The Physiological Functions and Polymorphisms of Type II Deiodinase
Article information
Abstract
Type II deiodinase (DIO2) is thought to provide triiodothyronine (T3) to the nucleus to meet intracellular needs by deiodinating the prohormone thyroxine. DIO2 is expressed widely in many tissues and plays an important role in a variety of physiological processes, such as controlling T3 content in developing tissues (e.g., bone, muscles, and skin) and the adult brain, and regulating adaptive thermogenesis in brown adipose tissue (BAT). However, the identification and cloning of DIO2 have been challenging. In recent years, several clinical investigations have focused on the Thr92Ala polymorphism, which is closely correlated with clinical syndromes such as type 2 diabetes, obesity, hypertension, and osteoarthritis. Thr92Ala-DIO2 was also found to be related to bone and neurodegenerative diseases and tumors. However, relatively few reviews have synthesized research on individual deiodinases, especially DIO2, in the past 5 years. This review summarizes current knowledge regarding the physiological functions of DIO2 in thyroid hormone signaling and adaptive thermogenesis in BAT and the brain, as well as the associations between Thr92Ala-DIO2 and bone and neurodegenerative diseases and tumors. This discussion is expected to provide insights into the physiological functions of DIO2 and the clinical syndromes associated with Thr92Ala-DIO2.
INTRODUCTION
Iodothyronine deiodinases (DIOs) are essential for maintaining appropriate levels of triiodothyronine (T3) in the circulation and ensuring its intracellular availability; this role is important since T3 has been implicated in the control of a variety of biological events including growth, development, and metabolism in vertebrates. DIOs have three isoforms, (DIO1, DIO2, and DIO3), which differ in their catalytic properties, tissue distribution, and substrate specificity [1]. These isoforms are expressed in a tissue-specific manner in fetal and adult life and selectively catalyze the activation or inactivation of thyroid hormones (THs) [2]. Although both DIO1 and DIO2 activate THs, their functions are different; specifically, DIO1 is a scavenger enzyme that recycles iodine to replenish the thyroid’s iodine reservoirs, while DIO2 provides T3 to the nucleus to meet intracellular needs [3]. DIO3 is the major TH-inactivating enzyme, which converts the prohormone thyroxine (T4) to reverse-T3 or T3 to T2. Therefore, the balance between the enzyme activity of DIO2 and DIO3 might determine the local concentration of T3. DIO1 and DIO3 are integral plasma membrane proteins. The DIO1 gene is expressed in the liver, thyroid, and kidney [4], while the DIO3 gene is mainly expressed in fetal tissues and the placenta, and its expression declines dramatically in adulthood, persisting primarily in the skin and brain, as well as in the uterus during pregnancy [3,5,6]. DIO2 is an endoplasmic reticulum-resident protein that is localized along radial glial cells, in brain barriers, in Cajal-Retzius cells, in migrating fibers of the brainstem, and in some neurons and glial cells with particular and complex spatiotemporal patterns [7]. Moreover, the DIO2 gene is expressed in the telencephalon [8], pituitary gland and hypothalamus [9,10], cochlea [11], muscles [12], heart [13], bone [14], brown adipose tissue (BAT) [15], glia, and astrocytes [16,17]. The expression of DIO2 in humans is less restricted than in rats, and about 70% of circulating serum T3 is derived from the extrathyroidal conversion from T4 to T3 catalyzed by DIO1 and DIO2 [5]. In two opposite pathological conditions, DIO2 is upregulated in patients with hypothyroidism and downregulated in those with hyperthyroidism. Changes in DIO2 expression or enzyme activity contribute to the general effort to maintain T3 homeostasis, both in the circulation and in specific tissues [18].
DIO2 expression or enzyme activity is essential for the maintenance of normal physiological function, including the central nervous system (CNS), BAT, and placenta. Its abnormal expression and enzyme activity are associated with various physiological and/or pathological processes, as listed in Table 1 [19-32]. A study showed that the overexpression of Dio2 could arrest trophoblast cell line proliferation at the G1 phase of the cell cycle by downregulating cyclin-D1 (Ccnd1) and proliferating cell nuclear antigen (Pcna), while promoting apoptosis via increased caspase-3 activity and inhibition of the Akt and extracellular signal‑regulated protein kinase (ERK1/2) signaling pathways [30]. These results indicate that DIO2 plays an indispensable role in many important physiological and/or pathological processes.

