Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach
Article information
Abstract
The fifth edition of the World Health Organization (WHO) histologic classification of thyroid neoplasms released in 2022 includes newly recognized tumor types, subtypes, and a grading system. Follicular cell-derived neoplasms are categorized into three families (classes): benign tumors, low-risk neoplasms, and malignant neoplasms. The terms “follicular nodular disease” and “differentiated high-grade thyroid carcinoma” are introduced to account for multifocal hyperplastic/neoplastic lesions and differentiated thyroid carcinomas with high-grade features, respectively. The term “Hürthle cells” is replaced with “oncocytic cells.” Invasive encapsulated follicular and cribriform morular variants of papillary thyroid carcinoma (PTC) are now redefined as distinct tumor types, given their different genetic alterations and clinicopathologic characteristics from other PTC subtypes. The term “variant” to describe a subclass of tumor has been replaced with the term “subtype.” Instead, the term “variant” is reserved to describe genetic alterations. A histologic grading system based on the mitotic count, necrosis, and/or the Ki67 index is used to identify high-grade follicular-cell derived carcinomas and medullary thyroid carcinomas. The 2022 WHO classification introduces the following new categories: “salivary gland-type carcinomas of the thyroid” and “thyroid tumors of uncertain histogenesis.” This review summarizes the major changes in the 2022 WHO classification and their clinical relevance.
INTRODUCTION
The World Health Organization (WHO) classification of tumors is updated regularly [1-4]. The fifth edition of the WHO Classification of Endocrine and Neuroendocrine Tumors was released as an online beta version (https://tumourclassification.iarc.who.int) in March 2022 [5]. Overview articles were published at the same time [2,6].
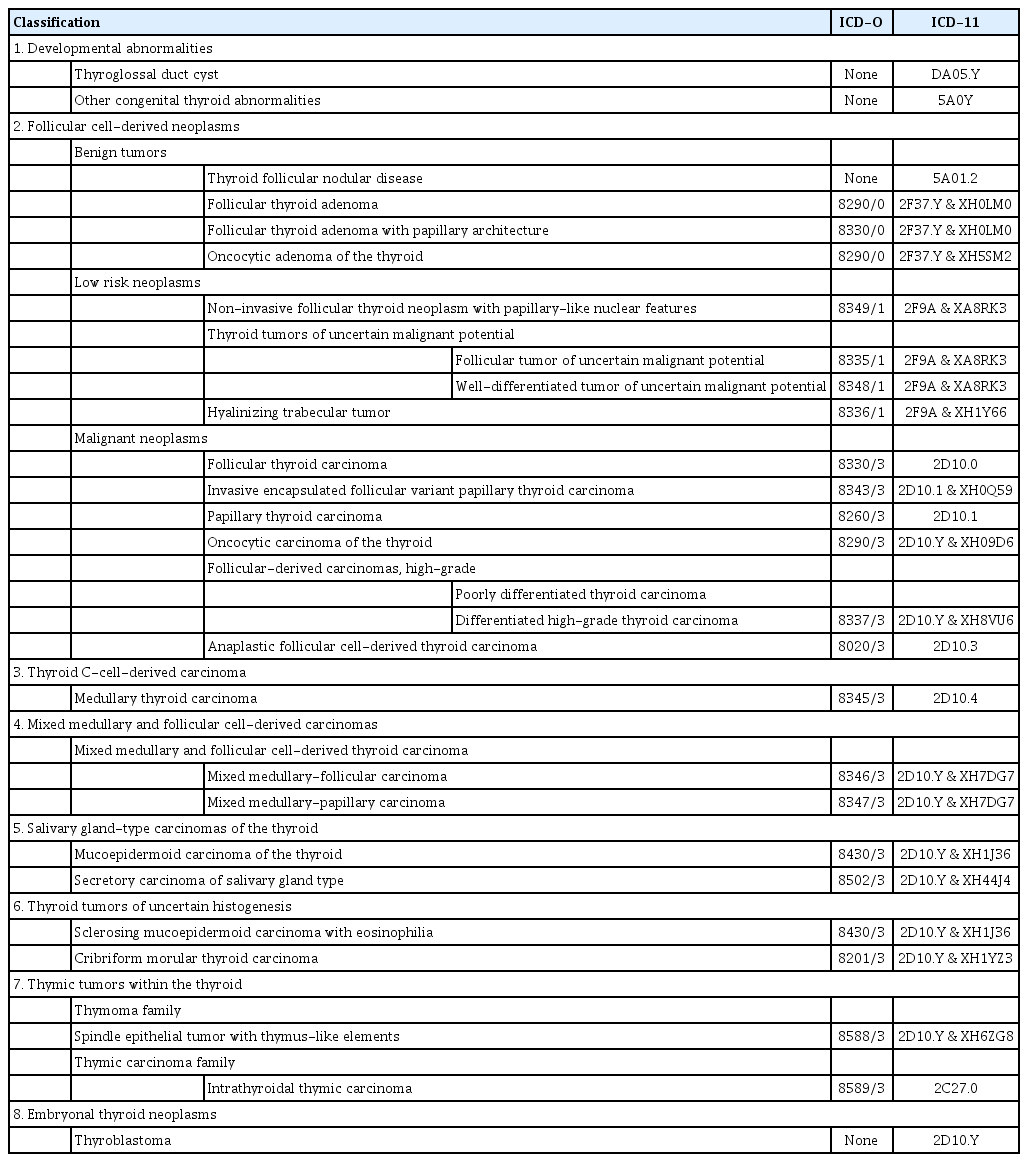
All volumes of WHO classification series organize tumors according to the anatomical site. In the fifth edition, each tumor is listed within a hierarchical taxonomic classification that is assigned based on the cell of origin, pathologic or molecular features, and biological behavior. The four main taxonomic ranks are category, family (class), type, and subtype. The majority of thyroid tumors are derived from follicular epithelial cells, while a small number arises from calcitonin-secreting C cells. Thymus, salivary gland, and germ cell tumors can present as primary thyroid neoplasms (Table 1). The fifth edition of the WHO classification includes newly recognized tumor types, subtypes, and a grading system. Follicular cell-derived neoplasms are categorized into benign tumors, low-risk neoplasms, and malignant neoplasms.
