Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
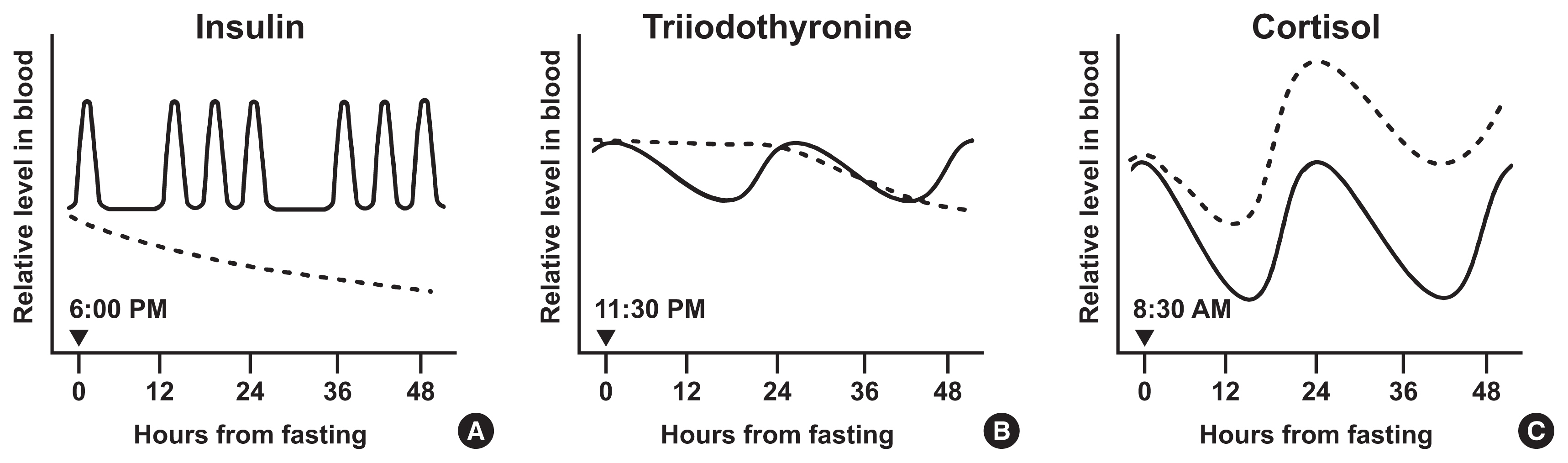
- Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones
- Bo Hye Kim, Yena Joo, Min-Seon Kim, Han Kyoung Choe, Qingchun Tong, Obin Kwon
- Endocrinol Metab. 2021;36(4):745-756. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.405
- 24,719 View
- 984 Download
- 29 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Intermittent fasting has become an increasingly popular strategy in losing weight and associated reduction in obesity-related medical complications. Overwhelming studies support metabolic improvements from intermittent fasting in blood glucose levels, cardiac and brain function, and other health benefits, in addition to weight loss. However, concerns have also been raised on side effects including muscle loss, ketosis, and electrolyte imbalance. Of particular concern, the effect of intermittent fasting on hormonal circadian rhythms has received little attention. Given the known importance of circadian hormonal changes to normal physiology, potential detrimental effects by dysregulation of hormonal changes deserve careful discussions. In this review, we describe the changes in circadian rhythms of hormones caused by intermittent fasting. We covered major hormones commonly pathophysiologically involved in clinical endocrinology, including insulin, thyroid hormones, and glucocorticoids. Given that intermittent fasting could alter both the level and frequency of hormone secretion, decisions on practicing intermittent fasting should take more considerations on potential detrimental consequences versus beneficial effects pertaining to individual health conditions.
