Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
Article information
Abstract
Background
Persistence with denosumab in male patients has not been adequately investigated, although poor denosumab persistence is associated with a significant risk of rebound vertebral fractures.
Methods
We retrospectively evaluated 294 Korean male osteoporosis patients treated with denosumab at three medical centers and examined their persistence with four doses of denosumab injection over 24 months of treatment. Persistence was defined as the extent to which a patient adhered to denosumab treatment in terms of the prescribed interval and dose, with a permissible gap of 8 weeks. For patients who missed their scheduled treatment appointment(s) during the follow-up period (i.e., no-shows), Cox proportional regression analysis was conducted to explore the factors associated with poor adherence. Several factors were considered, such as age, prior anti-osteoporotic drug use, the treatment provider’s medical specialty, the proximity to the medical center, and financial burdens of treatment.
Results
Out of 294 male patients, 77 (26.2%) completed all four sequential rounds of the denosumab treatment. Out of 217 patients who did not complete the denosumab treatment, 138 (63.6%) missed the scheduled treatment(s). Missing treatment was significantly associated with age (odds ratio [OR], 1.03), prior bisphosphonate use (OR, 0.76), and prescription by non-endocrinologists (OR, 2.24). Denosumab was stopped in 44 (20.3%) patients due to medical errors, in 24 (11.1%) patients due to a T-score improvement over –2.5, and in five (2.3%) patients due to expected dental procedures.
Conclusion
Our study showed that only one-fourth of Korean male osteoporosis patients were fully adherent to 24 months of denosumab treatment.
INTRODUCTION
Osteoporosis is a major disease affecting more than 200 million people worldwide [1]. Osteoporosis decreases bone strength, subsequently increasing the risk of fracture and leading to notable morbidity and diminished quality of life. Denosumab, the first-approved biologic agent for the treatment of osteoporosis, is a potent antiresorptive drug that can significantly reduce the risk of hip, vertebral, and non-vertebral fractures [2,3]. According to the most recent records of the Health Insurance Review and Assessment Service in South Korea, denosumab is the most frequently prescribed anti-osteoporosis drug, accounting for 30% of the entire market share of osteoporosis medications. However, concerns exist about the loss of gained bone mineral density (BMD) and rebound vertebral fractures associated with the discontinuation of denosumab injections [4-6]. Importantly, a short off-treatment period of 2 to 10 months has been reported to be enough to yield an elevated risk of fracture events [7]. Thus, persistence with denosumab is crucial for achieving therapeutic benefits.
The importance of drug persistence in treating osteoporosis to achieve the goal of fracture prevention is well-recognized and emphasized in several prior studies [8,9]. The issue of poor persistence not only directly impacts fracture rates and associated mortality, but also contributes to increasing healthcare costs [8, 10-12]. Persistence with osteoporosis-related therapy has been extensively studied in women, but there is a dearth of available data on men. Unlike women, men do not experience menopause, which is associated with rapid bone loss, but they lose substantial amounts of bone with aging. This results in low bone mass and microstructural worsening with a subsequently increased susceptibility to fracture. Globally, 39% of all osteoporotic fractures are estimated to occur in men over the age of 50 [13]. Moreover, the number of osteoporotic fractures in Korean men is increasing. However, eight out of 10 men with osteoporosis in Korea are not taking anti-osteoporosis medications [14]. Public awareness of the importance of persistence in osteoporosis treatment in men should be raised since mortality after hip fracture is higher in men than in women at any point in their life [15,16]. In this context, it is worth emphasizing that relatively few studies based on real-world data have evaluated the rate of persistence with denosumab in male osteoporosis patients. The underlying mechanisms of treatment adherence are not fully understood, but there are likely to be multiple contributing factors. Thus, the present study aimed to examine persistence with denosumab therapy in male osteoporosis patients and explore the factors associated with treatment adherence.
