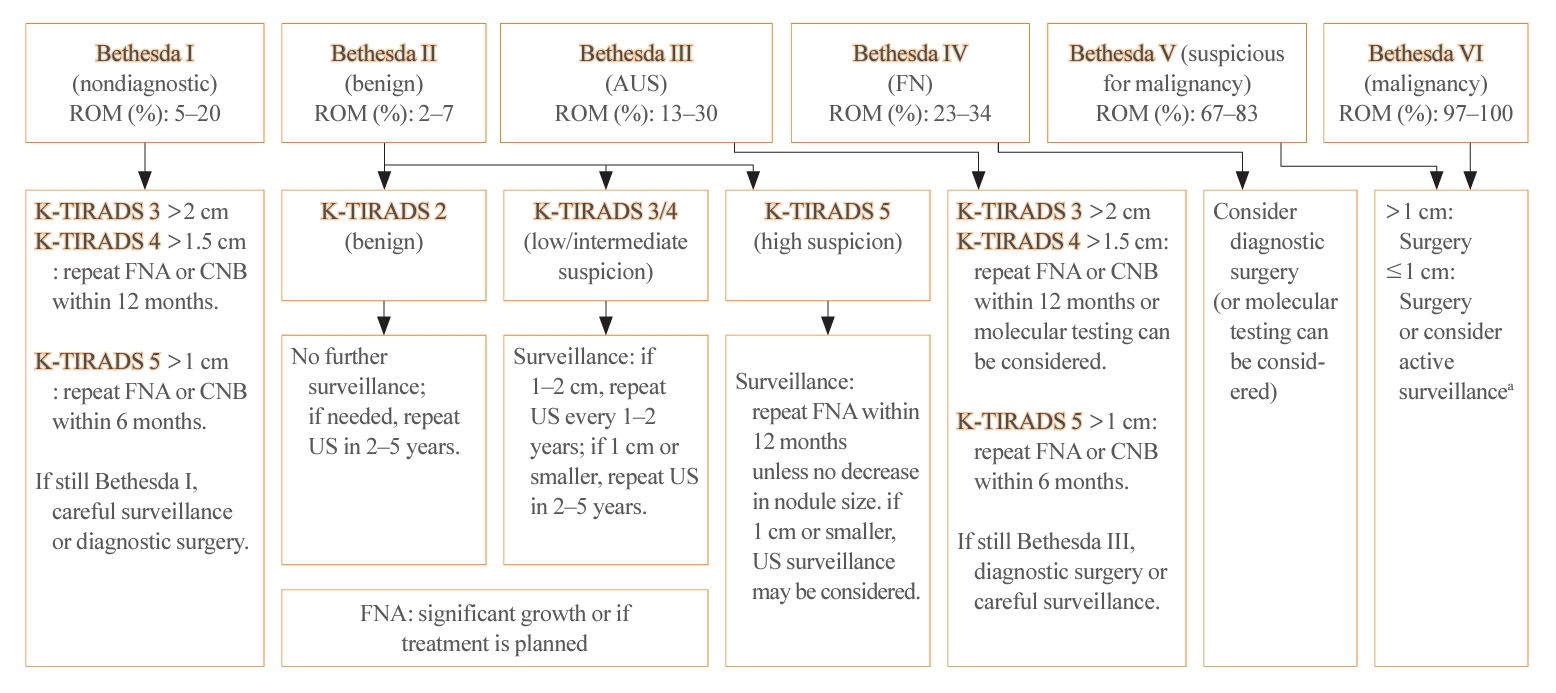
- The management of thyroid nodules should be based on US findings and a pathologic diagnosis. The combined use of US-based risk stratification and the Bethesda system after FNA may allow the early detection of thyroid cancer and assist in making optimal management decisions (Table 4) [1].
