Search
- Page Path
- HOME > Search
- Thyroid
Thyroid Cancer Screening - Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
- Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chul-Min Kim
- Endocrinol Metab. 2024;39(2):310-323. Published online April 9, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1870
- 996 View
- 12 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
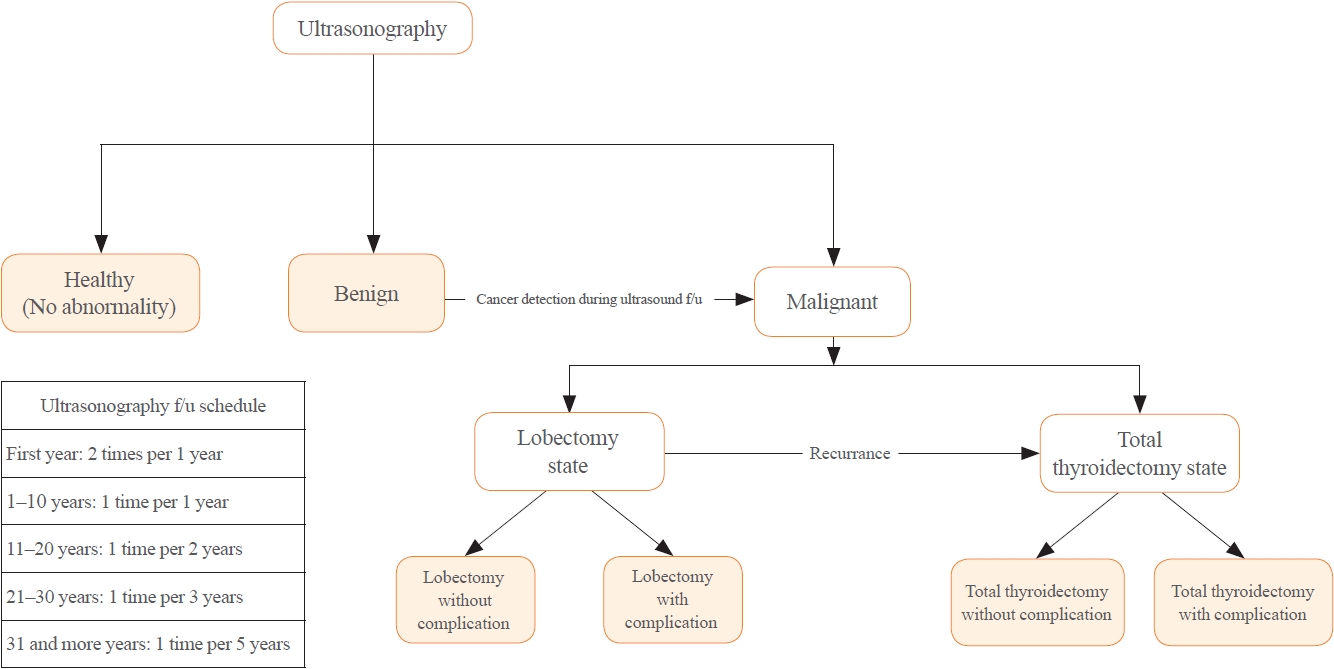
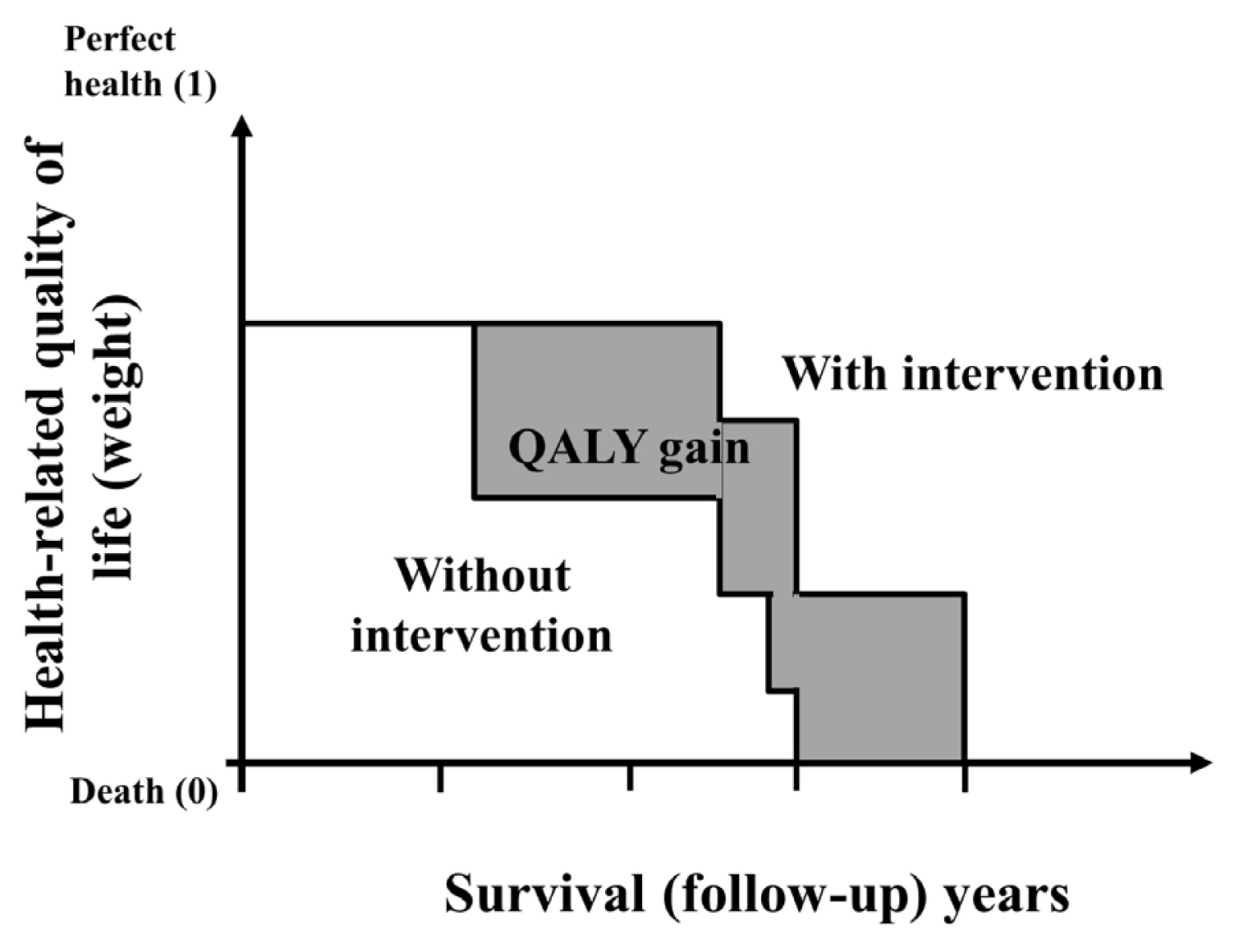
There is debate about ultrasonography screening for thyroid cancer and its cost-effectiveness. This study aimed to evaluate the cost-effectiveness of early screening (ES) versus symptomatic detection (SD) for differentiated thyroid cancer (DTC) in Korea.
Methods
A Markov decision analysis model was constructed to compare the cost-effectiveness of ES and SD. The model considered direct medical costs, health outcomes, and different diagnostic and treatment pathways. Input data were derived from literature and Korean population studies. Incremental cost-effectiveness ratio (ICER) was calculated. Willingness-to-pay (WTP) threshold was set at USD 100,000 or 20,000 per quality-adjusted life year (QALY) gained. Sensitivity analyses were conducted to address uncertainties of the model’s variables.
Results
In a base case scenario with 50 years of follow-up, ES was found to be cost-effective compared to SD, with an ICER of $2,852 per QALY. With WTP set at $100,000, in the case with follow-up less than 10 years, the SD was cost-effective. Sensitivity analysis showed that variables such as lobectomy probability, age, mortality, and utility scores significantly influenced the ICER. Despite variations in costs and other factors, all ICER values remained below the WTP threshold.
Conclusion
Findings of this study indicate that ES is a cost-effective strategy for DTC screening in the Korean medical system. Early detection and subsequent lobectomy contribute to the cost-effectiveness of ES, while SD at an advanced stage makes ES more cost-effective. Expected follow-up duration should be considered to determine an optimal strategy for DTC screening.

