Search
- Page Path
- HOME > Search
Review Articles
- Thyroid
- The Role of Thyroid Hormone in the Regulation of Cerebellar Development
- Sumiyasu Ishii, Izuki Amano, Noriyuki Koibuchi
- Endocrinol Metab. 2021;36(4):703-716. Published online August 9, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1150
- 4,585 View
- 170 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
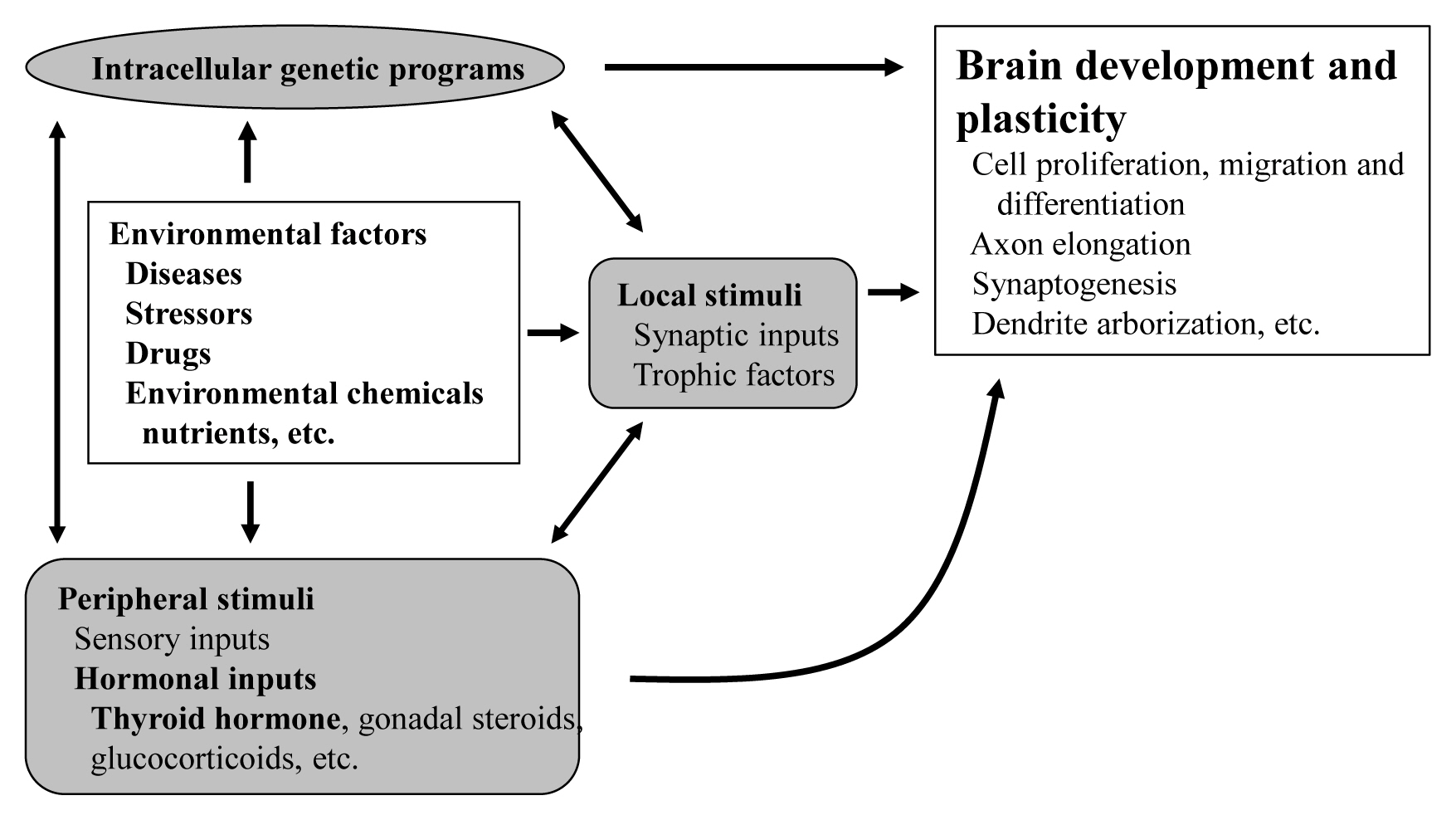
ePub - The proper organized expression of specific genes in time and space is responsible for the organogenesis of the central nervous system including the cerebellum. The epigenetic regulation of gene expression is tightly regulated by an intrinsic intracellular genetic program, local stimuli such as synaptic inputs and trophic factors, and peripheral stimuli from outside of the brain including hormones. Some hormone receptors are expressed in the cerebellum. Thyroid hormones (THs), among numerous circulating hormones, are well-known major regulators of cerebellar development. In both rodents and human, hypothyroidism during the postnatal developmental period results in abnormal morphogenesis or altered function. THs bind to the thyroid hormone receptors (TRs) in the nuclei and with the help of transcriptional cofactors regulate the transcription of target genes. Gene regulation by TR induces cell proliferation, migration, and differentiation, which are necessary for brain development and plasticity. Thus, the lack of TH action mediators may directly cause aberrant cerebellar development. Various kinds of animal models have been established in a bid to study the mechanism of TH action in the cerebellum. Interestingly, the phenotypes differ greatly depending on the models. Herein we summarize the actions of TH and TR particularly in the developing cerebellum.
-
Citations
Citations to this article as recorded by- Neuropeptides and Their Roles in the Cerebellum
Zi-Hao Li, Bin Li, Xiao-Yang Zhang, Jing-Ning Zhu
International Journal of Molecular Sciences.2024; 25(4): 2332. CrossRef - Exploring the underlying molecular mechanism of tri(1,3-dichloropropyl) phosphate-induced neurodevelopmental toxicity via thyroid hormone disruption in zebrafish by multi-omics analysis
Ying Xu, Lei Yang, Yanguo Teng, Jian Li, Na Li
Aquatic Toxicology.2023; 258: 106510. CrossRef - Association of Maternal TSH, FT4 With Children's BMI Trajectories, and Obesity: A Birth Cohort Study
Mengting Yang, Shanshan Zhang, Yuzhu Teng, Xue Ru, Linlin Zhu, Yan Han, Xingyong Tao, Hui Cao, Shuangqin Yan, Fangbiao Tao, Kun Huang
The Journal of Clinical Endocrinology & Metabolism.2023; 109(1): e190. CrossRef - Thyroid hormone receptor beta: Relevance in human health and diseases
Ghausiya Rehman, Neha Kumari, Farhad Bano, Rakesh K. Tyagi