Upregulation and Downregulation of DIO2 Associated with Various Physiological and Pathological Processes
In this review, we summarize the current knowledge on the physiological functions and polymorphisms of the DIO2 gene, thereby providing insights into the important roles of DIO2 in the pathogenesis of diseases.
MOLECULAR STRUCTURE OF DIO2
The identification and cloning of the three deiodinases were remarkably difficult and proved to be extremely challenging, particularly DIO2. Utilizing the relatively short but highly conserved regions between the known Dio1 and Dio3 cDNAs, Davey et al. [33] used a reverse transcription/polymerase chain reaction strategy to clone a cDNA for Dio2 in the amphibian species Rana catesbeiana. Subsequently, Croteau et al. [34] used the amphibian Dio2 cDNA to obtain the sequences in rats and humans. Dio2 in rats contains an open reading frame of 798 nucleotides that includes two in-frame TGA codons; this 798-nucleotide open reading frame is predicted to code for a protein of 266 amino acids with a molecular weight of 29.8 kDa. In humans, DIO2 contains an open reading frame of 819 nucleotides that codes for a protein of 273 amino acids with a molecular weight of 30.0 kDa. Moreover, fluorescence in situ hybridization confirmed that human DIO2 is located in chromosome 14q24.2→q24.3 [35]. An analysis of protein structure showed that a critical residue (the active center Sec) confers high catalytic activity to deiodinases [36]. The three-dimensional modeling of the DIO2 protein based on hydrophobic cluster analyses [37] identified a unique 18-residue “instability” loop in the Dio2 molecule, which could be recognized by the WD-40 propeller of WD repeat and socs box-containing 1 (WSB-1), a part of an E3 ubiquitin ligase [38,39]. Ubiquitination of DIO2 is a switch mechanism that controls DIO2 activity and intracellular T3 production, whereby T4 binding and/or T4 catalysis triggers DIO2 inactivation by ubiquitination, which is mediated by the E3 ubiquitin ligases WSB-1 and/or the yeast Doa10 mammalian ortholog TEB4. Ubiquitinated DIO2 could be either targeted for proteasomal degradation or reactivated by deubiquitination, a process that is mediated by the deubiquitinases ubiquitin-specific proteases 20/30 (USP20/33) and is important in adaptive thermogenesis [40]. The subcellular localization of Dio2 is usually in the endoplasmic reticulum; it is also closely associated with the cell nucleus, but not with the Golgi apparatus (Fig. 1) [41-43]. The subcellular localization of Dio2 in the Golgi apparatus could constitute a disease mechanism associated with the Thr92Ala polymorphism in DIO2 (Fig. 1) [44].

Schematic representation of the genomic actions of type II deiodinase (DIO2) in target cells, taking adipocytes as an example, as well as the occurrence of bone diseases caused by the Thr92Ala polymorphism in DIO2. Thyroxine (T4) is secreted by the thyroid gland and transported to the target tissue, such as adipose tissue (AT), through the blood. T4 then enters the cell via transport proteins including monocarboxylate transporter 8 (MCT8), MCT10, and organic anion-transporting polypeptide 1C1 (OATP1C1), which are located on the plasma and nuclear membranes. In the cytoplasm, DIO2 located in the endoplasmic reticulum catalyzes the conversion of T4 into active triiodothyronine (T3). Active T3 enters the nucleus via transport proteins and binds to thyroid hormone receptors (TRs) to regulate gene expression. However, when the subcellular location of DIO2 is in the Golgi apparatus in osteocytes, and the amino acid at position 92 of DIO2 changes from T (threonine) into A (alanine), bone diseases, such as osteoarthritis, osteoporosis, and Kashin-Beck disease (KBD), will occur. TRE, transcriptional regulatory element.
PHYSIOLOGICAL FUNCTIONS OF DIO2
As a deiodinase, the primary physiological function of DIO2 is to control the homeostasis of THs in the circulation and tissues, together with DIO1 and DIO3. It is also a key molecule for cold-adaptive thermogenesis in brown adipocytes, diet-induced thermogenic pathways, and it plays a metabolic role in humans [18]. The latest findings about DIO2 in TH signaling, adaptive thermogenesis in BAT, and the brain were summarized.
