Search
- Page Path
- HOME > Search
Original Articles
- Thyroid
- Efficacy and Safety of Long-Term Methimazole versus Radioactive Iodine in the Treatment of Toxic Multinodular Goiter
- Fereidoun Azizi, Navid Saadat, Mir Alireza Takyar, Hengameh Abdi, Ladan Mehran, Atieh Amouzegar
- Endocrinol Metab. 2022;37(6):861-869. Published online November 23, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1476

- 3,931 View
- 374 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study compared the degree of sustained control of hyperthyroidism in patients with toxic multinodular goiter (TMNG) treated with long-term methimazole (LT-MMI) or radioactive iodine (RAI).
Methods
In this clinical trial, 130 untreated patients with TMNG were randomized to either LT-MMI or RAI treatment. Both groups were followed for 108 to 148 months, with median follow-up durations of 120 and 132 months in the LT-MMI and RAI groups, respectively. Both groups of patients were followed every 1 to 3 months in the first year and every 6 months thereafter.
Results
After excluding patients in whom the treatment modality was changed and those who were lost to follow-up, 53 patients in the LT-MMI group and 54 in the RAI group completed the study. At the end of the study period, 50 (96%) and 25 (46%) patients were euthyroid, and two (4%) and 25 (46%) were hypothyroid in LT-MMI and RAI groups, respectively. In the RAI group, four (8%) patients had subclinical hyperthyroidism. The mean time to euthyroidism was 4.3±1.3 months in LT-MMI patients and 16.3± 15.0 months in RAI recipients (P<0.001). Patients treated with LT-MMI spent 95.8%±5.9% of the 12-year study period in a euthyroid state, whereas this proportion was 72.4%±14.8% in the RAI-treated patients (P<0.001). No major treatment-related adverse events were observed in either group.
Conclusion
In patients with TMNG, LT-MMI therapy is superior to RAI treatment, as shown by the earlier achievement of euthyroidism and the longer duration of sustained normal serum thyrotropin. -
Citations
Citations to this article as recorded by- Mechanism of Huatan Sanjie Fang in improving goiter in Graves' disease mice based on the Hippo signaling pathway