Endocrine and Metabolic Science.2023; 13: 100144. CrossRef - Targeting Thyroid Hormone/Thyroid Hormone Receptor Axis: An Attractive Therapy Strategy in Liver Diseases
Qianyu Tang, Min Zeng, Linxi Chen, Nian Fu
Frontiers in Pharmacology.2022;[Epub] CrossRef - Histone Deacetylase 3 Inhibitor Alleviates Cerebellar Defects in Perinatal Hypothyroid Mice by Stimulating Histone Acetylation and Transcription at Thyroid Hormone-Responsive Gene Loci
Alvin Susetyo, Sumiyasu Ishii, Yuki Fujiwara, Izuki Amano, Noriyuki Koibuchi
International Journal of Molecular Sciences.2022; 23(14): 7869. CrossRef - Selection-driven adaptation to the extreme Antarctic environment in the Emperor penguin
Federica Pirri, Lino Ometto, Silvia Fuselli, Flávia A. N. Fernandes, Lorena Ancona, Nunzio Perta, Daniele Di Marino, Céline Le Bohec, Lorenzo Zane, Emiliano Trucchi
Heredity.2022; 129(6): 317. CrossRef - Long-term depression–inductive stimulation causes long-term potentiation in mouse Purkinje cells with a mutant thyroid hormone receptor
Ayane Ninomiya, Izuki Amano, Michifumi Kokubo, Yusuke Takatsuru, Sumiyasu Ishii, Hirokazu Hirai, Nobutake Hosoi, Noriyuki Koibuchi
Proceedings of the National Academy of Sciences.2022;[Epub] CrossRef
- Neuropeptides and Their Roles in the Cerebellum

- Adrenal gland
- Embryonic Development and Adult Regeneration of the Adrenal Gland
- Ji-Hoon Kim, Man Ho Choi
- Endocrinol Metab. 2020;35(4):765-773. Published online December 23, 2020
- DOI: https://doi.org/10.3803/EnM.2020.403

- 8,155 View
- 370 Download
- 19 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - The adrenal gland plays a pivotal role in an organism’s health span by controlling the endocrine system. Decades of research on the adrenal gland have provided multiscale insights into the development and maintenance of this essential organ. A particularly interesting finding is that founder stem/progenitor cells participate in adrenocortical development and enable the adult adrenal cortex to regenerate itself in response to hormonal stress and injury. Since major advances have been made in understanding the dynamics of the developmental process and the remarkable regenerative capacity of the adrenal gland, understanding the mechanisms underlying adrenal development, maintenance, and regeneration will be of interest to basic and clinical researchers. Here, we introduce the developmental processes of the adrenal gland and discuss current knowledge regarding stem/progenitor cells that regulate adrenal cortex remodeling and regeneration. This review will provide insights into the fascinating ongoing research on the development and regeneration of the adrenal cortex.
-
Citations
Citations to this article as recorded by- Update on Adrenarche—Still a Mystery
Philipp Augsburger, Jani Liimatta, Christa E Flück
The Journal of Clinical Endocrinology & Metabolism.2024;[Epub] CrossRef - Aging induces region-specific dysregulation of hormone synthesis in the primate adrenal gland
Qiaoran Wang, Xuebao Wang, Beibei Liu, Shuai Ma, Feng Zhang, Shuhui Sun, Yaobin Jing, Yanling Fan, Yingjie Ding, Muzhao Xiong, Jiaming Li, Qiaocheng Zhai, Yandong Zheng, Chengyu Liu, Gang Xu, Jiayin Yang, Si Wang, Jinlin Ye, Juan Carlos Izpisua Belmonte,
Nature Aging.2024; 4(3): 396. CrossRef - Adrenal Dysfunction in Mitochondrial Diseases
Madeleine Corkery-Hayward, Louise A. Metherell
International Journal of Molecular Sciences.2023; 24(2): 1126. CrossRef - Effects of very low-calorie ketogenic diet on hypothalamic–pituitary–adrenal axis and renin–angiotensin–aldosterone system
L. Barrea, L. Verde, E. Camajani, A. S. Šojat, L. Marina, S. Savastano, A. Colao, M. Caprio, G. Muscogiuri
Journal of Endocrinological Investigation.2023; 46(8): 1509. CrossRef - Post‐hatching developmental changes in the adrenal gland of the Japanese quail (Coturnix coturnix japonica): Histological, immunohistochemical, and electron microscopic studies
Fatma M. Abdel‐maksoud, Saher Fadl, Ahmed Abou‐Elmagd, Abdelmohaimen M.M. Saleh
Microscopy Research and Technique.2023; 86(11): 1461. CrossRef - Interactive metabolic signatures of testicular testosterone with bilateral adrenalectomy in mice
Hae Lim Cho, Ji-Hoon Kim, Seuk-Min Ryu, Jongsung Noh, Sang Won Lee, Man Ho Choi
The Journal of Steroid Biochemistry and Molecular Biology.2023; 231: 106333. CrossRef - Construction of a novel clinical nomogram to predict cancer-specific survival in patients with primary malignant adrenal tumors: a large population-based retrospective study
Mingzhen Li, Xiaoying Duan, Di You, Linlin Liu
Frontiers in Medicine.2023;[Epub] CrossRef - The anti-platelet drug cilostazol enhances heart rate and interrenal steroidogenesis and exerts a scant effect on innate immune responses in zebrafish
Wei-Chun Chang, Mei-Jen Chen, Chung-Der Hsiao, Rong-Ze Hu, Yu-Shan Huang, Yu-Fu Chen, Tsai-Hua Yang, Guan-Yi Tsai, Chih-Wei Chou, Ren-Shiang Chen, Yung-Jen Chuang, Yi-Wen Liu, Mohammed Fouad El Basuini
PLOS ONE.2023; 18(10): e0292858. CrossRef - Regulation of Morphogenetic Processes during Postnatal Development and Physiological Regeneration of the Adrenal Medulla
S. S. Obernikhin, N. V. Yaglova, E. P. Timokhina, S. V. Nazimova, V. V. Yaglov
Bulletin of Experimental Biology and Medicine.2023; 175(4): 549. CrossRef - Distinct HAND2/HAND2-AS1 Expression Levels May Fine-Tune Mesenchymal and Epithelial Cell Plasticity of Human Mesenchymal Stem Cells
Rachel Vazana-Netzarim, Yishay Elmalem, Shachar Sofer, Hod Bruck, Naama Danino, Udi Sarig
International Journal of Molecular Sciences.2023; 24(22): 16546. CrossRef - An Update on Genetics of Adrenal Gland and Associated Disorders
Chester Gauss, Dustin Rowland, Berrin Ergun-Longmire
Endocrines.2022; 3(2): 187. CrossRef - Immune dysfunction after spinal cord injury – A review of autonomic and neuroendocrine mechanisms
Kyleigh A. Rodgers, Kristina A. Kigerl, Jan M. Schwab, Phillip G. Popovich
Current Opinion in Pharmacology.2022; 64: 102230. CrossRef - Clinical and Technical Aspects in Free Cortisol Measurement
Man Ho Choi
Endocrinology and Metabolism.2022; 37(4): 599. CrossRef - Surrénalectomies
Isabelle Valin, Dan Rosenberg
Le Nouveau Praticien Vétérinaire canine & féline.2022; 19(82): 50. CrossRef - Adrenal medulla development and medullary-cortical interactions
Nicole Bechmann, Ilona Berger, Stefan R. Bornstein, Charlotte Steenblock
Molecular and Cellular Endocrinology.2021; 528: 111258. CrossRef - Theory: Treatments for Prolonged ICU Patients May Provide New Therapeutic Avenues for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Dominic Stanculescu, Lars Larsson, Jonas Bergquist
Frontiers in Medicine.2021;[Epub] CrossRef - Unravelling Polycystic Ovary Syndrome and Its Comorbidities
Kyung-Wook Kim
Journal of Obesity & Metabolic Syndrome.2021; 30(3): 209. CrossRef
- Update on Adrenarche—Still a Mystery

Case Report
- A Case of Klinefelter's Syndrome with Rathke's Cleft Cyst.
- Hyun Joo Lee, Hyo Kyoung Park, Dae Jung Kim, Yu Mie Rhee, Chul Woo Ahn, Sang Soo Jung, Jae Hyun Nam, Bong Soo Cha, Young Duk Song, Sung Kil Lim, Kyung Rae Kim, Yong Koo Park, Hyun Chul Lee, Kap Bum Huh
- J Korean Endocr Soc. 2002;17(4):564-571. Published online August 1, 2002
- 1,080 View
- 23 Download
-
 Abstract
Abstract
 PDF
PDF - Klinefelter's syndrome is one of the most common forms of primary hypogonadism presenting with gynecomastia, azospermia and increased follicle-stimulating hormone. It is well known that this syndrome has an increased incidence of neoplasia, especially breast cancer and extragonadal germ cell tumors. However, it is rarely associated with an intracranial tumor of maldevelopmental origin, especially in the suprasellar area. We report, for the first time, a case of Klinefelter's syndrome, with a Rathke's cleft cyst is the patient was a 32-year-old male who was known to have an incidentaloma form brain computed tomography, which was clinically diagnosed as a suprasellar tumor. After operating, the suprasellar mass was confirmed as a Rathke's cleft cyst, and his hormonal abnormality, an elevated level of follicle-stimulating hormone, was not normalized. Therefore, we performed chromosomal analysis, and diagnosed Klinefelter's syndrome with the XXY karyotype.


 KES
KES

 First
First Prev
Prev