Role of DIO2 in TH signaling
Hypothyroidism is a state in which circulating TH levels are inadequate. It is commonly caused by autoimmune destruction or surgical removal of the thyroid gland (primary hypothyroidism). Therapy consisting of daily tablets of levothyroxine (LT4) to treat hypothyroidism is commonsensical and has become the standard of care for this disease [45,46]. Short-term LT4 treatment reduced the vestibular syndrome and significantly promoted vestibular compensation, and the observed presence of thyroid hormone receptors (TRs) and DIO2 in the vestibular nuclei supported the possibility that LT4 exerts local actions [47]. However, a small percentage of patients with hypothyroidism also experienced persistent symptoms despite LT4 therapy, with impaired cognition and tiredness [48]. These outcomes might be attributed to the lack of thyroid T3 secretion in LT4-treated hypothyroid patients. In patients with intrauterine adhesions, defective autophagy in the endometria has been shown to be associated with DIO2 downregulation, while overexpression of DIO2 or T3 treatment could restore autophagy and partly reverse the epithelial-mesenchymal transition in endometrial epithelial cells [28]. T3 is partially secreted by the thyroid gland, but mainly produced by DIO1 and DIO2 in various extrathyroidal tissues [49]. Therefore, several studies have investigated the efficiency of combined therapy with LT4+LT3. Shakir et al. [50] compared the treatment efficiency among LT4, LT4+LT3, and desiccated thyroid extract (DTE) and found similar outcomes among hypothyroid patients treated with LT4, LT4+LT3, and DTE; furthermore, the patients who were most symptomatic on LT4 preferred and responded positively to therapy with LT4+LT3 or DTE. A male patient with treatment-resistant depression and hypothyroidism responded to LT3/LT4 combination therapy, rather than LT4 alone, and he had a DIO2 polymorphism [51]. Wolff et al. [52] also addressed the problem of developing an optimal TH replacement strategy for hypothyroid patients and reported that LT3/LT4 combined therapy was slightly better than LT4 monotherapy for treating hypothyroidism. The European Thyroid Association guidelines state that LT4+LT3 combination therapy should be considered only as an experimental treatment modality in LT4-treated patients whose symptoms persist even though their serum thyroid-stimulating hormone levels are within the reference range [53]. The above results all indicate that the outcomes of combined therapy have shown progress in several fields, implying that DIO2 exerts an important effect in converting T4 to T3. However, debate continues regarding LT4+LT3 combination therapy in hypothyroidism patients. Drigo and Bianco [40] showed that DIO2 was associated with TH signaling in sensory organ development, skeletal development, regulation of the hypothalamic-pituitary-thyroid axis, adaptive thermogenesis, and metabolic control. The circulating TH levels remain fairly consistent during the entire adult life of healthy individuals. Therefore, deiodination carried out by deiodinases could control the important biological processes (growth, development, metabolism) regulated by THs despite no meaningful changes in plasma levels.
Role of DIO2 in adaptive thermogenesis in BAT
BAT is the main site of the sympathetic-mediated adaptive thermogenesis in human newborns and other small mammals, and the thermogenic pathway of BAT has been described in detail by Drigo et al. [54]. During cold exposure, DIO2 activity was found to show an acute approximately 50-fold increase in BAT, which accelerated the conversion from T4 to T3 [55]. Our understanding of the role of DIO2 in BAT physiology is based on the disruption of the Dio2 gene (Dio2−/−) in mice, which results in BAT-specific hypothyroidism in an otherwise euthyroid animal [56]. de Jesus et al. [15] demonstrated that the BAT of Dio2−/− mice had normal amounts of mitochondria and normal uncoupling protein 1 (UCP-1), which is a mitochondrial protein that shunts the energy derived from mitochondrial fatty acid oxidation from adenosine triphosphate formation to thermogenesis. In response to different adrenergic stimulants, Dio2−/− brown adipocytes exhibited a decreased cyclic adenosine monophosphate generation capacity, which might be the mechanism of impaired thermogenesis [15]. Another study also showed that cold exposure increased BAT sympathetic stimulation approximately 10-fold, with an increase in lipolysis as well as the mRNA levels of Ucp-1, guanosine monophosphate reductase (Gmpr), and peroxisome proliferator-activated receptor gamma coactivator 1 (Pgc-1) in Dio2−/− mice [57]. Recent research found that T3 increased fatty acid oxidation and mitochondrial respiration as well as autophagic flux, mitophagy, and mitochondrial biogenesis; however, no significant induction of intracellular reactive oxygen species was found despite high mitochondrial respiration and Ucp-1 induction by T3, indicating that T3 exerted direct effects on mitochondrial autophagy, activity, and turnover in BAT that were essential for thermogenesis [58]. These results indicate that DIO2 is an essential component of the thyroid-sympathetic synergism required for thermal homeostasis in human newborns and small mammals.