Huimin Yuan, Wenxin Ma, Yifei Song, Hang Wang, Shuxin Yan, Silan Hao, Xiaoyun Zhu, Yang Tang
Journal of Traditional Chinese Medical Sciences.2023; 10(3): 289. CrossRef
- Mechanism of Huatan Sanjie Fang in improving goiter in Graves' disease mice based on the Hippo signaling pathway

- Thyroid
- Clinical Outcomes of Repeated Radioactive Iodine Therapy for Graves’ Disease
- Min Joo Kim, Sun Wook Cho, Ye An Kim, Hoon Sung Choi, Young Joo Park, Do Joon Park, Bo Youn Cho
- Endocrinol Metab. 2022;37(3):524-532. Published online June 16, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1418
- 4,852 View
- 231 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
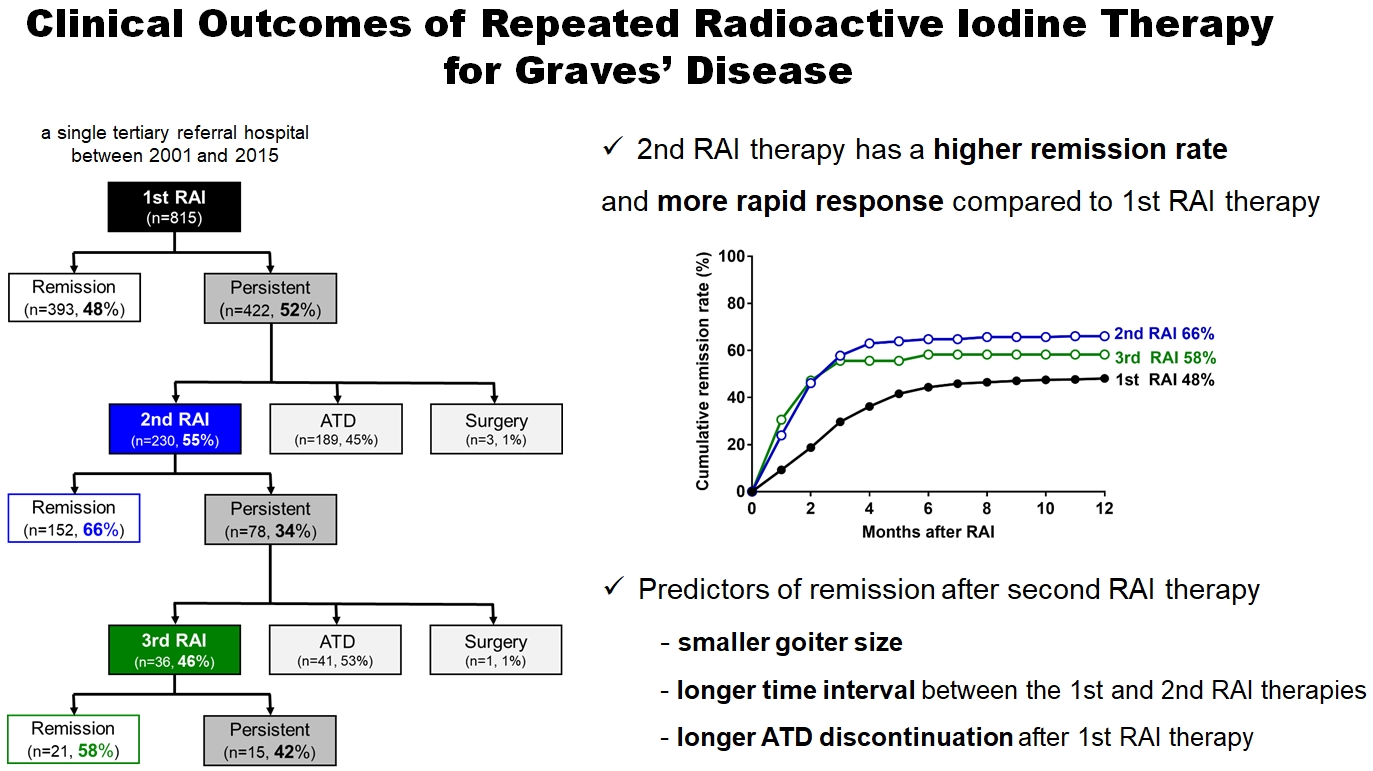
Radioactive iodine (RAI) therapy is a successful therapeutic modality for Graves’ disease. However, RAI therapy can fail, and RAI therapy after antithyroid drugs (ATDs) has a lower remission rate. Therefore, many patients require repeated RAI therapy. This study investigated the clinical outcomes of repeated RAI therapy for Graves’ disease.
Methods
Patients who underwent RAI therapy as second-line therapy after failure of ATD treatment between 2001 and 2015 were reviewed. Remission was defined as hypothyroid or euthyroid status without ATD, and with or without levothyroxine at 12 months after RAI therapy.
Results
The 1-year remission rate after 2nd RAI therapy (66%, 152/230) is significantly higher than that after 1st RAI therapy (48%, 393/815) or long-term ATD treatment after 1st RAI therapy failure (42%). The clinical response to 2nd RAI therapy was more rapid. The median time intervals from the 2nd RAI therapy to ATD discontinuation (1.3 months) and to the start of levothyroxine replacement (2.5 months) were significantly shorter than those for the 1st RAI therapy. A smaller goiter size, a longer time interval between the 1st and 2nd RAI therapies, and a longer ATD discontinuation period predicted remission after the 2nd RAI therapy. Finally, in 78 patients who failed the 2nd RAI therapy, the mean ATD dosage significantly reduced 5.1 mg over 12 months.