The classification of thyroid tumors has evolved based on classic histopathology and molecular pathogenesis. Most encapsulated/circumscribed thyroid tumors with a predominant follicular growth pattern exhibit a RAS-like molecular profile (Fig. 1) [7,8]. On the other hand, most thyroid tumors with a BRAFV600E-like molecular profile have papillary and/or infiltrative growth and florid nuclear atypia [7,8]. Both BRAFV600E-like and RAS-like thyroid cancers can gain additional genetic alterations and, as a result, progress to high-grade cancers [6].

Molecular classification and histopathological correlates in follicular cell-derived neoplasms. Thyroid neoplasms are classified as two molecular groups (BRAFV600E-like and RAS-like) or three groups (BRAFV600E-like, RAS-like, and non-BRAFV600E-/non-RAS-like) based on the mutational and gene expression profiles [7,8]. The BRAFV600E group is most commonly represented by papillary thyroid carcinoma (PTC). The BRAFV600E-like molecular profile includes the BRAFV600E mutation and gene fusions involving BRAF, RET, and neurotrophic receptor tyrosine kinase 1/3 (NTRK1/3). RAS-like molecular profiles include NRAS, HRAS, KRAS, EIF1AX, enhancer of zeste 1 polycomb repressive complex 2 subunit (EZH1), Dicer 1, ribonuclease III (DICER1), phosphatase and tensin homolog (PTEN) mutations, BRAFK601E, and gene fusions involving peroxisome proliferator-activated receptor gamma (PPARG) and THADA. When the three-group molecular classification is applied, PAX8::PPARG gene fusion and mutations of EIF1AX, EZH1, IDH1, SOS1, SPOP, DICER1, and PTEN genes are classified as a non-BRAFV600E-/non-RAS-like group [8]. Encapsulated/circumscribed thyroid tumors with a predominant follicular growth pattern generally have a RAS-like molecular profile. High grade is histologically defined as the presence of ≥5 mitoses per 2 mm2 and/or tumor necrosis. Y, yes; N, no; Q, questionable; PDTC, poorly differentiated thyroid carcinoma; ATC, anaplastic thyroid carcinoma; DHGTC, differentiated high-grade thyroid carcinoma; IEFVPTC, invasive encapsulated follicular variant of papillary thyroid carcinoma; WDT-UMP, well-differentiated tumor of uncertain malignant potential; NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclear features; FT-UMP, follicular tumor of uncertain malignant potential; FTC, follicular thyroid carcinoma; OCA, oncocytic carcinoma of the thyroid; TERT, telomerase reverse transcriptase; TP53, tumor protein p53; PAX8, paired box 8.
Thyroid mesenchymal and stromal tumors, hematolymphoid tumors, germ cell tumors, and metastasis to the thyroid have been moved from the thyroid chapter to the respective standalone chapters in the same volume combining these entities from all endocrine organs.
This review aimed to provide a summary of updates in the 2022 WHO classification of thyroid tumors.
MAIN CHANGES IN THE 2022 WHO CLASSIFICATION
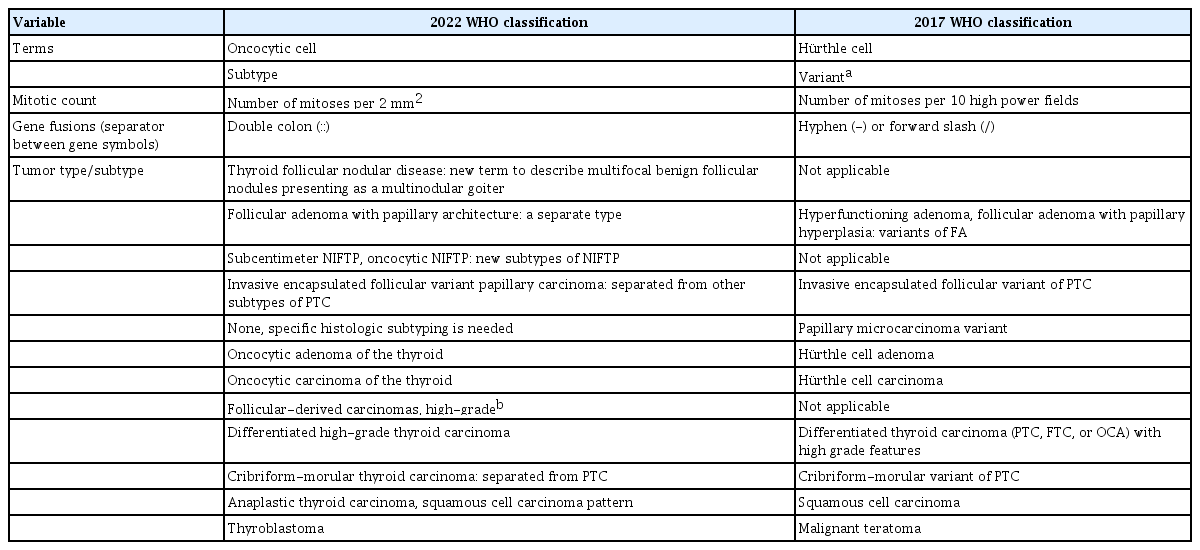
The main changes in the 2022 WHO classification compared against the previous edition are summarized in Table 2. The major revisions included modifications of the general terminology, the introduction of new entities, adoption of a grading system, and other changes specific to different tumor categories.
