Search
- Page Path
- HOME > Search
- Diabetes, Obesity and Metabolism
- Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia
- Joon Ho Moon, Kyuho Kim, Sung Hee Choi
- Endocrinol Metab. 2022;37(4):575-586. Published online August 29, 2022
- DOI: https://doi.org/10.3803/EnM.2022.402
- 7,835 View
- 440 Download
- 11 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
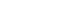
ePub - High levels of triglycerides (TG) and triglyceride-rich lipoproteins (TGRLs) confer a residual risk of cardiovascular disease after optimal low-density lipoprotein cholesterol (LDL-C)–lowering therapy. Consensus has been made that LDL-C is a non-arguable primary target for lipid lowering treatment, but the optimization of TGRL for reducing the remnant risk of cardiovascular diseases is urged. Omega-3 fatty acids and fibrates are used to reduce TG levels, but many patients still have high TG and TGRL levels combined with low high-density lipoprotein concentration that need to be ideally treated. Lipoprotein lipase (LPL) is a key regulator for TGs that hydrolyzes TGs to glycerol and free fatty acids in lipoprotein particles for lipid storage and consumption in peripheral organs. A deeper understanding of human genetics has enabled the identification of proteins regulating the LPL activity, which include the apolipoproteins and angiopoietin-like families. Novel therapeutic approach such as antisense oligonucleotides and monoclonal antibodies that regulate TGs have been developed in recent decades. In this article, we focus on the biology of LPL and its modulators and review recent clinical application, including genetic studies and clinical trials of novel therapeutics. Optimization of LPL activity to lower TG levels could eventually reduce incident atherosclerotic cardiovascular disease in conjunction with successful LDL-C reduction.
-
Citations
Citations to this article as recorded by- The chylomicron saga: time to focus on postprandial metabolism
Alejandro Gugliucci
Frontiers in Endocrinology.2024;[Epub] CrossRef - Sanghuangporus vaninii extract ameliorates hyperlipidemia in rats by mechanisms identified with transcriptome analysis
Ning Gao, Yuanzhen Liu, Guangjie Liu, Bo Liu, Yupeng Cheng
Food Science & Nutrition.2024;[Epub] CrossRef - Targeting host-specific metabolic pathways—opportunities and challenges for anti-infective therapy
Monika I. Konaklieva, Balbina J. Plotkin
Frontiers in Molecular Biosciences.2024;[Epub] CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the obesity medicine association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Obesity Pillars.2024; : 100108. CrossRef - Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
Chaicharn Deerochanawong, Sin Gon Kim, Yu-Cheng Chang
Diabetes & Metabolism Journal.2024; 48(2): 184. CrossRef - Xanthohumol, a prenylated chalcone, regulates lipid metabolism by modulating the LXRα/RXR-ANGPTL3-LPL axis in hepatic cell lines and high-fat diet-fed zebrafish models
Wan-Yun Gao, Pei-Yi Chen, Hao-Jen Hsu, Je-Wen Liou, Chia-Ling Wu, Ming-Jiuan Wu, Jui-Hung Yen
Biomedicine & Pharmacotherapy.2024; 174: 116598. CrossRef - Obesity, dyslipidemia, and cardiovascular disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024
Harold Edward Bays, Carol Kirkpatrick, Kevin C. Maki, Peter P. Toth, Ryan T. Morgan, Justin Tondt, Sandra Michelle Christensen, Dave Dixon, Terry A. Jacobson
Journal of Clinical Lipidology.2024;[Epub] CrossRef - High producer variant of lipoprotein lipase may protect from hepatocellular carcinoma in alcohol-associated cirrhosis
Franziska Schmalz, Janett Fischer, Hamish Innes, Stephan Buch, Christine Möller, Madlen Matz-Soja, Witigo von Schönfels, Benjamin Krämer, Bettina Langhans, Alexandra Klüners, Michael Soyka, Felix Stickel, Jacob Nattermann, Christian P. Strassburg, Thomas
JHEP Reports.2023; 5(4): 100684. CrossRef - Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations
Dieter Lütjohann, Hans-Ulrich Klör, Frans Stellaard
Nutrients.2023; 15(9): 2202. CrossRef - Influence of antipsychotic medications on hyperlipidemia risk in patients with schizophrenia: evidence from a population-based cohort study and in vitro hepatic lipid homeostasis gene expression
Tien-Yuan Wu, Ni Tien, Cheng-Li Lin, Yu-Cun Cheah, Chung Y. Hsu, Fuu-Jen Tsai, Yi-Jen Fang, Yun-Ping Lim
Frontiers in Medicine.2023;[Epub] CrossRef - Triglyceride-Rich Lipoprotein Metabolism: Key Regulators of Their Flux
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(13): 4399. CrossRef - Sugar and Dyslipidemia: A Double-Hit, Perfect Storm
Alejandro Gugliucci
Journal of Clinical Medicine.2023; 12(17): 5660. CrossRef - Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview
Sang Heon Suh, Soo Wan Kim
Diabetes & Metabolism Journal.2023; 47(5): 612. CrossRef - Peroxisome Proliferator-Activated Receptor α in Lipoprotein Metabolism and Atherosclerotic Cardiovascular Disease
Elena Valeria Fuior, Evangelia Zvintzou, Theodosios Filippatos, Katerina Giannatou, Victoria Mparnia, Maya Simionescu, Anca Violeta Gafencu, Kyriakos E. Kypreos
Biomedicines.2023; 11(10): 2696. CrossRef - Developing a model to predict the early risk of hypertriglyceridemia based on inhibiting lipoprotein lipase (LPL): a translational study
Julia Hernandez-Baixauli, Gertruda Chomiciute, Juan María Alcaide-Hidalgo, Anna Crescenti, Laura Baselga-Escudero, Hector Palacios-Jordan, Elisabet Foguet-Romero, Anna Pedret, Rosa M. Valls, Rosa Solà, Miquel Mulero, Josep M. Del Bas
Scientific Reports.2023;[Epub] CrossRef
- The chylomicron saga: time to focus on postprandial metabolism

- Diabetes, Obesity and Metabolism
Big Data Articles (National Health Insurance Service Database) - Association of High-Density Lipoprotein Cholesterol Phenotypes with the Risk of Cardiovascular Diseases and Mortality: A Cohort Study in Korea
- Ga Eun Nam, Youn Huh, Jin-Hyung Jung, Kyungdo Han, Seon Mee Kim, on Behalf of the Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity
- Endocrinol Metab. 2022;37(2):261-271. Published online April 25, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1259

- 3,514 View
- 141 Download
- 2 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
We investigated whether low high-density lipoprotein cholesterol (HDL-C) and isolated and non-isolated low HDL-C levels are associated with the risk of cardiovascular diseases and all-cause mortality among Korean adults.
Methods
We included 8,665,841 individuals aged ≥20 years who had undergone a health examination provided by the Korean National Health Insurance Service (NHIS) in 2009 and were followed up until the end of 2018. The hazard ratios (HRs) and 95% confidence intervals (CIs) for study outcomes were calculated using multivariable Cox proportional hazard regression analysis.
Results
During the 8.2 years of mean follow-up, myocardial infarction (MI), stroke, and all-cause mortality occurred in 81,431, 110,996, and 244,309 individuals, respectively. After adjusting for confounding variables (model 3), individuals with low HDL-C and lower HDL quartiles were associated with significantly increased risks of all three outcomes, compared to those with normal HDL-C and highest HDL-C quartile (all P<0.001), respectively. HRs for incident MI (1.28; 95% CI, 1.26 to 1.30), stroke (1.13; 95% CI, 1.11 to 1.15), and all-cause mortality (1.07; 95% CI, 1.05 to 1.08) increased in the non-isolated low HDL-C group compared to the normal HDL-C group. Isolated low HDL-C also showed an increase in the HRs of incident stroke (1.06; 95% CI, 1.04 to 1.08) and all-cause mortality (1.30; 95% CI, 1.28 to 1.32).
Conclusion
Low HDL-C and non-isolated low HDL-C were associated with increased risk of MI, stroke, and all-cause mortality, and isolated low HDL-C was associated with incident stroke and all-cause mortality risk. -
Citations
Citations to this article as recorded by- Association between HDL levels and stroke outcomes in the Arab population
Aizaz Ali, Omar Obaid, Naveed Akhtar, Rahul Rao, Syed Haroon Tora, Ashfaq Shuaib
Scientific Reports.2024;[Epub] CrossRef - Association of adiposity and fitness with triglyceride-to-high-density lipoprotein cholesterol ratio in youth
Danladi Ibrahim Musa, Abel Lamina Toriola, Nurudeen O Abubakar, Sunday Omachi, Victor B Olowoleni, Kolade B Ayodele
Annals of Pediatric Cardiology.2023; 16(3): 194. CrossRef - Association between cholesterol levels and dementia risk according to the presence of diabetes and statin use: a nationwide cohort study
You-Bin Lee, Min Young Kim, Kyungdo Han, Bongsung Kim, Jiyun Park, Gyuri Kim, Kyu Yeon Hur, Jae Hyeon Kim, Sang-Man Jin
Scientific Reports.2022;[Epub] CrossRef
- Association between HDL levels and stroke outcomes in the Arab population