Conclusion
Repeated RAI therapy can be a good therapeutic option, especially in patients with smaller goiters and those who are more responsive to the 1st RAI therapy. -
Citations
Citations to this article as recorded by- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
Endocrinology and Metabolism.2023; 38(3): 338. CrossRef - Effect of liver dysfunction on outcome of radioactive iodine therapy for Graves’ disease
Yuyang Ze, Fei Shao, Xuefeng Feng, Shanmei Shen, Yan Bi, Dalong Zhu, Xiaowen Zhang
BMC Endocrine Disorders.2022;[Epub] CrossRef
- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease

- Clinical Study
- Clinical Outcomes after Early and Delayed Radioiodine Remnant Ablation in Patients with Low-Risk Papillary Thyroid Carcinoma: Propensity Score Matching Analysis
- Jonghwa Ahn, Meihua Jin, Eyun Song, Min Ji Jeon, Tae Yong Kim, Jin-Sook Ryu, Won Bae Kim, Young Kee Shong, Ji Min Han, Won Gu Kim
- Endocrinol Metab. 2020;35(4):830-837. Published online November 18, 2020
- DOI: https://doi.org/10.3803/EnM.2020.747
- 4,240 View
- 132 Download
- 3 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
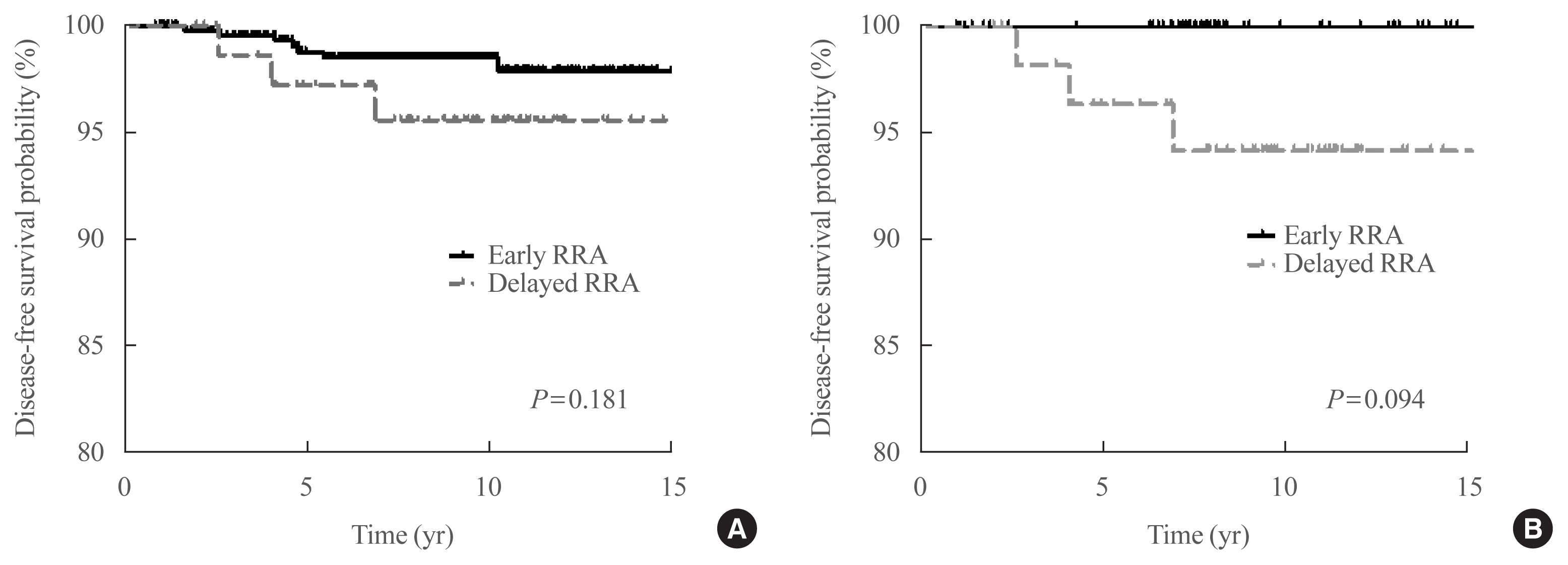
The clinical outcomes of delayed radioiodine remnant ablation (RRA) therapy in patients with low-risk papillary thyroid carcinoma (PTC) are unclear. We aimed to evaluate the clinical impact of the interval between total thyroidectomy (TT) and RRA therapy in patients with low-risk PTC.