- The 2023 guideline has adopted the recently updated third edition of The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) [5]. The third TBSRTC, which was released in 2023, has the goal of enhancing diagnostic clarity by discontinuing the use of several terms. In the previous edition, diagnostic categories I, III, and IV had interchangeable terms, which sometimes caused confusion [21]. The 3rd TBSRTC has streamlined the terminology by adopting a single, distinct name for each category to eliminate ambiguity. Category I is now called “nondiagnostic,” emphasizing the lack of diagnostic information due to inadequacy in the specimen’s cellular or colloid content. Category III is now solely referred to as “atypia of undetermined significance (AUS),” providing a clearer definition of the category’s nature. Similarly, category IV now exclusively uses “follicular neoplasm” to clarify the diagnostic category. An important development in the 3rd TBSRTC is the introduction of a two-level classification system within AUS: “nuclear” (previously “cytologic”) and “other.” This distinction is critical because AUS cases with nuclear atypia have a significantly higher risk of malignancy than those with other patterns, such as architectural or oncocytic atypia. In addition, the TBSRTC has been effectively adopted for use in pediatric thyroid cytopathology. Reflecting recent research, the risk of malignancy values for the six diagnostic categories have been specifically calculated for pediatric cases (Table 4).
- FNA is the main procedure used to determine whether a nodule requires excision by categorizing nodules according to their risk of malignancy, with minimal complications [22]. However, 2% to 3% of nodules yield nondiagnostic results (Bethesda I), and 7% to 14% are classified as indeterminate nodules (Bethesda III) [21]. In such cases, the necessity of a 3-month waiting period is questionable. A shorter waiting period might be justified, particularly when clinical or imaging findings are suggestive of malignancy.
- In nodules with nondiagnostic FNA results, repeating the FNA leads to nondiagnostic outcomes in 28.1% to 40.0% of cases. In contrast, CNB results in nondiagnostic findings in only 1.1% to 3.8% of cases. This suggests that CNB may be more effective in reducing the rate of nondiagnostic results compared to FNA. The pathologic classification of CNB, along with its advantages and potential risks, was introduced in the 2023 guideline. The pathologic diagnosis from CNB is categorized into six groups, similarly to the classification system used for FNA (Table 5). CNB is particularly valuable as it may yield more conclusive diagnostic outcomes for AUS nodules than subsequent FNA [23,24].
- CNB is recommended as a second-line procedure only if FNA yields nondiagnostic results after at least one attempt, according to the European Thyroid Association (ETA), or two attempts, as per the French Society of Endocrinology (SFE). This cautious approach is due to potential complications such as bleeding from CNB and the lack of established diagnostic pathology criteria in various countries. However, the risk of bleeding from CNB is only 2.5% to 4.9% [25,26]. Additionally, CNB offers the benefit of facilitating histological evaluation for a preoperative diagnosis using immunohistochemical (IHC) markers. IHC staining proves particularly useful in cases where poorly differentiated, anaplastic, or medullary thyroid carcinoma, differentiated high-grade thyroid carcinoma, or lymphoma are suspected. In Korea, where the application of molecular testing is limited by high costs, CNB has been included in the 2023 guideline. It is valued for its ability to provide critical information that aids in distinguishing malignant from benign thyroid nodules.
- The role of molecular testing is similar to that described in the previous 2016 guideline [3]. The high specificity of BRAF mutation for papillary thyroid carcinoma (PTC) has been updated in detail based on data from Asia, where the BRAF mutation is prevalent. The prognostic significance of molecular markers is substantial when a TERT promoter mutation co-occurs with other driver mutations (RAS, BRAF, etc.). A next-generation sequencing multi-gene panel is newly recommended in the 2023 guideline as an option with higher diagnostic accuracy and different malignancy risks depending on the region and population. Therefore, the 2023 guideline advised that molecular marker testing should be considered based on clinical risk factors, the risk of malignancy based on cytology and US findings, the feasibility of the procedures, and patient preference.
SECTION 3. THE PATHOLOGICAL DIAGNOSIS OF THYROID NODULES
- Follow-up surveillance is necessary even if a nodule is determined to be benign, because pathology tests for nodules have a false-negative rate of about 5%. Thyroid US should be performed to determine clinically meaningful size changes. Significant nodule growth is defined as a diameter increase of 20% or more that is greater than 2 mm in at least two dimensions, or a volume increase of 50% or more.