-
Citations
Citations to this article as recorded by- Common and divergent molecular mechanisms of fasting and ketogenic diets
Antonio Paoli, Grant M. Tinsley, Mark P. Mattson, Immaculata De Vivo, Ravi Dhawan, Tatiana Moro
Trends in Endocrinology & Metabolism.2024; 35(2): 125. CrossRef - Identifying Acss1, Mtfp1 and Oxct1 as key regulators and promising biomarkers of sarcopenia in various models
Hailong Cui, Die Hu, Yanling Liu, Jiejie Zhao
Gene.2024; 896: 148053. CrossRef - Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis—Molecular Mechanisms in Human Health
Cristina Manuela Drăgoi, Alina Crenguţa Nicolae, Anca Ungurianu, Denisa Marilena Margină, Daniela Grădinaru, Ion-Bogdan Dumitrescu
Cells.2024; 13(2): 138. CrossRef - Various types of fasting diet and possible benefits in nonalcoholic fatty liver: Mechanism of actions and literature update
Zahra Sadat Mirrazavi, Vahideh Behrouz
Clinical Nutrition.2024; 43(2): 519. CrossRef - Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
Diabetes & Metabolism Journal.2024; 48(1): 37. CrossRef - Genetics of Exercise and Diet-Induced Fat Loss Efficiency: A Systematic Review
Aleksandra Bojarczuk, Emiliya S. Egorova, Magdalena Dzitkowska-Zabielska, Ildus I. Ahmetov
Journal of Sports Science and Medicine.2024; : 236. CrossRef - Ramadan fasting in the third trimester of pregnancy and postpartum colostrum cortisol concentrations in Morocco
Meagan M. Guilfoyle
American Journal of Human Biology.2024;[Epub] CrossRef - Dietary factors in circadian rhythm modulation and their impact on metabolic diseases: a state of the science review
Malvika Dalvi, Srujana Medithi
Biological Rhythm Research.2024; : 1. CrossRef - Unlocking the Benefits of Fasting: A Review of its Impact on Various
Biological Systems and Human Health
Rawan Mackieh, Nadia Al-Bakkar, Milena Kfoury, Nathalie Okdeh, Hervé Pietra, Rabih Roufayel, Christian Legros, Ziad Fajloun, Jean-Marc Sabatier
Current Medicinal Chemistry.2024; 31(14): 1781. CrossRef - Fasting intervention and its clinical effects on the human host and microbiome
Sofia K. Forslund
Journal of Internal Medicine.2023; 293(2): 166. CrossRef - Umbrella review of time-restricted eating on weight loss, fasting blood glucose, and lipid profile
Han Shi Jocelyn Chew, Wei How Darryl Ang, Zhen Yang Abel Tan, Wen Wei Ang, Kin Sun Chan, Ying Lau
Nutrition Reviews.2023; 81(9): 1180. CrossRef - Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity
Melek Ece Öngel, Cennet Yildiz, Özge Başer, Bayram Yilmaz, Mustafa Özilgen
Entropy.2023; 25(2): 227. CrossRef - Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats
Cinthia García-Luna, Ixchel Prieto, Paulina Soberanes-Chávez, Elena Alvarez-Salas, Iván Torre-Villalvazo, Gilberto Matamoros-Trejo, Patricia de Gortari