Role of DIO2 in the brain
THs modulate the expression of a large number of genes in the CNS, and the expression of TH-targeted genes is regulated directly or indirectly through dynamic interactions of (non)ligand TRs with chromatin and DNA, in addition to epigenetic modifications. THs play a critical role in brain development, affecting neuronal migration, differentiation, and signal transduction, as well as myelin formation, neuronal cell proliferation, migration and maturation, synapse establishment and transmission [59]. In the brain, active T3 is either available directly from the circulation or is produced locally from T4 by Dio2, which is predominantly expressed in astrocytes [16,60]. Compared to hypothyroid mice, the T3 content in the brain of neonatal Dio2−/− mice was markedly reduced, while the mRNA levels of several T3-responsive genes were either unaffected or much less affected in the brain of the Dio2−/− mice, and the Dio2−/− mice exhibited a very mild neurological phenotype [59]. Notably, the Dio2−/− mice demonstrated no impairments in spatial learning and memory, but they displayed emotional alterations with increased anxiety-like behavior, as well as enhanced auditory-cued fear memory and spontaneous recovery of fear memory following extinction [61,62]. Another study indicated that Dio2 influenced working memory and verbal fluency in mice through neuropsychological testing [63]. Thus, the functions of Dio2 in learning and memory need further to be investigated. Studies have also shown that Dio2 expression is upregulated in a variety of neurological disorders. In mice that experienced 3 hours of status epilepticus (SE) caused by pilocarpine, the mRNA expression of Dio2 increased rapidly in the hippocampus, amygdala, and prefrontal cortex; however, the targeted disruption of Dio2 in astrocytes of mice had effects on highly induced genes in the hippocampus associated with inflammation, apoptosis, and cell death, suggesting that Dio2 induction caused by SE accelerated the production of T3 in different areas of the CNS and modified the hippocampal gene expression profile, affecting the balance between adaptive and maladaptive mechanisms [64]. Moreover, Dio2 mRNA expression significantly increased in the frontal cortex of two mandibular extension-treated rats compared with sham-operated rats, indicating the major involvement of Dio2 in an attempt to restore more physiological conditions and correct T3 levels, associated with normotensive status in the brain [65]. A recent result also showed that mice lacking both monocarboxylate transporter 8 (Mct8) and Dio2 presented peripheral and brain hypothyroidism; the severity of the brain hypothyroidism seemed permanent and varied across regions, with the striatum being a particularly affected area, and brain alterations were observed at the histological level compatible with TH deficiency and impaired motor skills [66]. Therefore, further investigations need to be carried out to understand the functional link between TH signaling and the role of Dio2 in different regions of the brain.
POLYMORPHISMS OF DIO2 LEAD TO MULTIPLE DISEASES
Polymorphisms of DIO2, including rs225014 (Thr92Ala), rs12885300, rs1352815, rs1388382, and rs955849187, have been shown to exert significant effects on physiological processes and diseases. Studies of polymorphisms of DIO2 in physiological and pathological processes or diseases are summarized in Table 2 [28,67-88]. Given its high prevalence in human populations (12% to 36%), the DIO2 rs225014 (Thr92Ala-DIO2) has been the most studied polymorphism in humans. It has also been researched in mouse and cell models [67], and has been found to be a potential risk factor for various diseases. Unfortunately, the clinical syndromes associated with DIO2 gene polymorphisms have not been reproduced in all population studies [67,89,90]. Maino et al. [53] summarized the clinical significance of the Thr92Ala-DIO2 polymorphism in patients with autoimmune or surgical hypothyroidism and in patients with physical/psychological disorders that could be associated with overt hypothyroidism, as well as severe type 2 diabetes mellitus or insulin resistance. Next, we focus on the latest findings on the role of Thr92Ala-DIO2 in bone diseases, neurodegenerative diseases, and other tumors.