Methods
We included 526 patients who underwent TT and RRA for low-risk PTC with a primary tumor size of >1 cm between 2000 and 2012. Patients were divided into the early (<90 days) and the delayed (≥90 days) RRA groups based on the interval between TT and RRA. The results of diagnostic whole-body scan (DxWBS), ongoing risk stratification (ORS; response to therapy), and disease-free survival (DFS) were evaluated before and after propensity score matching (PSM).
Results
Among the 526 patients, 75 (14.3%) patients underwent delayed RRA; they had more cervical lymph node metastasis and received a higher RRA dose than those who underwent early RRA. The median follow-up period was 9.1 years after initial therapy, and the structural recurrence rate was 1.9%. In DxWBS, 60 patients had focal iodine uptake limited in operative bed, with no significant difference between groups. According to ORS, 78%, 20%, 1%, and 1% patients were classified into excellent, indeterminate, biochemical incomplete, and structural incomplete response groups, respectively. There was no significant difference in ORS or DFS between groups before and after PSM.
Conclusion
The timing of the first RRA had no clinical impact in patients with low-risk PTC. Thus, the clinical decision for RRA can be determined >3 months after TT considering other prognostic factors. -
Citations
Citations to this article as recorded by- Dynamic risk assessment in patients with differentiated thyroid cancer
Erika Abelleira, Fernando Jerkovich
Reviews in Endocrine and Metabolic Disorders.2024; 25(1): 79. CrossRef - Ablation Rates and Long-Term Outcome Following Low-Dose Radioiodine for Differentiated Thyroid Cancer in the West of Scotland: A Retrospective Analysis
Kathryn Graham, Fay Tough, Helena Belikova, Irene Wotherspoon, David Colville, Nicholas Reed
Endocrine Practice.2024; 30(4): 327. CrossRef - Radioiodine ablation after thyroidectomy could be safely abandoned or postponed in selected stage I papillary thyroid carcinoma patients of low-risk group: an observational prospective study
S.M. Cherenko, A.Yu. Glagolieva, D.E. Makhmudov
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2024; 20(1): 7. CrossRef - Patient Preparation and Radiation Protection Guidance for Adult Patients Undergoing Radioiodine Treatment for Thyroid Cancer in the UK
J. Wadsley, N. Armstrong, V. Bassett-Smith, M. Beasley, R. Chandler, L. Cluny, A.J. Craig, K. Farnell, K. Garcez, N. Garnham, K. Graham, A. Hallam, S. Hill, H. Hobrough, F. McKiddie, M.W.J. Strachan
Clinical Oncology.2023; 35(1): 42. CrossRef - Delay of initial radioactive iodine therapy beyond 3 months has no effect on clinical responses and overall survival in patients with thyroid carcinoma: A cohort study and a meta‐analysis
Fang Cheng, Juan Xiao, Fengyan Huang, Chunchun Shao, Shouluan Ding, Canhua Yun, Hongying Jia
Cancer Medicine.2022; 11(12): 2386. CrossRef - Delayed (>3 Months) Postoperative Radioactive Iodine Ablation Does Not Impact Clinical Response or Survival in Differentiated Thyroid Cancers
Tatiana Fedorova, Lilah F. Morris-Wiseman
Clinical Thyroidology.2022; 34(10): 456. CrossRef
- Dynamic risk assessment in patients with differentiated thyroid cancer


 KES
KES

 First
First Prev
Prev