Nutrients.2023; 15(5): 1164. CrossRef - Possible homeostatic, glucose uptake mechanisms and hepato-pancreatic histological effects of intermittent fasting, exercise, starvation, and honey in streptozotocin-induced diabetes in rats
Ejime A. Chijiokwu, Eze K. Nwangwa, Mega O. Oyovwi, Benneth Ben-Azu, Alexander O. Naiho, Emuesiri Goodies Moke, Victor Emojevwe, Prosper A. Ehiwarior, Udoka S. Nwabuoku
Nutrire.2023;[Epub] CrossRef - Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice
Momoko Imamura, Hiroyuki Sasaki, Katsuki Hayashi, Shigenobu Shibata
Nutrients.2023; 15(7): 1679. CrossRef - All That Glitters Is Not Gold: The Same Sleep Time, but Different Diabetogenic Outcomes
Bohye Kim, Obin Kwon
Endocrinology and Metabolism.2023; 38(1): 78. CrossRef - The emerging role of circadian rhythms in the development and function of thermogenic fat
Xuemin Peng, Yong Chen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Time-restricted Feeding Changes as Inspiration for Drug Design
Zhangyuting He, Huayu Yang, Yilei Mao
Current Pharmaceutical Design.2023; 29(8): 559. CrossRef - Brain Dopamine–Clock Interactions Regulate Cardiometabolic Physiology: Mechanisms of the Observed Cardioprotective Effects of Circadian-Timed Bromocriptine-QR Therapy in Type 2 Diabetes Subjects
Anthony H. Cincotta
International Journal of Molecular Sciences.2023; 24(17): 13255. CrossRef - Adaptive Circadian Rhythms for Autonomous and Biologically Inspired Robot Behavior
Marcos Maroto-Gómez, María Malfaz, Álvaro Castro-González, Sara Carrasco-Martínez, Miguel Ángel Salichs
Biomimetics.2023; 8(5): 413. CrossRef - Intermittent Fasting on Human Health and Disease
Denisa Marilena Margină, Cristina Manuela Drăgoi
Nutrients.2023; 15(21): 4491. CrossRef - Synthetic augmentation of bilirubin metabolism in human pluripotent stem cell-derived liver organoids
Hasan Al Reza, Zishaan Farooqui, Abid Al Reza, Callen Conroy, Kentaro Iwasawa, Yasuhiro Ogura, Keisuke Okita, Kenji Osafune, Takanori Takebe
Stem Cell Reports.2023; 18(11): 2071. CrossRef - Average phenotype but not plasticity in two metabolic hormones covary in wild female bonobos (Pan paniscus)
Ruth Sonnweber, Gottfried Hohmann, Jeroen M. G. Stevens, Tobias Deschner, Barbara Fruth, Anna-Lena Fiedler, Niina O. Nurmi, Verena Behringer
Frontiers in Ecology and Evolution.2023;[Epub] CrossRef - Intermittent fasting, high-intensity interval training, or a combination of both have beneficial effects in obese mice with nonalcoholic fatty liver disease
Patrícia de Castro-de-Paiva, Thatiany de Souza Marinho, Carlos Alberto Mandarim-de-Lacerda, Marcia Barbosa Aguila
The Journal of Nutritional Biochemistry.2022; 104: 108997. CrossRef - Optimal Timing of Thyroid Hormone Replacement During Ramadan Fasting: A Randomized Controlled Trial in Patients with Prior Total Thyroidectomy