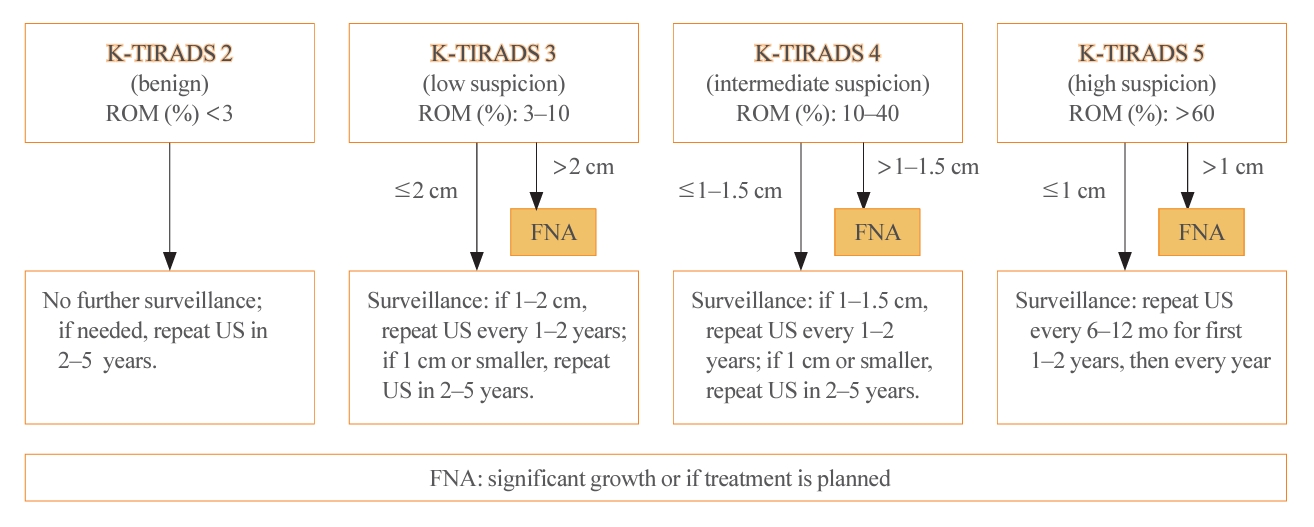
- The 2023 guideline described the follow-up strategies for two groups of thyroid nodules: pathologically confirmed benign nodules and those for which FNA is not indicated (Fig. 3). For biopsy-proven benign nodules, those identified as high suspicion on US should be followed with US surveillance within 12 months, and repeated FNA should be performed if they do not shrink. In the 2022 French consensus, US-suspicious thyroid nodules (European Thyroid Imaging Reporting and Data System [EU-TIRADS] 5) with a benign FNA result (Bethesda II) should be monitored every 1–2 years for 5 years after detection, and then monitoring should be discontinued if the nodules are stable [7]. The ETA recommends repeating FNA for US-suspicious thyroid nodules with benign FNA results if the nodule size is >10 mm [19].
- Regarding low to intermediate suspicion nodules on US, the 2023 KTA guideline recommend surveillance after 12 to 24 months and repeated FNA if significant nodule growth is observed [5]. The SFE recommends conducting surveillance after 1 to 2 years and repeating FNA only if the nodule characteristics warrant it [7]. The ETA recommends re-evaluating nodules (>20 mm for EU-TIRADS 3 or >15 mm for EU-TIRADS 4) in 3 to 5 years [19].
- For nodules that are not recommended for biopsy, the surveillance intervals have been extended compared to the 2016 guideline. The follow-up period for high suspicion nodules has increased from 6–12 to 12 months, and for low suspicion nodules, the recommendation has shifted from 1–2 years to no further surveillance. The ETA has based these surveillance intervals on the EU-TIRADS classification and the size of the nodules. Specifically, for EU-TIRADS 2–3 nodules that are less than 1 cm, no further evaluation is advised, whereas nodules larger than 1 cm should be reevaluated. For EU-TIRADS 4 nodules, the ETA suggests reevaluation after 1 year, and for EU-TIRADS 5 nodules, a follow-up is recommended within 6 to 12 months.
- The 2023 guideline now included AS as an alternative strategy for low-risk PTMC. If the pathological diagnosis is malignant, surgical treatment is usually indicated. However, AS may be considered in the following cases.
- (1) Very low-risk tumors (e.g., PTMC that is clinically free of metastases and local invasion and has no cytologic evidence of aggressive disease).
