Interplay of Vitamin D and CYP3A4 Polymorphisms in Endocrine Disorders and Cancer
Article information
Abstract
Vitamin D has received considerable optimistic attention as a potentially important factor in many pathological states over the past few decades. However, the proportion of the active form of vitamin D metabolites responsible for biological activity is highly questionable in disease states due to flexible alterations in the enzymes responsible for their metabolism. For instance, CYP3A4 plays a crucial role in the biotransformation of vitamin D and other drug substances. Food-drug and/or drug-drug interactions, the disease state, genetic polymorphism, age, sex, diet, and environmental factors all influence CYP3A4 activity. Genetic polymorphisms in CYP450-encoding genes have received considerable attention in the past few decades due to their extensive impact on the pharmacokinetic and dynamic properties of drugs and endogenous substances. In this review, we focused on CYP3A4 polymorphisms and their interplay with vitamin D metabolism and summarized the role of vitamin D in calcium homeostasis, bone diseases, diabetes, cancer, other diseases, and drug substances. We also reviewed clinical observations pertaining to CYP3A4 polymorphisms among the aforementioned disease conditions. In addition, we highlighted the future perspectives of studying the pharmacogenetics of CYP3A4, which may have potential clinical significance for developing novel diagnostic genetic markers that will ascertain disease risk and progression.
INTRODUCTION
Vitamin D overview
Vitamin D is classified as a hormone due to its unique ability to be synthesized endogenously in addition to exogenous supplementation as a vitamin from a wide variety of sources, including portabella mushrooms, fish, eggs, liver, and fortified foods. The physiological role of vitamin D in the maintenance of the kinetic and dynamic activities of calcium and its ultimate effects on bone tissue have been commonly discussed. However, the last few decades have witnessed an exponential rise in studies exploring the role of vitamin D in various diseases such as bone diseases, metabolic diseases/disorders, cardiovascular disease, cancer, immune diseases, and depression, underscoring the multidimensional landscape and importance of vitamin D in relation to health [1].
Globally, around 1 billion people are estimated to have vitamin D deficiency, whereas vitamin D insufficiency has been observed among half of the population [2]. The occurrence of vitamin D deficiency has increased. People with vitamin D insufficiency (i.e., serum 25-OH-D3 levels of 21 to 29 ng/mL) are generally asymptomatic, whereas in more advanced stages of deficiency (i.e., serum 25-OH-D3 levels at <20 ng/mL), detrimental effects may occur, including hypocalcemia and hyperthyroidism, which promote bone-related diseases (e.g., osteoporosis, recurrent fractures, and bone fragility), especially in the elderly [1-3]. The predisposing factors of vitamin D deficiency, along with altered vitamin D levels, are inadequate sun exposure and dietary intake. Recently, it has been recognized that clinical interactions could also alter serum 25-OH-D3 levels [4]. Studies over the past few years have primarily focused on the genetic inheritance of cytochrome P450 (CYP) enzymes and their effects on vitamin D metabolism [5]. In this context, the current review focused on the linkage between vitamin D levels and polymorphisms in its metabolizing enzyme CYP3A4, as well as probable future perspectives to develop putative targets and diagnostic genetic markers for vitamin D deficiency, bone diseases, cancer, and other conditions.
Vitamin D bioconversion
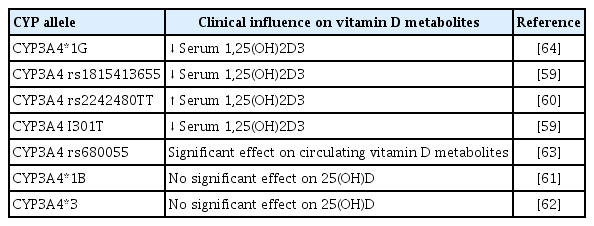
The bioconversion of vitamin D occurs at different sites of the body, and the skin, kidney, and liver play a major role in this process. In the following sections, we briefly describe the various steps of vitamin D metabolism (Fig. 1).