Changes in terminology
Historically, in thyroid pathology, the term “variant” has been used to describe the histologic subtype of a distinct tumor type, such as tall cell variant, columnar variant, hobnail variant of papillary thyroid carcinoma (PTC), clear cell variant of follicular thyroid carcinoma (FTC), papillary variant of medullary thyroid carcinoma (MTC), and so on. At the same time, “variant” is a commonly used genetic term, which means an alteration in the nucleotide sequence, and the genetic variant is increasingly being used in place of the term “mutation,” including for thyroid tumors. The new WHO classification uses “subtype” instead of “variant” in order to avoid confusion with genetic variants and to standardize the terminology across all the volumes of the fifth edition of the WHO classification. Therefore, the phrase “histologic variant” should be replaced with “histologic subtype.” When the term “variant” is used alone in the text of the WHO classification, readers should prioritize the genetic variant.
The term “Hürthle cell” is a historical misnomer [9]. In 1894, Karl Hürthle originally described the C cells of the thyroid gland as Hürthle cells in dogs. The new WHO classification has replaced “Hürthle cell” with “oncocytic cell.” As a result of this change, Hürthle cell adenoma and Hürthle cell carcinoma are now called oncocytic adenoma and oncocytic carcinoma (OCA), respectively.
For the designation of genes involved in gene fusions, the hyphen (-) or forward-slash (/) has been used as a separator between gene symbols, e.g., PAX8-PPARG (paired box 8-peroxisome proliferator-activated receptor gamma) or PAX8/PPARG. However, the separators should be replaced with a double colon (::) according to the HUGO Gene Nomenclature Committee recommendations [10], e.g., PAX8::PPARG.
New entities and reclassification
Before the fifth edition, the WHO classification of thyroid tumors included only neoplasms with morphology and behavior codes from the International Classification of Diseases for Oncology (ICD-O). Behavior codes of 0, 1, and 3 were assigned for benign tumors, borderline tumors (uncertain malignant potential), and malignancies, respectively. The 2022 WHO classification newly introduced tumor-like lesions for differential diagnosis purposes and convenience of the book readers (Table 1).
Developmental abnormalities include thyroglossal duct cyst, thyroid dysgenesis, and dyshormonogenetic goiter. The term “follicular nodular disease (FND)” was newly introduced to account for multifocal hyperplastic/neoplastic lesions occurring in the clinical setting of multinodular goiter. These lesions are not neoplastic and thus have no assigned ICD-O codes.
The new tumor type “high-grade follicular cell-derived carcinomas” has two histologic subtypes, traditional poorly differentiated thyroid carcinoma (PDTC) and a new subtype “differentiated high-grade thyroid carcinoma (DHGTC)” that arises from PTC, FTC, or OCA (Fig. 2). Another new diagnostic term is “thyroblastoma”; in particular, malignant teratoma or carcinosarcoma with Dicer 1, ribonuclease III (DICER1) mutation was renamed “thyroblastoma” (Table 1).

Decision tree for the differential diagnosis of follicular cell-derived neoplasms. The first step is to evaluate whether the tumor has nuclear features of papillary thyroid carcinoma (PTC). Tumors are then stratified according to the growth pattern, histologic differentiation, tumor capsular or vascular invasion, and high-grade histologic features. Y, yes; N, no; Q, questionable; FND, follicular nodular disease; FA, follicular adenoma; FA-P, follicular adenoma with papillary architecture; OA, oncocytic adenoma; FT-UMP, follicular tumor of uncertain malignant potential; IEFVPTC, invasive encapsulated follicular variant of papillary thyroid carcinoma; WDT-UMP, well-differentiated tumor of uncertain malignant potential; NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclear features; FTC, follicular thyroid carcinoma; OCA, oncocytic carcinoma of the thyroid; DHGTC, differentiated high-grade thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; ATC, anaplastic thyroid carcinoma.
Invasive encapsulated follicular variant of papillary thyroid carcinoma (IEFVPTC) is now considered a separate entity and no longer a subtype of PTC (Table 1, Fig. 2). IEFVPTC has a RAS-like mutational and transcriptomic profile similar to that of FA and FTC (Fig. 1), whereas classic PTC and the infiltrative follicular subtype of PTC have BRAFV600E-like molecular profiles [11-15]. IEFVPTCs have a fibrous capsule or well-defined border and lack the histologic features of infiltrative follicular PTC. Like FTC, IEFVPTC can invade vessels in the capsule and develop distant metastasis.
In the fourth edition of the WHO classification, cribriformmorular thyroid carcinoma (CMTC) was classified as a distinct variant/subtype of PTC. However, it is no longer considered PTC and is now listed as a cancer type among thyroid tumors of uncertain histogenesis.
Adoption of a grading system and high-grade thyroid carcinomas
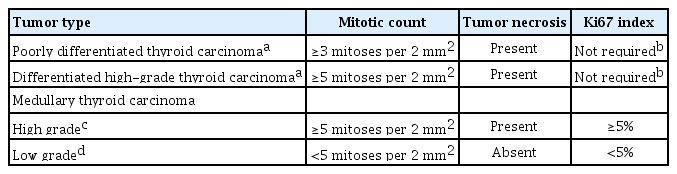
In differentiated follicular cell-derived carcinomas (PTC, FTC, and OCA) and MTC, high-grade histologic features include an increase in the mitotic activity and tumor necrosis (Fig. 2). Mitoses should be counted by expressing the number of mitoses per 2 mm2, which is roughly equivalent to 10 high-power fields (Fig. 3). The microscopic unit “high-power field” has been used for a long time. However, the high-power field is a non-standardized unit and should now be switched to square millimeters [16]. As the hotspot-counting method is recommended in thyroid pathology [17-19], the mitosis count should begin at the tumor region showing the highest mitotic activity.