- Thyroid
Big Data Articles (National Health Insurance Service Database) - Repeated Low High-Density Lipoprotein Cholesterol and the Risk of Thyroid Cancer: A Nationwide Population- Based Study in Korea
- Jinyoung Kim, Mee Kyoung Kim, Ki-Hyun Baek, Ki-Ho Song, Kyungdo Han, Hyuk-Sang Kwon
- Endocrinol Metab. 2022;37(2):303-311. Published online April 6, 2022
- DOI: https://doi.org/10.3803/EnM.2021.1332
- 4,652 View
- 154 Download
- 12 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
High-density lipoprotein cholesterol (HDL-C) plays an important role in the reverse cholesterol transport pathway and prevents atherosclerosis-mediated disease. It has also been suggested that HDL-C may be a protective factor against cancer. However, an inverse correlation between HDL-C and cancer has not been established, and few studies have explored thyroid cancer.
Methods
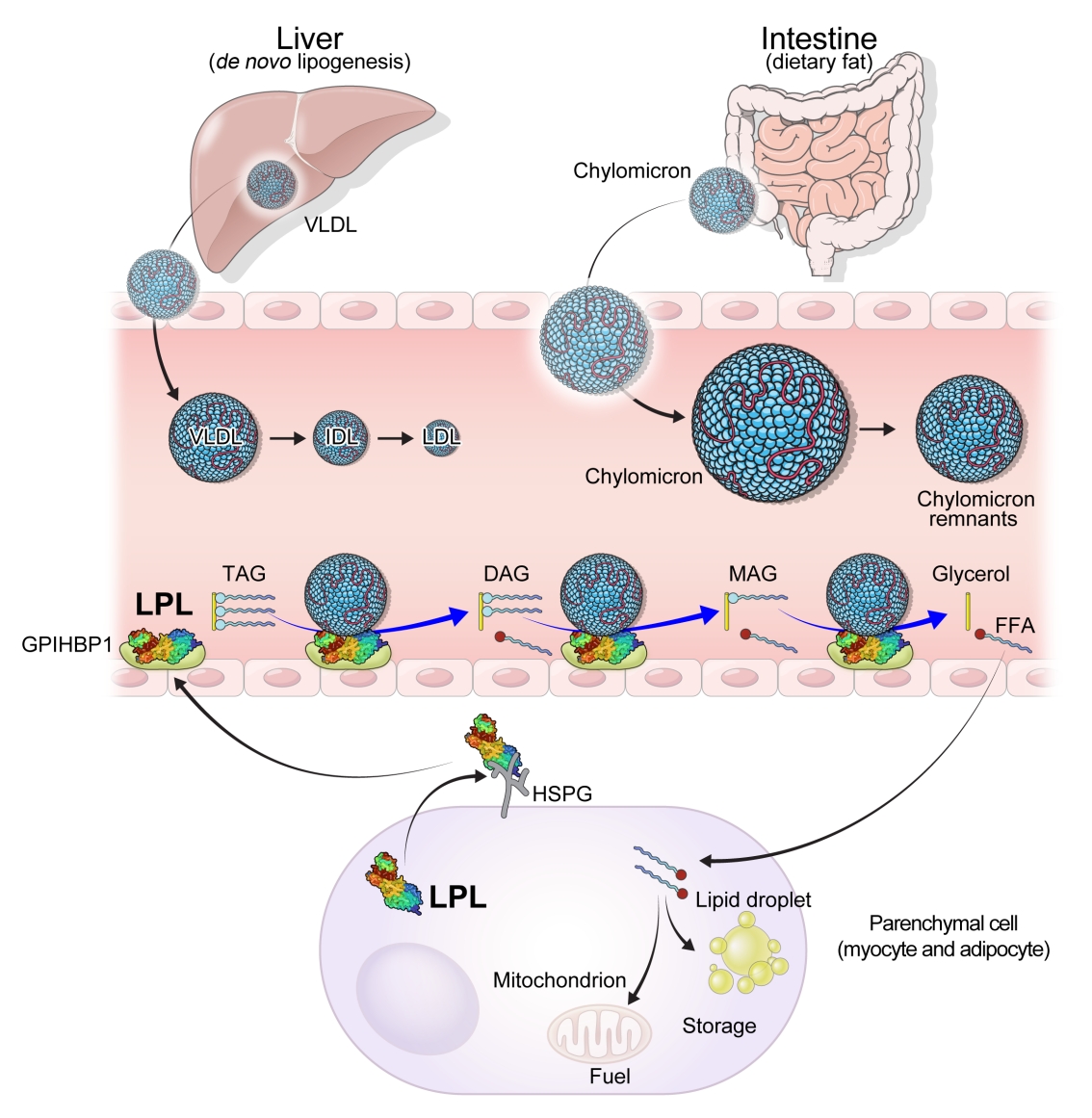
The study participants received health checkups provided by the Korean National Health Insurance Service from 2009 to 2013 and were followed until 2019. Considering the variability of serum HDL-C level, low HDL-C level was analyzed by grouping based on four consecutive health checkups. The data analysis was performed using univariate and multivariate Cox proportional hazard regression models.
Results
A total of 3,134,278 total study participants, thyroid cancer occurred in 16,129. In the crude model, the hazard ratios for the association between repeatedly measured low HDL-C levels and thyroid cancer were 1.243, 1.404, 1.486, and 1.680 (P for trend <0.01), respectively, which were significant even after adjusting for age, sex, lifestyle factors, and metabolic diseases. The subgroup analysis revealed that low HDL-C levels likely had a greater impact on the group of patients with central obesity (P for interaction= 0.062), high blood pressure (P for interaction=0.057), impaired fasting glucose (P for interaction=0.051), and hyperlipidemia (P for interaction=0.126).
Conclusion
Repeatedly measured low HDL-C levels can be considered a risk factor for cancer as well as vascular disease. Low HDL-C levels were associated with the risk of thyroid cancer, and this correlation was stronger in a metabolically unhealthy population. -
Citations
Citations to this article as recorded by- Association between total cholesterol levels and all-cause mortality among newly diagnosed patients with cancer
Seohyun Kim, Gyuri Kim, So Hyun Cho, Rosa Oh, Ji Yoon Kim, You-Bin Lee, Sang-Man Jin, Kyu Yeon Hur, Jae Hyeon Kim
Scientific Reports.2024;[Epub] CrossRef - Association between organophosphate flame retardant exposure and lipid metabolism: data from the 2013–2014 National Health and Nutrition Examination Survey
Fu-Jen Cheng, Kai-Fan Tsai, Kuo-Chen Huang, Chia-Te Kung, Wan-Ting Huang, Huey-Ling You, Shau-Hsuan Li, Chin-Chou Wang, Wen-Chin Lee, Hsiu-Yung Pan
Frontiers in Public Health.2024;[Epub] CrossRef - Low serum total cholesterol levels predict inferior prognosis of patients with POEMS syndrome
Jue Zhang, Ting Zhang, Ye Yao, Xuxing Shen, Yuanyuan Jin, Run Zhang, Lijuan Chen
Discover Oncology.2024;[Epub] CrossRef - Lipoprotein alterations in endocrine disorders - a review of the recent developments in the field
Michal Olejarz, Ewelina Szczepanek-Parulska, Marek Ruchala
Frontiers in Endocrinology.2024;[Epub] CrossRef - Carbohydrate, Lipid, and Apolipoprotein Biomarkers in Blood and Risk of Thyroid Cancer: Findings from the AMORIS Cohort
Xue Xiao, Yi Huang, Fetemeh Sadeghi, Maria Feychting, Niklas Hammar, Fang Fang, Zhe Zhang, Qianwei Liu
Cancers.2023; 15(2): 520. CrossRef - Altered serum lipid levels are associated with prognosis of diffuse large B cell lymphoma and influenced by utility of rituximab
Fei Wang, Luo Lu, HuiJuan Chen, Yanhua Yue, Yanting Sun, Feng Yan, Bai He, Rongrong Lin, Weiying Gu
Annals of Hematology.2023; 102(2): 393. CrossRef - Big Data Research in the Field of Endocrine Diseases Using the Korean National Health Information Database
Sun Wook Cho, Jung Hee Kim, Han Seok Choi, Hwa Young Ahn, Mee Kyoung Kim, Eun Jung Rhee
Endocrinology and Metabolism.2023; 38(1): 10. CrossRef - High-density lipoprotein cholesterol and carcinogenesis
Meijuan Tan, Shijie Yang, Xiequn Xu
Trends in Endocrinology & Metabolism.2023; 34(5): 303. CrossRef - Low Serum Cholesterol Level Is a Significant Prognostic Factor That Improves CLL-IPI in Chronic Lymphocytic Leukaemia
Rui Gao, Kaixin Du, Jinhua Liang, Yi Xia, Jiazhu Wu, Yue Li, Bihui Pan, Li Wang, Jianyong Li, Wei Xu
International Journal of Molecular Sciences.2023; 24(8): 7396. CrossRef - Do metabolic factors increase the risk of thyroid cancer? a Mendelian randomization study
Weiwei Liang, FangFang Sun
Frontiers in Endocrinology.2023;[Epub] CrossRef - Assessment of causal association between differentiated thyroid cancer and disordered serum lipid profile: a Mendelian randomization study
Qiang Ma, Yu Li, Lijuan An, Liang Guo, Xiaokang Liu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Risk factors and diagnostic prediction models for papillary thyroid carcinoma
Xiaowen Zhang, Yuyang Ze, Jianfeng Sang, Xianbiao Shi, Yan Bi, Shanmei Shen, Xinlin Zhang, Dalong Zhu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Exposure to multiple trace elements and thyroid cancer risk in Chinese adults: A case-control study
Jia-liu He, Hua-bing Wu, Wen-lei Hu, Jian-jun Liu, Qian Zhang, Wei Xiao, Ming-jun Hu, Ming Wu, Fen Huang
International Journal of Hygiene and Environmental Health.2022; 246: 114049. CrossRef
- Association between total cholesterol levels and all-cause mortality among newly diagnosed patients with cancer

- Diabetes, Obesity and Metabolism
- Lower High-Density Lipoprotein Cholesterol Concentration Is Independently Associated with Greater Future Accumulation of Intra-Abdominal Fat
- Sun Ok Song, You-Cheol Hwang, Han Uk Ryu, Steven E. Kahn, Donna L. Leonetti, Wilfred Y. Fujimoto, Edward J. Boyko
- Endocrinol Metab. 2021;36(4):835-844. Published online August 27, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1130
- 4,386 View
- 120 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Both intra-abdominal fat (IAF) and high-density lipoprotein cholesterol (HDL-C) are known to be associated with cardiometabolic health. We evaluated whether the accumulation of computed tomography (CT)-measured IAF over 5 years was related to baseline HDL-C concentration in a prospective cohort study.
Methods
All participants were Japanese-Americans between the ages of 34 and 74 years. Plasma HDL-C concentration and CT measurements of IAF, abdominal subcutaneous fat (SCF), and thigh SCF cross-sectional areas were assessed at baseline and at 5-year follow-up visits.
Results
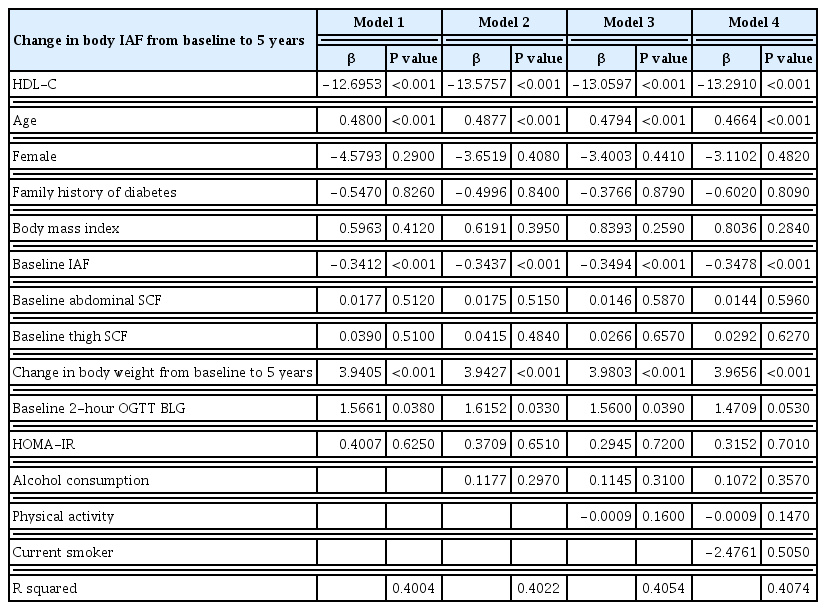
A total of 397 subjects without diabetes were included. The mean±standard deviation HDL-C concentration was 51.6±13.0 mg/dL in men and 66.0±17.0 mg/dL in women, and the IAF was 91.9±48.4 cm2 in men and 63.1±39.5 cm2 in women. The baseline plasma concentration of HDL-C was inversely associated with the change in IAF over 5 years using multivariable regression analysis with adjustment for age, sex, family history of diabetes, weight change over 5 years, and baseline measurements of body mass index, IAF, abdominal SCF, abdominal circumference, thigh SCF, and homeostatic model assessment for insulin resistance.
Conclusion
These results demonstrate that HDL-C concentration significantly predicts future accumulation of IAF over 5 years independent of age, sex, insulin sensitivity, and body composition in Japanese-American men and women without diabetes. -
Citations
Citations to this article as recorded by- Fenofibrate add-on to statin treatment is associated with low all-cause death and cardiovascular disease in the general population with high triglyceride levels
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Metabolism.2022; 137: 155327. CrossRef - The associations between lipid profiles and visceral obesity among gastrointestinal cancer patients: a cross-sectional study
Bo Gao, Xiangrui Li, Wenqing Chen, Shu’an Wang, Jian He, Yu Liu, Chao Ding, Xiaotian Chen
Lipids in Health and Disease.2022;[Epub] CrossRef
- Fenofibrate add-on to statin treatment is associated with low all-cause death and cardiovascular disease in the general population with high triglyceride levels

- Adrenal Gland
- Lipid Profiles in Primary Aldosteronism Compared with Essential Hypertension: Propensity-Score Matching Study
- Sun Joon Moon, Han Na Jang, Jung Hee Kim, Min Kyong Moon
- Endocrinol Metab. 2021;36(4):885-894. Published online August 10, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1012