METHODS
Data sources
We retrospectively evaluated male patients with osteoporosis receiving denosumab treatment for the first time from April 2019 to February 2022 at three medical centers: Seoul St. Mary’s Hospital, Yeouido St. Mary’s Hospital, and Uijeongbu St. Mary’s Hospital in South Korea. The first of those institutions is a tertiary hospital and the second is a secondary hospital, both of which are located in a megalopolis. The third is a secondary hospital located in the city of Uijeongbu.
Men aged 50 years or older diagnosed with osteoporosis were included in the present study. Initially, 319 male osteoporosis patients were eligible for the study. Among them, eight patients who stopped denosumab but received an alternative treatment without delay were excluded from the analysis. Five patients who died during the 24-month follow-up period were also excluded. Twelve patients were transferred to another medical center. The final sample included 294 male osteoporosis patients treated with denosumab. The study was approved by the Institutional Review Board of the Catholic University of Korea (XC22RIDI0016). Written informed consent by the patients was waived due to a retrospective nature of our study.
Persistence
We defined persistence according to the taxonomies of the International Society of Pharmacoeconomics and Outcomes Research [17] and Vrijens et al. [18] as follows: the extent to which a patient adheres to the denosumab treatment procedure in terms of the prescribed interval and dosing regimen. The outcomes included persistence over a 24-month period of follow-up for each patient. A permissible gap of up to 8 weeks between repeat injections was allowed, as in prior studies [19-23]. To explore the effects of different permissible gaps on denosumab persistence, we also performed a sensitivity analysis using 4-, 8-, and 12-week gaps. Doses given after the permissible gap were disregarded.
Potential factors contributing to the discontinuation of denosumab
Factors that were potentially associated with denosumab adherence included age, sex, proximity to the medical center, prior anti-osteoporotic drug use, any recorded medication-related side effect experience, medical center classification (i.e., secondary or tertiary), malignancy history, rural-urban classification of residence (i.e., megalopolis/metropolitan city or village or city), financial burden of the treatment, number of prior hospitalizations, and the treatment provider’s medical specialty (i.e., an endocrinologist or another specialist). The financial burden of the treatment was determined based on whether the patients were health insurance subscribers or Medical Aid beneficiaries. A “no-show” was operationalized as an occasion when a patient missed his or her scheduled treatment appointment(s), interrupting the continuous denosumab treatment procedure.
South Korean National Health Insurance reimbursement system
In South Korea, all citizens are automatically subscribed to the government-run National Health Insurance System [24]. Meanwhile, the Medical Aid program offers medical services to those with low income (medical care beneficiaries). For those beneficiaries, the co-pay is less than 2 U.S. dollars (USD) for each outpatient clinic visit. In South Korea, denosumab received insurance coverage as a primary treatment for osteoporosis in April 2019. For ordinary health insurance subscribers, the amount of co-pay for denosumab injection is approximately 100 USD. However, denosumab is no longer reimbursable in both groups once a patient’s condition improves to the point that BMD falls within the range indicating osteopenia, that is, a T-score >−2.5 at all skeletal sites.
Treatment schemes
Patients underwent dual-energy X-ray absorptiometry every 12 months after the first administration of denosumab. Denosumab (60 mg, Amgen Inc., Thousand Oaks, CA, USA) was administered subcutaneously in the upper arm every 6 months, with vitamin D and/or calcium supplementation. Patients’ persistence with the four doses of denosumab over the 24-month treatment procedure was investigated.
Statistical analysis
All data were statistically analyzed using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). All descriptive statistics are presented as means±standard deviations for continuous measures and as percentages for categorical measures. A P value <0.05 was considered to indicate statistical significance. The Kaplan-Meier estimator was used to explore and compare the persistence rate. To examine the factors associated with poor denosumab persistence in no-show patients, univariate and multivariate Cox proportional regression analyses were conducted, including no-shows and patients who received all four doses of denosumab.