- Thyroid
- Active Surveillance for Low-Risk Papillary Thyroid Carcinoma as an Acceptable Management Option with Additional Benefits: A Comprehensive Systematic Review
- Jee Hee Yoon, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2024;39(1):152-163. Published online January 22, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1794

- 1,184 View
- 42 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Active surveillance (AS) has been introduced as a management strategy for low-risk papillary thyroid carcinoma (PTC) due to its typically indolent nature. Despite this, the widespread adoption of AS has encountered several challenges. The aim of this systematic review was to evaluate the safety of AS related to disease progression and its benefits compared with immediate surgery (IS).
Methods
Studies related to AS in patients with low-risk PTC were searched through the Ovid MEDLINE, Embase, Cochrane Library, and KoreaMed databases. Studies on disease progression, surgical complication, quality of life (QoL), and cost-effectiveness were separately analyzed and narratively synthesized.
Results
In the evaluation of disease progression, the proportions of cases with tumor growth ≥3 mm and a volume increase >50% were 2.2%–10.8% and 16.0%–25.5%, respectively. Newly detected lymph node metastasis was identified in 0.0%–1.4% of patients. No significant difference was found between IS and delayed surgery in surgical complications, including vocal cord paralysis and postoperative hypoparathyroidism. AS was associated with better QoL than IS. Studies on the cost-effectiveness of AS reported inconsistent data, but AS was more cost-effective when quality-adjusted life years were considered.
Conclusion
AS is an acceptable management option for patients with low-risk PTC based on the low rate of disease progression and the absence of an increased mortality risk. AS has additional benefits, including improved QoL and greater QoL-based cost-effectiveness. -
Citations
Citations to this article as recorded by- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Kyeong Jin Kim
Endocrinology and Metabolism.2024; 39(1): 95. CrossRef
- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma

- Adrenal gland
Big Data Articles (National Health Insurance Service Database) - Mortality and Severity of Coronavirus Disease 2019 in Patients with Long-Term Glucocorticoid Therapy: A Korean Nationwide Cohort Study
- Eu Jeong Ku, Keeho Song, Kyoung Min Kim, Gi Hyeon Seo, Soon Jib Yoo
- Endocrinol Metab. 2023;38(2):253-259. Published online March 21, 2023
- DOI: https://doi.org/10.3803/EnM.2022.1607
- 2,633 View
- 103 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
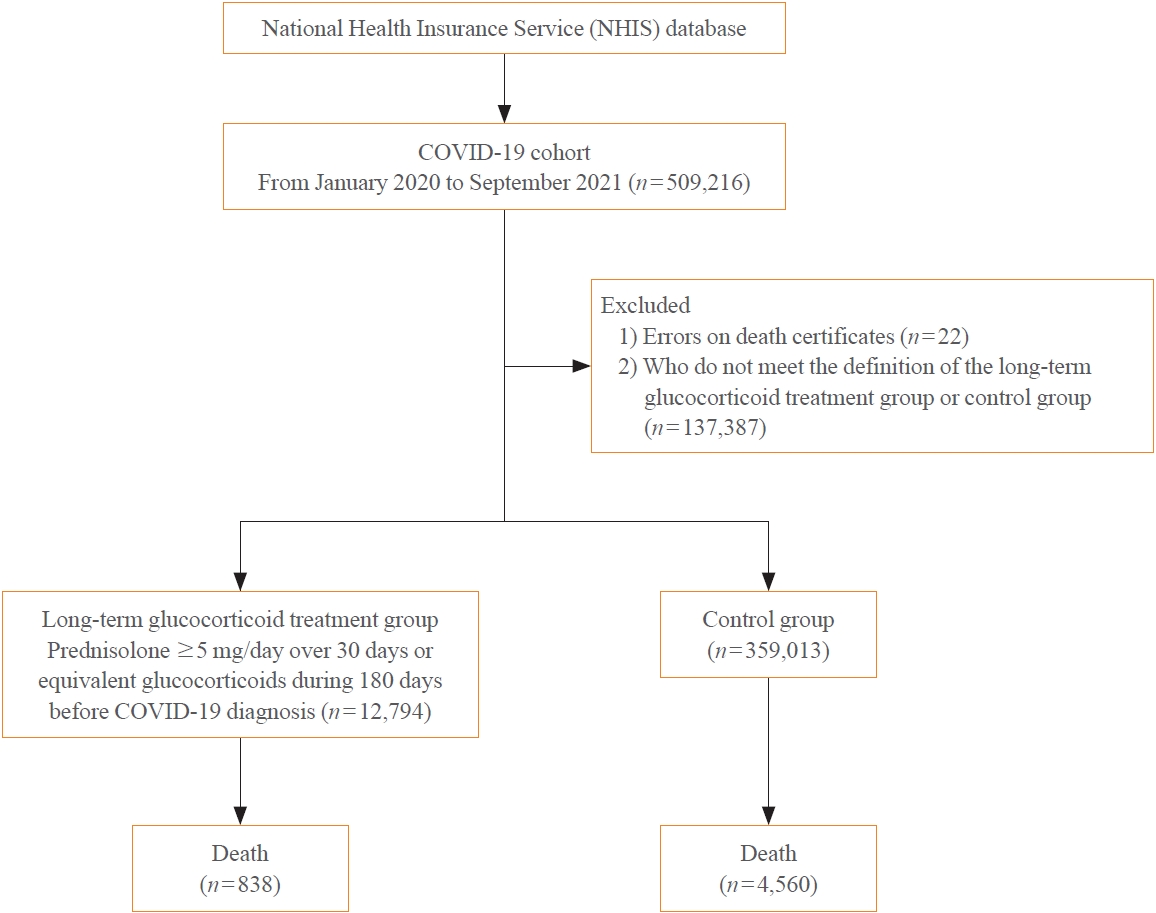
The severity of coronavirus disease 2019 (COVID-19) among patients with long-term glucocorticoid treatment (LTGT) has not been established. We aimed to evaluate the association between LTGT and COVID-19 prognosis.
Methods
A Korean nationwide cohort database of COVID-19 patients between January 2019 and September 2021 was used. LTGT was defined as exposure to at least 150 mg of prednisolone (≥5 mg/day and ≥30 days) or equivalent glucocorticoids 180 days before COVID-19 infection. The outcome measurements were mortality, hospitalization, intensive care unit (ICU) admission, length of stay, and mechanical ventilation.
Results
Among confirmed patients with COVID-19, the LTGT group (n=12,794) was older and had a higher proportion of comorbidities than the control (n=359,013). The LTGT group showed higher in-hospital, 30-day, and 90-day mortality rates than the control (14.0% vs. 2.3%, 5.9% vs. 1.1%, and 9.9% vs. 1.8%, respectively; all P<0.001). Except for the hospitalization rate, the length of stay, ICU admission, and mechanical ventilation proportions were significantly higher in the LTGT group than in the control (all P<0.001). Overall mortality was higher in the LTGT group than in the control group, and the significance remained in the fully adjusted model (odds ratio [OR], 5.75; 95% confidence interval [CI], 5.31 to 6.23) (adjusted OR, 1.82; 95% CI, 1.67 to 2.00). The LTGT group showed a higher mortality rate than the control within the same comorbidity score category.
Conclusion
Long-term exposure to glucocorticoids increased the mortality and severity of COVID-19. Prevention and early proactive measures are inevitable in the high-risk LTGT group with many comorbidities. -
Citations
Citations to this article as recorded by- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy
Eun-Hee Cho
Endocrinology and Metabolism.2023; 38(2): 223. CrossRef - Pituitary Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study
Jeonghoon Ha, Kyoung Min Kim, Dong-Jun Lim, Keeho Song, Gi Hyeon Seo
Journal of Clinical Medicine.2023; 12(14): 4799. CrossRef
- Glucocorticoids as a Double-Edged Sword in the Treatment of COVID-19: Mortality and Severity of COVID-19 in Patients Receiving Long-Term Glucocorticoid Therapy