- 3,190 View
- 138 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
There has been controversy regarding the association between primary aldosteronism (PA) and dyslipidemia and few studies considered the effects of diabetes and renal function on lipid metabolism. We analyzed lipid profiles of PA patients and compared them to propensity-score (PS)-matched essential hypertension (EH) patients adjusting for glycemic status and renal function.
Methods
Patients who were diagnosed with PA using a saline-infusion test at Seoul National University Hospital from 2000 to 2018 were retrospectively analyzed. EH patients who had aldosterone-renin ratio (ARR) results were selected as controls. Covariates, including diabetes, were PS-matched for patients with PA, lateralized PA, non-lateralized PA, and high ARR to EH patients, respectively.
Results
Among a total of 80 PA and 80 EH patients, total cholesterol (TC) and triglyceride (TG) levels were significantly lower in the PA patients than in the EH patients (least-squares mean±standard error: 185.5±4.4 mg/dL vs. 196.2±4.4 mg/dL, P=0.047, for TC; and 132.3±11.5 mg/dL vs. 157.4±11.4 mg/dL, P=0.035, for TG) in fully adjusted model (adjusting for multiple covariates, including diabetes status, glycosylated hemoglobin level, and estimated glomerular filtration rate). There were no significant differences in high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol levels between the two groups. According to increments in aldosterone levels, an increasing tendency of HDL-C and decreasing tendencies of TG and non-HDL-C were observed.
Conclusion
PA patients had lower TC and TG levels than EH patients, independent of glycemic status and renal function. -
Citations
Citations to this article as recorded by- Comparison of saline infusion test and captopril challenge test in the diagnosis of Chinese with primary aldosteronism in different age groups
Kaiwen Sun, Minghui Gong, Yang Yu, Minghui Yang, Ying Zhang, Yinong Jiang, Wei Song
Frontiers in Endocrinology.2024;[Epub] CrossRef - Meta‐analysis of blood parameters related to lipid and glucose metabolism between two subtypes of primary aldosteronism
Qiu‐Gen Zhu, Feng Zhu
The Journal of Clinical Hypertension.2023; 25(1): 13. CrossRef - 2023 Korean Endocrine Society Consensus Guidelines for the Diagnosis and Management of Primary Aldosteronism
Jeonghoon Ha, Jung Hwan Park, Kyoung Jin Kim, Jung Hee Kim, Kyong Yeun Jung, Jeongmin Lee, Jong Han Choi, Seung Hun Lee, Namki Hong, Jung Soo Lim, Byung Kwan Park, Jung-Han Kim, Kyeong Cheon Jung, Jooyoung Cho, Mi-kyung Kim, Choon Hee Chung
Endocrinology and Metabolism.2023; 38(6): 597. CrossRef - The differences of serum lipid profiles between primary aldosteronism and essential hypertension: a meta-analysis and systematic review
Worapaka Manosroi, Pitchaporn Phudphong, Pichitchai Atthakomol, Mattabhorn Phimphilai
BMC Endocrine Disorders.2022;[Epub] CrossRef
- Comparison of saline infusion test and captopril challenge test in the diagnosis of Chinese with primary aldosteronism in different age groups

- Clinical Study
- Efficacy and Safety of Pitavastatin in a Real-World Setting: Observational Study Evaluating SaFety in Patient Treated with Pitavastatin in Korea (PROOF Study)
- In-Kyung Jeong, Sung-Rae Kim
- Endocrinol Metab. 2020;35(4):882-891. Published online December 2, 2020
- DOI: https://doi.org/10.3803/EnM.2020.723
- 5,614 View
- 248 Download
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
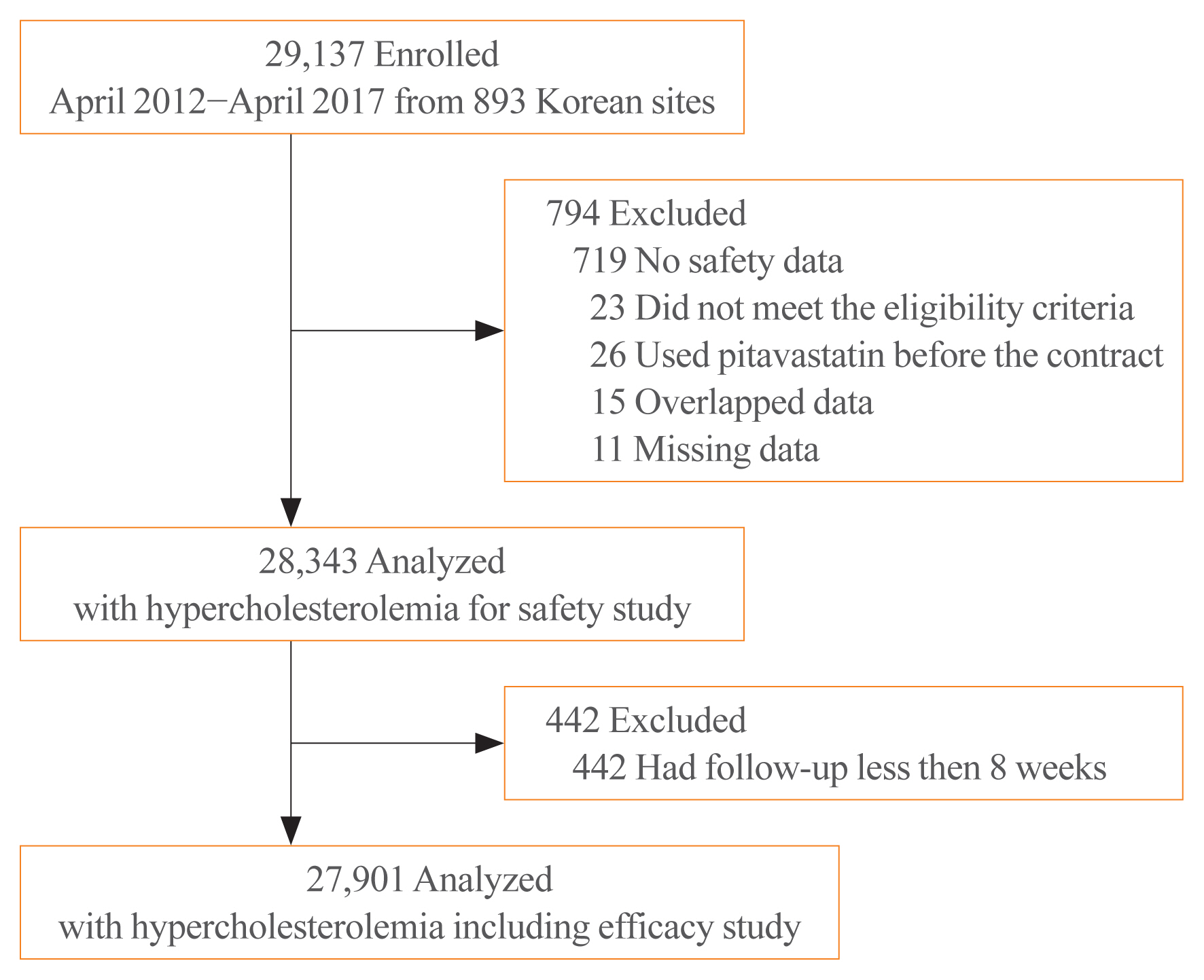
While randomized controlled trials provide useful information about drug safety and efficacy, they do not always reflect the observed results in the real world. The prospective, observational, non-comparative trial in South Korea was designed to evaluate the efficacy and safety of pitavastatin in clinical practice in 28,343 patients.
Methods
This study was conducted in 893 facilities in Korea from April 2, 2012 to April 1, 2017. This study was designed to administer 1, 2, or 4 mg pitavastatin to patients with hyperlipidemia at the age of 20 or older for at least 8 weeks.
Results
For 126 days of mean duration of administration of pitavastatin, the % change of low density lipoprotein cholesterol indicated a dose dependent reduction: –23.4%, –29.1%, and –35.2% in the 1, 2, and 4 mg groups, respectively in patients who have not been treated with lipid lowering medications prior to study. Only 1.74% (492/28,343) of pitavastatin-treated patients experienced adverse events, of which 0.43% (123/28,343) were adverse drug reactions. Less than 1% of patients experienced the grade 2 or more toxicity (Common Terminology Criteria for Adverse Events v4.03) in alanine aminotransferase, aspartate aminotransferase, serum creatinine, and serum creatine phosphokinase. Although there were no rhabdomyolysis in 28,343 patients, 0.04% of patients had been reported pitavastatin-related musculoskeletal disorders.
Conclusion
Overall, this observational study showed that pitavastatin was well tolerated and effectively modified the lipid profile, reducing cardiovascular and cerebrovascular risk in Korean patients with hypercholesterolemia in the real world. -
Citations
Citations to this article as recorded by- Low-Density Lipoprotein Cholesterol Level, Statin Use and Myocardial Infarction Risk in Young Adults
Heekyoung Jeong, Kyungdo Han, Soon Jib Yoo, Mee Kyoung Kim
Journal of Lipid and Atherosclerosis.2022; 11(3): 288. CrossRef
- Low-Density Lipoprotein Cholesterol Level, Statin Use and Myocardial Infarction Risk in Young Adults

- Clinical Study
- Achievement of LDL-C Targets Defined by ESC/EAS (2011) Guidelines in Risk-Stratified Korean Patients with Dyslipidemia Receiving Lipid-Modifying Treatments
- Ye Seul Yang, Seo Young Lee, Jung-Sun Kim, Kyung Mook Choi, Kang Wook Lee, Sang-Chol Lee, Jung Rae Cho, Seung-Jin Oh, Ji-Hyun Kim, Sung Hee Choi
- Endocrinol Metab. 2020;35(2):367-376. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.367