RESULTS
General baseline parameters
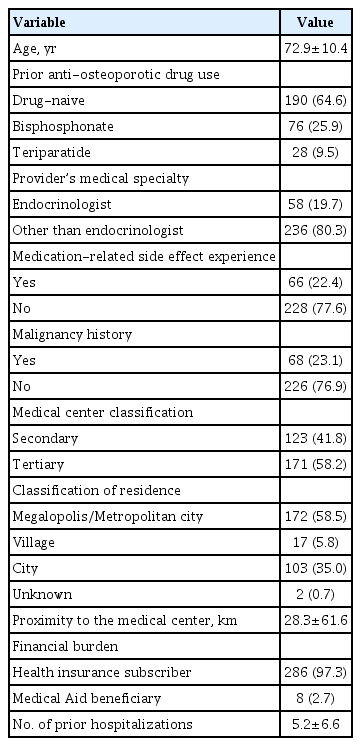
The baseline characteristics of the 294 patients are summarized in Table 1. All of them were male osteoporosis patients treated with denosumab. They were 50 to 96 years old, and the mean age was 72.9±10.4 years. Among them, 190 (64.6%) were antiosteoporotic drug-naïve, 76 (25.9%) were switched from prior bisphosphonate treatment to denosumab, and 28 (9.5%) were switched from teriparatide. Furthermore, 58 (29.7%) patients were prescribed denosumab by endocrinologists. Additionally, 286 (97.3%) patients were ordinary national health insurance subscribers, while eight (2.7%) patients were Medical Aid beneficiaries.
Persistence in the dosing schedule of denosumab therapy
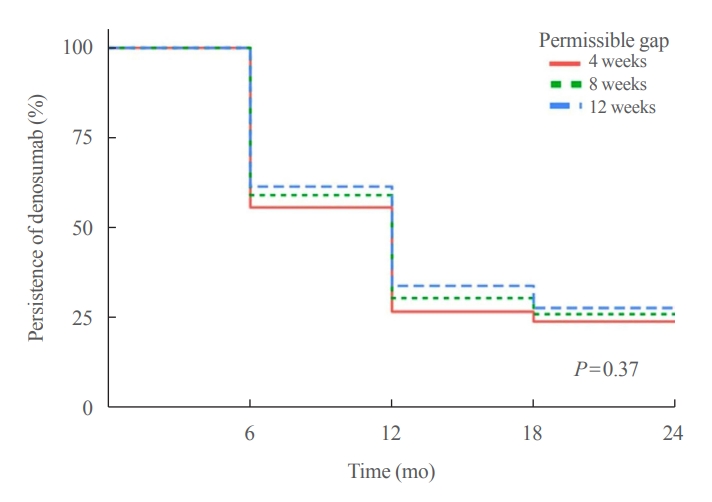
The percentages of patients who persisted with denosumab over 24 months (equal to receiving subsequent injections within 6 months±the permissible gap) are represented using Kaplan-Meier curves with permissible gaps of 4, 8, and 12 weeks (Fig. 1). Using a permissible gap of 8 weeks, 77 patients received all four doses of denosumab. The persistence rate of denosumab therapy was 59.2% at 12 months, 30.6% at 18 months, and 26.2% at 24 months. Using permissible gaps of 4 and 12 weeks, the persistence rates at months 12 and 24 were 55.8%–61.6% and 24.1%–27.9%, respectively (P=0.37). The reasons for the abrupt discontinuation of denosumab with a permissible gap of 8 weeks were analyzed following an extensive chart review. Patients (n=138, 63.6%) did not appear as scheduled at the medical center (no-shows). Denosumab was stopped without any alternative treatment in 44 (20.3%) patients due to an inadvertent mistake made by medical staff, in 24 (11.1%) patients due to improvement of the BMD T-score over –2.5, and in five (2.3%) patients due to expected dental procedures. Other reasons for denosumab discontinuation included patient refusal (n=3, 1.0%), poor general condition (n=2, 0.9%), and the occurrence of medication-related osteonecrosis of the jaw (n=1, 0.5%) (Fig. 2).