- (2) Patients at high-risk for surgery due to other comorbidities.
- (3) Patients expected to have a short life expectancy (e.g., those with severe cardiovascular disease, other malignancies, and advanced age).
- (4) Patients with concomitant medical or surgical comorbidities that need to be treated prior to thyroid surgery.
SECTION 4. LONG-TERM FOLLOW-UP OF THYROID NODULES
- In general, benign thyroid nodules do not require treatment. However, treatment may be considered if there are pressure symptoms, cosmetic concerns, or if the nodule exhibits autonomous functioning. When pressure symptoms or cosmetic concerns are present, imaging studies should be conducted to confirm that these symptoms are associated with a thyroid nodule, thereby distinguishing them from nonspecific symptoms.
- Benign thyroid nodules eligible for non-surgical treatment include: (1) those with two or more benign FNA or CNB results; (2) nodules with low suspicion (K-TIRADS 2) and autonomously functioning thyroid nodules that are expected to yield a benign FNA result; and (3) pure cysts classified as nondiagnostic by FNA. The 2022 SFE Consensus further recommends performing two FNAs for nodules classified as EU-TIRADS 3 and 4, and a single FNA for EU-TIRADS 2 nodules. However, the ETA does not specify non-surgical treatment as an indication.
- The treatment options for benign thyroid nodules include non-surgical ablation (ethanol, radiofrequency, laser ablation), radioactive iodine treatment, and surgery. The choice of treatment is determined by clinical characteristics, comorbidities, the advantages and disadvantages of each treatment modality, patient preference, and feasibility. Ethanol injection is typically the first-line treatment for cystic nodules that recur following aspiration, while thermal ablation, using either radiofrequency or laser, is preferred for solid nodules.
- CNB is recommended as a mandatory pathological test before non-surgical ablation, because CNB has a high diagnostic sensitivity for follicular neoplasms. Patients with two FNA results or a single CNB result indicating follicular neoplasm or malignancy, as well as those with high suspicion nodules (K-TIRADS 5) based on US are not candidates for non-surgical treatment. If a nodule increases in size or becomes symptomatic at follow-up after non-surgical treatment, a repeated pathological evaluation of the nodule is warranted.
SECTION 5. TREATMENT OF THYROID NODULES
- The size of the thyroid gland and nodules may increase during pregnancy due to the thyrotrophic effects of human chorionic gonadotropin and TSH stimulation, which occurs as a result of iodine store depletion, particularly in regions with low iodine intake [27,28]. However, pregnancy does not affect maternal or neonatal outcomes or cancer-specific survival [27]. Therefore, the approach to evaluating thyroid nodules in pregnant women is the same as in nonpregnant women. The clinical importance of thyroid nodules in pregnancy is primarily associated with two scenarios: toxic adenoma that causes hyperthyroidism, and thyroid cancer that carries a high-risk of recurrence and mortality [29].
- Evaluation of thyroid nodules during pregnancy involves TFT and US imaging. If the serum TSH level remains low without an associated rise in anti-TSH receptor antibodies beyond the first trimester, a functional thyroid nodule may be suspected. In such cases, a definitive pathological diagnosis can be postponed until after delivery.
- Based on the US findings (K-TIRADS), FNA could be performed starting in the second trimester, depending on the patient’s preference. However, there was no difference in recurrence and survival rates of thyroid cancer when surgery was performed during pregnancy compared to after delivery [30]. Another retrospective study indicated that delaying treatment for up to 1 year after a thyroid cancer diagnosis did not negatively impact patient outcomes [31]. In a Korean study, 19 pregnant women diagnosed with PTC just before or during pregnancy were monitored without surgery; the mean tumor size at diagnosis was 0.91 cm (interquartile range, 0.61 to 1.11), and the mean tumor size at 9.5 months post-diagnosis was 0.98 cm (interquartile range, 0.72 to 1.12). The change in tumor size by trimester was not significant, and there were no occurrences of new thyroid cancers or lymph node metastases during the follow-up period [32]. Therefore, the 2023 guideline suggests that surgery may be deferred until after delivery if there is no change in size by the second trimester or if the thyroid cancer is first diagnosed in the second trimester.