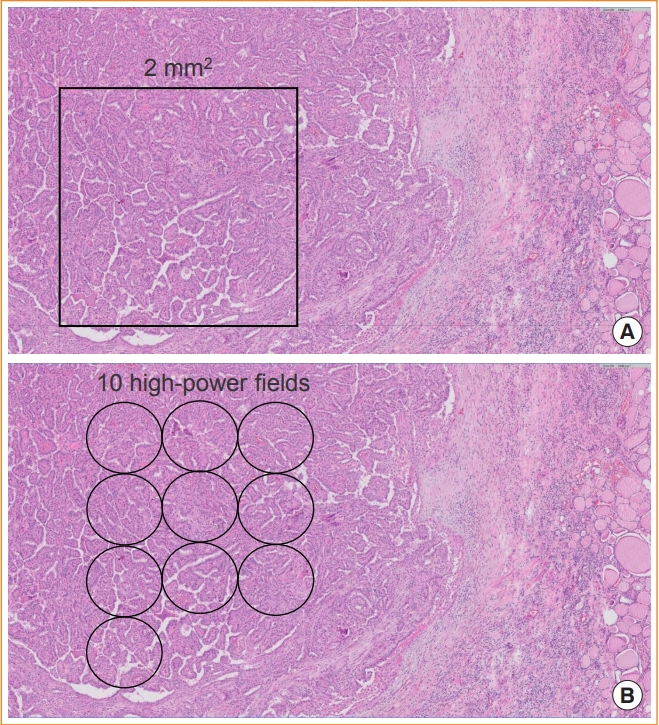
Counting mitoses in a hotspot. (A) The mitotic count is assessed by counting the number of tumor cells with mitosis per 2 mm2 in a hotspot (hematoxylin and eosin [H&E] stain, digital zoom ×10). (B) One high power-field of ×400 magnification using the ×40 objective lens and ×10 eyepiece has a field diameter of 0.49 to 0.53 mm in usual light microscopes (H&E stain, digital zoom ×10). Ten fields are approximately equivalent to 2 mm2.
DHGTC and PDTC are classified under the tumor type “follicular-derived carcinomas, high-grade” in the new 2022 WHO classification (Table 1). The new subtype DHGTC requires the presence of ≥5 mitoses per 2 mm2 and/or tumor necrosis (Table 3, Fig. 3). PTC, FTC, and OCA showing high-grade histologic features (mitotic count and tumor necrosis) are now classified as DHGTC (Fig. 2). The diagnostic criteria for PDTC have not changed. When an FTC has areas of solid or trabecular growth, it is important to examine the mitotic count and necrosis. When the mitotic count is ≥3 per 2 mm2 in solid or trabecular areas of the FTC, the tumor should be diagnosed as PDTC. When an FTC without solid or trabecular growth shows a mitotic count ≥ 5 per 2 mm2, the tumor should be diagnosed as DHGTC. Therefore, a prognostically relevant classification of thyroid carcinoma of follicular cells must take into account cellular differentiation and histologic grade (mitotic activity and/or necrosis).
The histologic grading scheme is newly applied to the diagnosis of high-grade MTC (Table 3). High-grade MTCs have at least one of the following three features: mitotic count ≥5 per 2 mm2, tumor necrosis, and/or Ki67 proliferation index ≥5% (Fig. 4). High-grade MTCs are associated with lower disease-specific survival and recurrence-free survival rates [19]. The Ki67 proliferative index is calculated as the percentage of positively stained tumor cells in a total of 500 to 2,000 tumor cells counted per tumor in hotspots [19]. Image analysis may assist in the accurate evaluation of the Ki67 index [18].

Increased mitotic activity and Ki67 index in high-grade thyroid cancers. Differentiated high-grade thyroid carcinomas can arise from hobnail papillary thyroid carcinoma (A, hematoxylin and eosin [H&E] stain, digital zoom ×100; B), tall cell papillary thyroid carcinoma (C, H&E stain, digital zoom ×100; D), and follicular thyroid carcinoma (E, H&E stain, digital zoom ×100; F). Immunohistochemical stains for Ki67 show proliferation index ≥5% (B, D, F; digital zoom ×40). High-grade medullary thyroid carcinoma shows mitotic count ≥5 per 2 mm2 (G, H&E stain, digital zoom ×100) and Ki67 proliferation index ≥5% (H, digital zoom ×40). Arrowheads indicate mitotic tumor cells.
Changes in benign follicular lesions
Follicular cell-derived neoplasms are divided into benign tumors, low-risk neoplasms, and malignant neoplasms. Benign follicular cell-derived tumors in the new WHO classification include thyroid FND (although not all of these lesions are true neoplasms), follicular adenoma, follicular adenoma with papillary architecture, and oncocytic adenomas.
Multifocal benign nodules are clinically known as multinodular goiter and generally considered hyperplastic and non-clonal lesions. However, some lesions are molecularly clonal (i.e., neoplastic) and morphologically look similar to adenoma. Histologic criteria cannot always differentiate hyperplastic from neoplastic lesions. Therefore, a new term (FND) was introduced for these lesions. Follicular nodules in FND have the characteristic histologic findings of nodular hyperplasia. FND can have encapsulated cellular nodules that morphologically look similar to follicular adenoma, and thus, they cannot be differentiated based on histologic features. Given the variety of historical terms behind the group entity of FND, the WHO classification allows the use of the following synonyms: adenomatous nodule, adenomatous hyperplasia, nodular hyperplasia, and multinodular goiter.