Results of Cox regression analysis
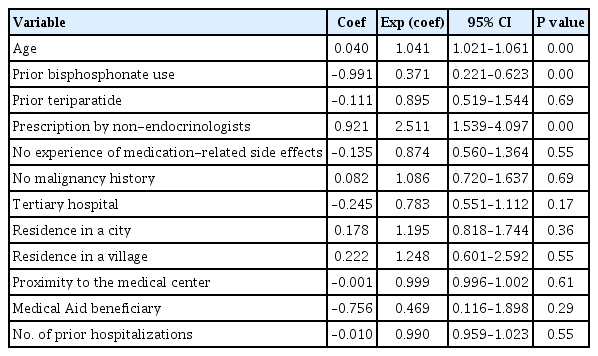
Table 2 shows the results of the univariate analysis of the no-show patients’ data. Factors that could potentially influence no-shows were investigated in the analysis, including age, prior anti-osteoporotic drug use, medication-related side effect experience, medical center classification, malignancy history, and the treatment provider’s medical specialty. Age (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.02 to 1.06), prior bisphosphonate use (OR, 0.37; 95% CI, 0.22 to 0.62), and prescription by non-endocrinologists (OR, 2.51; 95% CI, 1.54 to 4.01) were significantly associated with no-shows. Multivariate Cox regression analysis returned similar findings, according to which age (OR, 1.03; 95% CI, 1.01 to 1.05), prior bisphosphonate use (OR, 0.40; 95% CI, 0.24 to 0.68), and prescription by non-endocrinologists (OR, 2.24; 95% CI, 1.36 to 3.68) were the only significant factors associated with no-shows (Fig. 3).
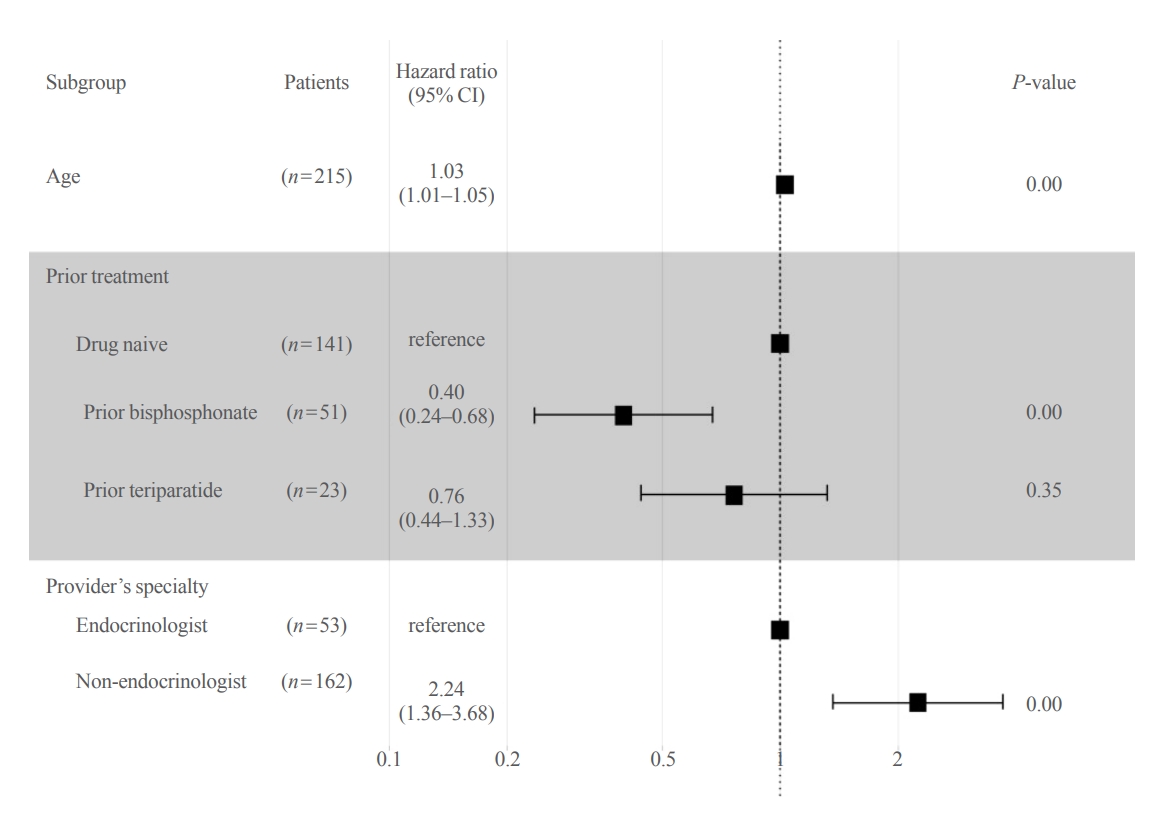
Forest plot of multivariate Cox proportional regression analysis for predicting no-shows of patients. The variables included in the analysis were age, prior anti-osteoporotic drug use, medication-related side effect experience, medical center classification, malignancy history, the treatment provider’s medical specialty, classification of residence, Medical Aid beneficiary status, number of prior hospitalizations, and the treatment provider’s medical specialty. CI, confidence interval.