Metabolism of vitamin D. The biotransformation of vitamin D3 (from ultraviolet irradiation) and vitamin D2 (from food sources) is carried out by cytochrome P450 (CYP) enzymes. The active 1,25(OH)2D3 formed from various metabolic conversions, binds to the vitamin D receptors, followed by retinoid X receptor (RXR) dimerization, which results in the formation of vitamin D responsive genes eventuating the respective physiological activities. Furthermore, the hormonally active 1,25(OH)2D3 is maintained by the negative/positive feedback mechanism of parathyroid hormone (PTH) [6-11]. UVB, ultraviolet B; DBP, vitamin D binding proteins; VDR, vitamin D receptor; VDRE, vitamin D responsive elements; FGF, fibroblast growth factor; CV, cardiovascular.
Skin
Vitamin D exists in two different forms: exogenously as vitamin D2, which is obtained from various sources like fish, cereals, and mushrooms, and endogenously, as vitamin D3 from ultraviolet B (UVB) light. The endogenous bioconversion of 7-dehydrocholesterol into vitamin D3 in the presence of UVB light occurs underneath the skin. Thereafter, vitamin D3 enters the circulation, binds to vitamin D binding protein (DBP), and reaches the liver for further metabolic conversions [6].
Liver
The liver is the major connecting site for many metabolic enzymes that catalyze various metabolic cascades. The metabolism of vitamin D occurs through the hepatic-renal pathway by a mixed-function oxidase system (CYP) [7]. Specifically, a group of 25-hydroxylases, including CYP2R1, CYP27A1, CYP3A4, and CYP2D25, is primarily found in the liver; these enzymes can induce the hydroxylation step of vitamin D3 [7,8]. Among these, the major enzymes that mediate the hydroxylated conversion of vitamin D3 into its 25-OH-D3 intermediate are CYP27A1 and CYP2R1, which are expressed to a greater extent in mitochondrial and microsomal sites of the liver [9]. Previous studies also revealed the expression of CYP27A1 in macrophages and dendritic cells, where it promotes the hydroxylation of vitamin D3 and promotes immune defense mechanisms [10].
Kidney
The intermediate 25-OH-D3 is available abundantly in the blood circulation, and 90% of 25-OH-D3 is bound to DBP [11]. The 25-OH-D3 and DBP complex is recognized and filtered by cell surface receptors of the proximal tubule of the kidney. The filtered intermediate 25-OH-D3 is then converted by 1α-hydroxylase (CYP27B1) into the physiologically active form 1,25-(OH)2D3. With the help of DBP, the active metabolite 1,25-(OH)2D3 reaches the target tissues, binds to the vitamin D receptor (VDR), and regulates the corresponding physiological genes [12]. Other renal hydroxylases, such as CYP24A1, serve as a local control mechanism for blood 1,25-(OH)2D3 levels. CYP24A1 counteracts vitamin D toxicity by promoting the catabolism of 1,25-(OH)2D3 into inactive metabolites, which are excreted as calcitroic acid in the urine and feces [11].
Role of CYP3A4 in the inactivation of vitamin D
CYP3A4 is a multifunctional microsomal enzyme abundantly found in the liver that participates in the xenobiotic transformation of many drugs and endogenous substances. The dual action of CYP3A4 promotes 24-hydroxylation and 25-hydroxylation of vitamin D3 and D2 [4]. CYP3A4 participates in the tissue-specific conversion of vitamin D metabolites into their respective inactive metabolites, such as 4β,25-OH-D3. It also promotes 23R- and 24S-mediated conversion of 1α,25(OH)2D3 into inactive 1α,23R,25(OH)2D3 and 1α,24S,25(OH)2D3. Therefore, studies focusing on CYP3A4 in connection with vitamin D metabolism have been extensively conducted [13,14]. Although vitamin D is inactivated by other extrarenal hydroxylases, such as CYP24A1, it is primarily biotransformed by CYP3A4. Therefore, any induction or inhibitory effect on CYP3A4 may lead to altered vitamin D levels. These changes may occur due to factors such as drugs that are substrate inhibitors or inducers of CYP3A4 enzyme or due to genetic polymorphisms of CYP3A4 gene which will alter the activity of CYP3A4 enzyme activity [15]. Collectively, studies have focused on the effects of genetic polymorphisms, drugs, herbal extracts, and natural flavonoids that may have the potential to alter CYP3A4 enzyme activity. However, the impact of altered enzyme activity due to genetic polymorphisms in the CYP3A4 gene on vitamin D levels in relation to bone-related diseases such as osteoporosis, osteomalacia, and other disease conditions needs to be investigated further, as this may provide valuable insights into developing potential therapeutic strategies [16].