Follicular adenoma with papillary architecture is generally an autonomous hyperfunctioning nodule and shows a characteristic intrafollicular papillary growth. In the previous edition of the WHO classification, this neoplasm was classified as hyperfunctioning adenoma (so-called toxic adenoma) within the category of follicular adenoma [1]. Follicular adenoma with papillary architecture and conventional follicular adenoma are different in that follicular adenomas with papillary architecture harbor mutations in the thyroid stimulating hormone receptor (TSHR), GNAS complex locus (GNAS), or enhancer of zeste 1 polycomb repressive complex 2 subunit (EZH1) genes while follicular adenomas usually harbor RAS mutations [20]. Among follicular adenomas that have a growth pattern of papillary hyperplasia, a tumor not associated with hyperfunction is called as follicular adenoma with papillary hyperplasia, which is classified within the follicular adenoma. It is important not to mistake these tumors for PTC. The papillae seen in follicular adenomas are broad edematous cores and lack PTC-type nuclei.
Changes in low-risk follicular cell-derived neoplasms
According to the new histopathological classification, low-risk neoplasms include non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), follicular tumor of uncertain malignant potential, well-differentiated tumor of uncertain malignant potential, and hyalinizing trabecular tumor. These neoplasms are regarded as borderline or uncertain behavior with ICD-O code of 1.
There were no changes in the diagnostic histologic criteria of these neoplasms. Although there was debate about the percentage (0% vs. <1%) of true papillae in the diagnostic criteria of NIFTP [21-24], the 2022 WHO classification maintained the original criterion (<1% papillae). If non-invasive follicular-patterned tumors fulfill the histologic criteria of NIFTP but have increased mitotic activity, the diagnosis differs depending on the mitotic count: <3 mitoses/2 mm2 does not change the diagnosis of NIFTP; 3 to 4 mitoses per 2 mm2 allow upgrading to encapsulated PTC with predominant follicular growth; and ≥5 mitoses per 2 mm2 qualified as non-invasive high-grade follicular variant of PTC that is DHGTC. Although diagnostic molecular tests are not mandatory (considered as “desirable diagnostic criteria”), the detection of BRAFV600E or telomerase reverse transcriptase (TERT) promoter mutations should exclude the diagnosis of NIFTP.
In the previous edition of the WHO classification, subcentimeter NIFTP and oncocytic NIFTP were not defined. However, tumors ≤1 cm in size and oncocytic tumors fulfilling the histologic criteria of NIFTP are now considered subtypes of NIFTP in the 2022 WHO classification [25,26]. The oncocytic subtype should be composed of 75% or more oncocytic tumor cells [25].
Encapsulated follicular-derived thyroid cancers