DISCUSSION
Our multicenter study based on real-world data showed that 26.2% of Korean male osteoporosis patients completed 24 months of denosumab treatment. Several prior retrospective studies have been conducted to investigate the persistence with denosumab (Supplemental Table S1) [19-23,25-34]. However, few studies have explored persistence with denosumab in the male population. The current understanding of the persistence with denosumab has been disproportionately based on limited studies in the female population.
The majority of the published studies on persistence with denosumab observed patients over a 24-month treatment period, and the persistence rates varied from 28.3% to 59.6% (Supplemental Table S1). This remarkable variation may be attributed to heterogeneity in study populations regarding age, culture, drug costs, and healthcare systems, as well as the size, design, and type of studies [23]. In Korea, national health insurance reimburses the cost of denosumab treatment only when patients’ lowest BMD T-score is –2.5 or lower. Therefore, it is improbable that patients who are undergoing follow-up at clinics would have switched to other practitioners without having their BMD measured again. For this reason, persistence with denosumab in the present study was unlikely to be underestimated. In this study, the persistence rate after 24 months was 26.2% overall in male patients, as defined by receiving five total injections, with an 8-week grace period.
The most frequent reason for sudden denosumab discontinuation was patients missing scheduled appointment(s), and some factors were found to be associated with no-shows. In our analysis, aging was significantly associated with poor denosumab persistence. Past studies examining adherence to osteoporosis treatment found conflicting results; namely, age was not associated with compromised persistence in some studies [20,35], while persistence declined with age in other studies [19,23, 36,37]. Arguably, making a trip to a healthcare facility to receive denosumab treatment every 6 months can be challenging for elderly patients.
A history of recent use of osteoporosis medications at baseline was significantly associated with persistence, in accordance with the findings of previous research [19,20,23]. In particular, our study replicated the findings of Tremblay et al. [19], who demonstrated that prior bisphosphonate treatment was associated with greater persistence with denosumab therapy. One viable explanation is that treatment-experienced patients, as opposed to treatment-naïve patients, may be more informed and knowledgeable about their disease and the need for their medication [19,25].
Patients were more likely to be adherent to denosumab therapy when it was prescribed by endocrinologists than they were prescribed by non-endocrinologists. The same finding was reported in a study by Chandran et al. [25]. Patients were 2.25 times more likely to adhere to denosumab when treated by endocrinologists than they were treated by non-endocrinologists over the 24 months of denosumab therapy. The underlying reason is unclear. The potential relationship between treatment providers’ specialty and the quality/effectiveness of communication with patients about the importance of denosumab persistence needs to be further explored. Patients with osteoporosis generally have poor awareness and knowledge regarding their condition [38]. Efforts should be made to prevent no-shows by educating patients.
We found that adherence to denosumab was not significantly related to the proximity to the medical center. A previous retrospective study revealed that a greater distance from one’s residence to a clinic was associated with compromised treatment persistence, although the relationship was not statistically significant [39]. In Korea, it takes less than a half-day for a round trip between most cities and we presume that the impact of the proximity to medical centers was not substantial in the context of our study.
