Search
- Page Path
- HOME > Search
- Thyroid
- Lobeglitazone, A Peroxisome Proliferator-Activated Receptor-Gamma Agonist, Inhibits Papillary Thyroid Cancer Cell Migration and Invasion by Suppressing p38 MAPK Signaling Pathway
- Jun-Qing Jin, Jeong-Sun Han, Jeonghoon Ha, Han-Sang Baek, Dong-Jun Lim
- Endocrinol Metab. 2021;36(5):1095-1110. Published online October 14, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1155
- 4,788 View
- 160 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) ligands have been widely shown to correlate with epithelial-mesenchymal transition (EMT) and cancer progression. Lobeglitazone (LGZ) is a novel ligand of PPAR-γ; and its role in EMT and metastasis in papillary thyroid carcinoma (PTC) is poorly understood. We aimed to investigate the role of LGZ in metastatic behavior of PTC cells.
Methods
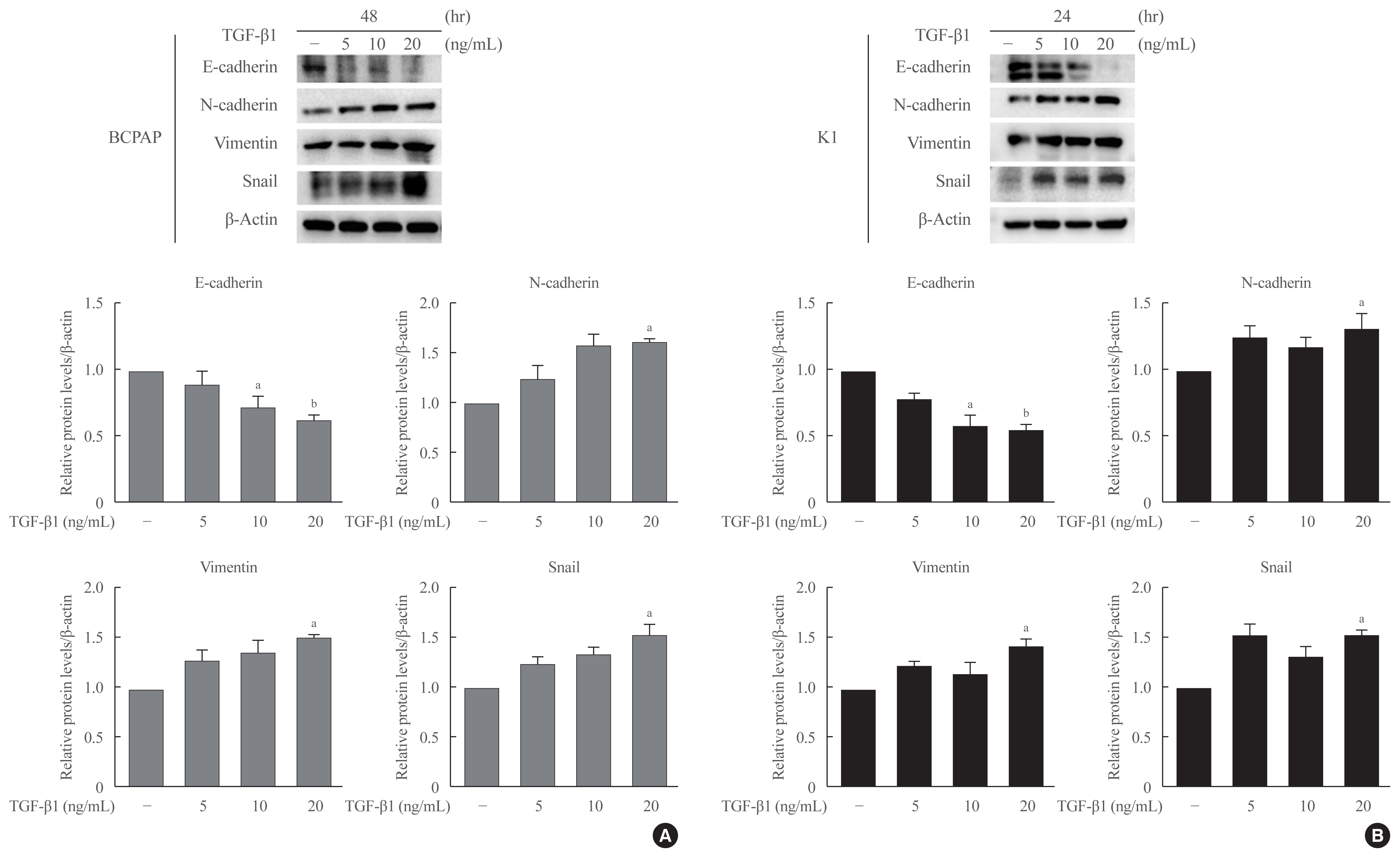
Half maximal inhibitory concentration (IC50) values of LGZ in BRAF-mutated PTC cell lines (BCPAP and K1) were determined using MTT assay. Rosiglitazone (RGZ), the PPAR-γ ligand was used as a positive control. The protein expression of PPAR-γ, cell-surface proteins (E-cadherin, N-cadherin), cytoskeletal protein (Vimentin), transcription factor (Snail), p38 mitogenactivated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) 1/2 pathway, and matrix metalloproteinase (MMP)-2 expression were measured using Western blotting. Changes in E-cadherin expression were also determined using immunocytochemistry. Cell migration and invasion were analyzed using wound healing and Matrigel invasion assays.