Clinical relevance of CYP3A4 enzymatic studies
CYP3A4 is the major class of enzyme that catalyzes the metabolism of 75% of therapeutic agents; thus, it has been broadly studied in various pathological conditions. Research has revealed that a combination of CYP3A4 inhibitors, such as clarithromycin, with FMS-like tyrosine kinase 3 inhibitors (FLT3 TKIs) led to functional improvement in the activity of FLT3 TKIs [17]. In contrast, a research study revealed that prolonged treatment of a few therapeutic drugs such as phenobarbital, hyperforin, carbamazepine, and rifampin showed an inductive effect only on CYP3A4, but not on other CYP enzymes such as CYP24A1 or CYP27B1. This type of interaction has become a notable factor in the etiology of drug-induced osteomalacia and/or decreased bone mineral density (BMD) caused by the CYP3A4-mediated inactivation of vitamin D metabolites, followed by rapid clearance [18,19].
In this context, the pleiotropic effects of CYP3A4 on various physiological conditions, especially vitamin D deficiency, need further attention [19]. Experimental studies have also stated that drugs and/or endogenous substances that are substrates of CYP3A4 may or may not have an impact (inhibitory/inducible) on active vitamin D (1,25-(OH)2D3), but impacts on active vitamin D levels can be achieved with a defined substrate concentration. For instance, tacrolimus acts as a substrate of CYP3A4 at a normal dose and does not induce/inhibit CYP3A4, but it was found to show an inhibitory effect on CYP3A4 at higher concentrations and exhibited an impact on vitamin D metabolism [20].
In this regard, the dose-dependent induction/inhibition achieved by CYP3A4 substrate inhibitors/inducers may sometimes exert beneficial as well as non-beneficial effects on various clinical conditions. Hence, vitamin D levels can be altered based on the concentration of the substrate, substrate specificity, site-specificity, and dosage regimens for developing novel therapeutic strategies focusing on CYP3A4 [21].
VITAMIN D AND ITS PHYSIOLOGICAL ROLE
Vitamin D has been broadly studied in various physiological conditions, as discussed below.
Calcium homeostasis
Calcium is a vital mineral that plays an imperative role in many physiological processes such as skeletal homeostasis, skeletal muscle contraction, neuronal impulse transmission, blood coagulation cascade, cardiac regulation, fluid, and electrolyte homeostasis in the cells, managing secondary messenger systems, hormonal and neurotransmitter release pathways [22]. Calcium transmission occurs via transcellular (active) or paracellular (passive) transport. Paracellular transport occurs as a result of differences in the electrochemical gradient, whereas vitamin D plays a role in transient receptor potential cation channel subfamily V member 5/6 (TRPV5/6)-mediated transcellular (active) transport [23,24].
Endocranially, the togetherness of vitamin D and calcium plays an essential role in maintaining skeletal homeostasis. The effects of vitamin D on calcium have been carefully explored, and it is generally understood that maintaining calcium homeostasis requires optimal levels of vitamin D. Overall, hormones such as parathyroid hormone (PTH) maintain calcium homeostasis in the gut, bone, and kidney through a negative feedback process that stimulates fibroblast growth factor, calcitonin, and vitamin D [25,26]. Any genetic mutation pertaining to PTH would alter the activity of vitamin D, which may also result in genetic hypercalcemia [27]. Similarly, genetic polymorphisms in vitamin D-related metabolic enzymes, such as CYP3A4, may have a meaningful impact on circulating vitamin D levels and thereafter on calcium homeostasis, which in turn plays a yet-to-be-determined role in bone health and the immune system [28,29].