Low socioeconomic status has been shown to be associated with non-persistence with oral medications in general [40], and previous research showed a decline in medication persistence when out-of-pocket drug costs were high [41]. However, our study showed that the difference in co-pay between health insurance subscribers and Medical Aid beneficiaries was not significantly associated with patients’ adherence to denosumab. In Korea, the amount of co-pay for denosumab injection does not exceed 100 USD even for ordinary health insurance subscribers. We view that the non-significant difference between the two groups can be attributed to the relatively low economic burden of denosumab. Unique healthcare systems and varying financial burdens in different countries should be considered when examining treatment persistence.
The second most frequent reason for sudden denosumab discontinuation was an inadvertent mistake made by medical practitioners. Nearly 15% of all patients discontinued denosumab due to physicians’ errors. Furthermore, 8.1% of the total patients stopped denosumab after their BMD improved, without other anti-osteoporosis medicine for consolidation treatment. As mentioned earlier, the Korean National Health Insurance System covers denosumab treatment only when patients’ annual BMD T-score is below –2.5. It is up to medical practitioners to persuade patients to continue lower-cost consolidation treatment even after they become ineligible for health insurance coverage for denosumab.
Some physicians discontinued prescribing denosumab before any expected dental procedures without switching to any other anti-osteoporosis therapy. Although there has been a report suggesting a time window of opportunity for dental procedures to reduce the risk of occurrence of medication-related osteonecrosis of the jaw [42] in patients receiving denosumab treatment, there is no consensus on the discontinuation of denosumab prior to dental procedures. Thus, denosumab discontinuation needs to be discouraged.
There are two main strengths of our study. To our best knowledge, this is the first study documenting persistence with denosumab therapy in male osteoporosis patients. Men have generally been considered to be less adherent to osteoporosis therapy than women. However, studies have reported conflicting findings, with men being less adherent to osteoporosis therapies than women in some studies [37,43-46], but not in others [47-49]. More studies are needed to clarify the gender difference in the persistence with denosumab and other osteoporosis treatments. Furthermore, the present study utilized real-world data from patients receiving denosumab treatment as part of routine clinical practice, rather than an exploration of subjects who volunteered for a clinical trial or consented to participate in a prospective study; thus, this study may have been susceptible to selection bias.
There are several limitations in this study as well. Our study period primarily overlapped with the coronavirus disease 2019 (COVID-19) pandemic, which may have been associated with patients’ hesitance to visit hospitals and clinics for the ongoing management of osteoporosis. According to the Korea National Health Insurance Review, the average number of visits to medical centers per month decreased in 2020 compared to the previous year. Likewise, a recent study conducted in Singapore reported a decrease in denosumab persistence during the COVID-19 pandemic [25]. The exact reasons for the decreased denosumab persistence rates during the COVID-19 pandemic are unknown. However, this decrease might be related to anxiety and fear of COVID-19 infection while visiting clinics and hospitals [25]. Second, this study was not designed to evaluate the association between denosumab persistence and the risk of incident fractures, nor did it assess the overall changes in BMD. Lastly, the present study was based on retrospective data, and information on some potential factors contributing to denosumab persistence was not available and not included in the analysis. Examples include socioeconomic status, educational level, and lifestyle factors. Future studies need to examine the roles of these factors.
In conclusion, as few as one-fourth of male patients were persistent with 24 months of denosumab treatment. Clinicians need to carefully address the issue of poor persistence with denosumab in male osteoporosis patients.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: K.H.B. Acquisition, analysis, or interpretation of data: C.J., J.L., J.K., J.H., K.J., Y.L., M.K.K., H.S.K., T.S.S., K.H.S., M.I.K. Drafting the work or revising: C.J., K.H.B. Final approval of the manuscript: C.J., J.L., J.K., J.H., K.J., Y.L., M.K.K., H.S.K., T.S.S., K.H.S., M.I.K., K.H.B.
Acknowledgements
The authors wish to acknowledge the financial support of The Catholic University of Korea, Uijeongbu St. Mary’s Hospital Clinical Research Laboratory Foundation made in the program year 2022.
SUPPLEMENTARY MATERIAL
Differences in Persistence with Denosumab in Prior Retrospective Study