- 7,992 View
- 144 Download
- 8 Web of Science
- 9 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
This study assessed the proportion of risk-stratified Korean patients with dyslipidemia achieving their low-density lipoprotein cholesterol (LDL-C) targets as defined by the European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) (2011) guidelines while receiving lipid-modifying treatments (LMTs).
Methods
In this multicenter, cross-sectional, observational study, we evaluated data from Korean patients aged ≥19 years who were receiving LMTs for ≥3 months and had an LDL-C value within the previous 12 months on the same LMT. Data were collected for demographics, cardiovascular (CV) risk factors, medical history, and healthcare consumption. Patients were risk-stratified according to the ESC Systematic COronary Risk Evaluation (SCORE) chart and LDL-C target achievement rate was assessed.
Results
Guideline-based risk-stratification of the 1,034 patients showed the majority (72.2%) to be in the very high-risk category. Investigators’ assessment of risk was underestimated in 71.6% compared to ESC/EAS guidelines. Overall LDL-C target achievement rate was 44.3%; target achievement was the highest (66.0%) in moderate-risk patients and the lowest (39.0%) in very high-risk patients. Overall 97.1% patients were receiving statin therapy, mostly as a single-agent (89.2%). High-intensity statins and the highest permissible dose of high-intensity statins had been prescribed to only 9.1% and 7.3% patients in the very high-risk group, respectively. Physician satisfaction with patients’ LDL-C levels was the primary reason for non-intensification of statin therapy.
Conclusion
Achievement of target LDL-C level is suboptimal in Korean patients with dyslipidemia, especially in those at very high-risk of CV events. Current practices in LMTs need to be improved based on precise CV risk evaluation posed by dyslipidemia. -
Citations
Citations to this article as recorded by- Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Journal of Lipid and Atherosclerosis.2023; 12(1): 12. CrossRef - Lipid Management in Korean People with Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement
Ye Seul Yang, Hack-Lyoung Kim, Sang-Hyun Kim, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 1. CrossRef - Bempedoic Acid for Lipid Management in the Indian Population: An Expert Opinion
Jagdish Hiremath, J C Mohan, Prakash Hazra, JP S Sawhney, Ashwani Mehta, Sadanand Shetty, Abraham Oomman, Mahesh K Shah, Ganapathi Bantwal, Rajeev Agarwal, Rajiv Karnik, Peeyush Jain, Saumitra Ray, Sambit Das, Vibhuti Jadhao, Sachin Suryawanshi, Hanmant B
Cureus.2023;[Epub] CrossRef - Optimal implementation of the 2019 ESC/EAS dyslipidaemia guidelines in patients with and without atherosclerotic cardiovascular disease across Europe: a simulation based on the DA VINCI study
Julia Brandts, Sarah Bray, Guillermo Villa, Alberico L. Catapano, Neil R. Poulter, Antonio J. Vallejo-Vaz, Kausik K. Ray
The Lancet Regional Health - Europe.2023; 31: 100665. CrossRef - Management of Dyslipidemia in Patients with Diabetes Mellitus
Kyung Ae Lee
The Journal of Korean Diabetes.2023; 24(3): 111. CrossRef - Target Low-Density Lipoprotein-Cholesterol and Secondary Prevention for Patients with Acute Myocardial Infarction: A Korean Nationwide Cohort Study
Ju Hyeon Kim, Jung-Joon Cha, Subin Lim, Jungseok An, Mi-Na Kim, Soon Jun Hong, Hyung Joon Joo, Jae Hyoung Park, Cheol Woong Yu, Do-Sun Lim, Kyeongmin Byeon, Sang-Wook Kim, Eun-Seok Shin, Kwang Soo Cha, Jei Keon Chae, Youngkeun Ahn, Myung Ho Jeong, Tae Hoo
Journal of Clinical Medicine.2022; 11(9): 2650. CrossRef - Current Status of Low-Density Lipoprotein Cholesterol Target Achievement in Patients with Type 2 Diabetes Mellitus in Korea Compared with Recent Guidelines
Soo Jin Yun, In-Kyung Jeong, Jin-Hye Cha, Juneyoung Lee, Ho Chan Cho, Sung Hee Choi, SungWan Chun, Hyun Jeong Jeon, Ho-Cheol Kang, Sang Soo Kim, Seung-Hyun Ko, Gwanpyo Koh, Su Kyoung Kwon, Jae Hyuk Lee, Min Kyong Moon, Junghyun Noh, Cheol-Young Park, Sung
Diabetes & Metabolism Journal.2022; 46(3): 464. CrossRef - There is urgent need to treat atherosclerotic cardiovascular disease risk earlier, more intensively, and with greater precision: A review of current practice and recommendations for improved effectiveness
Michael E. Makover, Michael D. Shapiro, Peter P. Toth
American Journal of Preventive Cardiology.2022; 12: 100371. CrossRef - Non-achievement of the Low-Density Lipoprotein Cholesterol Goal in Older Patients with Type 2 Diabetes Mellitus and a Very High Cardiovascular Disease Risk: A Multicenter Study in Vietnam
Huan Thanh Nguyen, Khang Pham Trong Ha, An Huu Nguyen, Thu Thanh Nguyen, Hang My Lam
Annals of Geriatric Medicine and Research.2021; 25(4): 278. CrossRef
- Lipid Management in Korean People With Type 2 Diabetes Mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis Consensus Statement

- Clinical Study
- Effects of a Portfolio-Mediterranean Diet and a Mediterranean Diet with or without a Sterol-Enriched Yogurt in Individuals with Hypercholesterolemia
- Yvelise Ferro, Elisa Mazza, Mariantonietta Salvati, Emma Santariga, Salvatore Giampà, Rocco Spagnuolo, Patrizia Doldo, Roberta Pujia, Adriana Coppola, Carmine Gazzaruso, Arturo Pujia, Tiziana Montalcini
- Endocrinol Metab. 2020;35(2):298-307. Published online June 24, 2020
- DOI: https://doi.org/10.3803/EnM.2020.35.2.298
- 6,768 View
- 140 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
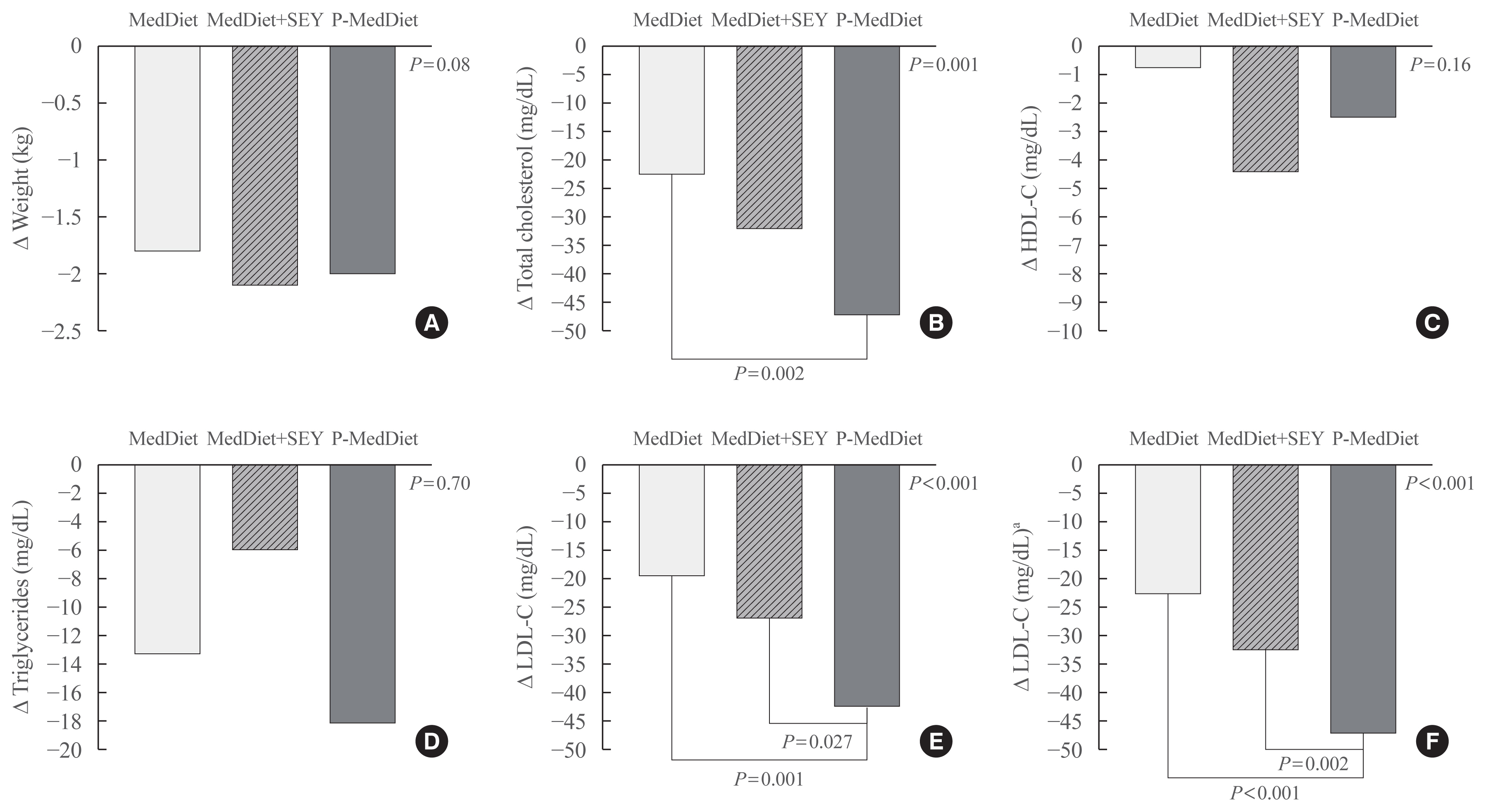
ePub - Background
A growing number of functional foods have been proposed to reduce cholesterol levels and the Portfolio Diet, which includes a combination of plant sterols, fibres, nuts, and soy protein, reduces low density lipoprotein cholesterol (LDL-C) from 20% to 30% in individuals with hyperlipidaemia. In this pilot study, the aim was to investigate whether a Mediterranean Diet incorporating a new and simple combination of cholesterol-lowering foods, excluding soy and nuts (namely the Portfolio-Mediterranean Diet), would reduce LDL-C levels, in the short-term, better than a Mediterranean Diet plus a sterol-enriched yogurt or a Mediterranean Diet alone.
Methods
We retrospectively evaluated 24 individuals on a Portfolio-Mediterranean Diet and 48 matched individuals on a Mediterranean Diet with or without a sterol-enriched yogurt (24 each groups) as controls.
Results
At follow-up (after 48±12 days), we observed an LDL reduction of 21±4, 23±4, and 44±4 mg/dL in the Mediterranean Diet alone, Mediterranean Diet plus yogurt and Portfolio-Mediterranean Diet respectively (P<0.001).
Conclusion
A Portfolio-Mediterranean Diet, incorporating a new combination of functional foods such as oats or barley, plant sterols, chitosan, and green tea but not soy and nuts, may reduce LDL of 25% in the short term in individuals with hypercholesterolemia. -
Citations
Citations to this article as recorded by- Intrinsic and environmental basis of aging: A narrative review
Carla Navarro, Juan Salazar, María P. Díaz, Maricarmen Chacin, Raquel Santeliz, Ivana Vera, Luis D′Marco, Heliana Parra, Mary Carlota Bernal, Ana Castro, Daniel Escalona, Henry García-Pacheco, Valmore Bermúdez
Heliyon.2023; 9(8): e18239. CrossRef - Application of small angle X‐ray scattering in exploring the effect of edible oils with different unsaturation FAs on bioaccessibility of stigmasterol oleate
Ying Wang, Tao Wang, Zhangtie Wang, Yiwen Guo, Ruijie Liu, Ming Chang
Journal of the Science of Food and Agriculture.2023; 103(15): 7764. CrossRef - Phyto-Enrichment of Yogurt to Control Hypercholesterolemia: A Functional Approach
Harsh Kumar, Kanchan Bhardwaj, Natália Cruz-Martins, Ruchi Sharma, Shahida Anusha Siddiqui, Daljeet Singh Dhanjal, Reena Singh, Chirag Chopra, Adriana Dantas, Rachna Verma, Noura S. Dosoky, Dinesh Kumar
Molecules.2022; 27(11): 3479. CrossRef - Familial Hypercholesterolemia and Its Current Diagnostics and Treatment Possibilities: A Literature Analysis
Kristina Zubielienė, Gintarė Valterytė, Neda Jonaitienė, Diana Žaliaduonytė, Vytautas Zabiela
Medicina.2022; 58(11): 1665. CrossRef - Mediterranean Diet a Potential Strategy against SARS-CoV-2 Infection: A Narrative Review
Yvelise Ferro, Roberta Pujia, Samantha Maurotti, Giada Boragina, Angela Mirarchi, Patrizia Gnagnarella, Elisa Mazza
Medicina.2021; 57(12): 1389. CrossRef
- Intrinsic and environmental basis of aging: A narrative review

- Efficacy of Fluvastatin in Patients with Hypercholesterolemia.
- Moon Ho Kang, Sung Gwang Lee, Jung Ho Youn, Tae Suk Kim, Seung Woon Ahn
- J Korean Endocr Soc. 1996;11(1):75-84. Published online November 7, 2019
- 1,115 View
- 26 Download
-
 Abstract
Abstract
 PDF
PDF - Background
Fluvastatin is the first entirely synthetic 3-hydroxy-3-methylglutaryl-coenzyme A(HMG-CoA) reductase inhibitor. Clinical data indicate that this agent exhibits the proven efficacy of its class and also has some theoretical advantages in safety for long-term use because of its unique pharmacololgic property consistent with hepatoselectivity(i.e., low systemic exposure). This study is to evaluate efficacy and safety of fluvastatin in hypercholesterolemic patients in Korea. Methods: An open clinical trial with fluvastatin was conducted in 31 subjects who continued to have high blood cholesterol levels of 6.21 mmol/L(240 mg/dl) or greater after 1 month of lipid-lowering diet plus single blind placebo period. Fluvastatin was administered for 8 weeks with the initial dose of 20 mg per day and if serum cholesterol levels did not fall below 5.20 mmol/L(200 mg/dl) after 4 weeks the dose was increased to 40 mg per day for the second 4 weeks. On each visit every 4 weeks they underwent interview and laboratory tests about side effects and tolerability. Results: The mean % changes in plasma total cholesterol and LDL-cholesterol from baseline were -14.6% and -20.2% at 4 week, and -19.5% and -24.7% at 8 week respectively(p<0.001). No significant change in plasma triglyceride was found in the overall group, but when analysis is confined to those with hypertriglycedemia combined(TG>- 2.26 mmol/L or 200 mg/dl), plasma triglyceride levels were significantly reduced by 23.3% at 8 week(p<0.05). There was no significant change in HDL-cholesterol during fluvastatin treatment. Three patients had mild gastrointestinal symptoms and one patient developed drowsiness, no symptoms were severe enough to discontinue the medication. Notable laboratory abnormalities including serum transaminase and creatine kinase elevations were not observed. Conclusion: This study suggests that fluvastatin is an effective, safe, and well-tolerated lipid lowering agent in the treatment of hypercholesterolemia. Controlled clinical studies on large scale and long-term basis should be followed.