SECTION 6. THYROID NODULES IN PREGNANT WOMEN
- Although thyroid nodules have a high prevalence, most are benign and have favorable outcomes. Nevertheless, the approach to diagnosing and managing these nodules can be significantly affected by socioeconomic factors, including healthcare costs. The 2023 guideline synthesizes expert consensus to ensure that individuals at high-risk are appropriately screened and that those requiring diagnosis receive timely and appropriate treatment. It also offers comprehensive guidance on the diagnosis and classification of thyroid nodules and will be updated to include specific action plans covering the continuum from diagnosis to follow-up, based on a review of the evidence. Furthermore, we anticipate that well-designed prospective studies will be conducted to address several key questions: (1) which patients should undergo screening for thyroid cancer; (2) the appropriate tests and intervals for monitoring non-cancerous nodules that are growing or symptomatic; (3) the use of molecular markers or IHC staining following core biopsy for nodules with indeterminate cytology; (4) the most cost-effective treatment strategies for patients with unique considerations (such as those in high-risk groups, pregnant women, and children); and (5) the development of shared decision-making processes that incorporate patient preferences.
CONCLUSIONS
-
CONFLICTS OF INTEREST
Young Joo Park is an editor-in-chief and Eun Kyung Lee is an associate editor of the journal. But they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Article information
-
Acknowledgements
- The authors would like to thank Dr. Mi Young Choi for kind support in developing the Korean Thyroid Association Management Guidelines for Thyroid Nodules. This research was supported by a research grant funded by National Cancer Center (grant number: 2112570) and a grant from the Korea Health Technology R&D Project through the Patient-Doctor Shared Decision Marking Research Center (grant number: HV23C1828) and PatientCentered Clinical Research Coordinating Center (grant number: HC19C0103), funded by the Ministry of Health & Welfare, Republic of Korea.
| Category | US patterns | Suggested malignancy risk, % | Nodule size threshold for biopsy, cmb |
|---|---|---|---|
| High suspicion (K-TIRADS 5) | Solid hypoechoic nodule with any of the three suspicious US featuresc | >60 | >1d |
| Intermediate suspicion (K-TIRADS 4)e | 1) Solid hypoechoic nodules without any of the three suspicious US features or | 10–40 | >1–1.5g |
| 2) Partially cystic or iso-/hyperechoic nodule with any of the three suspicious US features | |||
| 3) Entirely calcified nodulesf | |||
| Low suspicion (K-TIRADS 3) | Partially cystic or iso-/hyperechoic nodule without any of the three suspicious US features | 3–10 | >2 |
| Benign (K-TIRADS 2)h | 1) Iso-/hyperechoic spongiform | 3 | Not indicatedi |
| 2) Partially cystic nodule with intracystic echogenic foci and comet-tail artifact | |||
| 3) Pure cyst | |||
| No nodule (K-TIRADS 1) | - | - |
Modified from Ha et al. [1].
US, ultrasonography; K-TIRADS, Korean Thyroid Imaging Reporting and Data System.
a Biopsy should be performed regardless of the size of the most suspicious nodule in cases with poor prognostic factors, including suspected cervical lymph node metastases, obvious extrathyroidal extension to adjacent structures (trachea, larynx, pharynx, recurrent laryngeal nerve, or perithyroidal vessels), confirmed distant metastases, or suspected medullary thyroid cancer;
b Fine-needle aspiration (FNA) is the primary pathology test and core needle biopsy can be performed as an adjunctive pathology test to FNA; it should be performed by a trained operator [11,47];
c Suspicious US features of thyroid nodule: punctate echogenic foci, nonparallel orientation, and irregular margins;
d Biopsy is recommended for small (>0.5 and ≤1 cm) high suspicion (K-TIRADS 5) nodules with high-risk features, including attachment of nodules to the trachea or posteromedial capsule along the course of the recurrent laryngeal nerve considering the potentials of high-risk microcarcinomas requiring immediate surgery. Biopsy may be considered for small (>0.5 and ≤1 cm) K-TIRADS 5 nodules without high-risk features to decide the management plan in adults. In children, biopsy should be considered for small K-TIRADS 5 nodules (>0.5 and ≤1 cm) to decide the management plan considering the clinical context;
e Extensive parenchymal punctate echogenic foci (microcalcifications) without discrete nodules (suspicious for diffuse sclerosing variant of papillary thyroid carcinoma) and diffusely infiltrative lesions (suspicious for infiltrative malignancy, such as metastasis or lymphoma) are considered to be intermediate suspicion suspicion (K-TIRADS 4) nodules;
f Entirely calcified nodules with complete posterior acoustic shadowing, with no soft tissue component identified due to dense shadowing on US (isolated macrocalcification);
g Cutoff size for biopsy should be determined within the range of 1 and 1.5 cm, based on the US features, nodule location, clinical risk factors, and patient factors (age, comorbidities, and preferences);
h Regardless of coexisting suspicious US features (punctate echogenic foci, nonparallel orientation, or irregular margin);
i Although biopsy is not routinely indicated, it may be performed for nodules that demonstrate continuous and significant growth or for nodules prior to ablation therapy or surgery.