Thr92Ala alters the physical function of the DIO2 gene
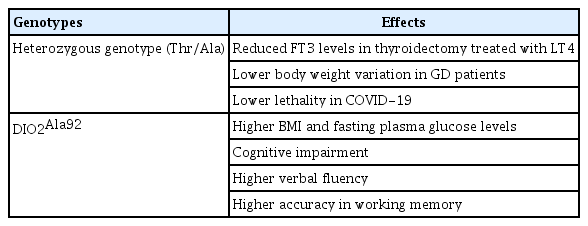
The subcellular localization of Thr92-Dio2 is usually in the endoplasmic reticulum, while Ala92-Dio2 accumulates in the Golgi apparatus, where its presence and/or ensuing oxidative stress disrupts basic cellular functions, including mitochondrial unbalancing and inflammation, and increases pre-apoptosis [43]. In patients with total thyroidectomy treated with LT4, heterozygous and rare homozygous patients carrying the Thr92Ala polymorphism in the DIO2 gene showed reduced free triiodothyronine (FT3) levels when data were analyzed assuming both dominant and recessive models, indicating that Thr92Ala-DIO2 might inhibit the conversion from T4 to T3 by DIO2 [91]. In Graves’ disease (GD) patients, the polymorphic inheritance (CC+CT genotype) of DIO2 rs225014 was associated with less body weight variation after GD treatment than in patients with the wild-type TT genotype, suggesting that DIO2 rs225014 genotyping might have an auxiliary role in predicting the posttreatment weight behavior of GD patients [92]. de Lima Beltrao et al. [93] investigated a possible association between the Thr92Ala-DIO2 polymorphism and in-hospital mortality from COVID-19 in adult patients admitted between June and August 2020, and the results showed lower lethality in people with the heterozygous genotype (Thr/Ala) than in those with homozygous genotypes (Thr/Thr and Ala/Ala), implying a protective role of Thr92Ala-DIO2 heterozygosity. An Ala92-Dio2 polymorphism-carrying mouse exhibited unfolded protein response and hypothyroidism in distinct brain areas, and the polymorphism-containing mice refrained from physical activity, slept more, and required additional time to memorize objects; however, LT3 treatment enhanced T3 signaling in the brain and improved cognition [94]. Compared with euthyroid noncarriers (Thr/Thr), euthyroid Ala92-DIO2 carriers showed higher body mass index values and fasting plasma glucose levels [95]. These results suggest that the Ala92-DIO2 might be deleterious for individuals with hypothyroidism and for euthyroid individuals.
Thr92Ala is associated with bone diseases
A study showed that the Tha92Ala-DIO2 polymorphism was associated with decreased femoral neck bone mineral density (BMD) and higher bone turnover independent of serum TH levels in patients with cured differentiated thyroid carcinoma, pointing to a potential functional role for Tha92Ala-DIO2 in bone metabolism [96]. In osteoporosis, female subjects carrying the Thr92Ala-DIO2 polymorphism had a significantly lower speed of sound and T-scores in the tibia than control participants, indicating a potential functional role of Thr92Ala-DIO2 in the maintenance of BMD [97]. However, the presence of the Thr92Ala-DIO2 polymorphism did not affect thyroid function tests in individuals who had a normal thyroid gland, but contradictory results were found in thyroidectomized patients kept on LT4 [90, 98,99]. In addition, the association between the Thr92Ala-DIO2 polymorphism and osteoarthritis was not reproduced by Kerkhof et al. [100] and a meta-analysis [101]. In contrast, Kerkhof et al. [100] showed that the T-allele of the rs12885300 single-nucleotide polymorphism in the DIO2 gene had a trend toward a protective effect for hip osteoarthritis. A recent study using CRISPR/Cas9 genome editing found that Dio2Thr92 mice had decreased cartilage volume and median thickness, with increased articular cartilage damage; in contrast, Dio2Ala92 mutants had no signs of osteoarthritis, indicating a protective role of the Ala92 polymorphism and providing the first functional evidence of a role for this candidate Dio2 polymorphism in vivo [102]. Notably, a meta-analysis found that the Thr92Ala-DIO2 polymorphism was also significantly associated with Kashin-Beck disease [103]. These results suggest that the Thr92Ala-DIO2 polymorphism might disrupt bone metabolism, and there might be an interaction between Thr92Ala-DIO2 and other polymorphisms of DIO2.