Encapsulated follicular-derived thyroid cancers include FTC, OCA, and IEFVPTC (Table 3, Fig. 3). For prognostic risk stratification of patients, all these tumors should be classified into minimally invasive (those with capsular invasion only), encapsulated angioinvasive, and widely invasive (those with entirely obliterated or focally intact tumor capsule and/or gross invasion through the gland) subtypes (Fig. 5). Encapsulated angioinvasive tumors are further divided into those with limited (<4 foci) or extensive (4 or more foci) vascular invasion. Extensive vascular invasion is often seen in widely invasive FTC, but extensive vascular invasion alone is not sufficient to diagnose widely invasive FTC.
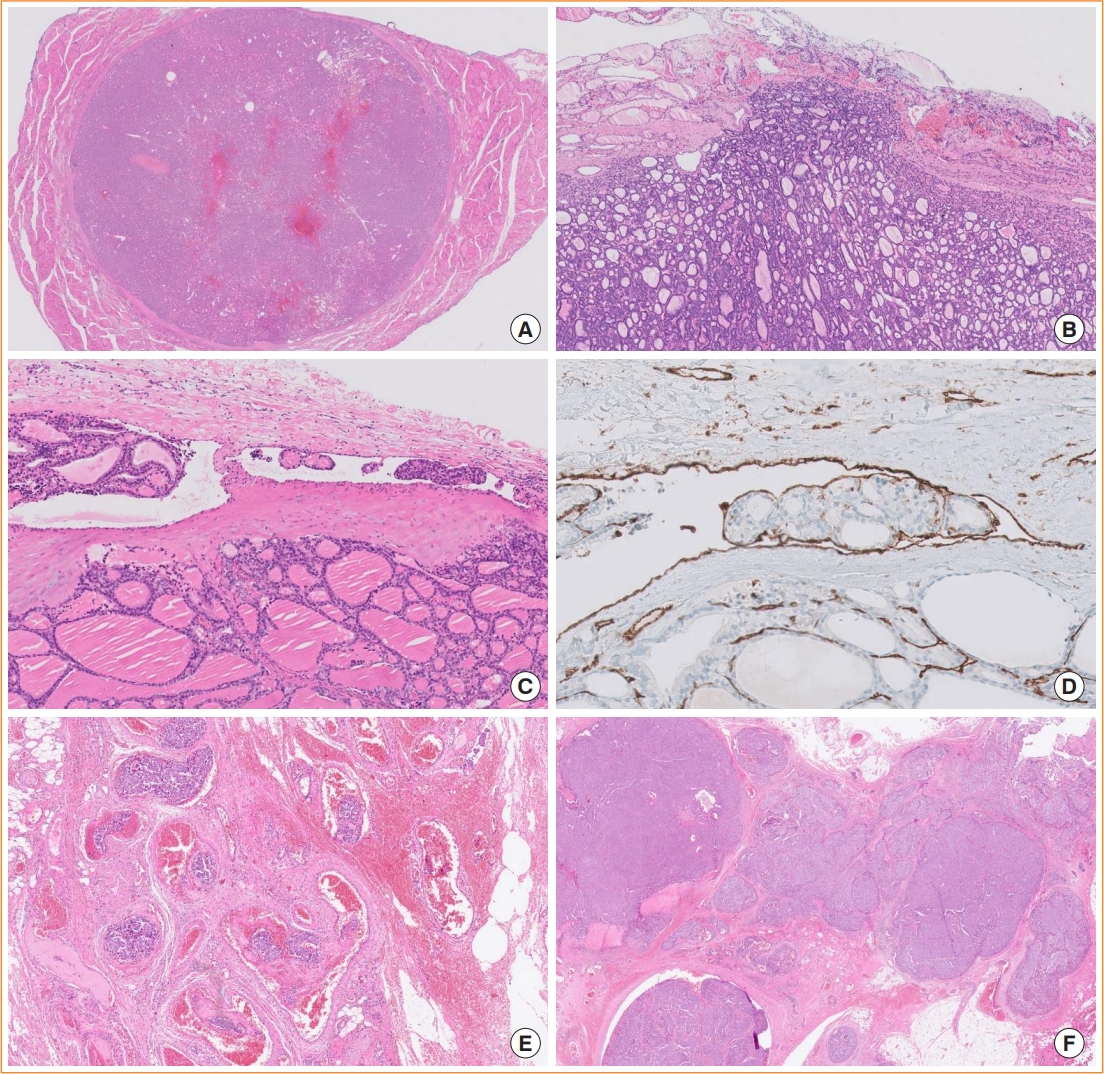
Subclassification of encapsulated follicular-derived thyroid tumors. Tumors with a non-invasive encapsulated follicular pattern include follicular adenoma and non-invasive follicular thyroid neoplasm with papillary-like nuclear features. (A) A follicular adenoma is shown (hematoxylin and eosin [H&E] stain, digital zoom ×1). (B) The minimally invasive subtype of follicular thyroid carcinoma has a tumor capsular invasion only (H&E stain, digital zoom ×10). (C) The encapsulated angioinvasive subtype is a cancer with vascular invasion regardless of capsular invasion status (H&E stain, digital zoom ×10). (D) CD31 immunostaining highlights endothelial-lined tumor emboli within the vessel (digital zoom ×40). (E) Extensive vascular invasion with ≥4 foci in extrathyroidal fibroadipose tissue is shown (H&E stain, digital zoom ×20). (F) The widely invasive subtype shows obliteration of the tumor capsule and invasion into extrathyroidal soft tissue (H&E stain, digital zoom ×10).
Changes in papillary thyroid carcinoma
PTC subtypes include classic, encapsulated classic, infiltrative follicular, diffuse sclerosing, solid/trabecular, tall cell, columnar cell, hobnail, clear cell, spindle cell, Warthin-like, oncocytic, and PTC with fibromatosis/fasciitis-like/desmoid-type stroma.
In the fourth edition of the WHO classification, the follicular variants (subtypes) of PTC included infiltrative, encapsulated with invasion, macrofollicular, and diffuse or multinodular follicular subtypes. A noninvasive form of encapsulated follicular PTC does not exist in the WHO classification because of the introduction of NIFTP. Infiltrative follicular PTCs have florid nuclear atypia of PTC, stromal fibrosis, and psammoma bodies, and often show lymphatic invasion and lymph node metastasis. These histologic features are different from those of IEFVPTC (Fig. 6). In the 2022 WHO classification, there is no mention of the macrofollicular and multinodular follicular subtypes.
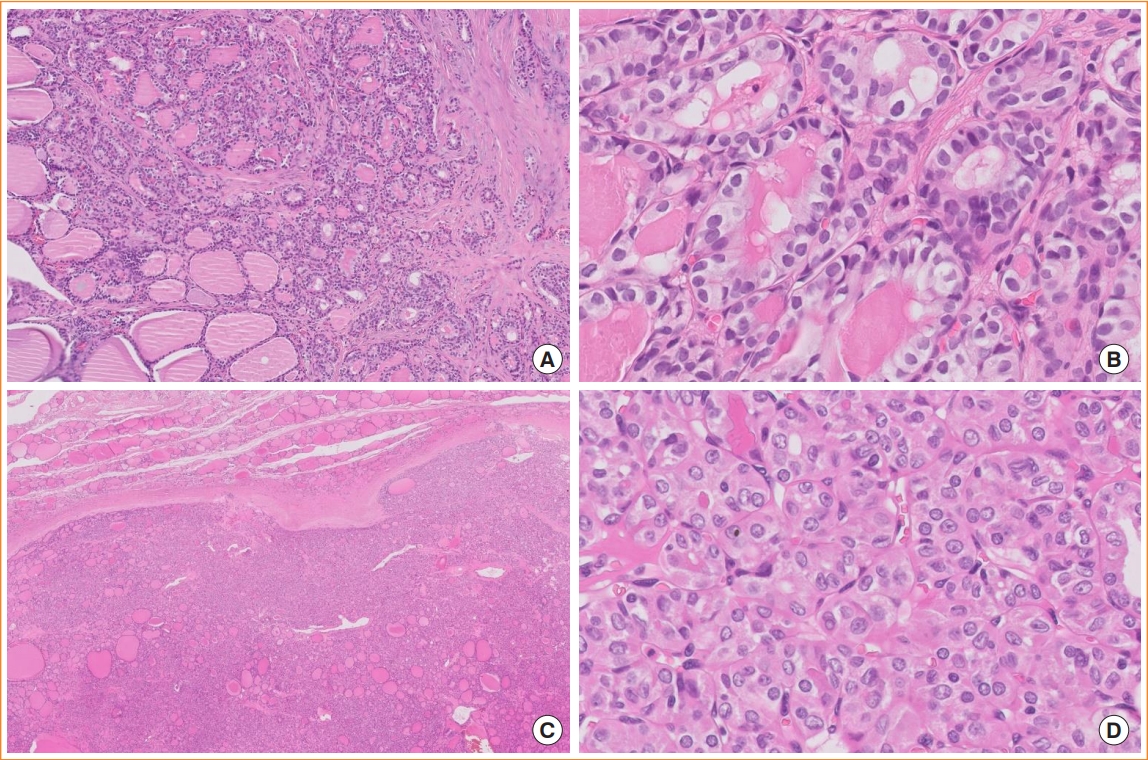
Follicular subtypes of papillary thyroid carcinoma (PTC). (A) Infiltrative follicular PTC shows an ill-defined infiltrative margin, follicular growth, and fibrotic stroma (digital zoom ×10). (B) Tumor cells have well-developed PTC nuclear features (digital zoom ×100). (C) Invasive encapsulated follicular variant PTC, which is now considered a separate entity and not a PTC subtype, invades through the fibrous capsule and shows a predominant follicular growth pattern (digital zoom ×10). (D) The nuclear features of invasive encapsulated follicular variant PTC are less developed than those of infiltrative follicular PTC; that is, the nuclei are rounder and more uniform (digital zoom ×100).