Furthermore, clinical studies have revealed that supplementation of calcium and vitamin D in normal individuals without any pathological conditions cannot prevent future vitamin D-related complications and seems to be inappropriate, as it may lead to various consequences, such as the formation of kidney stones and bloating. Instead, supplementation shows an appropriate physiological effect only in individuals with vitamin D deficiency [30]. Another randomized controlled trial among children revealed the combination of vitamin D and limestone, a rich source of calcium, showed a beneficial effect in children with calcium-deficient rickets [31].
Skeletal homeostasis
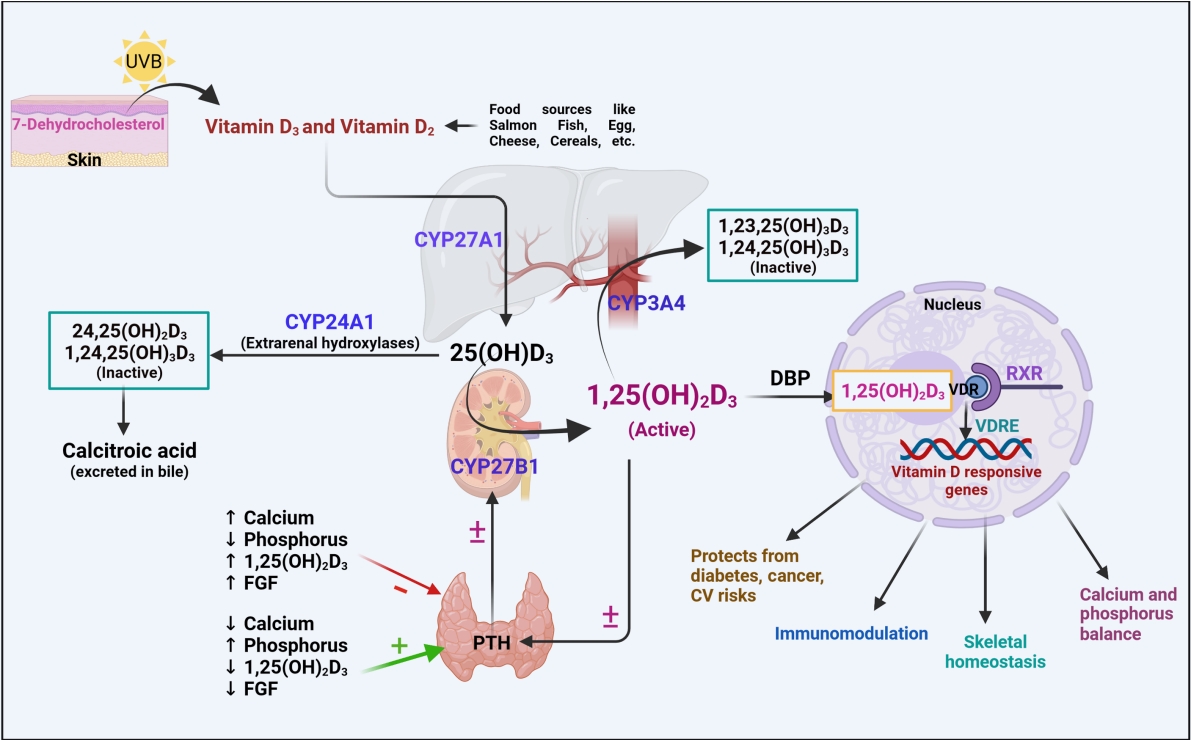
Bone is the only organ that undergoes continuous formation and simultaneous resorption [32]. The factors that are involved in the formation of high-mass bones are vitamin D, calcium, phosphorus, magnesium, and other micro-nutrients. The imbalance between bone resorption and bone formation leads to bone-related disorders [33]. Osteoblasts (bone-forming cells), osteoclasts (bone resorption cells), and osteocytes (mature osteoblasts inside the bone) are the three main cell types responsible for bone homeostasis [32]. In general, the dynamic effects of active vitamin D are mediated by the transcriptional activity of VDR, a nuclear receptor, which leads to dimerization of the retinoid X receptor (RXR) and promotes the respective genomic activities (bone homeostasis) (Fig. 2) [34]. VDR has diverse expression in various cellular sites, including bone formation cells, which promotes the proliferation of proteins such as collagen, alkaline phosphatase, and osteoclastin. In addition, vitamin D has a positive regulatory effect on RANKL, promotes bone resorption cells (osteoclasts) for rapid removal of the calcified matrix, and maintains homeostasis [35]. In vitro findings revealed that 1,25(OH)2D3 administered to cultured osteoblasts showed positive and negative regulation on osteoblastic markers. This demonstrates the modulatory and pleiotropic effect of 1,25(OH)2D3 on osteoblastic cells during growth and maturation [36]. In vivo findings revealed that VDR mutant mice showed a definite impact on calcium availability and imbalances in skeletal formation, which are recognized as a major risk factor for the onset of rickets [37,38]. In this context, the variable mutations or polymorphisms in VDR and RANKL have been widely studied to understand the development of bone-related diseases such as osteoporosis.