- Miscellaneous
- Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
- Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim, The Committee of Clinical Practice Guideline of the Korean Endocrine Society
- Endocrinol Metab. 2022;37(6):839-850. Published online December 26, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1627
- 3,525 View
- 321 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
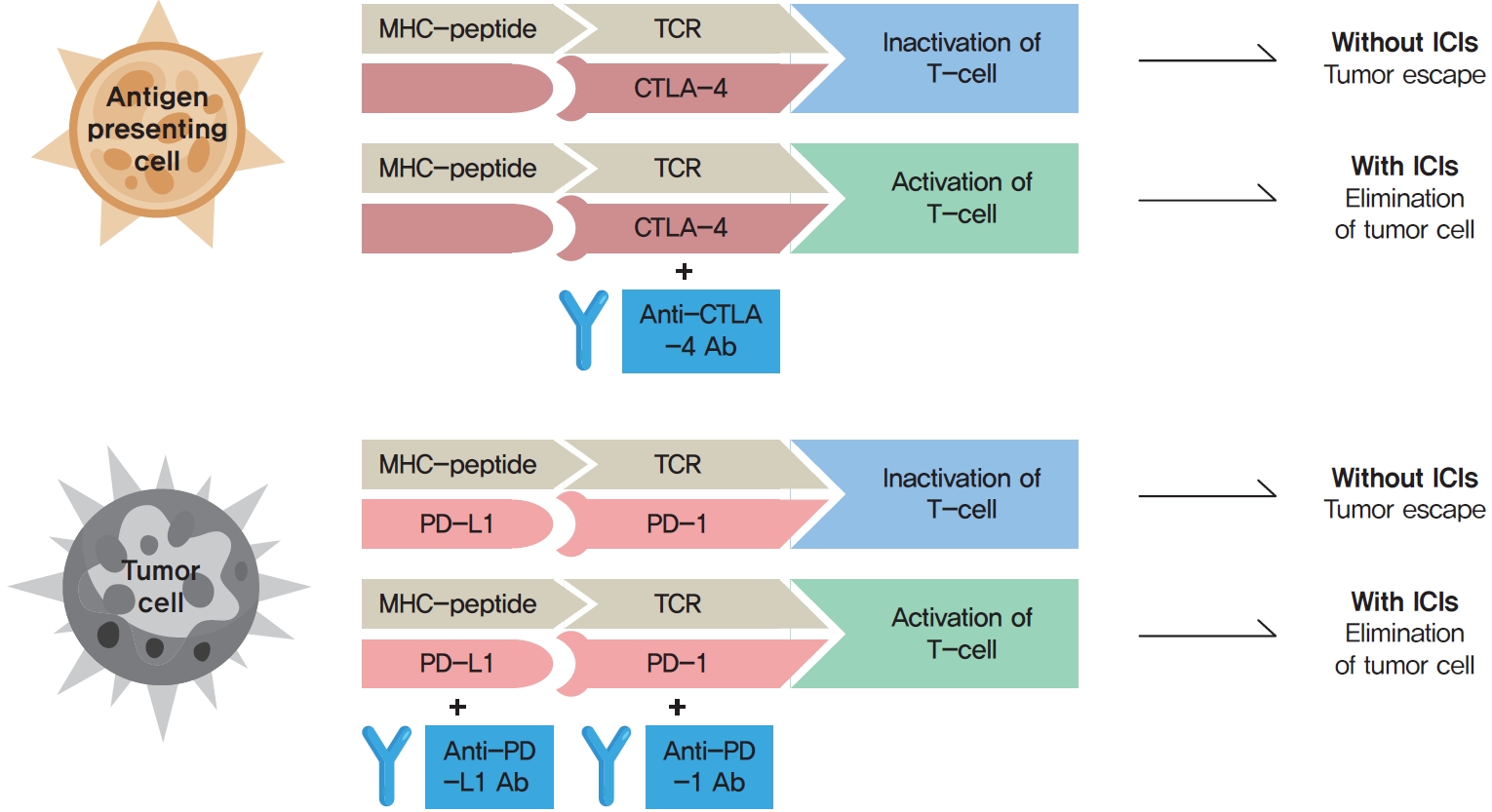
ePub - Immune checkpoint inhibitors (ICIs) including an anti-cytotoxic T-lymphocyte-associated antigen 4 inhibitor, anti-programmed cell death protein 1 (PD-1) inhibitors, and anti-PD-ligand 1 inhibitors are representative therapeutics for various malignancies. In oncology, the application of ICIs is currently expanding to a wider range of malignancies due to their remarkable clinical outcomes. ICIs target immune checkpoints which suppress the activity of T-cells that are specific for tumor antigens, thereby allowing tumor cells to escape the immune response. However, immune checkpoints also play a crucial role in preventing autoimmune reactions. Therefore, ICIs targeting immune checkpoints can trigger various immune-related adverse events (irAEs), especially in endocrine organs. Considering the endocrine organs that are frequently involved, irAEs associated endocrinopathies are frequently life-threatening and have unfavorable clinical implications for patients. However, there are very limited data from large clinical trials that would inform the development of clinical guidelines for patients with irAEs associated endocrinopathies. Considering the current clinical situation, in which the scope and scale of the application of ICIs are increasing, position statements from clinical specialists play an essential role in providing the appropriate recommendations based on both medical evidence and clinical experience. As endocrinologists, we would like to present precautions and recommendations for the management of immune-related endocrine disorders, especially those involving the adrenal, thyroid, and pituitary glands caused by ICIs.
-
Citations
Citations to this article as recorded by- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population
Ryo Fujiwara, Takeshi yuasa, kenichi kobayashi, tetsuya yoshida, susumu kageyama
Expert Review of Anticancer Therapy.2023; 23(5): 461. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef
- Pembrolizumab plus lenvatinib for radically unresectable or metastatic renal cell carcinoma in the Japanese population

- Adrenal Gland
Big Data Articles (National Health Insurance Service Database) - Epidemiology and Long-Term Adverse Outcomes in Korean Patients with Congenital Adrenal Hyperplasia: A Nationwide Study
- Jung Hee Kim, Sunkyu Choi, Young Ah Lee, Juneyoung Lee, Sin Gon Kim
- Endocrinol Metab. 2022;37(1):138-147. Published online February 28, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1328
- 3,370 View
- 149 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Previous studies on the epidemiology and complications of congenital adrenal hyperplasia (CAH) were conducted in Western countries and in children/adolescents. We aimed to explore the epidemiology of CAH, as well as the risk of comorbidities and mortality, in a Korean nationwide case-control study.
Methods
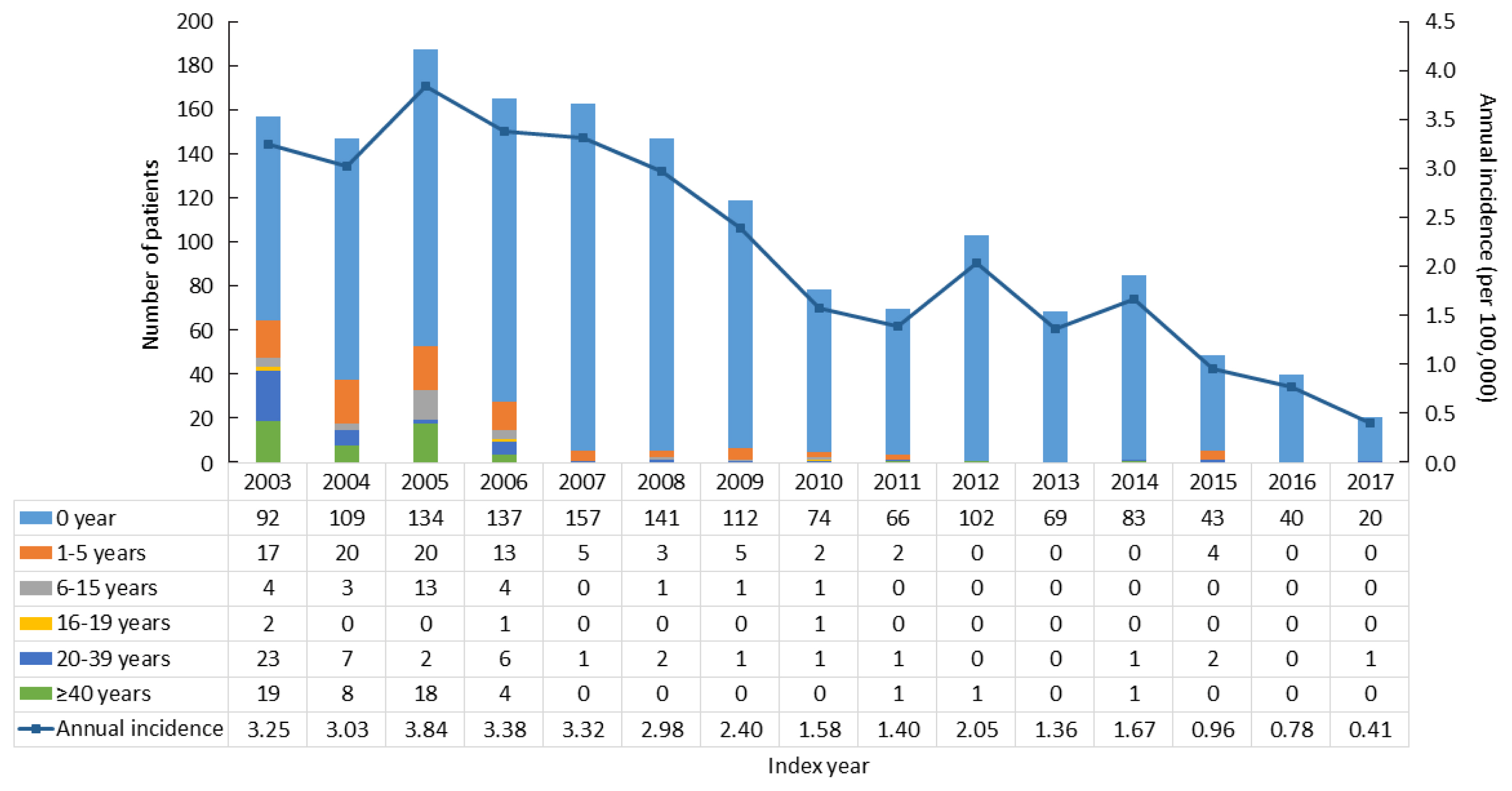
CAH patients (n=2,840) were included between 2002 and 2017 from the National Health Insurance Service database and the Rare Intractable Disease program. CAH patients were compared, at a 1:10 ratio, with age-, sex-, and index year-matched controls (n=28,400).
Results
The point prevalence of CAH patients in Korea was 1 in 18,745 persons in 2017. The annual incidence rate declined between 2003 and 2017 from 3.25 to 0.41 per 100,000 persons. CAH patients were at elevated risk for cardiovascular disease (odds ratio [OR], 1.6; 95% confidence interval [CI], 1.4 to 1.9), stroke (OR, 1.7; 95% CI, 1.3 to 2.0), diabetes mellitus (OR, 2.8; 95% CI, 2.6 to 3.1), dyslipidemia (OR, 2.4; 95% CI, 2.2 to 2.6), and psychiatric disorders (OR, 1.5; 95% CI, 1.3 to 1.6). Fracture risk increased in CAH patients aged over 40 years (OR, 1.4; 95% CI, 1.1 to 1.7). CAH patients were at higher risk of mortality than controls (hazard ratio, 1.6; 95% CI, 1.3 to 2.0).
Conclusion
Our nationwide study showed a recent decline in the incidence of CAH and an elevated risk for cardiovascular, metabolic, skeletal, and psychiatric disorders in CAH patients. Lifelong management for comorbidity risk is a crucial component of treating CAH patients. -
Citations
Citations to this article as recorded by- Hyperandrogenism and Cardiometabolic Risk in Pre- and Postmenopausal Women—What Is the Evidence?
Angelica Lindén Hirschberg
The Journal of Clinical Endocrinology & Metabolism.2024; 109(5): 1202. CrossRef - Predictors of Cardiovascular Morbidities in Adults With 21-Hydroxylase Deficiency Congenital Adrenal Hyperplasia
Suranut Charoensri, Richard J Auchus
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1133. CrossRef - Case report: Development of central precocious puberty in a girl with late-diagnosed simple virilizing congenital adrenal hyperplasia complicated with Williams syndrome
Eun Young Joo, Myung Ji Yoo, Su Jin Kim, Woori Jang, Ji-Eun Lee
Frontiers in Endocrinology.2024;[Epub] CrossRef - Анализ распространенности и заболеваемости надпочечниковой недостаточностью в мире
М. Ю. Юкина, Н. Ф. Нуралиева, Е. А. Трошина
Ateroscleroz.2023; 18(4): 426. CrossRef - Big Data Research in the Field of Endocrine Diseases Using the Korean National Health Information Database
Sun Wook Cho, Jung Hee Kim, Han Seok Choi, Hwa Young Ahn, Mee Kyoung Kim, Eun Jung Rhee
Endocrinology and Metabolism.2023; 38(1): 10. CrossRef - Long-term cardiometabolic morbidity in young adults with classic 21-hydroxylase deficiency congenital adrenal hyperplasia
Beatrice Righi, Salma R. Ali, Jillian Bryce, Jeremy W. Tomlinson, Walter Bonfig, Federico Baronio, Eduardo C. Costa, Guilherme Guaragna-Filho, Guy T’Sjoen, Martine Cools, Renata Markosyan, Tania A. S. S. Bachega, Mirela C. Miranda, Violeta Iotova, Henrik
Endocrine.2023; 80(3): 630. CrossRef - Serum steroid profile captures metabolic phenotypes in adults with classic congenital adrenal hyperplasia
Chang Ho Ahn, Jaeyoon Shim, Han Na Jang, Young Ah Lee, Sang-Won Lee, Man Ho Choi, Jung Hee Kim
The Journal of Steroid Biochemistry and Molecular Biology.2023; 234: 106374. CrossRef - Long‐term health consequences of congenital adrenal hyperplasia
Riccardo Pofi, Xiaochen Ji, Nils P. Krone, Jeremy W. Tomlinson
Clinical Endocrinology.2023;[Epub] CrossRef - Multiplexed Serum Steroid Profiling Reveals Metabolic Signatures of Subtypes in Congenital Adrenal Hyperplasia
Jaeyoon Shim, Chang Ho Ahn, Seung Shin Park, Jongsung Noh, Chaelin Lee, Sang Won Lee, Jung Hee Kim, Man Ho Choi
Journal of the Endocrine Society.2023;[Epub] CrossRef - Long-Term Outcomes of Congenital Adrenal Hyperplasia
Anna Nordenström, Svetlana Lajic, Henrik Falhammar
Endocrinology and Metabolism.2022; 37(4): 587. CrossRef
- Hyperandrogenism and Cardiometabolic Risk in Pre- and Postmenopausal Women—What Is the Evidence?