- The Role of Macrophage Lipophagy in Reverse Cholesterol Transport
- Se-Jin Jeong, Mi-Ni Lee, Goo Taeg Oh
- Endocrinol Metab. 2017;32(1):41-46. Published online March 20, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.41
- 4,426 View
- 73 Download
- 31 Web of Science
- 34 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Macrophage cholesterol efflux is a central step in reverse cholesterol transport, which helps to maintain cholesterol homeostasis and to reduce atherosclerosis. Lipophagy has recently been identified as a new step in cholesterol ester hydrolysis that regulates cholesterol efflux, since it mobilizes cholesterol from lipid droplets of macrophages via autophagy and lysosomes. In this review, we briefly discuss recent advances regarding the mechanisms of the cholesterol efflux pathway in macrophage foam cells, and present lipophagy as a therapeutic target in the treatment of atherosclerosis.
-
Citations
Citations to this article as recorded by- Bile salt signaling and bile salt-based therapies in cardiometabolic disease
Claire C.J. Groenen, Thuc-Anh Nguyen, Coen C. Paulusma, Stan F.J. van de Graaf
Clinical Science.2024; 138(1): 1. CrossRef - Huazhuotongmai decoction exerts anti-atherosclerotic effects by modulating the expression of ABCA1/SR-B1/PPAR-γ in vivo and in vitro
Ya-ru Yan, Zi-jun Jia, Ya Wang, Feng-qin Xu, Qing-bing Zhou
Phytomedicine Plus.2023; 3(2): 100436. CrossRef - The SGLT2 Inhibitor Canagliflozin Reduces Atherosclerosis by Enhancing Macrophage Autophagy
Hongping Chen, Da Teng, Bowen Xu, Chunxiao Wang, Hua Wang, Wenjuan Jia, Lei Gong, Haibin Dong, Lin Zhong, Jun Yang
Journal of Cardiovascular Translational Research.2023; 16(5): 999. CrossRef - FURIN suppresses the progression of atherosclerosis by promoting macrophage autophagy
Hongping Chen, Lihui Zhang, Shaohua Mi, Hua Wang, Chunxiao Wang, Wenjuan Jia, Lei Gong, Haibin Dong, Bowen Xu, Yanyan Jing, Peipei Ge, Zhigang Pei, Lin Zhong, Jun Yang
The FASEB Journal.2023;[Epub] CrossRef - Unbalanced Redox With Autophagy in Cardiovascular Disease
Se-Jin Jeong, Goo Taeg Oh
Journal of Lipid and Atherosclerosis.2023; 12(2): 132. CrossRef - Edible Bird’s Nest Effectively Attenuates Atherosclerosis through Modulation of Cholesterol Metabolism via Activation of PPARγ/LXRα Signaling Pathway In Vivo
Nurul Nadiah Mohamad Nasir, Ramlah Mohamad Ibrahim, Rozi Mahmud, Nor Asma Ab Razak, Norsharina Ismail, Kim Wei Chan, Md Zuki Abu Bakar, Akhilesh K. Verma
Journal of Food Biochemistry.2023; 2023: 1. CrossRef - The mitochondrial translocator protein (TSPO, 18 kDa): A key multifunctional molecule in liver diseases
Yuchang Li, Liting Chen, Vassilios Papadopoulos
Biochimie.2023;[Epub] CrossRef - The Differential Metabolomes in Cumulus and Mural Granulosa Cells from Human Preovulatory Follicles
Er-Meng Gao, Bongkoch Turathum, Ling Wang, Di Zhang, Yu-Bing Liu, Rong-Xin Tang, Ri-Cheng Chian
Reproductive Sciences.2022; 29(4): 1343. CrossRef - PHLPP1 promotes neutral lipid accumulation through AMPK/ChREBP-dependent lipid uptake and fatty acid synthesis pathways
Keerthana Balamurugan, Raghavender Medishetti, Jyothi Kotha, Parameshwar Behera, Kanika Chandra, Vijay Aditya Mavuduru, Manjunath B. Joshi, Ramesh Samineni, Madhumohan R. Katika, Writoban Basu Ball, Manjunatha Thondamal, Anil Challa, Kiranam Chatti, Kisho
iScience.2022; 25(2): 103766. CrossRef - Hydrochloride Berberine ameliorates alcohol-induced liver injury by regulating inflammation and lipid metabolism
Xiumei Ke, Ruoyu Zhang, Pan Li, Ling Zuo, Meng Wang, Junxuan Yang, Jianwei Wang
Biochemical and Biophysical Research Communications.2022; 610: 49. CrossRef - Metabolic Regulation of Macrophage Activation
Ourania Kolliniati, Eleftheria Ieronymaki, Eleni Vergadi, Christos Tsatsanis
Journal of Innate Immunity.2022; 14(1): 51. CrossRef - Emerging Roles of Lipophagy in Cancer Metastasis
Haimeng Yin, Ying Shan, Tian Xia, Yan Ji, Ling Yuan, Yiwen You, Bo You
Cancers.2022; 14(18): 4526. CrossRef - Genetic Factors for Coronary Heart Disease and Their Mechanisms: A Meta-Analysis and Comprehensive Review of Common Variants from Genome-Wide Association Studies
Khairul Anwar Zarkasi, Noraidatulakma Abdullah, Nor Azian Abdul Murad, Norfazilah Ahmad, Rahman Jamal
Diagnostics.2022; 12(10): 2561. CrossRef - Caveolin-1 in autophagy: A potential therapeutic target in atherosclerosis
Kai Hou, Shuai Li, Meng Zhang, Xuping Qin
Clinica Chimica Acta.2021; 513: 25. CrossRef - Contradictory regulation of macrophages on atherosclerosis based on polarization, death and autophagy
Jing Zhang, Chuan-Rui Ma, Yun-Qing Hua, Lan Li, Jing-Yu Ni, Yu-Ting Huang, Sophia Esi Duncan, Sheng Li, Shan Gao, Guan-Wei Fan
Life Sciences.2021; 276: 118957. CrossRef - A meta-analysis of HDL cholesterol efflux capacity and concentration in patients with rheumatoid arthritis
Binbin Xie, Jiang He, Yong Liu, Ting Liu, Chaoqun Liu
Lipids in Health and Disease.2021;[Epub] CrossRef - Rosmarinic Acid Increases Macrophage Cholesterol Efflux through Regulation of ABCA1 and ABCG1 in Different Mechanisms
Jean-Baptiste Nyandwi, Young Shin Ko, Hana Jin, Seung Pil Yun, Sang Won Park, Hye Jung Kim
International Journal of Molecular Sciences.2021; 22(16): 8791. CrossRef - FGF21 induces autophagy‐mediated cholesterol efflux to inhibit atherogenesis via RACK1 up‐regulation
Lin Xiaolong, Guo Dongmin, Mihua Liu, Wang Zuo, Hu Huijun, Tan Qiufen, Hu XueMei, Lin Wensheng, Pan Yuping, Lin Jun, Zeng Zhaolin
Journal of Cellular and Molecular Medicine.2020; 24(9): 4992. CrossRef - Lipophagy in atherosclerosis
Qing Liu, Yuan-Mei Wang, Hong-Feng Gu
Clinica Chimica Acta.2020; 511: 208. CrossRef - Lysosomotropic Features and Autophagy Modulators among Medical Drugs: Evaluation of Their Role in Pathologies
Tatiana A. Korolenko, Thomas P. Johnston, Vaclav Vetvicka
Molecules.2020; 25(21): 5052. CrossRef - LncRNA MALAT1 Enhances ox-LDL-Induced Autophagy through the SIRT1/MAPK/NF-κB Pathway in Macrophages
Jiaqi Yang, Xuze Lin , Liangshan Wang, Tienan Sun, Qi Zhao, Qian Ma, Yujie Zhou
Current Vascular Pharmacology.2020; 18(6): 652. CrossRef -
CTRP13 inhibits atherosclerosis
via
autophagy‐lysosome‐dependent degradation of CD36
Cheng Wang, Wenjing Xu, Minglu Liang, Dan Huang, Kai Huang
The FASEB Journal.2019; 33(2): 2290. CrossRef - Subclinical atherosclerosis and its progression are modulated by PLIN2 through a feed‐forward loop between LXR and autophagy
P. Saliba‐Gustafsson, M. Pedrelli, K. Gertow, O. Werngren, V. Janas, S. Pourteymour, D. Baldassarre, E. Tremoli, F. Veglia, R. Rauramaa, A.J. Smit, P. Giral, S. Kurl, M. Pirro, U. de Faire, S.E. Humphries, A. Hamsten, I. Gonçalves, M. Orho‐Melander, A. Fr
Journal of Internal Medicine.2019; 286(6): 660. CrossRef - PCSK9: A new participant in lipophagy in regulating atherosclerosis?
Jun Xiao, Yi-Min Deng, Xiang-Rui Liu, Jian-Ping Cao, Min Zhou, Ya-Ling Tang, Wen-Hao Xiong, Zhi-Sheng Jiang, Zhi-Han Tang, Lu-Shan Liu
Clinica Chimica Acta.2019; 495: 358. CrossRef - Autophagy-Mediated Cholesterol Trafficking Controls Steroid Production
Michael J. Texada, Alina Malita, Christian F. Christensen, Kathrine B. Dall, Nils J. Faergeman, Stanislav Nagy, Kenneth A. Halberg, Kim Rewitz
Developmental Cell.2019; 48(5): 659. CrossRef - Lipophagy in nonliver tissues and some related diseases: Pathogenic and therapeutic implications
Kebing Zhou, Pingbo Yao, Jun He, Hong Zhao
Journal of Cellular Physiology.2019; 234(6): 7938. CrossRef - Autophagy differentially regulates macrophage lipid handling depending on the lipid substrate (oleic acid vs. acetylated-LDL) and inflammatory activation state
Sapir Hadadi-Bechor, Yulia Haim, Tal Pecht, Roni Gat, Tanya Tarnovscki, Martin Gericke, Assaf Rudich
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2019; 1864(12): 158527. CrossRef - Autophagy and Age-Related Eye Diseases
Xue Yang, Xinan Pan, Xiaorui Zhao, Jin Luo, Mingpu Xu, Daoming Bai, Yan Hu, Xu Liu, Qiongfang Yu, Dian Gao
BioMed Research International.2019; 2019: 1. CrossRef - Foam cell formation and cholesterol trafficking and metabolism disturbances in atherosclerosis
Alexandrina Volobueva, Dongwei Zhang, Andrey V. Grechko, Alexander N. Orekhov
Cor et Vasa.2019; 61(1): 48. CrossRef - Oxidative Stress, Lipid Peroxidation, and Loss of Hyaluronic Acid in the Human Vitreous Affected by Synchysis Scintillans
Loredana Bergandi, Oleksii A Skorokhod, Rosalba La Grotta, Evelin Schwarzer, Raffaele Nuzzi
Journal of Ophthalmology.2019; 2019: 1. CrossRef - Programmed cell death protein 4 deficiency suppresses foam cell formation by activating autophagy in advanced glycation end‐product low‐density lipoprotein–induced macrophages
Shan Li, Guangdong Gao, Fuyun Wu, Dan Liu, Hongyan Zhao, Jing Ke, Ying Liu, Fei Li, Jian Li, Zongyun Chen, Zhiming Tang, Lei Bai, Jinxuan Zhang, Wei Zheng, Xin Chen
Journal of Cellular Biochemistry.2019; 120(5): 7689. CrossRef - Mindin deficiency in macrophages protects against foam cell formation and atherosclerosis by targeting LXR-β
Cheng Zhang, Juan-Juan Qin, Fu-Han Gong, Jing-Jing Tong, Wen-Lin Cheng, Haiping Wang, Yan Zhang, Xueyong Zhu, Zhi-Gang She, Hao Xia, Li-Hua Zhu
Clinical Science.2018; 132(11): 1199. CrossRef - LJ-1888, a selective antagonist for the A3 adenosine receptor, ameliorates the development of atherosclerosis and hypercholesterolemia in apolipoprotein E knock-out mice
Jong-Gil Park, Se-Jin Jeong, Jinha Yu, Gyudong Kim, Lak Shin Jeong, Goo Taeg Oh
BMB Reports.2018; 51(10): 520. CrossRef - Intracellular and Plasma Membrane Events in Cholesterol Transport and Homeostasis
Dmitry Y. Litvinov, Eugeny V. Savushkin, Alexander D. Dergunov
Journal of Lipids.2018; 2018: 1. CrossRef
- Bile salt signaling and bile salt-based therapies in cardiometabolic disease