Thr92Ala is associated with neurodegenerative diseases
Previous research showed that both the Thr92Ala-DIO2 carriers and Ala92-Dio2-expressing HEK-293 cells exhibited a transcriptional fingerprint that included sets of genes involved in CNS diseases, ubiquitin, mitochondrial dysfunction, inflammation, apoptosis, DNA repair, and growth factor signaling [43]. Subsequent studies pointed out that the Thr92Ala-DIO2 polymorphism in neurodegenerative diseases was related to racial differences. For instance, compared with European Americans, African Americans with Thr92Ala-DIO2 had greater odds of developing Alzheimer disease (AD), dementia, or cognitive impairment without dementia [104]. In older adults, the outcomes of a standard questionnaire and evaluations of thyroid function were similar regardless of genotyping results for the Thr92Ala-DIO2 polymorphism, suggesting that the Thr92Ala-DIO2 polymorphism may not be associated with relevant cognitive impairment in older adults [16]. In light of these inconsistent findings, the role of Thr92Ala-DIO2 in cognitive impairment in individuals from different populations remains to be elucidated. Moreover, neuropsychological testing revealed that homozygous Ala92-carriers showed both higher verbal fluency and higher accuracy in working memory [63], suggesting improved executive function in homozygous Ala92-carriers. In autistic spectrum disorder (ASD), the minor allele (Ala92) frequency was not significantly different in ASD children, but carriers of the Thr92Ala-DIO2 polymorphism exhibited higher adaptive behavior, such as daily living skills and communication [105]. Those results indicate the importance of Thr92Ala-DIO2 in neurodegenerative diseases, such as AD, dementia, and ASD. However, more evidence is needed in large populations to demonstrate whether Thr92Ala-DIO2 is a risk factor for neurodegenerative diseases, and it is not clear whether the localization of Ala92-DIO2 is associated with those diseases.
Thr92Ala is associated with tumors
Only one study has described the relationship between Thr92Ala and tumors; that study showed that there was a 1.99-fold higher risk of developing endometrial cancer in CC homozygotes, reflecting a DIO2 (rs225014) polymorphism, than in TT homozygotes, indicating that carriers of the DIO2 polymorphism might be predisposed to the development of endometrial cancer [106]. Because altered expression of DIO2 was correlated with thyroid, pituitary and brain tumors, we speculate that the Thr92Ala might also have a relationship with these tumors.
CONCLUSIONS
Although the deiodinase family, which consists of DIO1, DIO2, and DIO3, is a dynamic system, DIO2 plays an essential role in TH signaling and adaptive thermogenesis in BAT and the brain by converting T4 to T3 in the target tissues. This review emphasizes the physiological functions and polymorphisms of the DIO2 gene, which is closely related to physiological/pathological processes and tumors; in particular, Thr92Ala-DIO2 has been shown to play deleterious or protective roles in different diseases (Table 3, Fig. 1). However, these results were based only on measurements of expression levels in several specific populations, and the reasons underlying these alterations in expression and associations with polymorphisms of the DIO2 gene have rarely been explored. Future studies need to be carried out to explore the pathogenic mechanisms by collectively analyzing subcellular localization, expression alterations, protein structure, and polymorphisms in the DIO2 gene. Considering the importance of the physiological functions of DIO2 and the clinical significance of polymorphisms of DIO2, it might be considered as a potential therapeutic target for metabolic diseases (bone metabolism), neurodegenerative diseases (AD, dementia, and ASD), and tumors (thyroid, pituitary, and brain tumors). The present review provides insights and orientations for future research on DIO2.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31871031, 32170968), the Fund of the Key Laboratory of Medical Electrophysiology in 2021 (KeyME-2021-01) and the China Postdoctoral Science Foundation (2021M692700).