- Thyroid
- The Concept of Economic Evaluation and Its Application in Thyroid Cancer Research
- Kyungsik Kim, Mijin Kim, Woojin Lim, Bo Hyun Kim, Sue K. Park
- Endocrinol Metab. 2021;36(4):725-736. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1164
- 4,382 View
- 147 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Economic evaluation is a type of comparative analysis between interventions in terms of both their resource use and health outcomes. Due to the good prognosis of thyroid cancer (TC), the socioeconomic burden of TC patients post-diagnosis is increasing. Therefore, economic evaluation studies focusing on TC are recommended. This study aimed to describe the concept and methods of economic evaluation and reviewed previous TC studies. Several previous studies compared the costs of interventions or evaluated recurrence, complications, or quality of life as measures of their effectiveness. Regarding costs, most studies focused on direct costs and applied hypothetical models. Cost-minimization analysis should be distinguished from simple cost analysis. Furthermore, due to the universality of the term “cost-effectiveness analysis” (CEA), several studies have not distinguished CEA from cost-utility analysis; this point needs to be considered in future research. Cost-benefit analyses have not been conducted in previous TC research. Since TC has a high survival rate and good prognosis, the need for economic evaluations has recently been pointed out. Therefore, correct concepts and methods are needed to obtain clear economic evaluation results. On this basis, it will be possible to provide appropriate guidelines for TC treatment and management in the future.
-
Citations
Citations to this article as recorded by- Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population
Han-Sang Baek, Jeonghoon Ha, Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, Sungju Kim, Dong-Jun Lim, Chul-Min Kim
Endocrinology and Metabolism.2024; 39(2): 310. CrossRef - Role of Prehabilitation and Rehabilitation on Functional Recovery and Quality of Life in Thyroid Cancer Patients: A Comprehensive Review
Lorenzo Lippi, Alessio Turco, Stefano Moalli, Marco Gallo, Claudio Curci, Antonio Maconi, Alessandro de Sire, Marco Invernizzi
Cancers.2023; 15(18): 4502. CrossRef - Sex-specific Associations between Body Mass Index and Thyroid Cancer Incidence among Korean Adults
Kyoung-Nam Kim, Kyungsik Kim, Sangjun Lee, Sue K. Park
Cancer Epidemiology, Biomarkers & Prevention.2023; 32(9): 1227. CrossRef - Active Surveillance Versus Immediate Surgery for Low-Risk Papillary Thyroid Microcarcinoma Patients in South Korea: A Cost-Minimization Analysis from the MAeSTro Study
Kyungsik Kim, June Young Choi, Su-jin Kim, Eun Kyung Lee, Young Ki Lee, Jun Sun Ryu, Kyu Eun Lee, Jae Hoon Moon, Young Joo Park, Sun Wook Cho, Sue K. Park
Thyroid.2022; 32(6): 648. CrossRef - A Systematic Review of Economic Evaluation of Thyroid Cancer
Mijin Kim, Woojin Lim, Kyungsik Kim, Ja Seong Bae, Byung Joo Lee, Bon Seok Koo, Eun Kyung Lee, Eu Jeong Ku, June Young Choi, Bo Hyun Kim, Sue K. Park
International Journal of Thyroidology.2022; 15(2): 74. CrossRef
- Cost-Utility Analysis of Early Detection with Ultrasonography of Differentiated Thyroid Cancer: A Retrospective Study on a Korean Population

- Clinical Study
- Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors
- Jee Hee Yoon, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2021;36(2):413-423. Published online April 6, 2021
- DOI: https://doi.org/10.3803/EnM.2020.906
- 5,687 View
- 218 Download
- 23 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
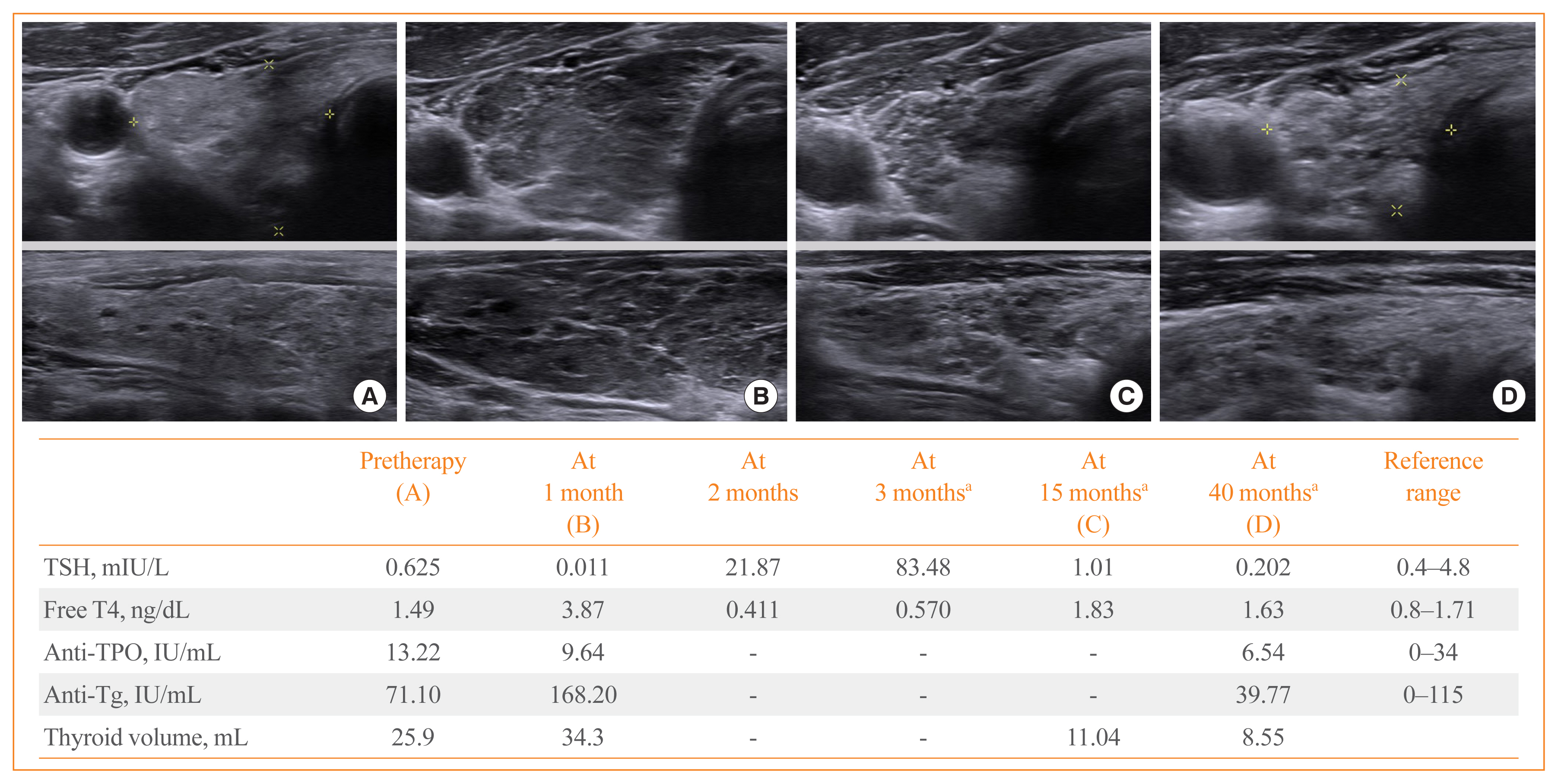
Thyroid immune-related adverse events (IRAEs) have been reported in patients treated with programmed cell death protein-1 (PD-1) and programmed cell death protein-ligand 1 (PD-L1) inhibitors. We investigated the incidence and clinical course of PD-1/PD-L1 inhibitor-induced thyroid IRAEs, and identified predictable clinical risk factors of thyroid IRAEs, in particular, overt hypothyroidism (OH).
Methods
We retrospectively reviewed the medical records of 325 cancer patients receiving PD-1/PD-L1 inhibitor in a tertiary referral center.
Results
A total of 50.5% (164/325) of patients experienced at least one abnormal thyroid function following PD-1/PD-L1 inhibitor. Eighty-four patients (51.2%) of them recovered to normal thyroid function during follow-up. In overall population, 25 patients (7.7%) required thyroid hormone replacement therapy due to PD-1/PD-L1 inhibitor-induced OH. Patients who progressed to OH showed significantly higher baseline thyroid stimulating hormone level and longer duration of PD-1/PD-L1 inhibitor therapy than those without thyroid dysfunction or OH (both P<0.001). Median time interval to the development of OH was 3 months after the therapy. OH was significantly associated with positive anti-thyroid peroxidase antibody at baseline and anti-thyroglobulin antibody during the therapy than those without thyroid dysfunction or OH (P=0.015 and P=0.005, respectively). We observed no patients with OH who were able to stop levothyroxine replacement after the cessation of PD-1/PD-L1 inhibitor therapy.
Conclusion
PD-1/PD-L1 inhibitor-induced thyroid dysfunctions are considerably reversible; however, OH is irreversible requiring levothyroxine replacement even after stopping the therapy. Positive thyroid autoantibodies may predict the progression to OH. -
Citations
Citations to this article as recorded by- Thyroid Dysfunction after Atezolizumab and Bevacizumab Is Associated with Favorable Outcomes in Hepatocellular Carcinoma
Young Shin Song, Hannah Yang, Beodeul Kang, Jaekyung Cheon, Ilhwan Kim, Hyeyeong Kim, Won Suk Lee, Yun Beom Sang, Sanghoon Jung, Ho Yeong Lim, Vincent E. Gaillard, Chan Kim, Hong Jae Chon
Liver Cancer.2024; 13(1): 89. CrossRef - Thyroid dysfunction (TD) induced by PD-1/PD-L1 inhibitors in advanced lung cancer
Yanling Wang, Xiaoxuan Yang, Jia Ma, Shenglan Chen, Ping Gong, Ping Dai
Heliyon.2024; 10(5): e27077. CrossRef - Non-Invasive Predictive Biomarkers for Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors
Ben Ponvilawan, Abdul Wali Khan, Janakiraman Subramanian, Dhruv Bansal
Cancers.2024; 16(6): 1225. CrossRef - Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy
David Dora, Syeda Mahak Zahra Bokhari, Kenan Aloss, Peter Takacs, Juliane Zsuzsanna Desnoix, György Szklenárik, Patrick Deniz Hurley, Zoltan Lohinai
International Journal of Molecular Sciences.2023; 24(3): 2769. CrossRef - Immune checkpoint inhibitor-associated toxicity in advanced non-small cell lung cancer: An updated understanding of risk factors
Xiangxiao Hu, Lina Wang, Bin Shang, Junren Wang, Jian Sun, Bin Liang, Lili Su, Wenjie You, Shujuan Jiang
Frontiers in Immunology.2023;[Epub] CrossRef - Immune-related adverse events induced by programmed death protein-1 inhibitors from the perspective of lymphoma immunotherapy
Yong-Zhe Hou, Qin Zhang, Hai Bai, Tao Wu, Ya-Jie Chen
World Journal of Clinical Cases.2023; 11(7): 1458. CrossRef - Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events
Iñigo Les, Mireia Martínez, Inés Pérez-Francisco, María Cabero, Lucía Teijeira, Virginia Arrazubi, Nuria Torrego, Ana Campillo-Calatayud, Iñaki Elejalde, Grazyna Kochan, David Escors
Cancers.2023; 15(5): 1629. CrossRef - Immune-related thyroid dysfunctions during anti PD-1/PD-L1 inhibitors: new evidence from a single centre experience
Alice Nervo, Matteo Ferrari, Giovanni Gruosso, Enrica Migliore, Sara Basile, Valentina D’Angelo, Anna Roux, Alessandro Piovesan, Emanuela Arvat
Clinical and Experimental Medicine.2023; 23(8): 4817. CrossRef - RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies
Rena Pollack, Joshua Stokar, Natan Lishinsky, Irina Gurt, Naomi Kaisar-Iluz, Merav E. Shaul, Zvi G. Fridlender, Rivka Dresner-Pollak
International Journal of Molecular Sciences.2023; 24(13): 10526. CrossRef - Immune‐related adverse events after immune check point inhibitors: Understanding the intersection with autoimmunity
Namrata Singh, Anne M. Hocking, Jane H. Buckner
Immunological Reviews.2023; 318(1): 81. CrossRef - Endocrine Side Effects in Patients Treated with Immune Checkpoint Inhibitors: A Narrative Review
Nicia I. Profili, Roberto Castelli, Antonio Gidaro, Alessandro Merella, Roberto Manetti, Giuseppe Palmieri, Margherita Maioli, Alessandro P. Delitala
Journal of Clinical Medicine.2023; 12(15): 5161. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef - PD-1/PD-L1 Inhibitors in Patients With Preexisting Autoimmune Diseases
Ke Zhang, Xiangyi Kong, Yuan Li, Zhongzhao Wang, Lin Zhang, Lixue Xuan
Frontiers in Pharmacology.2022;[Epub] CrossRef - Association between the type of thyroid dysfunction induced by immune checkpoint inhibitors and prognosis in cancer patients
Han-sang Baek, Chaiho Jeong, Kabsoo Shin, Jaejun Lee, Heysun Suh, Dong-Jun Lim, Moo Il Kang, Jeonghoon Ha
BMC Endocrine Disorders.2022;[Epub] CrossRef - A Successful Case of Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab with Multisystem Immune-related Adverse Events
Hidemi Hayashi, Koji Sawada, Takumu Hasebe, Shunsuke Nakajima, Jun Sawada, Yuri Takiyama, Yumi Takiyama, Toshikatsu Okumura, Mikihiro Fujiya
Internal Medicine.2022; 61(23): 3497. CrossRef - Immune Related Adverse Events of the Thyroid – A Narrative Review
Christopher A. Muir, Venessa H. M. Tsang, Alexander M. Menzies, Roderick J. Clifton-Bligh
Frontiers in Endocrinology.2022;[Epub] CrossRef - Thyroid-related adverse events induced by immune checkpoint inhibitors
Alexandra Chera, Andreea Lucia Stancu, Octavian Bucur
Frontiers in Endocrinology.2022;[Epub] CrossRef - Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors
Adithya Chennamadhavuni, Laith Abushahin, Ning Jin, Carolyn J. Presley, Ashish Manne
Frontiers in Immunology.2022;[Epub] CrossRef - Case 5: A 41-Year-Old Woman With Palpitation
Jiwon Yang, Kabsoo Shin, Jeongmin Lee, Jeonghoon Ha, Dong-Jun Lim, Han-Sang Baek
Journal of Korean Medical Science.2022;[Epub] CrossRef - Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim
Endocrinology and Metabolism.2022; 37(6): 839. CrossRef - Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario
Calogera Claudia Spagnolo, Giuseppe Giuffrida, Salvatore Cannavò, Tindara Franchina, Nicola Silvestris, Rosaria Maddalena Ruggeri, Mariacarmela Santarpia
Cancers.2022; 15(1): 246. CrossRef - Antineoplastics
Reactions Weekly.2021; 1874(1): 31. CrossRef
- Thyroid Dysfunction after Atezolizumab and Bevacizumab Is Associated with Favorable Outcomes in Hepatocellular Carcinoma