Modified from Ali et al. [21], with permission from Mary Ann Liebert, Inc.
ROM, risk of malignancy; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features.
Modified from Jung [24].
ROM, risk of malignancy; CNB, core needle biopsy; NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features.
- 1. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol 2021;22:2094–123.ArticlePubMedPMCPDF
- 2. Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation 1999;99:2829–48.ArticlePubMed
- 3. Yi KH. The revised 2016 Korean Thyroid Association guidelines for thyroid nodules and cancers: differences from the 2015 American Thyroid Association guidelines. Endocrinol Metab (Seoul) 2016;31:373–8.ArticlePubMedPMCPDF
- 4. Guyatt GH, Schunemann HJ, Djulbegovic B, Akl EA. Guideline panels should not GRADE good practice statements. J Clin Epidemiol 2015;68:597–600.ArticlePubMed
- 5. Park YJ, Lee EK, Song YS, Kang SH, Koo BS, Kim SW, et al. 2023 Korean Thyroid Association management guidelines for patients with thyroid nodules. Int J Thyroidol 2023;16:1–31.Article
- 6. Nose V, Lazar AJ. Update from the 5th edition of the World Health Organization classification of head and neck tumors: familial tumor syndromes. Head Neck Pathol 2022;16:143–57.ArticlePubMedPMCPDF
- 7. Borson-Chazot F, Buffet C, Decaussin-Petrucci M, Cao CD, Drui D, Leboulleux S, et al. SFE-AFCE-SFMN 2022 consensus on the management of thyroid nodules: synthesis and algorithms. Ann Endocrinol (Paris) 2022;83:440–53.PubMed
- 8. Pazaitou-Panayiotou K, Michalakis K, Paschke R. Thyroid cancer in patients with hyperthyroidism. Horm Metab Res 2012;44:255–62.ArticlePubMed
- 9. Song Y, Fu L, Wang P, Sun N, Qiu X, Li J, et al. Effect of Graves’ disease on the prognosis of differentiated thyroid carcinoma: a meta-analysis. Endocrine 2020;67:516–25.ArticlePubMedPDF
- 10. Hu X, Wang X, Liang Y, Chen X, Zhou S, Fei W, et al. Cancer risk in Hashimoto’s thyroiditis: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2022;13:937871.ArticlePubMedPMC
- 11. Yang G, Su X, Huang Y, Luo G, Wang Z, Cai P, et al. Intensive cycles of neoadjuvant camrelizumab combined with chemotherapy in locally advanced esophageal squamous cell carcinoma: a single-arm, phase II trial. J Transl Med 2023;21:411.ArticlePubMedPMCPDF
- 12. Ma J, Mao Z, Yao Y, Lu Y, Wang H, Yang Y, et al. Coexistence of papillary thyroid carcinoma in secondary hyperparathyroidism. BMC Surg 2021;21:335.ArticlePubMedPMCPDF
- 13. Jeong C, Kwon HI, Baek H, Kim HS, Lim DJ, Baek KH, et al. Association of hyperparathyroidism and papillary thyroid cancer: a multicenter retrospective study. Endocrinol Metab (Seoul) 2020;35:925–32.PubMedPMC
- 14. Klubo-Gwiezdzinska J, Yang L, Merkel R, Patel D, Nilubol N, Merino MJ, et al. Results of screening in familial non-medullary thyroid cancer. Thyroid 2017;27:1017–24.ArticlePubMedPMC
- 15. Lee YM, Jeon MJ, Kim WW, Chung KW, Baek JH, Shong YK, et al. Comparison between familial and sporadic non-medullary thyroid carcinoma: a retrospective individual risk factor-matched cohort study. Ann Surg Oncol 2021;28:1722–30.ArticlePubMedPDF
- 16. Wang X, Cheng W, Li J, Su A, Wei T, Liu F, et al. Endocrine tumours: familial nonmedullary thyroid carcinoma is a more aggressive disease: a systematic review and meta-analysis. Eur J Endocrinol 2015;172:R253–62.ArticlePubMed