- Prevalence and Clinical Characteristics of Dyslipidemia in Koreans
- Jee-Sun Jeong, Hyuk-Sang Kwon
- Endocrinol Metab. 2017;32(1):30-35. Published online March 20, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.1.30
- 4,980 View
- 46 Download
- 22 Web of Science
- 25 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader The prevalence of hypercholesterolemia in Koreans 30 years old and over was 19.5% in 2015 according to the Korean Nutrition and Health Examination Survey, which means that one-fifth of adults had hypercholesterolemia. The prevalence of hypertriglyceridemia in adults 30 years of age and older was 16.8% in 2015, and men had a 2-fold higher prevalence of hypertriglyceridemia than women (23.9% vs. 10.4%). The awareness of hypercholesterolemia in Koreans was higher in women than among men (62.4% vs. 51.4%). It increased with age; the level of awareness in participants 30 to 49 years of age (32.1% in men and 32.6% in women) was less than half of that observed among respondents ≥65 years old (77.5% in men and 78.0% in women). Regular check-ups for dyslipidemia and the active management thereof are urgent in Korean men aged 30 to 49. In women, the perimenopausal period is crucial for the prevention and management of metabolic syndrome, including dyslipidemia. Overall, improvements in awareness and treatment in the age group of 30 to 49 years in both men and women remain necessary.
-
Citations
Citations to this article as recorded by- Association between weekend catch-up sleep and dyslipidemia among Korean workers
Ye Seul Jang, Yu Shin Park, Kyungduk Hurh, Eun-Cheol Park, Sung-In Jang
Scientific Reports.2023;[Epub] CrossRef - The association between meat intake and the risk of coronary heart disease in Korean men using the Framingham risk score: A prospective cohort study
Jiwon Jeong, Kyungjoon Lim, Sangah Shin
Nutrition, Metabolism and Cardiovascular Diseases.2023; 33(6): 1158. CrossRef - New-onset dyslipidemia in adult cancer survivors from medically underserved areas: a 10-year retrospective cohort study
Yun Hwa Jung, IL Yun, Eun-Cheol Park, Sung-In Jang
BMC Cancer.2023;[Epub] CrossRef - A Paradigm Shift in Dyslipidemia Management in Primary Care: A 12-Month Cohort Study
Jun Hwa Hong, Ung Jeon, Won-Yong Shin, Weon Kim, Kayeon Seong, Sang-Ho Park, Hee-dong Kim, Joong-Wha Chung, Jaehyuk Choi
Clinical Therapeutics.2022; 44(5): 698. CrossRef - Temporal trends in heart failure over 11 years in the aging Korean population: A retrospective study using the national health insurance database
Dong-Hyuk Cho, Chan Joo Lee, Jung-Woo Son, Jimi Choi, Jinseub Hwang, Byung-Su Yoo, Yajing Wang
PLOS ONE.2022; 17(12): e0279541. CrossRef - Cardiovascular risk and undertreatment of dyslipidemia in lung cancer survivors: A nationwide population-based study
In Young Cho, Kyungdo Han, Dong Wook Shin, Sang Hyun Park, Dong Woog Yoon, Sujeong Shin, Su-Min Jeong, Jong Ho Cho
Current Problems in Cancer.2021; 45(1): 100615. CrossRef - Association Between Blood Heavy Metal Concentrations and Dyslipidemia in the Elderly
Xingmeng Zhu, Yong Fan, Jie Sheng, Ling Gu, Qi Tao, Rui Huang, Kaiyong Liu, Linsheng Yang, Guimei Chen, Hongjuan Cao, Kaichun Li, Fangbiao Tao, Sufang Wang
Biological Trace Element Research.2021; 199(4): 1280. CrossRef - The prevalence of hypercholesterolemia and associated risk factors in Al-Kharj population, Saudi Arabia: a cross-sectional survey
Jamaan Al-Zahrani, Mamdouh M. Shubair, Sameer Al-Ghamdi, Abdullah A. Alrasheed, Abdulrahman A. Alduraywish, Fayez Saud Alreshidi, Saeed Mastour Alshahrani, Majid Alsalamah, Badr F. Al-Khateeb, Aljawharah Ibraheem Ashathri, Ashraf El-Metwally, Khaled K. Al
BMC Cardiovascular Disorders.2021;[Epub] CrossRef - Prevalence, awareness, treatment, and control of dyslipidemia among diabetes mellitus patients and predictors of optimal dyslipidemia control: results from the Korea National Health and Nutrition Examination Survey
Seung Jae Kim, Oh. Deog Kwon, Kyung-Soo Kim
Lipids in Health and Disease.2021;[Epub] CrossRef - Case Report of a Patient Diagnosed with Fatty Liver Accompanied by Hypertriglyceridemia
Soyoung Hur, Soyeon An, Eujin Kim, Cho-Hyun Hwang, Eungyeong Jang, Youngchul Kim, Jang-Hoon Lee
The Journal of Internal Korean Medicine.2021; 42(2): 207. CrossRef - Changes in Cardiovascular Risk Factors in Residents of the Siberian Region (According to Epidemiological Studies)
G. V. Artamonova, S. A. Maksimov, D. P. Tsygankova, E. D. Bazdyrev, E. V. Indukaeva, T. A. Mulerova, E. B. Shapovalova, A. S. Agienko, O. V. Nakhratova, O. L. Barbarash
Rational Pharmacotherapy in Cardiology.2021; 17(3): 362. CrossRef - Pre-existing Depression among Newly Diagnosed Dyslipidemia Patients and Cardiovascular Disease Risk
Jihoon Andrew Kim, Seulggie Choi, Daein Choi, Sang Min Park
Diabetes & Metabolism Journal.2020; 44(2): 307. CrossRef - Low-density lipoprotein cholesterol goal attainment rates in high-risk patients with cardiovascular diseases and diabetes mellitus in Korea: a retrospective cohort study
Ye Seul Yang, Bo Ram Yang, Mi-Sook Kim, Yunji Hwang, Sung Hee Choi
Lipids in Health and Disease.2020;[Epub] CrossRef - Risk factors for cardiovascular disease in ethnic Europeans and Koreans living in the Primorsky Krai
D. Yu. Bogdanov, V. A. Nevzorova, V. B. Shumatov, E. A. Kondrashova, E. Yu. Shestopalov
Cardiovascular Therapy and Prevention.2020; 19(1): 40. CrossRef - Effect of Smartphone-Based Lifestyle Coaching App on Community-Dwelling Population With Moderate Metabolic Abnormalities: Randomized Controlled Trial
So Mi Jemma Cho, Jung Hyun Lee, Jee-Seon Shim, Hyungseon Yeom, Su Jin Lee, Yong Woo Jeon, Hyeon Chang Kim
Journal of Medical Internet Research.2020; 22(10): e17435. CrossRef - Risk factors for cardiovascular disease in ethnic Europeans and Koreans living in the Primorsky Krai
D. Yu. Bogdanov, V. A. Nevzorova, V. B. Shumatov, E. A. Kondrashova, E. Yu. Shestopalov
Cardiovascular Therapy and Prevention.2020; 19(1): 40. CrossRef - The effect of healthy Nordic diet on cardio-metabolic markers: a systematic review and meta-analysis of randomized controlled clinical trials
Nahid Ramezani-Jolfaie, Mohammad Mohammadi, Amin Salehi-Abargouei
European Journal of Nutrition.2019; 58(6): 2159. CrossRef - Association between Low-Intensity Smoking and Metabolic Syndrome in Korean Men
Minji Park, Seran Min, Yu Jin Cho, Sunwoo Kim, Hyuktae Kwon, Hee-Kyung Joh, Bumjo Oh, Seung-Won Oh, Ho Chun Choi, Cheol Min Lee
Journal of the Korean Society for Research on Nicotine and Tobacco.2019; 10(2): 89. CrossRef - Efficacy and safety of alirocumab in Korean patients with hypercholesterolemia and high cardiovascular risk: subanalysis of the ODYSSEY-KT study
Chang-Wook Nam, Dong-Soo Kim, Jianyong Li, Marie T. Baccara-Dinet, Ivy Li, Ji-Hyun Kim, Chong-Jin Kim
The Korean Journal of Internal Medicine.2019; 34(6): 1252. CrossRef - Progress of Short-term Herbal Medicine Administration for Hypertriglyceridemia: a Case Report
Bo-min Kim, Hee-geun Jo
The Journal of Internal Korean Medicine.2019; 40(3): 517. CrossRef - Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ki Hoon Han, Jimi Choi, Juneyoung Lee, Sin Gon Kim
BMJ.2019; : l5125. CrossRef - The risk of herpes zoster virus infection in patients with depression
Hyo Geun Choi, Eui-Joong Kim, Young Kyung Lee, Miyoung Kim
Medicine.2019; 98(40): e17430. CrossRef - Effect of Change in Total Cholesterol Levels on Cardiovascular Disease Among Young Adults
Su‐Min Jeong, Seulggie Choi, Kyuwoong Kim, Sung Min Kim, Gyeongsil Lee, Seong Yong Park, Yeon‐Yong Kim, Joung Sik Son, Jae‐Moon Yun, Sang Min Park
Journal of the American Heart Association.2018;[Epub] CrossRef - Efficacy and Safety of Ezetimibe and Rosuvastatin Combination Therapy Versus Those of Rosuvastatin Monotherapy in Patients With Primary Hypercholesterolemia
Woohyeun Kim, Yeonyee E. Yoon, Sung-Hee Shin, Jang-Whan Bae, Bum-Kee Hong, Soon Jun Hong, Ki Chul Sung, Seung Hwan Han, Weon Kim, Moo-Yong Rhee, Sang-Hyun Kim, Sang Eun Lee, Min Su Hyon, Gyo-Seung Hwang, Jang Won Son, Jang-Young Kim, Min Kyu Kim, Sang Woo
Clinical Therapeutics.2018; 40(6): 993. CrossRef - Prevalence of dyslipidemia among the population of a large region of Eastern Siberia and its association with sociodemographic and behavioral factors
Yu. I. Grinshtein, V. V. Shabalin, R. R. Ruf, M. M. Petrova, S. A. Shal'nova
Profilakticheskaya meditsina.2018; 21(5): 63. CrossRef
- Association between weekend catch-up sleep and dyslipidemia among Korean workers

- Clinical Study
- Eligibility for Statin Treatment in Korean Subjects with Reduced Renal Function: An Observational Study
- Byung Sub Moon, Jongho Kim, Ji Hyun Kim, Young Youl Hyun, Se Eun Park, Hyung-Geun Oh, Cheol-Young Park, Won-Young Lee, Ki-Won Oh, Kyu-Beck Lee, Hyang Kim, Sung-Woo Park, Eun-Jung Rhee
- Endocrinol Metab. 2016;31(3):402-409. Published online August 26, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.3.402
- 3,928 View
- 33 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The purpose of this study was to investigate the relationship between statin eligibility and the degree of renal dysfunction using the Adult Treatment Panel (ATP) III and the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines in Korean adults.
Methods Renal function was assessed in 18,746 participants of the Kangbuk Samsung Health Study from January 2011 to December 2012. Subjects were divided into three groups according to estimated glomerular filtration rate (eGFR): stage 1, eGFR ≥90 mL/min/1.73 m2; stage 2, eGFR 60 to 89 mL/min/1.73 m2; and stages 3 to 5, eGFR <60 mL/min/1.73 m2. Statin eligibility in these groups was determined using the ATP III and ACC/AHA guidelines, and the risk for 10-year atherosclerotic cardiovascular disease (ASCVD) was calculated using the Framingham Risk Score (FRS) and Pooled Cohort Equation (PCE).
Results There were 3,546 (18.9%) and 4,048 (21.5%) statin-eligible subjects according to ATP III and ACC/AHA guidelines, respectively. The proportion of statin-eligible subjects increased as renal function deteriorated. Statin eligibility by the ACC/AHA guidelines showed better agreement with the Kidney Disease Improving Global Outcomes (KDIGO) recommendations compared to the ATP III guidelines in subjects with stage 3 to 5 chronic kidney disease (CKD) (κ value, 0.689 vs. 0.531). When the 10-year ASCVD risk was assessed using the FRS and PCE, the mean risk calculated by both equations significantly increased as renal function declined.
Conclusions The proportion of statin-eligible subjects significantly increased according to worsening renal function in this Korean cohort. ACC/AHA guideline showed better agreement for statin eligibility with that recommended by KDIGO guideline compared to ATP III in subjects with CKD.
-
Citations
Citations to this article as recorded by- Association between atherosclerotic cardiovascular diseases risk and renal outcome in patients with type 2 diabetes mellitus
Honghong Ren, Lijun Zhao, Yutong Zou, Yiting Wang, Junlin Zhang, Yucheng Wu, Rui Zhang, Tingli Wang, Jiali Wang, Yitao Zhu, Ruikun Guo, Huan Xu, Lin Li, Mark E. Cooper, Fang Liu
Renal Failure.2021; 43(1): 477. CrossRef - Long-term effects of various types of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on changes in glomerular filtration rate in Korea
Seo Yeon Baik, Hyunah Kim, So Jung Yang, Tong Min Kim, Seung-Hwan Lee, Jae Hyoung Cho, Hyunyong Lee, Hyeon Woo Yim, Kun-Ho Yoon, Hun-Sung Kim
Frontiers of Medicine.2019; 13(6): 713. CrossRef - Analysis and comparison of the cost-effectiveness of statins according to the baseline low-density lipoprotein cholesterol level in Korea
Y. J. Jeong, H. Kim, S. J. Baik, T. M. Kim, S. J. Yang, S.-H. Lee, J.-H. Cho, H. Lee, H. W. Yim, I. Y. Choi, K.-H. Yoon, H.-S. Kim
Journal of Clinical Pharmacy and Therapeutics.2017; 42(3): 292. CrossRef - Comparison between Atorvastatin and Rosuvastatin in Renal Function Decline among Patients with Diabetes
Eugene Han, Gyuri Kim, Ji-Yeon Lee, Yong-ho Lee, Beom Seok Kim, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
Endocrinology and Metabolism.2017; 32(2): 274. CrossRef
- Association between atherosclerotic cardiovascular diseases risk and renal outcome in patients with type 2 diabetes mellitus

- Obesity and Metabolism
- High-Density Lipoprotein, Lecithin: Cholesterol Acyltransferase, and Atherosclerosis
- Alice Ossoli, Chiara Pavanello, Laura Calabresi
- Endocrinol Metab. 2016;31(2):223-229. Published online June 10, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.2.223
- 5,562 View
- 77 Download
- 36 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Epidemiological data clearly show the existence of a strong inverse correlation between plasma high-density lipoprotein cholesterol (HDL-C) concentrations and the incidence of coronary heart disease. This relation is explained by a number of atheroprotective properties of HDL, first of all the ability to promote macrophage cholesterol transport. HDL are highly heterogeneous and are continuously remodeled in plasma thanks to the action of a number of proteins and enzymes. Among them, lecithin:cholesterol acyltransferase (LCAT) plays a crucial role, being the only enzyme able to esterify cholesterol within lipoproteins. LCAT is synthetized by the liver and it has been thought to play a major role in reverse cholesterol transport and in atheroprotection. However, data from animal studies, as well as human studies, have shown contradictory results. Increased LCAT concentrations are associated with increased HDL-C levels but not necessarily with atheroprotection. On the other side, decreased LCAT concentration and activity are associated with decreased HDL-C levels but not with increased atherosclerosis. These contradictory results confirm that HDL-C levels
per se do not represent the functionality of the HDL system.-
Citations
Citations to this article as recorded by- Association between Mycoplasma pneumoniae infection, high‑density lipoprotein metabolism and cardiovascular health (Review)
Tao Shen, Yanfang Li, Tingting Liu, Yunzhi Lian, Luke Kong
Biomedical Reports.2024;[Epub] CrossRef - The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients
Roxana Nartea, Brindusa Ilinca Mitoiu, Ioana Ghiorghiu
Current Issues in Molecular Biology.2023; 45(4): 3146. CrossRef - Mannose-Coated Reconstituted Lipoprotein Nanoparticles for the Targeting of Tumor-Associated Macrophages: Optimization, Characterization, and In Vitro Evaluation of Effectiveness
Akpedje S. Dossou, Morgan E. Mantsch, Ammar Kapic, William L. Burnett, Nirupama Sabnis, Jeffery L. Coffer, Rance E. Berg, Rafal Fudala, Andras G. Lacko
Pharmaceutics.2023; 15(6): 1685. CrossRef - Abdominal obesity negatively influences key metrics of reverse cholesterol transport
Jennifer Härdfeldt, Marica Cariello, Sara Simonelli, Alice Ossoli, Natasha Scialpi, Marilidia Piglionica, Emanuela Pasculli, Alessia Noia, Elsa Berardi, Patrizia Suppressa, Giuseppina Piazzolla, Carlo Sabbà, Laura Calabresi, Antonio Moschetta
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids.2022; 1867(2): 159087. CrossRef - Effects of Lactobacillus paracasei N1115 on dyslipidaemia: A randomized controlled study
Hua Jiang, Shengjie Tan, Ke Ning, Hao Li, Wenzhi Zhao, Ai Zhao, Hong Zhu, Shijie Wang, Peiyu Wang, Yumei Zhang
Journal of Functional Foods.2022; 89: 104956. CrossRef - LCAT- targeted therapies: Progress, failures and future
Kaixu Yang, Junmin Wang, Hongjiao Xiang, Peilun Ding, Tao Wu, Guang Ji
Biomedicine & Pharmacotherapy.2022; 147: 112677. CrossRef - Cyclodextrin boostered-high density lipoprotein for antiatherosclerosis by regulating cholesterol efflux and efferocytosis
Yanyan Wang, Hai Gao, Xinya Huang, Zhaoan Chen, Pengyu Kang, Yunyi Zhou, Danhua Qin, Wenli Zhang, Jianping Liu
Carbohydrate Polymers.2022; 292: 119632. CrossRef - Lipidomic Approaches to Study HDL Metabolism in Patients with Central Obesity Diagnosed with Metabolic Syndrome
Gabriele Mocciaro, Simona D’Amore, Benjamin Jenkins, Richard Kay, Antonio Murgia, Luis Vicente Herrera-Marcos, Stefanie Neun, Alice P. Sowton, Zoe Hall, Susana Alejandra Palma-Duran, Giuseppe Palasciano, Frank Reimann, Andrew Murray, Patrizia Suppressa, C
International Journal of Molecular Sciences.2022; 23(12): 6786. CrossRef - rHDL modeling and the anchoring mechanism of LCAT activation
Tommaso Laurenzi, Chiara Parravicini, Luca Palazzolo, Uliano Guerrini, Elisabetta Gianazza, Laura Calabresi, Ivano Eberini
Journal of Lipid Research.2021; 62: 100006. CrossRef - Vasculoprotective properties of plasma lipoproteins from brown bears (Ursus arctos)
Matteo Pedrelli, Paolo Parini, Jonas Kindberg, Jon M. Arnemo, Ingemar Bjorkhem, Ulrika Aasa, Maria Westerståhl, Anna Walentinsson, Chiara Pavanello, Marta Turri, Laura Calabresi, Katariina Öörni, Gérman Camejo, Ole Fröbert, Eva Hurt-Camejo
Journal of Lipid Research.2021; 62: 100065. CrossRef - The Fate of Dietary Cholesterol in the Kissing Bug Rhodnius prolixus
Petter F. Entringer, David Majerowicz, Katia C. Gondim
Frontiers in Physiology.2021;[Epub] CrossRef - Excesive consumption of unsaturated fatty acids leads to oxidative and inflammatory instability in Wistar rats
Jelica D. Grujić-Milanović, Zoran Z. Miloradović, Nevena D. Mihailović-Stanojević, Vojislav V. Banjac, Strahinja Vidosavljević, Milan S. Ivanov, Danijela J. Karanović, Una-Jovana V. Vajić, Djurdjica M. Jovović
Biomedicine & Pharmacotherapy.2021; 139: 111691. CrossRef - Cholesterol Metabolic Reprogramming in Cancer and Its Pharmacological Modulation as Therapeutic Strategy
Isabella Giacomini, Federico Gianfanti, Maria Andrea Desbats, Genny Orso, Massimiliano Berretta, Tommaso Prayer-Galetti, Eugenio Ragazzi, Veronica Cocetta
Frontiers in Oncology.2021;[Epub] CrossRef - Association between Serum Concentrations of Apolipoprotein A-I (ApoA-I) and Alzheimer’s Disease: Systematic Review and Meta-Analysis
Marco Zuin, Carlo Cervellati, Alessandro Trentini, Angelina Passaro, Valentina Rosta, Francesca Zimetti, Giovanni Zuliani
Diagnostics.2021; 11(6): 984. CrossRef - Dapagliflozin reduces thrombin generation and platelet activation: implications for cardiovascular risk reduction in type 2 diabetes mellitus
Christina Kohlmorgen, Stephen Gerfer, Kathrin Feldmann, Sören Twarock, Sonja Hartwig, Stefan Lehr, Meike Klier, Irena Krüger, Carolin Helten, Petra Keul, Sabine Kahl, Amin Polzin, Margitta Elvers, Ulrich Flögel, Malte Kelm, Bodo Levkau, Michael Roden, Jen
Diabetologia.2021; 64(8): 1834. CrossRef - Impact of Dietary Lipids on the Reverse Cholesterol Transport: What We Learned from Animal Studies
Bianca Papotti, Joan Carles Escolà-Gil, Josep Julve, Francesco Potì, Ilaria Zanotti
Nutrients.2021; 13(8): 2643. CrossRef - Supramolecular copolymer modified statin-loaded discoidal rHDLs for atherosclerotic anti-inflammatory therapy by cholesterol efflux and M2 macrophage polarization
Qiqi Zhang, Jianhua He, Fengfei Xu, Xinya Huang, Yanyan Wang, Wenli Zhang, Jianping Liu
Biomaterials Science.2021; 9(18): 6153. CrossRef - Association between Small Dense Low-Density Lipoproteins and High-Density Phospolipid Content in Patients with Coronary Artery Disease with or without Diabetes
Hanene Aoua, Ymène Nkaies, Ali Ben Khalfallah, Mohsen Sakly, Ezzedine Aouani, Nebil Attia
Laboratory Medicine.2020; 51(3): 271. CrossRef - Biomechanical and biochemical investigation of erythrocytes in late stage human leptospirosis
J.A.X. Silva, A.V.P. Albertini, C.S.M. Fonseca, D.C.N. Silva, V.C.O. Carvalho, V.L.M. Lima, A. Fontes, E.V.L. Costa, R.A. Nogueira
Brazilian Journal of Medical and Biological Research.2020;[Epub] CrossRef - Cardioprotective Properties of HDL: Structural and Functional Considerations
Eleni Pappa, Moses S. Elisaf, Christina Kostara, Eleni Bairaktari, Vasilis K. Tsimihodimos
Current Medicinal Chemistry.2020; 27(18): 2964. CrossRef - Relationship between non–high-density lipoprotein cholesterol/apolipoprotein A-I and monocyte/high-density lipoprotein cholesterol ratio and coronary heart disease
Ya Li, Shu Li, Yulin Ma, Jialing Li, Mingying Lin, Jing Wan
Coronary Artery Disease.2020; 31(7): 623. CrossRef - Lipid transfer to high‐density lipoproteins in coronary artery disease patients with and without previous cerebrovascular ischemic events
Carlos J.D.G. Barbosa, Raul C. Maranhão, Renata S. Barreiros, Fatima R. Freitas, André Franci, Célia M.C. Strunz, Flávia B.B. Arantes, Thauany M. Tavoni, José A.F. Ramires, Roberto Kalil Filho, José C. Nicolau
Clinical Cardiology.2019; 42(11): 1100. CrossRef - Antibodies Against the C-Terminus of ApoA-1 Are Inversely Associated with Cholesterol Efflux Capacity and HDL Metabolism in Subjects with and without Type 2 Diabetes Mellitus
Robin Dullaart, Sabrina Pagano, Frank Perton, Nicolas Vuilleumier
International Journal of Molecular Sciences.2019; 20(3): 732. CrossRef - The effect of chronic kidney disease on lipid metabolism
Neris Dincer, Tuncay Dagel, Baris Afsar, Adrian Covic, Alberto Ortiz, Mehmet Kanbay
International Urology and Nephrology.2019; 51(2): 265. CrossRef - Biological Consequences of Dysfunctional HDL
Angela Pirillo, Alberico Luigi Catapano, Giuseppe Danilo Norata
Current Medicinal Chemistry.2019; 26(9): 1644. CrossRef - Plasma lecithin:cholesterol acyltransferase and phospholipid transfer protein activity independently associate with nonalcoholic fatty liver disease
Karlijn J. Nass, Eline H. van den Berg, Eke G. Gruppen, Robin P. F. Dullaart
European Journal of Clinical Investigation.2018;[Epub] CrossRef - An integrated metabolomic strategy for the characterization of the effects of Chinese yam and its three active components on septic cardiomyopathy
Ning Zhou, Meng-Nan Zeng, Kai Li, Yan-Yun Yang, Zhi-Yao Bai, Xiao-Ke Zheng, Wei-Sheng Feng
Food & Function.2018; 9(9): 4989. CrossRef - Effect of Rosuvastatin on Cholesterol Efflux Capacity and Endothelial Function in Type 2 Diabetes Mellitus and Dyslipidemia
Kyong Yeun Jung, Kyoung Min Kim, Sun Kyoung Han, Han Mi Yun, Tae Jung Oh, Sung Hee Choi, Kyong Soo Park, Hak Chul Jang, Soo Lim
Circulation Journal.2018; 82(5): 1387. CrossRef - Association Between Serum LDL-C and ApoB and SYNTAX Score in Patients With Stable Coronary Artery Disease
Taiwu Lin, Luzhao Wang, Jingbin Guo, Peng Liu, Liheng Chen, Mengqiu Wei, Gongxin Li
Angiology.2018; 69(8): 724. CrossRef - Influence of i.v. lipid emulsion on lipoprotein subclass in preterm infants
Hiroki Suganuma, Naho Ikeda, Natsuki Ohkawa, Hiromichi Shoji, Toshiaki Shimizu
Pediatrics International.2018; 60(9): 839. CrossRef - The HDL cholesterol/apolipoprotein A-I ratio: an indicator of cardiovascular disease
Eun-Jung Rhee, Christopher D. Byrne, Ki-Chul Sung
Current Opinion in Endocrinology, Diabetes & Obesity.2017; 24(2): 148. CrossRef - Moringa Leaves Prevent Hepatic Lipid Accumulation and Inflammation in Guinea Pigs by Reducing the Expression of Genes Involved in Lipid Metabolism
Manal Almatrafi, Marcela Vergara-Jimenez, Ana Murillo, Gregory Norris, Christopher Blesso, Maria Fernandez
International Journal of Molecular Sciences.2017; 18(7): 1330. CrossRef
- Association between Mycoplasma pneumoniae infection, high‑density lipoprotein metabolism and cardiovascular health (Review)