Khalid M. Al-Qahtani, Ibraheem Ahmed Aldeeri, Amal M. Alshaibi, Norah Salman Alshabib, Rakan M. Barghouthi, Ebtihal Y. Alyusuf, Anwar Ali Jammah
Thyroid.2022; 32(9): 1029. CrossRef - Exploring the Effects of Energy Constraints on Performance, Body Composition, Endocrinological/Hematological Biomarkers, and Immune System among Athletes: An Overview of the Fasting State
Hadi Nobari, Saber Saedmocheshi, Eugenia Murawska-Ciałowicz, Filipe Manuel Clemente, Katsuhiko Suzuki, Ana Filipa Silva
Nutrients.2022; 14(15): 3197. CrossRef - Alternate day fasting and time-restricted feeding may confer similar neuroprotective effects during aging in male rats
Sukanya Bhoumik, Rashmi Kesherwani, Raushan Kumar, Syed Ibrahim Rizvi
Biogerontology.2022; 23(6): 757. CrossRef - Intermittent Fasting—A Healthy Dietary Pattern for Diabetic Nephropathy
Ming Yang, Wei Chen, Liyu He, Di Liu, Li Zhao, Xi Wang
Nutrients.2022; 14(19): 3995. CrossRef - β-hydroxybutyrate as an Anti-Aging Metabolite
Lian Wang, Peijie Chen, Weihua Xiao
Nutrients.2021; 13(10): 3420. CrossRef
- Common and divergent molecular mechanisms of fasting and ketogenic diets

- Clinical Study
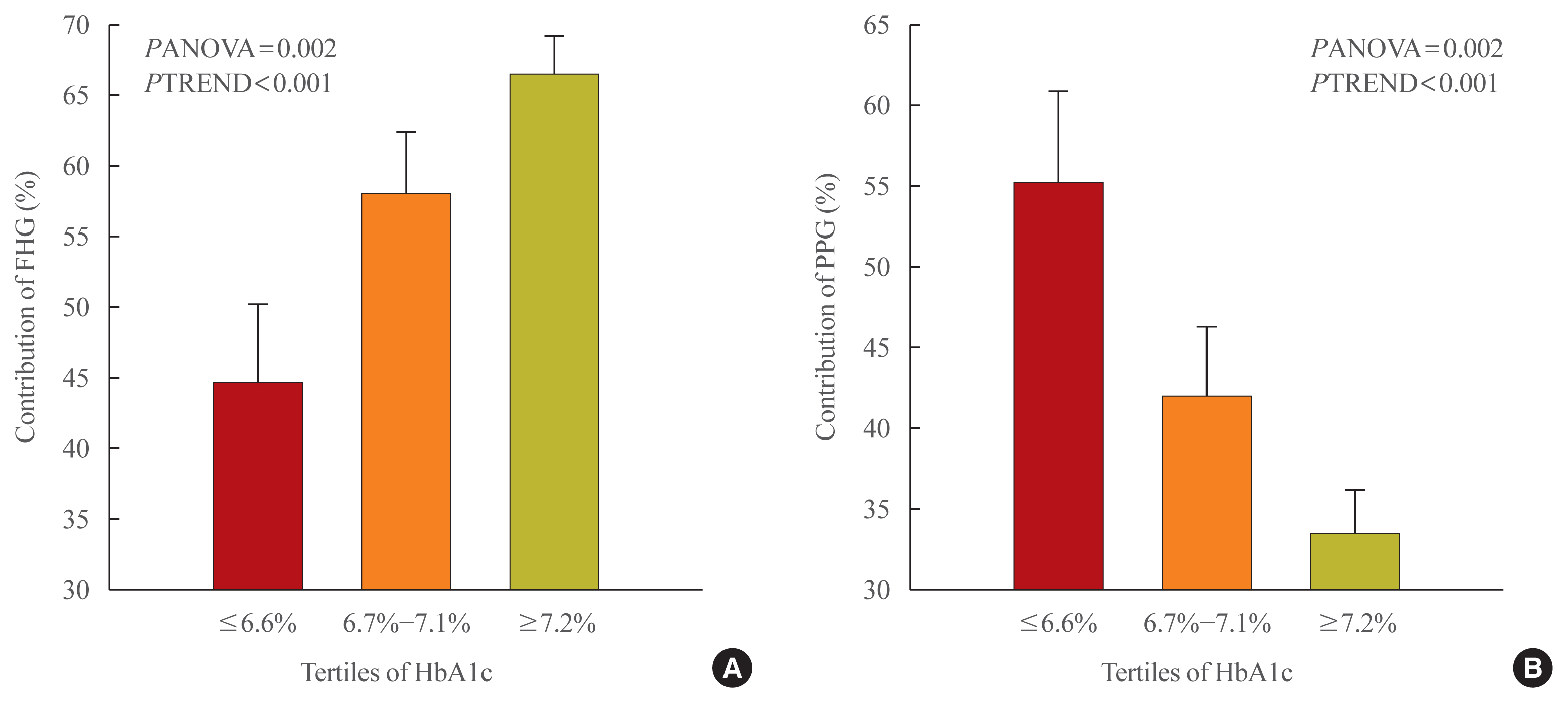
- Fasting and Postprandial Hyperglycemia: Their Predictors and Contributions to Overall Hyperglycemia in Korean Patients with Type 2 Diabetes
- Jaecheol Moon, Ji Young Kim, Soyeon Yoo, Gwanpyo Koh
- Endocrinol Metab. 2020;35(2):290-297. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.290
- 6,891 View
- 201 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
This study aimed to identify factors that affect fasting hyperglycemia (FHG) and postprandial hyperglycemia (PPG) and their contributions to overall hyperglycemia in Korean patients with type 2 diabetes mellitus (T2DM).
Methods
This was a retrospective study conducted on 194 Korean T2DM patients with 7-point self-monitoring blood glucose (SMBG) profiles plotted in 4 days in 3 consecutive months. We calculated the areas corresponding to FHG and PPG (area under the curve [AUC]FHG and AUCPPG) and contributions (%) in the graph of the 7-point SMBG data. The levels of glycated hemoglobin (HbA1c) were categorized by tertiles, and the contributions of FHG and PPG were compared.
Results
The relative contribution of FHG increased (44.7%±5.6%, 58.0%±4.4%, 66.5%±2.8%; PANOVA=0.002, PTREND <0.001), while that of PPG decreased (55.3%±5.5%, 42.0%±4.4%, 33.5%±2.8%; PANOVA=0.002, PTREND <0.001) with the elevated HbA1c. Multivariate analysis showed that HbA1c (β=0.615, P<0.001), waist circumference (β=0.216, P=0.042), and triglyceride (β=0.121, P=0.048) had a significant association with AUCFHG. Only HbA1c (β=0.231, P=0.002) and age (β=0.196, P=0.009) was significantly associated with AUCPPG.
Conclusion
The data suggested that in Korean T2DM patients, FHG predominantly contributed to overall hyperglycemia at higher HbA1c levels, whereas it contributed to PPG at lower HbA1c levels. It is recommended that certain factors, namely age, degree of glycemic control, obesity, or triglyceride levels, should be considered when prescribing medications for T2DM patients. -
Citations
Citations to this article as recorded by- Prospective study of the association between chronotype and cardiometabolic risk among Chinese young adults
Tingting Li, Yang Xie, Shuman Tao, Liwei Zou, Yajuan Yang, Fangbiao Tao, Xiaoyan Wu
BMC Public Health.2023;[Epub] CrossRef - Effects of mulberry twig alkaloids(Sangzhi alkaloids) and metformin on blood glucose fluctuations in combination with premixed insulin-treated patients with type 2 diabetes
Ziyu Meng, Chengye Xu, Haoling Liu, Xinyuan Gao, Xinyu Li, Wenjian Lin, Xuefei Ma, Changwei Yang, Ming Hao, Kangqi Zhao, Yuxin Hu, Yi Wang, Hongyu Kuang
Frontiers in Endocrinology.2023;[Epub] CrossRef - Evaluating Triglyceride and Glucose Index as a Simple and Easy-to-Calculate Marker for All-Cause and Cardiovascular Mortality
Kyung-Soo Kim, Sangmo Hong, You-Cheol Hwang, Hong-Yup Ahn, Cheol-Young Park
Journal of General Internal Medicine.2022; 37(16): 4153. CrossRef - A new approach for investigating the relative contribution of basal glucose and postprandial glucose to HbA1C
Jing Ma, Hua He, Xiaojie Yang, Dawei Chen, Cuixia Tan, Li Zhong, Qiling Du, Xiaohua Wu, Yunyi Gao, Guanjian Liu, Chun Wang, Xingwu Ran
Nutrition & Diabetes.2021;[Epub] CrossRef - The Clinical Characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Endocrinology and Metabolism.2021; 36(3): 628. CrossRef
- Prospective study of the association between chronotype and cardiometabolic risk among Chinese young adults

- Diabetes
- Association between White Blood Cell Counts within Normal Range and Hemoglobin A1c in a Korean Population
- Jae Won Hong, Jung Hyun Noh, Dong-Jun Kim
- Endocrinol Metab. 2018;33(1):79-87. Published online January 30, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.1.79
- 4,588 View
- 51 Download
- 5 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background We examined whether white blood cell (WBC) count levels within normal range, could be associated with hemoglobin A1c (HbA1c) levels.
Methods Among the 11,472 people (≥19 years of age) who participated in the 2011 to 2012 Korea National Health and Nutrition Examination, subjects with chronic disease or illness, including 807 patients with diabetes currently taking anti-diabetic medications and/or 1,149 subjects with WBC levels <4,000 or >10,000/µL were excluded.
Results Overall, adjusted HbA1c levels increased across the WBC quartiles (5.55%±0.01%, 5.58%±0.01%, 5.60%±0.01%, and 5.65%±0.01%,
P <0.001) after adjusting for confounding factors, such as age, gender, fasting plasma glucose, college graduation, smoking history, waist circumference, presence of hypertension, serum total cholesterol, serum triglyceride, and presence of anemia. The adjusted proportions (%) of HbA1c levels of ≥5.7%, ≥6.1%, and ≥6.5% showed significant increases across WBC quartiles (P <0.001,P =0.002, andP =0.022, respectively). Logistic regression analyses of WBC quartiles for the risk of HbA1c levels of ≥5.7%, ≥6.1%, and ≥6.5%, using the variables above as covariates, showed that the odds ratios of the fourth quartile of WBCs were 1.59 (95% confidence interval [CI], 1.35 to 1.89;P <0.001), 1.78 (95% CI, 1.31 to 2.42;P <0.001), and 2.03 (95% CI, 1.13 to 3.64;P =0.018), using the first quartile of WBCs as the reference.Conclusion HbA1c levels were positively associated with WBC levels within normal range in a general adult population.
-
Citations
Citations to this article as recorded by- Vitamin D supplementation modulates glycated hemoglobin (HBA1c) in diabetes mellitus
Asma Akhter, Sultan Alouffi, Uzma Shahab, Rihab Akasha, Mohd Fazal-Ur-Rehman, Mohamed E. Ghoniem, Naved Ahmad, Kirtanjot Kaur, Ramendra Pati Pandey, Ahmed Alshammari, Firoz Akhter, Saheem Ahmad
Archives of Biochemistry and Biophysics.2024; 753: 109911. CrossRef - Glucose indices as inflammatory markers in children with acute surgical abdomen: a cross-sectional study
Hoda Atef Abdelsattar Ibrahim, Sherif Kaddah, Sara Mohamed Elkhateeb, Abeer Aboalazayem, Aya Ahmed Amin, Mahmoud Marei Marei
Annals of Medicine.2023;[Epub] CrossRef - Factors associated with relative muscle strength in patients with type 2 diabetes mellitus
Chiao-Nan Chen, Ting-Chung Chen, Shiow-Chwen Tsai, Chii-Min Hwu
Archives of Gerontology and Geriatrics.2021; 95: 104384. CrossRef - Non-vascular contributing factors of diabetic foot ulcer severity in national referral hospital of Indonesia
Em Yunir, Dicky L. Tahapary, Tri Juli Edi Tarigan, Dante Saksono Harbuwono, Yoga Dwi Oktavianda, Melly Kristanti, Eni Iswati, Angela Sarumpaet, Pradana Soewondo
Journal of Diabetes & Metabolic Disorders.2021; 20(1): 805. CrossRef - Association between Inflammatory Markers and Glycemic Control in Korean Diabetic Patients
Min Kang, Seok-Joon Sohn
Journal of Health Informatics and Statistics.2021; 46(3): 247. CrossRef - Prediabetes Is Independently Associated with Subclinical Carotid Atherosclerosis: An Observational Study in a Non-Urban Mediterranean Population
Maria Belén Vilanova, Josep Franch-Nadal, Mireia Falguera, Josep Ramon Marsal, Sílvia Canivell, Esther Rubinat, Neus Miró, Àngels Molló, Manel Mata-Cases, Mònica Gratacòs, Esmeralda Castelblanco, Dídac Mauricio
Journal of Clinical Medicine.2020; 9(7): 2139. CrossRef
- Vitamin D supplementation modulates glycated hemoglobin (HBA1c) in diabetes mellitus

- Adrenal gland
- Association between the Growth Hormone Receptor Exon 3 Polymorphism and Metabolic Factors in Korean Patients with Acromegaly
- Hye Yoon Park, In Ryang Hwang, Jung Bum Seo, Su Won Kim, Hyun Ae Seo, In Kyu Lee, Jung Guk Kim
- Endocrinol Metab. 2015;30(3):312-317. Published online January 5, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.3.312
- 3,900 View
- 35 Download
- 7 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background This study investigated the association between the frequency of growth hormone receptor (GHR) exon 3 polymorphism (exon 3 deletion; d3-GHR) and metabolic factors in patients with acromegaly in Korea.
Methods DNA was extracted from the peripheral blood of 30 unrelated patients with acromegaly. GHR genotypes were evaluated by polymerase chain reaction and correlated with demographic data and laboratory parameters.
Results No patient had the d3/d3 genotype, while four (13.3%) had the d3/fl genotype, and 26 (86.7%) had the fl/fl genotype. Body mass index (BMI) in patients with the d3/fl genotype was significantly higher than in those with the fl/fl genotype (
P =0.001). Age, gender, blood pressure, insulin-like growth factor-1, growth hormone, fasting plasma glucose, triglycerides, high density lipoprotein cholesterol, and low density lipoprotein cholesterol levels showed no significant differences between the two genotypes.Conclusion The d3-GHR polymorphism may be associated with high BMI but not with other demographic characteristics or laboratory parameters.
-
Citations
Citations to this article as recorded by- The Exon 3-Deleted Growth Hormone Receptor (d3GHR) Polymorphism—A Favorable Backdoor Mechanism for the GHR Function
Ghadeer Falah, Lital Sharvit, Gil Atzmon
International Journal of Molecular Sciences.2023; 24(18): 13908. CrossRef - Gender Specificity and Local Socioeconomic Influence on Association of GHR fl/d3 Polymorphism With Growth and Metabolism in Children and Adolescents
Xiaotian Chen, Chunlan Liu, Song Yang, Yaming Yang, Yanchun Chen, Xianghai Zhao, Weiguang Zhu, Qihui Zhao, Chuan Ni, Xiangyuan Huang, Weili Yan, Chong Shen, Harvest F. Gu
Frontiers in Pediatrics.2022;[Epub] CrossRef - Exon 3-deleted growth hormone receptor isoform is not related to worse bone mineral density or microarchitecture or to increased fracture risk in acromegaly
J. Pontes, M. Madeira, C. H. A. Lima, L. L. Ogino, F. de Paula Paranhos Neto, L. M. C. de Mendonça, M. L. F. Farias, L. Kasuki, M. R. Gadelha
Journal of Endocrinological Investigation.2020; 43(2): 163. CrossRef - MECHANISMS IN ENDOCRINOLOGY: Lessons from growth hormone receptor gene-disrupted mice: are there benefits of endocrine defects?
Reetobrata Basu, Yanrong Qian, John J Kopchick
European Journal of Endocrinology.2018; 178(5): R155. CrossRef - MECHANISMS IN ENDOCRINOLOGY: Clinical and pharmacogenetic aspects of the growth hormone receptor polymorphism
Cesar L Boguszewski, Edna J L Barbosa, Per-Arne Svensson, Gudmundur Johannsson, Camilla A M Glad
European Journal of Endocrinology.2017; 177(6): R309. CrossRef
- The Exon 3-Deleted Growth Hormone Receptor (d3GHR) Polymorphism—A Favorable Backdoor Mechanism for the GHR Function

- Changes in Growth Hormone-Axis Function in Nutrient Excess or Deprivation.
- Seungjoon Park
- Endocrinol Metab. 2011;26(4):279-284. Published online December 1, 2011
- DOI: https://doi.org/10.3803/EnM.2011.26.4.279
- 1,577 View
- 34 Download
-
 Abstract
Abstract
 PDF
PDF - Growth hormone (GH) is produced in a select population of cells, somatotropes, located in the anterior pituitary gland. GH is released into the general circulation where it interacts with multiple peripheral tissues through its receptor, GH receptor, to regulate growth and metabolic function. GH-releasing hormone (GHRH) and somatostatin are the primary positive and negative regulators of GH secretion, respectively. More recently, ghrelin has emerged as an additional stimulatory hormone for GH release. In humans, GH levels decrease in states of nutrient excess, such as obesity, and increase in response to nutrient deprivation, such as fasting, type 1 diabetes, and anorexia nervosa. Considering that GH regulates metabolism of carbohydrate, lipid, and protein, clarifying the mechanisms by which metabolic changes alter pituitary GH synthesis and secretion will increase our knowledge on the pathophysiology and treatment of metabolic diseases. In this review, the effect of nutrient excess and nutrient deficiency on GH-axis function in humans and other mammals will be summarized, with particular emphasis on studies exploring the direct effects of systemic signals, including insulin-like growth factor 1 (IGF-1) and insulin, on somatotrope function. Additionally, new mouse models with somatotrope-specific knockout of IGF-1 and insulin receptors generated by using the Cre/loxP system will be discussed.

- The Effect of Leptin Level Fluctuations by a Repeated Fasting/Refeeding on the Leptin Sensitivity in OLETF Rats.
- Sung Chul Park, Yong Hoon Park, So Young Park, Jong Yeon Kim, Yoon Ki Park, Tae Hyung Lee, Kyu Chang Won, Yong Woon Kim
- J Korean Endocr Soc. 2008;23(5):310-318. Published online October 1, 2008
- DOI: https://doi.org/10.3803/jkes.2008.23.5.310
- 1,941 View
- 30 Download
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Leptin resistance is a common feature in obese subjects and animals, and this is commonly accompanied with hyperleptinemia. We speculated that one of the causes of leptin resistance is a persistently elevated leptin concentration and then we hypothesized that fluctuations of serum leptin would increase leptin sensitivity in the leptin-resistant state. METHODS: We used a repeated fasting and refeeding (RFR) protocol to produce fluctuation in leptin levels in 7 month-old Otsuka Long-Evans Tokushima Fatty (OLETF) rats and Long-Evans Tokushima Otsuka (LETO) rats, We then measured the leptin sensitivity following an intracerebroventricular (i.c.v.) infusion of leptin. RESULTS: The OLETF rats exhibited severe visceral fat deposition, hyperleptinemia and leptin resistance. However, in the OLETF-RFR rats, the anorexic effect following i.c.v. leptin infusion was restored. Moreover, the visceral fat mass and serum leptin levels decreased, while the serum adiponectin levels were elevated in the OLETF-RFR rats compared to the OLETF-Control rats. The leptin receptor content in the hypothalamus increased in the OLETF-RFR rats compared to the OLETF-Control rats, and the leptin receptor content in the OLETF-RFR rats decreased compared to that in the the LETO-Control rats. CONCLUSION: These results suggest that the intermittent suppression of the serum leptin level reversed the leptin resistance in OLEFT rats, and this may have occurred due to an increased number of leptin receptors in the hypothalamus. -
Citations
Citations to this article as recorded by- Reduced Striatal Dopamine Transporter Availability and Heightened Response to Natural and Pharmacological Stimulation in CCK-1R-Deficient Obese Rats
Sevag Hamamah, Andras Hajnal, Mihai Covasa
International Journal of Molecular Sciences.2023; 24(11): 9773. CrossRef - Improvement of Leptin Resistance
Yong Woon Kim
Yeungnam University Journal of Medicine.2013; 30(1): 4. CrossRef - The Effect of Food Restriction on Appetite Regulating Hormones and Adiponectin Activity
Ki Hoon Kim, Hyun Kook Kim
Korean Journal of Nutrition.2012; 45(1): 5. CrossRef - The Effect of Leptin Level Fluctuations by a Repeated Fasting/Refeeding on the Leptin Sensitivity in OLETF Rats
Min Seon Kim
Journal of Korean Endocrine Society.2008; 23(5): 298. CrossRef
- Reduced Striatal Dopamine Transporter Availability and Heightened Response to Natural and Pharmacological Stimulation in CCK-1R-Deficient Obese Rats

- Changes in Hypothalamic-pituitary-growth Hormone (GH) Axis by Fasting: Study on the Differences between Male and Female Rats.
- Sookjin Sohn, Mina Lee, Seungjoon Park
- J Korean Endocr Soc. 2002;17(4):473-485. Published online August 1, 2002
- 918 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Fasting has a profound impact on GH synthesis, and is released in all mammalian species that have been studied. The male rat has long been used as a model to determine the mechanism on how fasting mediates these changes. However, many aspects of GH synthesis, release and function are known to be gender-dependent. This study was conducted in order to determine if changes in the GH-axis, in response to fasting, differs between the sexes. METHODS: Male and female rats (8~9 weeks; n=5/group) were fasted for 72h, or supplied food ad libitum. The mean circulating serum GH and IGF-I concentrations were measured by radioimmunoassay. The levels of hypothalamic GH-releasing hormone (GHRH), somatostatin (SRIF), neuropeptide Y (NPY) and pituitary GH mRNA were measured using an RNase protection assay. The levels of pituitary GHRH receptor (GHRH-R), GH secretagogue (GHS) receptor (GHS-R) and SRIF receptor (sst1-5) mRNA were measured by reverse transcription-polymerase chain reaction (RT-PCR). RESULTS: Fasting resulted in a comparable weight loss in both the males and the females, (18.0+/-0.9%) and (17.0 0.8%), respectively. In the fasted males, there was a characteristic decrease in the serum GH (98 60 vs. 7 4 ng/mL) and IGF-I (367 35 vs 152 12 ng/mL), associated with a decrease in the hypothalamic GHRH, and an increase in the NPY mRNA, levels of 52 6% and 138 6%, respectively, compared to those of the fed controls (p<0.05). In spite of the reduction in the GHRH, fasting did not alter the levels of the pituitary GH mRNA, and in fact increased the expression of the pituitary receptors, GHRH-R and GHS-R, to 185 15 and 169 25%, respectively, to those of the fed controls. In contrast to the positive impact of fasting on the GH-stimulatory receptors, fasting led to a dramatic decrease in the expressions of the somatostatin receptor subtypes, sst2 (29+/-5% of Fed) and sst4 (60+/-7% of Fed). Fasting had comparable effects on the GH-axis of the female rats, with two notable exceptions; first, fasting did not suppress the mean circulating GH levels (16 3 vs. 38 28 ng/mL) and second, did not alter the sst2 and sst4 expressions. CONCLUSION: These results corroborate the other reports regarding the effects of fasting on the expressions of hypothalamic neuropeptides, pituitary GHRH-R and sst2, in male rats. This is the first report demonstrating that fasting stimulates the expression of pituitary GHS-R in both sexes. This is of great interest given the fact that ghrelin, the putative GHS-R ligand, is also elevated by fasting. We propose that the upregulation of both ghrelin and GHS-R may play important roles in increasing the sensitivity of the pituitary to GHRH, in that these GH-stimulatory systems work synergistically. These changes may compensate for the fasting-induced suppression of hypothalamic GHRH input. We might speculate that such compensatory mechanisms are dominant in the female rat, in that circulating GH levels are not suppressed by fasting.


 KES
KES

 First
First Prev
Prev