- 17. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 2015;25:716–59.ArticlePubMedPMC
- 18. Clement SC, Kremer LC, Verburg FA, Simmons JH, Goldfarb M, Peeters RP, et al. Balancing the benefits and harms of thyroid cancer surveillance in survivors of childhood, adolescent and young adult cancer: recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer Treat Rev 2018;63:28–39.ArticlePubMed
- 19. Durante C, Hegedus L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association clinical practice guidelines for thyroid nodule management. Eur Thyroid J 2023;12:e230067.ArticlePubMedPMC
- 20. Chung SR, Choi YJ, Suh CH, Kim HJ, Lee JJ, Kim WG, et al. Thyroid incidentalomas detected on 18F-fluorodeoxyglucose positron emission tomography with computed tomography: malignant risk stratification and management plan. Thyroid 2018;28:762–8.ArticlePubMed
- 21. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid 2023;33:1039–44.ArticlePubMed
- 22. Hsiao V, Massoud E, Jensen C, Zhang Y, Hanlon BM, Hitchcock M, et al. Diagnostic accuracy of fine-needle biopsy in the detection of thyroid malignancy: a systematic review and meta-analysis. JAMA Surg 2022;157:1105–13.ArticlePubMedPMC
- 23. Jung CK, Baek JH, Na DG, Oh YL, Yi KH, Kang HC. 2019 Practice guidelines for thyroid core needle biopsy: a report of the clinical practice guidelines development committee of the Korean Thyroid Association. J Pathol Transl Med 2020;54:64–86.ArticlePubMedPMCPDF
- 24. Jung CK. Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy. J Pathol Transl Med 2023;57:208–16.ArticlePubMedPMCPDF
- 25. Hong MJ, Na DG, Lee H. Diagnostic efficacy and safety of core needle biopsy as a first-line diagnostic method for thyroid nodules: a prospective cohort study. Thyroid 2020;30:1141–9.ArticlePubMed
- 26. Park JY, Choi W, Hong AR, Yoon JH, Kim HK, Kang HC. A comprehensive assessment of the harms of fine-needle aspiration biopsy for thyroid nodules: a systematic review. Endocrinol Metab (Seoul) 2023;38:104–16.ArticlePubMedPMCPDF
- 27. Lee SY, Pearce EN. Assessment and treatment of thyroid disorders in pregnancy and the postpartum period. Nat Rev Endocrinol 2022;18:158–71.ArticlePubMedPMCPDF
- 28. Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am J Obstet Gynecol 2003;189:1128–35.ArticlePubMed
- 29. Drui D, Briet C, Guerin C, Lugat A, Borson-Chazot F, Grunenwald S. SFE-AFCE-SFMN 2022 Consensus on the management of thyroid nodules: thyroid nodules and pregnancy. Ann Endocrinol (Paris) 2022;83:435–9.ArticlePubMed
- 30. Moosa M, Mazzaferri EL. Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab 1997;82:2862–6.ArticlePubMed
- 31. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418–28.ArticlePubMed
- 32. Oh HS, Kim WG, Park S, Kim M, Kwon H, Jeon MJ, et al. Serial neck ultrasonographic evaluation of changes in papillary thyroid carcinoma during pregnancy. Thyroid 2017;27:773–7.ArticlePubMed
References
Figure & Data
References
Citations

- 2023 Update of the Korean Thyroid Association Guidelines for the Management of Thyroid Nodules
Eun Kyung Lee, Young Joo Park
Clinical Thyroidology®.2024; 36(4): 153. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite