Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- Association of Shift Work with Normal-Weight Obesity in Community-Dwelling Adults
- Chul Woo Ahn, Sungjae Shin, Seunghyun Lee, Hye-Sun Park, Namki Hong, Yumie Rhee
- Endocrinol Metab. 2022;37(5):781-790. Published online October 25, 2022
- DOI: https://doi.org/10.3803/EnM.2022.1532
- 3,311 View
- 190 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Shift work is associated with obesity and metabolic syndrome. However, this association in the normal-weight population remains unclear. This study aimed to investigate whether shift work is associated with normal-weight obesity (NWO).
Methods
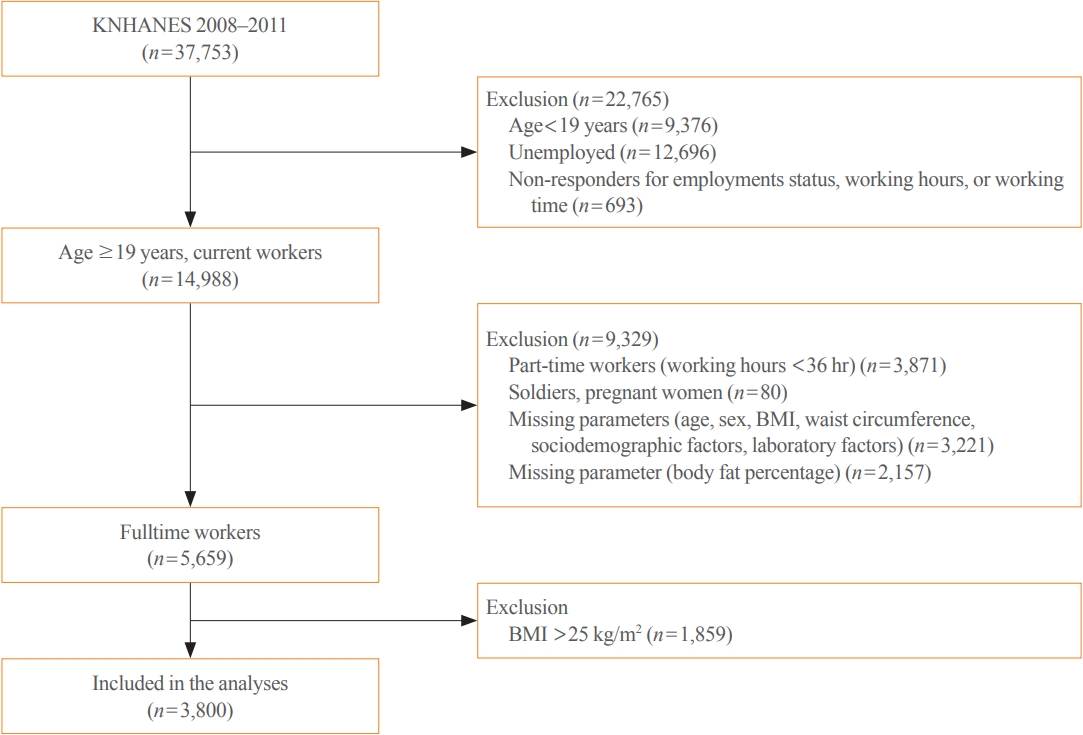
From the nationally representative Korea National Health and Nutrition Examination Survey (KNHANES) dataset (2008 to 2011), 3,800 full-time workers aged ≥19 years with a body mass index (BMI) ≤25 kg/m2 were analysed. We defined NWO as BMI ≤25 kg/m2 and body fat percentage ≥25% in men and ≥37% in women. Working patterns were classified into “daytime,” “other than daytime,” and “shift.” Multivariable logistic regression analysis was performed to evaluate the relationship between shift work and NWO.
Results
Shift work was associated with higher odds of NWO than daytime work (adjusted odds ratio [aOR], 1.47; 95% confidence interval [CI], 1.04 to 2.09) and night/evening work (aOR, 1.87; 95% CI, 1.11 to 3.14) after adjustment for type of work, working hours, age, sex, BMI, 25-hydroxyvitamin D levels, homeostatic model assessment for insulin resistance, and other sociodemographic factors. In subgroup analyses, the association between shift work and NWO was more robust in those aged ≥60 years and those working ≥56 hours/week.
Conclusion
Shift work was associated with NWO in community-dwelling Korean adults, independent of age, sex, BMI, and other covariates. -
Citations
Citations to this article as recorded by- Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work
Sorina Hohor, Cristina Mandanach, Andreea Maftei, Corina Aurelia Zugravu, Marina Ruxandra Oțelea
Antioxidants.2023; 12(4): 959. CrossRef - Normal-Weight Obesity and Metabolic Syndrome in Korean Adults: A Population-Based Cross-Sectional Study
Jeonghyeon Kim, Seamon Kang, Hyunsik Kang
Healthcare.2023; 11(16): 2303. CrossRef - You Can’t Avoid Shift Work? Then Focus on Body Fat Rather than Weight
Eun Kyung Lee
Endocrinology and Metabolism.2022; 37(5): 756. CrossRef
- Impaired Melatonin Secretion, Oxidative Stress and Metabolic Syndrome in Night Shift Work

- Diabetes, Obesity and Metabolism
- Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones
- Bo Hye Kim, Yena Joo, Min-Seon Kim, Han Kyoung Choe, Qingchun Tong, Obin Kwon
- Endocrinol Metab. 2021;36(4):745-756. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.405
- 24,884 View
- 986 Download
- 29 Web of Science
- 29 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
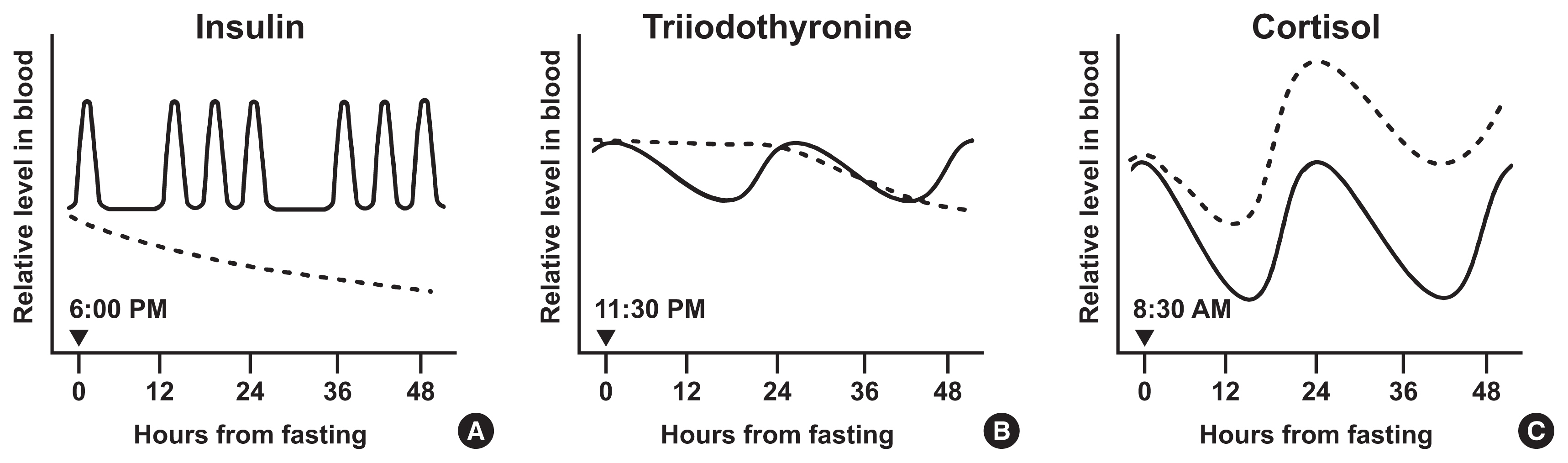
ePub - Intermittent fasting has become an increasingly popular strategy in losing weight and associated reduction in obesity-related medical complications. Overwhelming studies support metabolic improvements from intermittent fasting in blood glucose levels, cardiac and brain function, and other health benefits, in addition to weight loss. However, concerns have also been raised on side effects including muscle loss, ketosis, and electrolyte imbalance. Of particular concern, the effect of intermittent fasting on hormonal circadian rhythms has received little attention. Given the known importance of circadian hormonal changes to normal physiology, potential detrimental effects by dysregulation of hormonal changes deserve careful discussions. In this review, we describe the changes in circadian rhythms of hormones caused by intermittent fasting. We covered major hormones commonly pathophysiologically involved in clinical endocrinology, including insulin, thyroid hormones, and glucocorticoids. Given that intermittent fasting could alter both the level and frequency of hormone secretion, decisions on practicing intermittent fasting should take more considerations on potential detrimental consequences versus beneficial effects pertaining to individual health conditions.
-
Citations
Citations to this article as recorded by- Common and divergent molecular mechanisms of fasting and ketogenic diets
Antonio Paoli, Grant M. Tinsley, Mark P. Mattson, Immaculata De Vivo, Ravi Dhawan, Tatiana Moro
Trends in Endocrinology & Metabolism.2024; 35(2): 125. CrossRef - Identifying Acss1, Mtfp1 and Oxct1 as key regulators and promising biomarkers of sarcopenia in various models
Hailong Cui, Die Hu, Yanling Liu, Jiejie Zhao
Gene.2024; 896: 148053. CrossRef - Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis—Molecular Mechanisms in Human Health
Cristina Manuela Drăgoi, Alina Crenguţa Nicolae, Anca Ungurianu, Denisa Marilena Margină, Daniela Grădinaru, Ion-Bogdan Dumitrescu
Cells.2024; 13(2): 138. CrossRef - Various types of fasting diet and possible benefits in nonalcoholic fatty liver: Mechanism of actions and literature update
Zahra Sadat Mirrazavi, Vahideh Behrouz
Clinical Nutrition.2024; 43(2): 519. CrossRef - Attention to Innate Circadian Rhythm and the Impact of Its Disruption on Diabetes
Da Young Lee, Inha Jung, So Young Park, Ji Hee Yu, Ji A Seo, Kyeong Jin Kim, Nam Hoon Kim, Hye Jin Yoo, Sin Gon Kim, Kyung Mook Choi, Sei Hyun Baik, Nan Hee Kim
Diabetes & Metabolism Journal.2024; 48(1): 37. CrossRef - Genetics of Exercise and Diet-Induced Fat Loss Efficiency: A Systematic Review
Aleksandra Bojarczuk, Emiliya S. Egorova, Magdalena Dzitkowska-Zabielska, Ildus I. Ahmetov
Journal of Sports Science and Medicine.2024; : 236. CrossRef - Ramadan fasting in the third trimester of pregnancy and postpartum colostrum cortisol concentrations in Morocco
Meagan M. Guilfoyle
American Journal of Human Biology.2024;[Epub] CrossRef - Dietary factors in circadian rhythm modulation and their impact on metabolic diseases: a state of the science review
Malvika Dalvi, Srujana Medithi
Biological Rhythm Research.2024; : 1. CrossRef - Unlocking the Benefits of Fasting: A Review of its Impact on Various
Biological Systems and Human Health
Rawan Mackieh, Nadia Al-Bakkar, Milena Kfoury, Nathalie Okdeh, Hervé Pietra, Rabih Roufayel, Christian Legros, Ziad Fajloun, Jean-Marc Sabatier
Current Medicinal Chemistry.2024; 31(14): 1781. CrossRef - Fasting intervention and its clinical effects on the human host and microbiome
Sofia K. Forslund
Journal of Internal Medicine.2023; 293(2): 166. CrossRef - Umbrella review of time-restricted eating on weight loss, fasting blood glucose, and lipid profile
Han Shi Jocelyn Chew, Wei How Darryl Ang, Zhen Yang Abel Tan, Wen Wei Ang, Kin Sun Chan, Ying Lau
Nutrition Reviews.2023; 81(9): 1180. CrossRef - Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity
Melek Ece Öngel, Cennet Yildiz, Özge Başer, Bayram Yilmaz, Mustafa Özilgen
Entropy.2023; 25(2): 227. CrossRef - Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats
Cinthia García-Luna, Ixchel Prieto, Paulina Soberanes-Chávez, Elena Alvarez-Salas, Iván Torre-Villalvazo, Gilberto Matamoros-Trejo, Patricia de Gortari
Nutrients.2023; 15(5): 1164. CrossRef - Possible homeostatic, glucose uptake mechanisms and hepato-pancreatic histological effects of intermittent fasting, exercise, starvation, and honey in streptozotocin-induced diabetes in rats
Ejime A. Chijiokwu, Eze K. Nwangwa, Mega O. Oyovwi, Benneth Ben-Azu, Alexander O. Naiho, Emuesiri Goodies Moke, Victor Emojevwe, Prosper A. Ehiwarior, Udoka S. Nwabuoku
Nutrire.2023;[Epub] CrossRef - Mid-Point of the Active Phase Is Better to Achieve the Natriuretic Effect of Acute Salt Load in Mice
Momoko Imamura, Hiroyuki Sasaki, Katsuki Hayashi, Shigenobu Shibata
Nutrients.2023; 15(7): 1679. CrossRef - All That Glitters Is Not Gold: The Same Sleep Time, but Different Diabetogenic Outcomes
Bohye Kim, Obin Kwon
Endocrinology and Metabolism.2023; 38(1): 78. CrossRef - The emerging role of circadian rhythms in the development and function of thermogenic fat
Xuemin Peng, Yong Chen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Time-restricted Feeding Changes as Inspiration for Drug Design
Zhangyuting He, Huayu Yang, Yilei Mao
Current Pharmaceutical Design.2023; 29(8): 559. CrossRef - Brain Dopamine–Clock Interactions Regulate Cardiometabolic Physiology: Mechanisms of the Observed Cardioprotective Effects of Circadian-Timed Bromocriptine-QR Therapy in Type 2 Diabetes Subjects
Anthony H. Cincotta
International Journal of Molecular Sciences.2023; 24(17): 13255. CrossRef - Adaptive Circadian Rhythms for Autonomous and Biologically Inspired Robot Behavior
Marcos Maroto-Gómez, María Malfaz, Álvaro Castro-González, Sara Carrasco-Martínez, Miguel Ángel Salichs
Biomimetics.2023; 8(5): 413. CrossRef - Intermittent Fasting on Human Health and Disease
Denisa Marilena Margină, Cristina Manuela Drăgoi
Nutrients.2023; 15(21): 4491. CrossRef - Synthetic augmentation of bilirubin metabolism in human pluripotent stem cell-derived liver organoids
Hasan Al Reza, Zishaan Farooqui, Abid Al Reza, Callen Conroy, Kentaro Iwasawa, Yasuhiro Ogura, Keisuke Okita, Kenji Osafune, Takanori Takebe
Stem Cell Reports.2023; 18(11): 2071. CrossRef - Average phenotype but not plasticity in two metabolic hormones covary in wild female bonobos (Pan paniscus)
Ruth Sonnweber, Gottfried Hohmann, Jeroen M. G. Stevens, Tobias Deschner, Barbara Fruth, Anna-Lena Fiedler, Niina O. Nurmi, Verena Behringer
Frontiers in Ecology and Evolution.2023;[Epub] CrossRef - Intermittent fasting, high-intensity interval training, or a combination of both have beneficial effects in obese mice with nonalcoholic fatty liver disease
Patrícia de Castro-de-Paiva, Thatiany de Souza Marinho, Carlos Alberto Mandarim-de-Lacerda, Marcia Barbosa Aguila
The Journal of Nutritional Biochemistry.2022; 104: 108997. CrossRef - Optimal Timing of Thyroid Hormone Replacement During Ramadan Fasting: A Randomized Controlled Trial in Patients with Prior Total Thyroidectomy
Khalid M. Al-Qahtani, Ibraheem Ahmed Aldeeri, Amal M. Alshaibi, Norah Salman Alshabib, Rakan M. Barghouthi, Ebtihal Y. Alyusuf, Anwar Ali Jammah
Thyroid.2022; 32(9): 1029. CrossRef - Exploring the Effects of Energy Constraints on Performance, Body Composition, Endocrinological/Hematological Biomarkers, and Immune System among Athletes: An Overview of the Fasting State
Hadi Nobari, Saber Saedmocheshi, Eugenia Murawska-Ciałowicz, Filipe Manuel Clemente, Katsuhiko Suzuki, Ana Filipa Silva
Nutrients.2022; 14(15): 3197. CrossRef - Alternate day fasting and time-restricted feeding may confer similar neuroprotective effects during aging in male rats
Sukanya Bhoumik, Rashmi Kesherwani, Raushan Kumar, Syed Ibrahim Rizvi
Biogerontology.2022; 23(6): 757. CrossRef - Intermittent Fasting—A Healthy Dietary Pattern for Diabetic Nephropathy
Ming Yang, Wei Chen, Liyu He, Di Liu, Li Zhao, Xi Wang
Nutrients.2022; 14(19): 3995. CrossRef - β-hydroxybutyrate as an Anti-Aging Metabolite
Lian Wang, Peijie Chen, Weihua Xiao
Nutrients.2021; 13(10): 3420. CrossRef
- Common and divergent molecular mechanisms of fasting and ketogenic diets

- Obesity and Metabolism
- Distinct Ultradian Rhythms in Plasma Clusterin Concentrations in Lean and Obese Korean Subjects
- Jong Han Choi, Eunheui Jeong, Byung Soo Youn, Min-Seon Kim
- Endocrinol Metab. 2018;33(2):245-251. Published online May 4, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.2.245
- 4,527 View
- 42 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Blood levels of many hormones show rhythmic fluctuations with variable duration of cycles. Clusterin/apolipoprotein J is a glycoprotein which is highly expressed in the plasma and has modulatory roles in immune and inflammatory reactions, neurobiology, lipid metabolism, and leptin signaling. In this study, we examined the diurnal fluctuations of plasma clusterin concentrations in lean and obese young men.
Methods For the study, 14 subjects (five lean and five obese men; two lean and two obese women) were admitted to the research ward and blood samples were drawn every 30 minutes during light-on period (6:00 AM to 10:00 PM) and every hour during light-off period.
Results Notably, plasma clusterin concentrations displayed a unique ultradian rhythm with five cycles a day in both men and women. During the light-on period, circulating clusterin levels showed fluctuating curves with 4 hours regular intervals with sharp peaks and troughs. In contrast, single oscillation curve during light-off exhibited a smoothened/lower peak and longer (8-hour) duration. In obese men, these cycles were phase-advanced by approximately 1 hour, and had reduced amplitude of fluctuating curves and blunted diurnal pattern. Cyclic fluctuations of plasma clusterin were preserved under fasting and unexpected meal condition, suggesting that rhythmic oscillations in plasma clusterin levels are not generated by meal-related cues.
Conclusion These findings firstly demonstrate a novel pattern of plasma clusterin fluctuations with extremely regular cycles.
-
Citations
Citations to this article as recorded by- Clusterin and Related Scoring Index as Potential Early Predictors of Response to Sorafenib in Hepatocellular Carcinoma
Satoshi Narahara, Takehisa Watanabe, Katsuya Nagaoka, Nahoko Fujimoto, Yoki Furuta, Kentaro Tanaka, Takayuki Tokunaga, Takeshi Kawasaki, Yoko Yoshimaru, Hiroko Setoyama, Kentaro Oniki, Junji Saruwatari, Masakuni Tateyama, Hideaki Naoe, Motohiko Tanaka, Ya
Hepatology Communications.2022; 6(5): 1198. CrossRef - The role of circadian rhythm in choroid plexus functions
Telma Quintela, André Furtado, Ana C. Duarte, Isabel Gonçalves, Jihwan Myung, Cecília R.A. Santos
Progress in Neurobiology.2021; 205: 102129. CrossRef - A Novel Multi-Biomarker Assay for Non-Invasive Quantitative Monitoring of Kidney Injury
Drew Watson, Joshua Y. C. Yang, Reuben D. Sarwal, Tara K. Sigdel, Juliane M. Liberto, Izabella Damm, Victoria Louie, Shristi Sigdel, Devon Livingstone, Katherine Soh, Arjun Chakraborty, Michael Liang, Pei-Chen Lin, Minnie M. Sarwal
Journal of Clinical Medicine.2019; 8(4): 499. CrossRef
- Clusterin and Related Scoring Index as Potential Early Predictors of Response to Sorafenib in Hepatocellular Carcinoma

- Effect of Mefloquine, a Gap Junction Blocker, on Circadian Period2 Gene Oscillation in the Mouse Suprachiasmatic Nucleus Ex Vivo
- Jinmi Koo, Han Kyoung Choe, Hee-Dae Kim, Sung Kook Chun, Gi Hoon Son, Kyungjin Kim
- Endocrinol Metab. 2015;30(3):361-370. Published online December 9, 2014
- DOI: https://doi.org/10.3803/EnM.2015.30.3.361
- 3,641 View
- 37 Download
- 5 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background In mammals, the master circadian pacemaker is localized in an area of the ventral hypothalamus known as the suprachiasmatic nucleus (SCN). Previous studies have shown that pacemaker neurons in the SCN are highly coupled to one another, and this coupling is crucial for intrinsic self-sustainability of the SCN central clock, which is distinguished from peripheral oscillators. One plausible mechanism underlying the intercellular communication may involve direct electrical connections mediated by gap junctions.
Methods We examined the effect of mefloquine, a neuronal gap junction blocker, on circadian Period 2 (
Per2 ) gene oscillation in SCN slice cultures prepared from Per2::luciferase (PER2::LUC) knock-in mice using a real-time bioluminescence measurement system.Results Administration of mefloquine causes instability in the pulse period and a slight reduction of amplitude in cyclic PER2::LUC expression. Blockade of gap junctions uncouples PER2::LUC-expressing cells, in terms of phase transition, which weakens synchrony among individual cellular rhythms.
Conclusion These findings suggest that neuronal gap junctions play an important role in synchronizing the central pacemaker neurons and contribute to the distinct self-sustainability of the SCN master clock.
-
Citations
Citations to this article as recorded by- High-Throughput Screening Assay for Detecting Drug-Induced Changes in Synchronized Neuronal Oscillations and Potential Seizure Risk Based on Ca2+ Fluorescence Measurements in Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuronal 2D and 3D Cultures
Hua-Rong Lu, Manabu Seo, Mohamed Kreir, Tetsuya Tanaka, Rie Yamoto, Cristina Altrocchi, Karel van Ammel, Fetene Tekle, Ly Pham, Xiang Yao, Ard Teisman, David J. Gallacher
Cells.2023; 12(6): 958. CrossRef - The role of gap junctions in cell death and neuromodulation in the retina
Gergely Szarka, Márton Balogh, ÁdámJ Tengölics, Alma Ganczer, Béla Völgyi, Tamás Kovács-Öller
Neural Regeneration Research.2021; 16(10): 1911. CrossRef - Programming effects of maternal stress on the circadian system of adult offspring
Seongsik Yun, Eun Jeong Lee, Han Kyoung Choe, Gi Hoon Son, Kyungjin Kim, Sooyoung Chung
Experimental & Molecular Medicine.2020; 52(3): 473. CrossRef - Kisspeptin Neuron-Specific and Self-Sustained Calcium Oscillation in the Hypothalamic Arcuate Nucleus of Neonatal Mice: Regulatory Factors of its Synchronization
Doyeon Kim, Sangwon Jang, Jeongah Kim, Inah Park, Kyojin Ku, Mijung Choi, Sukwon Lee, Won Do Heo, Gi Hoon Son, Han Kyoung Choe, Kyungjin Kim
Neuroendocrinology.2020; 110(11-12): 1010. CrossRef
- High-Throughput Screening Assay for Detecting Drug-Induced Changes in Synchronized Neuronal Oscillations and Potential Seizure Risk Based on Ca2+ Fluorescence Measurements in Human Induced Pluripotent Stem Cell (hiPSC)-Derived Neuronal 2D and 3D Cultures


 KES
KES

 First
First Prev
Prev