Among PTC subtypes, aggressive histologic subtypes include tall cell, columnar cell, and hobnail PTCs. The aggressive PTCs usually occur at an older age, are often associated with angiovascular invasion and advanced pathologic stage, and show increased mitotic activity. However, such aggressive PTCs with an indolent clinical course occur at a young age, are usually encapsulated or well circumscribed, and show a low mitotic activity and low Ki67 labeling index [27-31].
In the previous WHO classification, papillary thyroid microcarcinoma was a distinct subtype/variant of PTC. However, in the 2022 WHO classification, it is no longer considered a subtype because the microcarcinoma implies any histologic subtype of PTCs measuring ≤1 cm. Aggressive subtypes can occur as microcarcinoma. Therefore, subcentimeter PTCs require further histologic subtyping according to their histologic characteristics, such as classic papillary microcarcinoma, tall cell papillary microcarcinoma, and so on. The 2022 WHO classification reworded the term “encapsulated variant” into “encapsulated classic subtype” of PTC.
The criteria of tall cell PTC have been redefined. In the previous edition, tall cells were defined as cells that are two to three times as tall as they are wide. However, the newly adopted diagnostic criteria require that tall cells must have a height of at least three timestheir width and show dense eosinophilic cytoplasm and distinct cell membranes (Fig. 7A, B). The tall cell subtype should be composed of at least 30% of tumor cells qualified as tall. In a Japanese study, disease-free survival was shorter for tall cell PTC patients with a high Ki67 labeling index (≥5%) than in those with a lower Ki67 labeling index [27].

Tall cell and hobnail subtypes of papillary thyroid carcinoma (PTC) and encapsulated classic PTC with hobnail-like morphology. (A) Tall cell PTC shows an elongated and closely packed papillary pattern (hematoxylin and eosin [H&E] stain, digital zoom ×20). (B) The height of tumor cells is at least three times greater than their width (H&E stain, digital zoom ×100). Tumor cells have abundant eosinophilic cytoplasm and distinct cell membranes. (C) Hobnail PTC has papillary or micropapillary structures (H&E stain, digital zoom ×20). (D) Hobnail cells show enlarged hyperchromatic nuclei with reverse polarity (H&E stain, digital zoom ×100). (E) Encapsulated classic PTC shows cystic spaces and papillae lined with tumor cells having hobnail-like morphology (H&E stain, digital zoom ×20). (F) The papillary structure shows hyalinized and edematous stroma and cells with hobnailing cytomorphology (H&E stain, digital zoom ×100). The nuclear features look similar to those of classic PTC. These histologic findings are associated with ischemic and degenerative changes, and should not be diagnosed as hobnail PTC, which is an aggressive subtype.
Hobnail PTC shows a mixed papillary and micropapillary structure and dyscohesive tumor cells. Hobnail cells have more pronounced nuclear atypia than those seen in classic PTC (Fig. 7C, D). It is important for pathologists to recognize the histologic findings mimicking features of hobnail PTC. The histologic mimic “hobnail-like morphology” can be found in more than 30% of classic PTCs [28]. Encapsulated or well-circumscribed classic PTCs often show cystic changes and have edematous and hyalinized papillae lined by hobnailing tumor cells (Fig. 7E, F). The hobnail-like morphology is considered degenerative atypia and not a true hobnail subtype. Patients with PTC showing hobnail-like morphology are younger and have a lower rate of aggressive clinicopathologic features and a better prognosis than those with hobnail PTC [28]. Therefore, a diagnosis of hobnail PTC in the absence of aggressive clinical and pathologic features (high mitotic count, prominent extrathyroidal extension, etc.) should be rendered with confidence only after excluding low-risk PTC.
The tumor previously known as the “cribriform-morular variant of PTC” is no longer a PTC subtype. A new term, CMTC, is now listed among the tumors of uncertain histogenesis.
Changes in anaplastic thyroid carcinoma
Anaplastic thyroid carcinoma (ATC) may show variety of histologic patterns, including epithelioid cell, spindle cell, giant cell, pleomorphic cell, small cell, squamous cell, rhabdoid, angiomatoid, or paucicellular; however, the WHO classification has not defined subtypes of ATC based on the predominant pattern.