Results
Treatment with LGZ or RGZ significantly inhibited transforming growth factor-beta1 (TGF-β1)-induced EMT-associated processes such as fibroblast-like morphological changes, EMT-related protein expression, and increased cell migration and invasion in BCPAP and K1 cells. LGZ restored TGF-β1-induced loss of E-cadherin, as observed using immunocytochemistry. Furthermore, LGZ and RGZ suppressed TGF-β1-induced MMP-2 expression and phosphorylation of p38 MAPK, but not ERK1/2. Although there was no change in PPAR-γ expression after treatment with LGZ or RGZ, the effect of downstream processes mediated by LGZ was hampered by GW9662, a PPAR-γ antagonist.
Conclusion
LGZ inhibits TGF-β1-induced EMT, migration, and invasion through the p38 MAPK signaling pathway in a PPAR-γ-dependent manner in PTC cells. -
Citations
Citations to this article as recorded by- Diabetes Mellitus and Thyroid Cancers: Risky Correlation, Underlying Mechanisms and Clinical Prevention
Rongqian Wu, Junping Zhang, Guilin Zou, Shanshan Li, Jinying Wang, Xiaoxinlei Li, Jixiong Xu
Diabetes, Metabolic Syndrome and Obesity.2024; Volume 17: 809. CrossRef - Clinicopathological Evaluation of Papillary Thyroid Microcarcinoma
Ando Takahito, Kimihito Fujii, Hirona Banno, Masayuki Saito, Yukie Ito, Mirai Ido, Manami Goto, Yukako Mouri, Junko Kousaka, Tsuneo Imai, Shogo Nakano
Cureus.2024;[Epub] CrossRef - Pioglitazone, a peroxisome proliferator‑activated receptor γ agonist, induces cell death and inhibits the proliferation of hypoxic HepG2 cells by promoting excessive production of reactive oxygen species
Guohao Huang, Mengfan Zhang, Manzhou Wang, Wenze Xu, Xuhua Duan, Xinwei Han, Jianzhuang Ren
Oncology Letters.2024;[Epub] CrossRef - The Activation of PPARγ by (2Z,4E,6E)-2-methoxyocta-2,4,6-trienoic Acid Counteracts the Epithelial–Mesenchymal Transition Process in Skin Carcinogenesis
Enrica Flori, Sarah Mosca, Giorgia Cardinali, Stefania Briganti, Monica Ottaviani, Daniela Kovacs, Isabella Manni, Mauro Truglio, Arianna Mastrofrancesco, Marco Zaccarini, Carlo Cota, Giulia Piaggio, Mauro Picardo
Cells.2023; 12(7): 1007. CrossRef - Cumulative exposure to metabolic syndrome increases thyroid cancer risk in young adults: a population-based cohort study
Jinyoung Kim, Kyungdo Han, Mee Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Hyuk-Sang Kwon
The Korean Journal of Internal Medicine.2023; 38(4): 526. CrossRef - Drug repositioning in thyroid cancer treatment: the intriguing case of anti-diabetic drugs
Alessia Greco, Francesca Coperchini, Laura Croce, Flavia Magri, Marsida Teliti, Mario Rotondi
Frontiers in Pharmacology.2023;[Epub] CrossRef - Fish and the Thyroid: A Janus Bifrons Relationship Caused by Pollutants and the Omega-3 Polyunsaturated Fatty Acids
Salvatore Benvenga, Fausto Famà, Laura Giovanna Perdichizzi, Alessandro Antonelli, Gabriela Brenta, Francesco Vermiglio, Mariacarla Moleti
Frontiers in Endocrinology.2022;[Epub] CrossRef - Identifying and categorizing compounds that reduce corneal transforming growth factor beta induced protein levels: a scoping review
Gabriella Guo Sciriha, Janet Sultana, Joseph Borg
Expert Review of Clinical Pharmacology.2022; 15(12): 1423. CrossRef
- Diabetes Mellitus and Thyroid Cancers: Risky Correlation, Underlying Mechanisms and Clinical Prevention

- Endocrine Research
- Mechanism of Lipid Accumulation through PAR2 Signaling in Diabetic Male Mice
- Dae Hyun Kim, Ye Ra Kim, EunJin Bang, Sugyeong Ha, Sang Gyun Noh, Byeong Moo Kim, Seong Ho Jeong, Hee Jin Jung, Ji Young Lee, Hae Young Chung
- Endocrinol Metab. 2021;36(1):171-184. Published online February 24, 2021
- DOI: https://doi.org/10.3803/EnM.2020.850
- 4,630 View
- 135 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
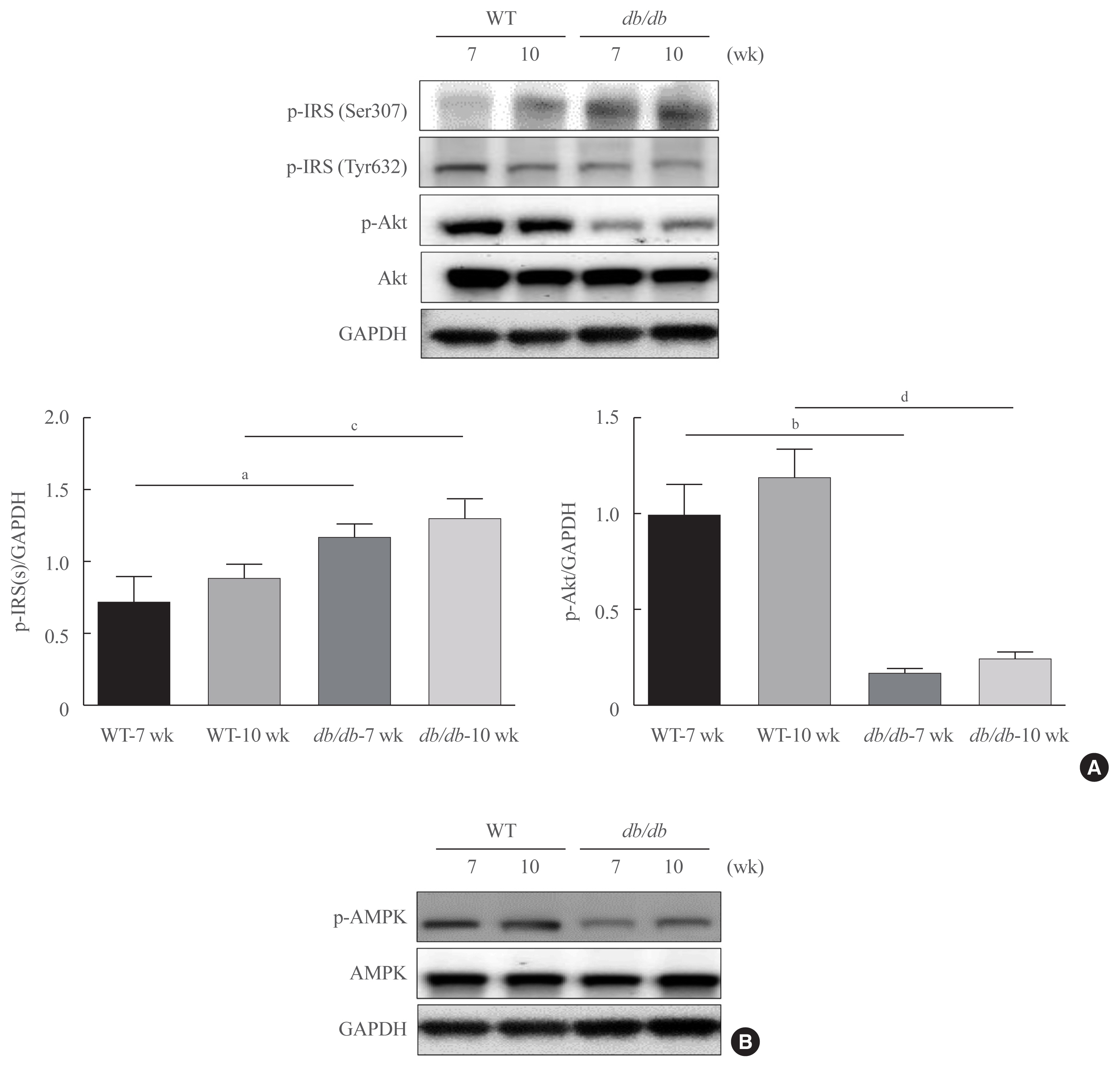
Protease-activated protein-2 (PAR2) has been reported to regulate hepatic insulin resistance condition in type 2 diabetes mice. However, the mechanism of lipid metabolism through PAR2 in obesity mice have not yet been examined. In liver, Forkhead box O1 (FoxO1) activity induces peroxisome proliferator-activated receptor γ (PPARγ), leading to accumulation of lipids and hyperlipidemia. Hyperlipidemia significantly influence hepatic steatoses, but the mechanisms underlying PAR2 signaling are complex and have not yet been elucidated.
Methods
To examine the modulatory action of FoxO1 and its altered interaction with PPARγ, we utilized db/db mice and PAR2-knockout (KO) mice administered with high-fat diet (HFD).
Results
Here, we demonstrated that PAR2 was overexpressed and regulated downstream gene expressions in db/db but not in db+ mice. The interaction between PAR2/β-arrestin and Akt was also greater in db/db mice. The Akt inhibition increased FoxO1 activity and subsequently PPARγ gene in the livers that led to hepatic lipid accumulation. Our data showed that FoxO1 was negatively controlled by Akt signaling and consequently, the activity of a major lipogenesis-associated transcription factors such as PPARγ increased, leading to hepatic lipid accumulation through the PAR2 pathway under hyperglycemic conditions in mice. Furthermore, the association between PPARγ and FoxO1 was increased in hepatic steatosis condition in db/db mice. However, HFD-fed PAR2-KO mice showed suppressed FoxO1-induced hepatic lipid accumulation compared with HFD-fed control groups.
Conclusion
Collectively, our results provide evidence that the interaction of FoxO1 with PPARγ promotes hepatic steatosis in mice. This might be due to defects in PAR2/β-arrestin-mediated Akt signaling in diabetic and HFD-fed mice. -
Citations
Citations to this article as recorded by- Biochanin‐A has antidiabetic, antihyperlipidemic, antioxidant, and protective effects on diabetic nephropathy via suppression of TGF‐β1 and PAR‐2 genes expression in kidney tissues of STZ‐induced diabetic rats
Jamal Amri, Mona Alaee, Rasool Babaei, Zahra Salemi, Reza Meshkani, Ali Ghazavi, Ahmad Akbari, Mehdi Salehi
Biotechnology and Applied Biochemistry.2022; 69(5): 2112. CrossRef - Delineation of the healthy rabbit liver by immunohistochemistry – A technical note
Gabriella Meier Bürgisser, Olivera Evrova, Dorothea M. Heuberger, Julia Rieber, Pietro Giovanoli, Maurizio Calcagni, Johanna Buschmann
Acta Histochemica.2021; 123(7): 151795. CrossRef
- Biochanin‐A has antidiabetic, antihyperlipidemic, antioxidant, and protective effects on diabetic nephropathy via suppression of TGF‐β1 and PAR‐2 genes expression in kidney tissues of STZ‐induced diabetic rats

- Thyroid
- Mutation Profile of Well-Differentiated Thyroid Cancer in Asians
- Young Shin Song, Jung Ah Lim, Young Joo Park
- Endocrinol Metab. 2015;30(3):252-262. Published online September 22, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.3.252
- 5,198 View
- 64 Download
- 60 Web of Science
- 52 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Recent advances in molecular diagnostics have led to significant insights into the genetic basis of thyroid tumorigenesis. Among the mutations commonly seen in thyroid cancers, the vast majority are associated with the mitogen-activated protein kinase pathway. B-Raf proto-oncogene (
BRAF ) mutations are the most common mutations observed in papillary thyroid cancers (PTCs), followed byRET/PTC rearrangements andRAS mutations, while follicular thyroid cancers are more likely to harborRAS mutations orPAX8 /peroxisome proliferator-activated receptor γ (PPARγ ) rearrangements. Beyond these more common mutations, alterations in the telomerase reverse transcriptase (TERT ) promoter have recently been associated with clinicopathologic features, disease prognosis, and tumorigenesis in thyroid cancer. While the mutations underlying thyroid tumorigenesis are well known, the frequency of these mutations is strongly associated with geography, with clear differences reported between Asian and Western countries. Of particular interest is the prevalence ofBRAF mutations, with Korean patients exhibiting the highest rate ofBRAF -associated thyroid cancers in the world. Here, we review the prevalence of each of the most common mutations in Asian and Western countries, and identify the characteristics of well-differentiated thyroid cancer in Asians.-
Citations
Citations to this article as recorded by- BRAF V600E Mutation Lacks Association with Poorer Clinical Prognosis in Papillary Thyroid Carcinoma
Hon-Fan Lai, Jen-Fan Hang, Po-Chung Kuo, Chin-Sung Kuo, San-Fan Yao, Jui-Yu Chen, Chen-Hsen Lee
Annals of Surgical Oncology.2024; 31(5): 3495. CrossRef - Clinical value of multi-gene testing in distinguishing benign and malignant thyroid nodules
Murui Zhang, Xiaotong Hu, Lunming Liu, Yihong Wang, Junchang Jiang, Hui Li, Weiqiang Fei, Tingting Zhong, Zhinong Jiang
Medicine.2024; 103(4): e35960. CrossRef - The Association of Socioeconomic Factors and Well-Differentiated Thyroid Cancer
Andrew Bonner, Brendon Herring, Rongzhi Wang, Andrea Gillis, Polina Zmijewski, Brenessa Lindeman, Jessica Fazendin, Herbert Chen
Journal of Surgical Research.2023; 283: 973. CrossRef - BRAFV600E and TERT promoter C228T mutations on ThyroSeq v3 analysis of delayed skin metastasis from papillary thyroid cancer: a case report and literature review
Jee-Hye Choi, Hyeong Won Yu, Ja Kyung Lee, Woochul Kim, June Young Choi, Hee Young Na, So Yeon Park, Chang Ho Ahn, Jae Hoon Moon, Sang Il Choi, Ho-Young Lee, Won Woo Lee, Wonjae Cha, Woo-Jin Jeong
World Journal of Surgical Oncology.2023;[Epub] CrossRef - Less is more meets do more with less: Exploring differences in thyroid FNA molecular testing between Asian and Western practices
Michiya Nishino
Cancer Cytopathology.2023; 131(7): 421. CrossRef - Histological and Genetic Diversity in Ovarian Mucinous Carcinomas: A Pilot Study
Sultana Razia, Kentaro Nakayama, Hitomi Yamashita, Tomoka Ishibashi, Masako Ishikawa, Kosuke Kanno, Seiya Sato, Satoru Kyo
Current Oncology.2023; 30(4): 4052. CrossRef - Effective Use of microRNA, BRAF and Sonographic Risk Assessment in Bethesda III Thyroid Nodules Requires a Different Approach to Nodules with Features of Nuclear Atypia and Other Types of Atypia
Dorota Słowińska-Klencka, Bożena Popowicz, Dominika Kulczycka-Wojdala, Bożena Szymańska, Joanna Duda-Szymańska, Martyna Wojtaszek-Nowicka, Krzysztof Kaczka, Mariusz Klencki
Cancers.2023; 15(17): 4287. CrossRef - Long-Term Changes in the Mortality Rates of Thyroid Cancer in Korea: Analysis of Korean National Data from 1985 to 2020
Yun Mi Choi, Min-Ju Kim, Jiwoo Lee, Mi Kyung Kwak, Min Ji Jeon, Tae Yong Kim, Eun-Gyoung Hong, Won Bae Kim, Won Gu Kim
Endocrinology and Metabolism.2023; 38(5): 588. CrossRef - 遺伝子から頭頸部がんを診る : 甲状腺癌 (分化癌を中心に)
季吉 森谷
Nippon Jibiinkoka Tokeibugeka Gakkai Kaiho(Tokyo).2023; 126(12): 1277. CrossRef - Toward Systems-Level Metabolic Analysis in Endocrine Disorders and Cancer
Aliya Lakhani, Da Hyun Kang, Yea Eun Kang, Junyoung O. Park
Endocrinology and Metabolism.2023; 38(6): 619. CrossRef - Risk and Prognostic Factors for BRAFV600E Mutations in Papillary Thyroid Carcinoma
Xiaojing Wei, Xiaodong Wang, Jie Xiong, Chen Li, Yixuan Liao, Yongjun Zhu, Jingxin Mao, Gitana Maria Aceto
BioMed Research International.2022; 2022: 1. CrossRef - Disparities in the impact of the AJCC 8th edition staging system on differentiated thyroid cancer outcomes
Juan A. Santamaria‐Barria, Amanda N. Graff‐Baker, Shu‐Ching Chang, Adam Khader, Anthony J. Scholer, Mary Garland‐Kledzik, Melanie Goldfarb
Head & Neck.2022; 44(10): 2129. CrossRef - Relationship Between The BRAF V600E And Tumor Size, Lymph Node, And Distant Metastasis In Papillary Thyroid Carcinoma
Edmond Rukmana Wikanta, Yan Wisnu Prajoko, Benny Issakh, Hermawan Istiadi, Dik Puspasari
Russian Open Medical Journal.2022;[Epub] CrossRef - Lactate Dehydrogenase A as a Potential New Biomarker for Thyroid Cancer
Eun Jeong Ban, Daham Kim, Jin Kyong Kim, Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Kee-Hyun Nam, Woong Youn Chung, Kunhong Kim
Endocrinology and Metabolism.2021; 36(1): 96. CrossRef - Association of Hyperparathyroidism and Papillary Thyroid Cancer: A Multicenter Retrospective Study (Endocrinol Metab 2020;35:925-32, Chaiho Jeong et al.)
Chaiho Jeong, Jeonghoon Ha, Moo Il Kang
Endocrinology and Metabolism.2021; 36(1): 205. CrossRef - Molecular pathogenesis of pediatric thyroid carcinoma
Norisato Mitsutake, Vladimir Saenko
Journal of Radiation Research.2021; 62(Supplement): i71. CrossRef - The Incidence of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features: A Meta-Analysis Assessing Worldwide Impact of the Reclassification
Chanchal Rana, Huy Gia Vuong, Thu Quynh Nguyen, Hoang Cong Nguyen, Chan Kwon Jung, Kennichi Kakudo, Andrey Bychkov
Thyroid.2021;[Epub] CrossRef - The Association Between Radioiodine Refractory in Papillary Thyroid Carcinoma, Sodium/Iodide Symporter Expression, and BRAFV600E Mutation
Tauangtham Anekpuritanang, Maythad Uataya, Apichaya Claimon, Natthawadee Laokulrath, Warut Pongsapich, Paveena Pithuksurachai
OncoTargets and Therapy.2021; Volume 14: 3959. CrossRef - Genomic landscape of metastatic papillary thyroid carcinoma and novel biomarkers for predicting distant metastasis
Xiabin Lan, Hua Bao, Xinyang Ge, Jun Cao, Xiaojun Fan, Qihong Zhang, Kaihua Liu, Xian Zhang, Zhuo Tan, Chuanming Zheng, Ao Wang, Chao Chen, Xin Zhu, Jiafeng Wang, Jiajie Xu, Xuhang Zhu, Xue Wu, Xiaonan Wang, Yang Shao, Minghua Ge
Cancer Science.2020; 111(6): 2163. CrossRef - Did Introducing a New Category of Thyroid Tumors (Non-invasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features) Decrease the Risk of Malignancy for the Diagnostic Categories in the Bethesda System for Reporting Thyroid Cytopathology?
Janusz Kopczyński, Agnieszka Suligowska, Kornelia Niemyska, Iwona Pałyga, Agnieszka Walczyk, Danuta Gąsior-Perczak, Artur Kowalik, Kinga Hińcza, Ryszard Mężyk, Stanisław Góźdź, Aldona Kowalska
Endocrine Pathology.2020; 31(2): 143. CrossRef - The Significance of Transcriptomic Signatures in the Multifocal Papillary Thyroid Carcinoma: Two mRNA Expression Patterns with Distinctive Clinical Behavior from The Cancer Genome Atlas (TCGA) Database
Yea Eun Kang, Boyoung Hwang, Ju Hee Lee, Minho Shong, Hyon-Seung Yi, Bon Seok Koo, Dong Jin Lee
International Journal of Thyroidology.2020; 13(1): 1. CrossRef - High Genetic Diversity and No Evidence of Clonal Relation in Synchronous Thyroid Carcinomas Associated with Hashimoto’s Thyroiditis: A Next-Generation Sequencing Analysis
Csaba Molnár, Emese Sarolta Bádon, Attila Mokánszki, Anikó Mónus, Lívia Beke, Ferenc Győry, Endre Nagy, Gábor Méhes
Diagnostics.2020; 10(1): 48. CrossRef - Low Prevalence of TERT Promoter, BRAF and RAS Mutations in Papillary Thyroid Cancer in the Greek Population
Marilena Argyropoulou, Aristidis S. Veskoukis, Pagona-Maria Karanatsiou, Aikaterini Manolakelli, Ifigenia Kostoglou-Athanassiou, George Vilaras, Andreas Karameris, Kalliopi Liadaki
Pathology & Oncology Research.2020; 26(1): 347. CrossRef - Predominant DICER1 Pathogenic Variants in Pediatric Follicular Thyroid Carcinomas
Young Ah Lee, Sun-Wha Im, Kyeong Cheon Jung, Eun-Jae Chung, Choong Ho Shin, Jong-Il Kim, Young Joo Park
Thyroid.2020; 30(8): 1120. CrossRef - BRAF and KRAS mutations in papillary thyroid carcinoma in the United Arab Emirates
Suhail Al-Salam, Charu Sharma, Bachar Afandi, Khaled Al Dahmani, Ali S. Al-Zahrani, Amal Al Shamsi, Juma Al Kaabi, Paula Soares
PLOS ONE.2020; 15(4): e0231341. CrossRef - VE1 Immunohistochemistry Improves the Limit of Genotyping for Detecting BRAFV600E Mutation in Papillary Thyroid Cancer
Sonam Choden, Somboon Keelawat, Chan Kwon Jung, Andrey Bychkov
Cancers.2020; 12(3): 596. CrossRef - Highly Sensitive and Specific Molecular Test for Mutations in the Diagnosis of Thyroid Nodules: A Prospective Study of BRAF-Prevalent Population
Yoon Young Cho, So Young Park, Jung Hee Shin, Young Lyun Oh, Jun-Ho Choe, Jung-Han Kim, Jee Soo Kim, Hyun Sook Yim, Yoo-Li Kim, Chang-Seok Ki, Tae Hyuk Kim, Jae Hoon Chung, Sun Wook Kim
International Journal of Molecular Sciences.2020; 21(16): 5629. CrossRef - Is there adenoma-carcinoma sequence between benign adenoma and papillary cancer of thyroid: A genomic linkage study
Ramesh Bangaraiahgari, Ramakanth Bhargav Panchangam, Pradeep Puthenveetil, Sabaretnam Mayilvaganan, Rajesh Bangaraiahgari, Rajkiran reddy Banala, Poongkodi Karunakaran, Rafi Md
Annals of Medicine and Surgery.2020; 60: 695. CrossRef - Genomic Characterization of Differentiated Thyroid Carcinoma
Young Shin Song, Young Joo Park
Endocrinology and Metabolism.2019; 34(1): 1. CrossRef - Association between BRAFV600E Mutations and Clinicopathological Features of Papillary Thyroid Microcarcinoma (PTMC)
Sung Min Lee, Cho Rok Lee, Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Kee-Hyun Nam, Woong Youn Chung, Cheong Soo Park
Journal of Endocrine Surgery.2019; 19(3): 76. CrossRef - Combined quantitation of HMGA2 mRNA, microRNAs, and mitochondrial-DNA content enables the identification and typing of thyroid tumors in fine-needle aspiration smears
Sergei E. Titov, Mikhail K. Ivanov, Pavel S. Demenkov, Gevork A. Katanyan, Eugenia S. Kozorezova, Anastasia V. Malek, Yulia A. Veryaskina, Igor F. Zhimulev
BMC Cancer.2019;[Epub] CrossRef - Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer
Young Shin Song, Seong-Keun Yoo, Hwan Hee Kim, Gyeongseo Jung, Ah-Reum Oh, Ji-Young Cha, Su-jin Kim, Sun Wook Cho, Kyu Eun Lee, Jeong-Sun Seo, Young Joo Park
Endocrine-Related Cancer.2019; 26(6): 629. CrossRef - Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice: Perspectives for Surgical Pathology and Cytopathology
Andrey Bychkov, Chan Kwon Jung, Zhiyan Liu, Kennichi Kakudo
Endocrine Pathology.2018; 29(3): 276. CrossRef - Understanding Malignancies of the Thyroid Gland: Institutional Experience
Jaimanti Bakshi, Sourabha Kumar Patro, Navjot Kaur, Naresh Kumar Panda, Grace Budhiraja
Indian Journal of Otolaryngology and Head & Neck Surgery.2018; 70(4): 482. CrossRef - Case–Control Study of Papillary Thyroid Carcinoma on Urinary and Dietary Iodine Status in South Korea
Joon‐Hyop Lee, Ra‐Yeong Song, Jin Wook Yi, Hyeong Won Yu, Hyungju Kwon, Su‐jin Kim, Young Jun Chai, June Young Choi, Jae Hoon Moon, Kyu Eun Lee, Young Joo Park, Sue K. Park
World Journal of Surgery.2018; 42(5): 1424. CrossRef - Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multi-institutional study in five Asian countries
Andrey Bychkov, Somboon Keelawat, Shipra Agarwal, Deepali Jain, Chan Kwon Jung, SoonWon Hong, Chiung-Ru Lai, Shinya Satoh, Kennichi Kakudo
Pathology.2018; 50(4): 411. CrossRef - Genetic landscape of papillary thyroid carcinoma in the Chinese population
Jialong Liang, Wanshi Cai, Dongdong Feng, Huajing Teng, Fengbiao Mao, Yi Jiang, Shanshan Hu, Xianfeng Li, Yujie Zhang, Baoguo Liu, Zhong Sheng Sun
The Journal of Pathology.2018; 244(2): 215. CrossRef - Aberrant expression of CD20 in thyroid cancer and its clinicopathologic significance
Andrey Bychkov, Chan Kwon Jung
Human Pathology.2018; 71: 74. CrossRef - Molecular markers in well-differentiated thyroid cancer
Anil K. D’Cruz, Richa Vaish, Abhishek Vaidya, Iain J. Nixon, Michelle D. Williams, Vincent Vander Poorten, Fernando López, Peter Angelos, Ashok R. Shaha, Avi Khafif, Alena Skalova, Alessandra Rinaldo, Jennifer L. Hunt, Alfio Ferlito
European Archives of Oto-Rhino-Laryngology.2018; 275(6): 1375. CrossRef - Meta-Analysis Confirms the Deleterious Effects of CombinedBRAFV600Eand Telomerase Reverse Transcriptase Promoter Mutations on the Course and Mortality of Papillary Thyroid Carcinoma
Charles H. Emerson
Clinical Thyroidology.2017; 29(5): 176. CrossRef - Genetic Alterations and Their Clinical Implications in High-Recurrence Risk Papillary Thyroid Cancer
Min-Young Lee, Bo Mi Ku, Hae Su Kim, Ji Yun Lee, Sung Hee Lim, Jong-Mu Sun, Se-Hoon Lee, Keunchil Park, Young Lyun Oh, Mineui Hong, Han-Sin Jeong, Young-Ik Son, Chung-Hwan Baek, Myung-Ju Ahn
Cancer Research and Treatment.2017; 49(4): 906. CrossRef - Frequent BRAF
V600E
and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan
Naoki Oishi, Tetsuo Kondo, Tadao Nakazawa, Kunio Mochizuki, Tomohiro Inoue, Kazunari Kasai, Ippei Tahara, Tomonori Yabuta, Mitsuyoshi Hirokawa, Akira Miyauchi, Ryohei Katoh
Endocrine Pathology.2017; 28(2): 103. CrossRef - Predicting Factors for Bilaterality in Papillary Thyroid Carcinoma with Tumor Size <4 cm
Seo Ki Kim, Inhye Park, Jung-Woo Woo, Jun Ho Lee, Jun-Ho Choe, Jung-Han Kim, Jee Soo Kim
Thyroid.2017; 27(2): 207. CrossRef - Low Rate of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features in Asian Practice
Andrey Bychkov, Mitsuyoshi Hirokawa, Chan Kwon Jung, Zhiyan Liu, Yun Zhu, Soon Won Hong, Shinya Satoh, Chiung-Ru Lai, Lien Huynh, Kennichi Kakudo
Thyroid.2017; 27(7): 983. CrossRef - Changes in the clinicopathological characteristics and genetic alterations of follicular thyroid cancer
Young Shin Song, Jung Ah Lim, Hye Sook Min, Min Joo Kim, Hoon Sung Choi, Sun Wook Cho, Jae Hoon Moon, Ka Hee Yi, Do Joon Park, Bo Youn Cho, Young Joo Park
European Journal of Endocrinology.2017; 177(6): 465. CrossRef - Effects of CoexistentBRAFV600EandTERTPromoter Mutations on Poor Clinical Outcomes in Papillary Thyroid Cancer: A Meta-Analysis
Shinje Moon, Young Shin Song, Ye An Kim, Jung Ah Lim, Sun Wook Cho, Jae Hoon Moon, Seokyung Hahn, Do Joon Park, Young Joo Park
Thyroid.2017; 27(5): 651. CrossRef - TERT Promoter Mutation in an Aggressive Cribriform Morular Variant of Papillary Thyroid Carcinoma
Eun Ji Oh, Sohee Lee, Ja Seong Bae, Yourha Kim, Sora Jeon, Chan Kwon Jung
Endocrine Pathology.2017; 28(1): 49. CrossRef - TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer
Xue Yang, Jiao Li, Xiaoyi Li, Zhiyong Liang, Wen Gao, Jun Liang, Shujun Cheng, Yansong Lin
Journal of Nuclear Medicine.2017; 58(2): 258. CrossRef - Molecular Diagnosis Using Residual Liquid-Based Cytology Materials for Patients with Nondiagnostic or Indeterminate Thyroid Nodules
Hyemi Kwon, Won Gu Kim, Markus Eszlinger, Ralf Paschke, Dong Eun Song, Mijin Kim, Suyeon Park, Min Ji Jeon, Tae Yong Kim, Young Kee Shong, Won Bae Kim
Endocrinology and Metabolism.2016; 31(4): 586. CrossRef - TERT promoter mutations and long-term survival in patients with thyroid cancer
Tae Hyuk Kim, Young-Eun Kim, Soomin Ahn, Ji-Youn Kim, Chang-Seok Ki, Young Lyun Oh, Kyunga Kim, Jae Won Yun, Woong-Yang Park, Jun-Ho Choe, Jung-Han Kim, Jee Soo Kim, Sun Wook Kim, Jae Hoon Chung
Endocrine-Related Cancer.2016; 23(10): 813. CrossRef - Reply:
Y. Che
American Journal of Neuroradiology.2016; 37(1): E9. CrossRef - Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients
Young Shin Song, Jung Ah Lim, Hoonsung Choi, Jae‐Kyung Won, Jae Hoon Moon, Sun Wook Cho, Kyu Eun Lee, Young Joo Park, Ka Hee Yi, Do Joon Park, Jeong‐Sun Seo
Cancer.2016; 122(9): 1370. CrossRef
- BRAF V600E Mutation Lacks Association with Poorer Clinical Prognosis in Papillary Thyroid Carcinoma


 KES
KES

 First
First Prev
Prev