- Effects of Incretin-Based Therapies on Diabetic Microvascular Complications
- Yu Mi Kang, Chang Hee Jung
- Endocrinol Metab. 2017;32(3):316-325. Published online September 18, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.3.316
- 4,597 View
- 55 Download
- 10 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The morbidity and mortality associated with diabetic complications impose a huge socioeconomic burden worldwide. Therefore, the ultimate goal of managing diabetes mellitus (DM) is to lower the risk of macrovascular complications and highly morbid microvascular complications such as diabetic nephropathy (DN) and diabetic retinopathy (DR). Potential benefits of incretin-based therapies such as glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and dipeptidyl peptidase-4 (DPP-4) inhibitors on the diabetic macrovascular complications have been recently suggested, owing to their pleiotropic effects on multiple organ systems. However, studies primarily investigating the role of these therapies in diabetic microvascular complications are rare. Nevertheless, preclinical and limited clinical data suggest the potential protective effect of incretin-based agents against DN and DR via their anti-inflammatory, antioxidative, and antiapoptotic properties. Evidence also suggests that these incretin-dependent and independent beneficial effects are not necessarily associated with the glucose-lowering properties of GLP-1 RAs and DPP-4 inhibitors. Hence, in this review, we revisit the preclinical and clinical evidence of incretin-based therapy for DR and DN, the two most common, morbid complications in individuals with DM. In addition, the review discusses a few recent studies raising concerns of aggravating DR with the use of incretin-based therapies.
-
Citations
Citations to this article as recorded by- Efficacy and Safety of the Utilization of Dipeptidyl Peptidase IV Inhibitors in Diabetic Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials
Moeber Mahzari, Muhannad Alqirnas, Moustafa Alhamadh, Faisal Alrasheed, Abdulrahman Alhabeeb, Wedad Al Madani, Hussain Aldera
Diabetes, Metabolic Syndrome and Obesity.2024; Volume 17: 1425. CrossRef - Anti-Inflammatory Effects of GLP-1R Activation in the Retina
Alessandra Puddu, Davide Maggi
International Journal of Molecular Sciences.2022; 23(20): 12428. CrossRef - Diabetes and Its Complications: Therapies Available, Anticipated and Aspired
Anu Grover, Komal Sharma, Suresh Gautam, Srishti Gautam, Monica Gulati, Sachin Kumar Singh
Current Diabetes Reviews.2021; 17(4): 397. CrossRef - SGLT2 Inhibitors, GLP-1 Agonists, and DPP-4 Inhibitors in Diabetes and Microvascular Complications: A Review
Christopher El Mouhayyar, Ruba Riachy, Abir Bou Khalil, Asaad Eid, Sami Azar
International Journal of Endocrinology.2020; 2020: 1. CrossRef - Novel therapeutic agents for the treatment of diabetic kidney disease
Rachel E. Hartman, P.S.S. Rao, Mariann D. Churchwell, Susan J. Lewis
Expert Opinion on Investigational Drugs.2020; 29(11): 1277. CrossRef - Nationwide Trends in Pancreatitis and Pancreatic Cancer Risk Among Patients With Newly Diagnosed Type 2 Diabetes Receiving Dipeptidyl Peptidase 4 Inhibitors
Minyoung Lee, Jiyu Sun, Minkyung Han, Yongin Cho, Ji-Yeon Lee, Chung Mo Nam, Eun Seok Kang
Diabetes Care.2019; 42(11): 2057. CrossRef - Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Jae Hyun Bae, Sunhee Kim, Eun-Gee Park, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Endocrinology and Metabolism.2019; 34(1): 80. CrossRef - Serum adipocytokines are associated with microalbuminuria in patients with type 1 diabetes and incipient chronic complications
Tomislav Bulum, Marijana Vučić Lovrenčić, Martina Tomić, Sandra Vučković-Rebrina, Vinko Roso, Branko Kolarić, Vladimir Vuksan, Lea Duvnjak
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(1): 496. CrossRef - Protective Effects of Incretin Against Age-Related Diseases
Di Zhang, Mingzhu Ma, Yueze Liu
Current Drug Delivery.2019; 16(9): 793. CrossRef - The role of dipeptidylpeptidase-4 inhibitors in management of cardiovascular disease in diabetes; focus on linagliptin
Annayya R. Aroor, Camila Manrique-Acevedo, Vincent G. DeMarco
Cardiovascular Diabetology.2018;[Epub] CrossRef
- Efficacy and Safety of the Utilization of Dipeptidyl Peptidase IV Inhibitors in Diabetic Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Clinical Trials