Primary squamous cell carcinoma of the thyroid, listed separately in the previous edition of the WHO tumor classification, is now considered a histologic pattern of ATC. ATC with the squamous cell carcinoma pattern has more frequent BRAFV600E mutation rate and PAX8 immunopositivity than other ATCs [32,33]. Squamous differentiation can be seen not only in ATC, but also in other types of thyroid tumors, including PTC, FTC, PDTC, MTC, mucoepidermoid carcinoma, and intrathyroidal thymic carcinoma [34]. In particular, the histopathologic features of intrathyroidal thymic carcinoma may mimic those of ATC with the squamous cell carcinoma pattern or metastatic squamous cell carcinoma. These tumors must be differentiated from intrathyroidal thymic carcinoma because the latter has better clinical outcomes, with a 10-year survival rate of more than 80% [35].
Thyroid carcinomas of salivary gland-type and uncertain histogenesis
One important change in the 2022 WHO classification is the addition of a new category, thyroid tumors of uncertain histogenesis, including sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) and CMTC. In the 2017 WHO classification, SMECE was considered a subtype of salivary gland-type carcinomas of the thyroid gland.
A category “salivary gland-type carcinomas of the thyroid” in the 2022 WHO classification includes mucoepidermoid carcinoma and secretory carcinoma previously known as mammary analogue secretory carcinoma.
Fusion genes are hallmarks of salivary gland-type carcinomas of the thyroid. Secretory carcinoma and mucoepidermoid carcinoma are characterized by specific fusion genes (ETV6:: NTRK3 [ETS variant transcription factor 6::neurotrophic receptor tyrosine kinase 3] and CRTC1 [CREB regulated transcription coactivator 1] or CRTC3::MAML2 [CREB regulated transcription coactivator 3::mastermind like transcriptional coactivator 2], respectively) [36,37]. However, these gene fusions are typically not found in SMECE [38-40]. SMECE is morphologically different from mucoepidermoid carcinoma of the thyroid, although epidermoid and mucous cells are observed in both tumor types. SMECE is always associated with chronic lymphocytic thyroiditis showing a marked sclerotic background and infiltration of lymphocytes and eosinophils. The lack of common mutations found in differentiated thyroid tumors (BRAFV600E, RAS, and other mutations or gene fusions) further supports the proposal that SMECE is distinct in origin from salivary glandtype carcinoma and follicular cell or C cell-derived carcinomas [38]. Both solid cell nests and SMECE share immunophenotypic characteristics (p63-positive and thyroglobulin-negative) as shown in Table 4. SMECEs are therefore presumed to arise from solid cell nests (ultimobranchial body remnants) [38,40-42]. Genetic alterations found in some cases of SMECE include MET hyperploidy and mutations in the APC regulator of WNT signaling pathway (APC), neurotrophic receptor tyrosine kinase 3 (NTRK3), and neurofibromin 1 (NF1) genes [38,40,43].
CMTC was classified as a distinct subtype of PTC in the previous WHO classification because its papillary pattern and nuclear features are similar to those of classic PTC. However, CMTC has a different molecular profile from that of PTC. CMTC has genetic alterations involved in the Wnt/beta-catenin pathway, such as mutations in the APC and CTNNB1 (catenin beta 1) genes [44,45]. There have been no cases of CMTC with BRAFV600E. In terms of immunohistochemistry, tumor cells show nuclear expression of beta-catenin and express estrogen receptor and progesterone receptor [44,46,47]. These tumors have no colloid formation and are often negative for markers of thyroid follicular cell differentiation, such as thyroglobulin and PAX8 [45]. The cribriform component shows thyroid transcription factor 1 (TTF1) positivity, but morulae are negative.
Other tumors
Changes pertinent to PDTC and MTC have been described in the section “adoption of a grading system and high-grade thyroid carcinomas” above. Thymic tumors arising within the thyroid gland include thymoma, intrathyroid thymic carcinoma, and spindle epithelial tumor with thymus-like elements (SETTLE). There are no changes in the diagnostic criteria of these tumors. Thyroblastoma is an exceptionally rare embryonal high-grade thyroid neoplasm composed of primitive follicular cells, small cells, and mesenchymal stroma. The most common genetic alterations found in thyroblastoma are somatic DICER1 mutations [48,49]. Thyroblastoma is a new term for malignant teratoma and carcinosarcoma associated with DICER1 mutations in the thyroid gland [48-50].
REMARKS FOR CLINICIANS
For clinicians, the most important change in the 2022 WHO classification is the introduction of the two-tiered grading system, allowing the distinction of high-grade cancers from well-differentiated follicular cell-derived carcinomas and MTC. DHGTCs exhibit more advanced histopathologic stages and lower radioactive iodine avidity than well-differentiated thyroid cancers (PTC, FTC, and OCA) [51-53]. The overall prognosis of DHGTC is similar to that of PDTC [52]. High-grade MTCs occur in approximately 25% of cases and have worse prognosis than low-grade MTC [19]. Therefore, patients with DHGTC and high-grade MTC require more intense treatments and should be more closely followed up for recurrence.
Clinicians and pathologists need to be aware of new terminologies and classification schemes. The updated WHO classification and nomenclature will have a further impact on the upcoming releases of clinical guidelines, cancer staging protocols, thyroid histopathology reporting standards, and cytology reporting systems.
CONCLUSIONS
Recent advances in the molecular pathogenesis of thyroid tumors have improved our understanding of tumor origin and evolution and impacted their diagnostic criteria. Thyroid tumors are now classified based on the tumor cell of origin and molecular profile. The concept of low-risk neoplasm and histologybased grading systems help guide personalized therapeutic decisions for patients at different levels of risk. These changes are continuing, and new updates in the WHO classification of endocrine neoplasms are expected in the next 5 years.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a grant (NRF-2020R1F1A1070028) from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT. This research was also supported by a grant (HI21C0940) from the Korean Health Technology R&D Project, Ministry of Health &Welfare, Republic of Korea.