- Endocrine Research
- Thyroid Hormone Regulates the mRNA Expression of Small Heterodimer Partner through Liver Receptor Homolog-1
- Hwa Young Ahn, Hwan Hee Kim, Ye An Kim, Min Kim, Jung Hun Ohn, Sung Soo Chung, Yoon-Kwang Lee, Do Joon Park, Kyong Soo Park, David D. Moore, Young Joo Park
- Endocrinol Metab. 2015;30(4):584-592. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.584
- 3,764 View
- 39 Download
- 4 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Expression of hepatic cholesterol 7α-hydroxylase (CYP7A1) is negatively regulated by orphan nuclear receptor small heterodimer partner (SHP). In this study, we aimed to find whether thyroid hormone regulates SHP expression by modulating the transcriptional activities of liver receptor homolog-1 (LRH-1).
Methods We injected thyroid hormone (triiodothyronine, T3) to C57BL/6J wild type. RNA was isolated from mouse liver and used for microarray analysis and quantitative real-time polymerase chain reaction (PCR). Human hepatoma cell and primary hepatocytes from mouse liver were used to confirm the effect of T3
in vitro . Promoter assay and electrophoretic mobility-shift assay (EMSA) were also performed using human hepatoma cell lineResults Initial microarray results indicated that SHP expression is markedly decreased in livers of T3 treated mice. We confirmed that T3 repressed SHP expression in the liver of mice as well as in mouse primary hepatocytes and human hepatoma cells by real-time PCR analysis. LRH-1 increased the promoter activity of SHP; however, this increased activity was markedly decreased after thyroid hormone receptor β/retinoid X receptor α/T3 administration. EMSA revealed that T3 inhibits specific LRH-1 DNA binding.
Conclusion We found that thyroid hormone regulates the expression of SHP mRNA through interference with the transcription factor, LRH-1.
-
Citations
Citations to this article as recorded by- Bile acid and receptors: biology and drug discovery for nonalcoholic fatty liver disease
Ting-ying Jiao, Yuan-di Ma, Xiao-zhen Guo, Yun-fei Ye, Cen Xie
Acta Pharmacologica Sinica.2022; 43(5): 1103. CrossRef - Loperamide induces excessive accumulation of bile acids in the liver of mice with different diets
Zili Lei, Hedong Rong, Yanhong Yang, Siping Yu, Tianle Zhang, Lei Chen, Ya Nie, Qi Song, Qing Hu, Jiao Guo
Toxicology.2022; 477: 153278. CrossRef - Pathogenesis of hypothyroidism-induced NAFLD is driven by intra- and extrahepatic mechanisms
Giuseppe Ferrandino, Rachel R. Kaspari, Olga Spadaro, Andrea Reyna-Neyra, Rachel J. Perry, Rebecca Cardone, Richard G. Kibbey, Gerald I. Shulman, Vishwa Deep Dixit, Nancy Carrasco
Proceedings of the National Academy of Sciences.2017;[Epub] CrossRef
- Bile acid and receptors: biology and drug discovery for nonalcoholic fatty liver disease

- APOA5 Polymorphism Is Associated with Metabolic Syndrome in Korean Postmenopausal Women.
- Doh Hee Kim, Seung Hee Lee, Kyung Hoon Han, Chae Bong Kim, Kwan Young Song, Sook Cho, Kye Heui Lee
- Endocrinol Metab. 2012;27(4):276-281. Published online December 20, 2012
- DOI: https://doi.org/10.3803/EnM.2012.27.4.276
- 4,654 View
- 23 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Menopause is an independent risk factor in metabolic syndrome which induced an alteration of the lipid metabolism by hormonal changes. Apolipoprotein A5 gene (APOA5) was related to the regulation of triglyceride and high density lipoprotein cholesterol (HDL-C) level with biosynthesis and decomposition. This study was conducted to investigate the relationship between APOA5 polymorphism and metabolic syndrome in Korean postmenopausal women. METHODS: This study included 307 postmenopausal women with anthropometric and biochemical measurement in 2010-2011. The polymorphism of APOA5 was analyzed by polymerase chain reaction-restriction fragment length polymorphism method with MseI restriction enzyme. RESULTS: The metabolic syndrome prevalence with TT genotype was significantly lower than the frequency in those with TC/CC (27.09%, 38.46%, and 45.71% for TT, TC, and CC, respectively; P < 0.05). Multiple regression analysis of metabolic syndrome risk factors indicated that postmenopausal women with CC genotype had a higher risk with 3 times than that in TT genotype (P < 0.05). APOA5 C carriers showed an increased risk of triglyceride level (odd ratio, 2.93 and 1.85 for CC and TC+CC, respectively; P < 0.05). Interestingly, HDL-C was related to triglyceride directly in comparison to APOA5. CONCLUSION: The results of this study indicate that APOA5 has an influence on serum triglyceride and HDL-C, which contribute to metabolic syndrome in Korean postmenopausal women. -
Citations
Citations to this article as recorded by- Effects of a 3-year dietary intervention on age-related changes in triglyceride and apolipoprotein A-V levels in patients with impaired fasting glucose or new-onset type 2 diabetes as a function of the APOA5 -1131 T > C polymorphism
Minjoo Kim, Jey Sook Chae, Miri Kim, Sang-Hyun Lee, Jong Ho Lee
Nutrition Journal.2014;[Epub] CrossRef - APOA5Polymorphism Is Associated with Metabolic Syndrome in Korean Postmenopausal Women
Mi Hae Seo, Won Young Lee
Endocrinology and Metabolism.2012; 27(4): 274. CrossRef
- Effects of a 3-year dietary intervention on age-related changes in triglyceride and apolipoprotein A-V levels in patients with impaired fasting glucose or new-onset type 2 diabetes as a function of the APOA5 -1131 T > C polymorphism


 KES
KES

 First
First Prev
Prev