- Obesity and Metabolism
- Cardiovascular Effects of Glucagon-Like Peptide-1 Receptor Agonists
- Yu Mi Kang, Chang Hee Jung
- Endocrinol Metab. 2016;31(2):258-274. Published online April 25, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.258
- 6,404 View
- 92 Download
- 31 Web of Science
- 33 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Glucagon-like peptide-1 (GLP-1) is a member of the proglucagon incretin family, and GLP-1 receptor agonists (RAs) have been introduced as a new class of antidiabetic medications in the past decade. The benefits of GLP-1 RAs are derived from their pleiotropic effects, which include glucose-dependent insulin secretion, suppressed glucagon secretion, and reduced appetite. Moreover, GLP-1 RAs also exert beneficial roles on multiple organ systems in which the GLP-1 receptors exist, including the cardiovascular system. Cardiovascular effects of GLP-1 RAs have been of great interest since the burden from cardiovascular diseases (CVD) has been unbearably increasing in a diabetic population worldwide, despite strict glycemic control and advanced therapeutic techniques to treat CVD. Preclinical studies have already demonstrated the beneficial effects of GLP-1 on myocardium and vascular endothelium, and many clinical studies evaluating changes in surrogate markers of CVD have suggested potential benefits from the use of GLP-1 RAs. Data from numerous clinical trials primarily evaluating the antihyperglycemic effects of multiple GLP-1 RAs have also revealed that changes in most CVD risk markers reported as secondary outcomes have been in favor of GLP-1 RAs treatment. However, to date, there is only one randomized clinical trial of GLP-1 RAs (the ELIXA study) evaluating major cardiovascular events as their primary outcomes, and in this study, a neutral cardiovascular effect of lixisenatide was observed in high-risk diabetic subjects. Therefore, the results of ongoing CVD outcome trials with the use of GLP-1 RAs should be awaited to elucidate the translation of benefits previously seen in CVD risk marker studies into large clinical trials with primary cardiovascular outcomes.
-
Citations
Citations to this article as recorded by- Cardioprotective Actions of a Glucagon‐like Peptide‐1 Receptor Agonist on Hearts Donated After Circulatory Death
Sachiko Kadowaki, M. Ahsan Siraj, Weiden Chen, Jian Wang, Marlee Parker, Anita Nagy, Chun‐Po Steve Fan, Kyle Runeckles, Jing Li, Junko Kobayashi, Christoph Haller, Mansoor Husain, Osami Honjo
Journal of the American Heart Association.2023;[Epub] CrossRef - The role of dipeptidyl peptidase-IV in abdominal aortic aneurysm pathogenesis: A systematic review
Elisha Ngetich, Pierfrancesco Lapolla, Anirudh Chandrashekar, Ashok Handa, Regent Lee
Vascular Medicine.2022; 27(1): 77. CrossRef - Glucagon-like Peptide-1 Receptor Agonists in the Management of Type 2 Diabetes Mellitus and Obesity: The Impact of Pharmacological Properties and Genetic Factors
Jasna Klen, Vita Dolžan
International Journal of Molecular Sciences.2022; 23(7): 3451. CrossRef - Glucagon-like peptide-1 (GLP-1) receptor agonists and cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of double-blind, randomized, placebo-controlled clinical trials
Jing Qin, Li Song
BMC Endocrine Disorders.2022;[Epub] CrossRef - Role of G-protein coupled receptor (GPCRs)/(GPR-120) as an agonists in diabetic wound healing
Jagat Pal Yadav, Dinesh Kumar Patel, Prateek Pathak, Maria Grishina
Obesity Medicine.2022; 36: 100466. CrossRef - Protection against stroke with glucagon-like peptide-1 receptor agonists: a comprehensive review of potential mechanisms
Bruno Vergès, Victor Aboyans, Denis Angoulvant, Pierre Boutouyrie, Bertrand Cariou, Fabien Hyafil, Kamel Mohammedi, Pierre Amarenco
Cardiovascular Diabetology.2022;[Epub] CrossRef - Changing Fields-Diabetes Medications Invading the Cardiovascular Space
Lauren D. Breite, Mackenzie Steck, Brandon Tate Cutshall, Samarth P. Shah, Brandon E. Cave
Current Problems in Cardiology.2021; 46(3): 100736. CrossRef - PEGDA/HA mineralized hydrogel loaded with Exendin4 promotes bone regeneration in rat models with bone defects by inducing osteogenesis
Wei Liu, Xiaowei Jing, Zhiwen Xu, Chong Teng
Journal of Biomaterials Applications.2021; 35(10): 1337. CrossRef - Metabolite G-Protein Coupled Receptors in Cardio-Metabolic Diseases
Derek Strassheim, Timothy Sullivan, David C. Irwin, Evgenia Gerasimovskaya, Tim Lahm, Dwight J. Klemm, Edward C. Dempsey, Kurt R. Stenmark, Vijaya Karoor
Cells.2021; 10(12): 3347. CrossRef - PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice
Marie K. Holt, Daniel R. Cook, Daniel I. Brierley, James E. Richards, Frank Reimann, Alexander V. Gourine, Nephtali Marina, Stefan Trapp
Molecular Metabolism.2020; 39: 101024. CrossRef - A glycosylated Fc‐fused glucagon‐like peptide‐1 receptor agonist exhibits equivalent glucose lowering to but fewer gastrointestinal side effects than dulaglutide
In Bok An, Mi Sun Byun, Sang In Yang, Yuri Choi, Jung Won Woo, Hak Chul Jang, Young Chul Sung
Diabetes, Obesity and Metabolism.2020; 22(8): 1455. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists in Adult Patients With Type 2 Diabetes: Review of Cardiovascular Outcome Trials
Elodie M. Varin, Brent A. McLean, Julie A. Lovshin
Canadian Journal of Diabetes.2020; 44(1): 68. CrossRef - Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors
Alexandros Sachinidis, Dragana Nikolic, Anca Pantea Stoian, Nikolaos Papanas, Omer Tarar, Ali A. Rizvi, Manfredi Rizzo
Metabolism.2020; 111: 154343. CrossRef - GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction
Lili Zhang, Jiali Tian, Sujuan Diao, Guowei Zhang, Mochao Xiao, Dong Chang
Chemico-Biological Interactions.2020; 332: 109252. CrossRef - Predictors of Effectiveness of Glucagon-Like Peptide-1 Receptor Agonist Therapy in Patients with Type 2 Diabetes and Obesity
Alina Yu. Babenko, Daria A. Savitskaya, Yulia A. Kononova, Aleksandra Yu. Trofimova, Anna V. Simanenkova, Elena Yu. Vasilyeva, Evgeny V. Shlyakhto
Journal of Diabetes Research.2019; 2019: 1. CrossRef - Predictors of effectiveness of glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes and obesity
Ekaterina V. Tikhonenko, Alina Y. Babenko, Evgeny V. Shlyakhto
Obesity and metabolism.2019; 15(4): 22. CrossRef - Asian Subpopulations May Exhibit Greater Cardiovascular Benefit from Long-Acting Glucagon-Like Peptide 1 Receptor Agonists: A Meta-Analysis of Cardiovascular Outcome Trials
Yu Mi Kang, Yun Kyung Cho, Jiwoo Lee, Seung Eun Lee, Woo Je Lee, Joong-Yeol Park, Ye-Jee Kim, Chang Hee Jung, Michael A. Nauck
Diabetes & Metabolism Journal.2019; 43(4): 410. CrossRef - Diabetes, Incretin Therapy and Thoracic Aortic Aneurysm – What Does the Evidence Show?
Camilla Krizhanovskii , Anders Franco-Cereceda
Current Vascular Pharmacology.2019; 17(5): 432. CrossRef - Cardiovascular Effects of Different GLP-1 Receptor Agonists in Patients with Type 2 Diabetes
Gül Bahtiyar, Jean Pujals-Kury, Alan Sacerdote
Current Diabetes Reports.2018;[Epub] CrossRef - Efficacy From Strange Sources
Lawrence J. Lesko
Clinical Pharmacology & Therapeutics.2018; 103(2): 253. CrossRef - Exogenous SERP1 attenuates restenosis by restoring GLP-1 receptor activity in diabetic rats following vascular injury
Lishuai Feng, Jianbo Wang, Xu Ma
Biomedicine & Pharmacotherapy.2018; 103: 290. CrossRef - Exenatide exhibits anti‐inflammatory properties and modulates endothelial response to tumor necrosis factor α‐mediated activation
Wojciech Garczorz, Enrique Gallego‐Colon, Agnieszka Kosowska, Agnieszka Kłych‐Ratuszny, Michał Woźniak, Wiesław Marcol, K.J. Niesner, Tomasz Francuz
Cardiovascular Therapeutics.2018;[Epub] CrossRef - Molecular and clinical roles of incretin-based drugs in patients with heart failure
Bassant Orabi, Rasha Kaddoura, Amr S. Omar, Cornelia Carr, Abdulaziz Alkhulaifi
Heart Failure Reviews.2018; 23(3): 363. CrossRef - The effects of Exendin-4 on bone marrow-derived mesenchymal cells
Paola Luciani, Benedetta Fibbi, Benedetta Mazzanti, Cristiana Deledda, Lara Ballerini, Alessandra Aldinucci, Susanna Benvenuti, Riccardo Saccardi, Alessandro Peri
Endocrine.2018; 60(3): 423. CrossRef - Real-world clinical experience of Xultophy in the management of patients with type 2 diabetes in a secondary care clinic
David M. Williams, Natasha Shrikrishnapalasuriyar, Waheeba Syed, Win L. Yin, Richard Chudleigh, Stephen C. Bain, David E. Price, Jeffrey W. Stephens
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(6): 1079. CrossRef - Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome
Maja Ðanić, Bojan Stanimirov, Nebojša Pavlović, Svetlana Goločorbin-Kon, Hani Al-Salami, Karmen Stankov, Momir Mikov
Frontiers in Pharmacology.2018;[Epub] CrossRef - Cardiovascular Outcome Trials of Diabetes and Obesity Drugs: Implications for Conditional Approval and Early Phase Clinical Development
Andrew J. Krentz, Gerardo Rodriguez-Araujo
Pharmaceutical Medicine.2017; 31(6): 399. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - GLP-1R as a Target for the Treatment of Diabetic Retinopathy: Friend or Foe?
Rafael Simó, Cristina Hernández
Diabetes.2017; 66(6): 1453. CrossRef - GLP-1 receptor agonists and heart failure in diabetes
André J. Scheen
Diabetes & Metabolism.2017; 43: 2S13. CrossRef - Effects of Incretin-Based Therapies on Diabetic Microvascular Complications
Yu Mi Kang, Chang Hee Jung
Endocrinology and Metabolism.2017; 32(3): 316. CrossRef - Historique des études cardiovasculaires : de l’UGDP… aux dernières études
A.-J. Scheen
Médecine des Maladies Métaboliques.2017; 11: 2S15. CrossRef - Cardiovascular safety and benefits of GLP-1 receptor agonists
Niels B. Dalsgaard, Andreas Brønden, Tina Vilsbøll, Filip K. Knop
Expert Opinion on Drug Safety.2017; 16(3): 351. CrossRef
- Cardioprotective Actions of a Glucagon‐like Peptide‐1 Receptor Agonist on Hearts Donated After Circulatory Death

- Clinical Study
- Economic Evaluation of Recombinant Human Thyroid Stimulating Hormone Stimulation vs. Thyroid Hormone Withdrawal Prior to Radioiodine Ablation for Thyroid Cancer: The Korean Perspective
- Seo Young Sohn, Hye Won Jang, Yoon Young Cho, Sun Wook Kim, Jae Hoon Chung
- Endocrinol Metab. 2015;30(4):531-542. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.531
- 3,545 View
- 43 Download
- 9 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Previous studies have suggested that recombinant human thyroid stimulating hormone (rhTSH) stimulation is an acceptable alternative to thyroid hormone withdrawal (THW) when radioiodine remnant ablation is planned for thyroid cancer treatment, based on superior short-term quality of life with non-inferior remnant ablation efficacy. This study evaluated the cost-effectiveness of radioiodine remnant ablation using rhTSH, compared with the traditional preparation method which renders patients hypothyroid by THW, in Korean perspective.
Methods This economic evaluation considered the costs and benefits to the Korean public healthcare system. Clinical experts were surveyed regarding the current practice of radioiodine ablation in Korea and their responses helped inform assumptions used in a cost effectiveness model. Markov modelling with 17 weekly cycles was used to assess the incremental costs per quality-adjusted life year (QALY) associated with rhTSH. Clinical inputs were based on a multi-center, randomized controlled trial comparing remnant ablation success after rhTSH preparation with THW. The additional costs associated with rhTSH were considered relative to the clinical benefits and cost offsets.
Results The additional benefits of rhTSH (0.036 QALY) are achieved with an additional cost of Korean won ₩961,105, equating to cost per QALY of ₩26,697,361. Sensitivity analyses had only a modest impact upon cost-effectiveness, with one-way sensitivity results of approximately ₩33,000,000/QALY.
Conclusion The use of rhTSH is a cost-effective alternative to endogenous hypothyroid stimulation prior to radioiodine ablation for patients who have undergone thyroidectomy in Korea.
-
Citations
Citations to this article as recorded by- Comparison of Recombinant Human Thyroid-Stimulating Hormone and Thyroid Hormone Withdrawal for 131I Therapy in Patients With Intermediate- to High-Risk Thyroid Cancer
Sohyun Park, Ji-In Bang, Keunyoung Kim, Youngduk Seo, Ari Chong, Chae Moon Hong, Dong-Eun Lee, Miyoung Choi, Sang-Woo Lee, So Won Oh
Clinical Nuclear Medicine.2024; 49(3): e96. CrossRef - Thyroid Hormone Withdrawal versus Recombinant Human TSH as Preparation for I-131 Therapy in Patients with Metastatic Thyroid Cancer: A Systematic Review and Meta-Analysis
Luca Giovanella, Maria Luisa Garo, Alfredo Campenní, Petra Petranović Ovčariček, Rainer Görges
Cancers.2023; 15(9): 2510. CrossRef - Health-related quality of life of thyroid cancer patients undergoing radioiodine therapy: a cohort real-world study in a reference public cancer hospital in Brazil
Jayda Eiras Ramim, Marcella Araugio Soares Cardoso, Gessen Lopes Carneiro de Oliveira, Maria Luisa Gomes, Tiago Teixeira Guimarães, Rossana Corbo Ramalho de Mello, Anke Bergmann, Priscilla Brunelli Pujatti
Supportive Care in Cancer.2020; 28(8): 3771. CrossRef - Predictive factors determining incomplete response to radioiodine therapy in patients with differentiated thyroid cancer
Ewelina Szczepanek-Parulska, Magdalena Wojewoda-Korbelak, Martyna Borowczyk, Malgorzata Kaluzna, Barbara Brominska, Katarzyna Ziemnicka, Rafal Czepczynski, Maciej Baczyk, Marek Ruchala
The Quarterly Journal of Nuclear Medicine and Molecular Imaging.2020;[Epub] CrossRef - Initial Adoption of Recombinant Human Thyroid-Stimulating Hormone Following Thyroidectomy in the Medicare Thyroid Cancer Patient Population
Michaela A. Dinan, Yanhong Li, Shelby D. Reed, Julie Ann Sosa
Endocrine Practice.2019; 25(1): 31. CrossRef - Triennial Report ofEndocrinology and Metabolism, 2015 to 2017
Eun-Jung Rhee, Hey Yeon Jang, Won-Young Lee
Endocrinology and Metabolism.2018; 33(2): 195. CrossRef - Recombinant human TSH stimulated thyroglobulin levels at remnant ablation predict structural incomplete response to treatment in patients with differentiated thyroid cancer
Jeonghoon Ha, Min Hee Kim, Kwanhoon Jo, Yejee Lim, Ja Seong Bae, Sohee Lee, Moo Il Kang, Bong Yun Cha, Dong Jun Lim
Medicine.2017; 96(29): e7512. CrossRef - Does the Risk of Metabolic Syndrome Increase in Thyroid Cancer Survivors?
Min-Hee Kim, Jin-young Huh, Dong-jun Lim, Moo-Il Kang
Thyroid.2017; 27(7): 936. CrossRef
- Comparison of Recombinant Human Thyroid-Stimulating Hormone and Thyroid Hormone Withdrawal for 131I Therapy in Patients With Intermediate- to High-Risk Thyroid Cancer

- High-Dose Hook Effect in Patients with Macroprolactinoma.
- Sung Yeon Kim, Chul Gu Park, Young Ju Choi, Eui Sil Hong, Sang Wan Kim, Chan Soo Shin, Hak Chul Jang, Seong Yeon Kim, Bo Youn Cho, Hong Kyu Lee
- J Korean Endocr Soc. 2005;20(2):148-153. Published online April 1, 2005
- DOI: https://doi.org/10.3803/jkes.2005.20.2.148
- 1,982 View
- 20 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Large amounts of antigen may produce false low values on immunoradiometric assays due to the so-called high-dose hook effect. The physicians' awareness of the possibility of the "high-dose hook effect" will prevent preoperative misdiagnosis. The study was designed to identify the frequency and clinical features of patients with pituitary macroadenomas in whom a high-dose PRL hook effect was documented. METHODS: Our retrospective study involved 42 patients with non-functioning pituitary adenomas (tumor diameter >30mm) who underwent transsphenoidal microsurgery from between Jan 1999 to Aug 2004, and 6 patients with non-functioning pituitary adenoma(tumor diameter>30mm) were selected for prospective study from Sep 2003 to Feb 2004. Our retrospective study also involved 13 patients with macroprolactinoma for the comparison of the clinical features. RESULTS: 1) The presence of a high-dose hook effect was retrospectively suggested when the PRL levels increased in 4 out of the 42 patients with non- functioning adenomas(tumor diameter >30mm) after surgery. Post-operative immunohistochemical staining of their pituitary specimens revealed the tumors to be prolactinoma. 2) Prospectively, dilution testing of the specimens obtained before surgery was done in the 6 patients, and one patient presented with a case of the hook effect. The patient's prolactin level was measured at 53.1ng/mL before dilution and this was increased up to 22,600ng/mL upon the 1:1000 dilution. 3) Conclusively, the hook effect was seen in 5 of the 48 patients(10.4%) with non-functioning pituitary adenoma(tumor diameter >30mm) 4) Compared with other 2 patient groups(the macroprolactinoma(N=13) group, and the non-functioning pituitary tumor(N=43) group), the high-dose PRL hook effect is more likely to be observed in male patients with large pituitary tumors. CONCLUSION: In order to avoid the high-dose hook effect, PRL should be assayed at 1:100~1:200 or even higher dilutions of serum from all patients(and especially the male patients) with large pituitary tumors -
Citations
Citations to this article as recorded by- Multiple Endocrine Neoplasia Type 1 Presenting with an Invasive Giant Prolactinoma
Jinhoon Cha, Jin Seo Kim, Jung Suk Han, Yeon Won Park, Min Joo Kim, Yun Hyi Ku, Hong Il Kim
The Korean Journal of Medicine.2016; 91(3): 300. CrossRef
- Multiple Endocrine Neoplasia Type 1 Presenting with an Invasive Giant Prolactinoma

- Fos Expression Induced by Combined Injection of Leptin and Cholecystokinin in the Rat Brain.
- Young Uck Kim, Kyung Suk You, Ho Suck Kang, Choon Hee Chung, Tae Sun Hwang
- J Korean Endocr Soc. 2002;17(4):486-500. Published online August 1, 2002
- 994 View
- 18 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Several studies have reported that cholecystokinin (CCK), a short-term meal related satiety signal, and leptin, long-term signal for controlling feeding behaviour and body weight, act synergistically to inhibit food intake. However the mechanism and neuroanatomical basis for this response remain unclear. To clarify the neuronal mechanisms underlying the synergistic interaction between leptin and CCK, we examined the neuron activated by single or combined injection of leptin and CCK in fasted rats using immunohistochemistry for Fos. The expression of Fos can be used to trace neuronal activation pathways. METHODS: The rats were divided into 4 groups; Tris solution-saline, Tris solution-CCK, leptin-saline, leptin-CCK. Rats were received a single intracerebroventricular injection of either 3mul Tris solution or 3microgram leptin, and a single intraperitoneal injection of either 2mul saline or 2microgram/kg sulfated CCK-8. The changes of the Fos expression were investigated in the paraventricular nucleus (Pa), retrochiasmatic area (RCh), lateral hypothalamic nucleus (LH), central nucleus of amygdala(Ce), supraoptic nucleus (SO), arcuate nucleus (Arc), ventromedial hypothalamic nucleus(VMH),dorsomedial hypothalamic nucleus (DM), ventral premammillary nucleus (PMV), superior lateral subdivision of parabrachial nucleus (LPBS), external lateral subdivision of parabrachial nucleus (LPBE), supragenual nucleus (SGe), area postrema (AP), medial area (SolM) and commissural area (SolC) of nucleus of the solitary tract nuclei. RESULTS: CCK increased the Fos expression in the Pa, RCh, LH, Ce, SO, Arc, VMH, DM, PMV, LPBS, LPBE and SolM. Leptin increased the Fos expression in the Pa, RCh, LH, SO, Arc, VMH, DM, PMV, LPBS, LPBE, SGe, AP and SolM. Injections of leptin and CCK significantly enhanced the Fos expression in the Pa, RCh, VMH, DM, LPBS, and SolM compared with those induced by leptin or CCK alone. CONCLUSION: Our results suggest that the Pa, RCh, VMH, DM, LPBS and SolM may be essential sites mediating the synergistic effect of leptin and CCK to regulate food intake.

- Antioxidative Effect of Melatonin in Streptozotocin-Induced Diabetic Rats.
- Hyung Joon Yoo, Do Ho Moon, Hong Bae Chung, Myung Soo Ahn, Kwang Sik Yoon, Byoung Jin Ahn, Jin Shin, An Chul Chung, Young Joong Cho, Hong Woo Nahm
- J Korean Endocr Soc. 1998;13(1):45-51. Published online January 1, 2001
- 987 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
An increase in oxidative stress has been suggested to play major roles in the complications of diabetes. The bulk of the experimental data favors enhanced free radicals in diabetes and antioxidant defense mechanisms may be reduced in diabetes. Melatonin, the major secretory product of the pineal gland has been shown to be a potent and specific hydroxyl radical scavenger. The purpose of our study was to determine the antioxidative effeet of melatonin in streptozotocin-induced diabetic rats. METHODS: Sprague-Dawley rats weighing 200-240 g were divided into 3 groups: normal controls(n-7), diabetic contmls(n-9), melatonin-treated diabetic animals(n-9). Diabetes was induced by intraperitoneal injection of streptozotoein(55 mg/kg body weight) and melatonin(6 mg/kg body weight) was orally administered for 20 days. At day 20 after streptozotocin administration, blood was collected for the assay of glucose, albumin and cholesterol. Erythrocyte membrane lipid peroxidation was determined by malonyldialdehyde(MDA) reactivity. RESULTS: 1) The MDA resctivity of erytbrocyte membrane in melatonin-treated diabetic animals (meanstandard deviation: 5.52+-1.52nmol/ml packed cells) were lower(p<0.05) than that in diabetic controls(7.68+-1.16nmol/mL packed cells). But, there was no significant difference between melatonin-treated diabetic animals and normal contls(4.93+-1.19 nmol/mL packed cells). 2) There were no significant differences of blood glucose and body weight between diabetic controls and melatonin-treated diabetic animals. CONCLUSION: These results show the antioxidative effect of melatonin in streptozotocin-induced diabetic rats. Further clinical and long-term experimental studies are needed to assess the effect of melatonin on development and progression of diabetic complications.


 KES
KES

 First
First Prev
Prev