Vitamin D and its effects on calcium and skeletal homeostasis, focusing on the role of 1,25(OH)2D3. Calcium from the food sources is absorbed by transcellular transport by transient receptor potential cation channel subfamily V member 5/6 (TRPV5/6) channels into enterocytes, where it forms a calcium-calbindin complex. The formed calcium complex enters the blood circulation via paracellular and/or transcellular transport and is used for a wide range of physiological processes. Based on the increased or decreased levels of calcium, parathyroid hormone (PTH)/fibroblast growth factor-23 (FGF23) shows the following activities: stimulating or inhibiting 1,25(OH)2D3, which further promotes vitamin D receptor (VDR)-retinoid X receptor (RXR) dimerization leads to the expression of vitamin D-responsive genes, eliciting effects on calcium transport channels and maintaining an optimum calcium concentration in enterocytes as depicted; balancing bone resorption and calcification; and promoting the CYP27B1 enzyme, which eventually increases the formation of 1,25(OH)2D3 in the kidney [24,25,28,35,36]. ↑ indicates increase; ↓ indicates decrease. NCX1, sodium-calcium exchanger-1; PCMA, plasma membrane Ca2+ ATPase; RANKL, receptor activator of nuclear factor kappa-Β ligand.
Furthermore, the supplementation of vitamin D has shown a prominent effect on improving skeletal health. In contrast, recent randomized clinical trials have revealed that individuals aged 55 to 70 years showed low BMD when treated with high-dose of vitamin D (10,000 IU/day) for 3 years in comparison with the optimal dosage of 400 IU/day, supporting the fact that the physiological effects of vitamin D vary with dosage. Contrarily, studies on healthy middle-aged men who received 20,000 IU/day for 12 weeks found no significant effect on BMD [39]. Many natural products have shown a profound positive impact on vitamin D levels. However, further studies need to be focused on dose-dependent safety and efficacy of vitamin D.
Immunomodulation
The mammalian immune system plays a major role in defense against various infectious diseases and plays a crucial role in developing immune-mediated diseases/disorders. Vitamin D plays a notable function in managing both the innate and adaptive immune systems [40]. Studies have demonstrated that 1,25(OH)2D3 is influential on the adaptive immune system, as VDR is expressed in almost all cell types, such as T-cells, B-cells, macrophages, monocytes, and dendritic cells (antigen-presenting cells) [41]. Deficient vitamin D levels are eventually observed in most acquired immune-mediated diseases. However, vitamin D has shown unfavorable effects in systemic lupus erythematosus in connection with B-cells [42]. Evidence has also revealed that active vitamin D promotes T-cell activation and facilitates the production of inflammatory cytokines, which strengthen the autocrine functions of vitamin D [43].
The significance of vitamin D in immune cell generation and proliferation, as well as the link between active vitamin D and other antigen-presenting cells, has been addressed [44]. However, systemic studies on insufficient vitamin D levels need to be further conducted, since vitamin D metabolic enzymes are also expressed in immune cells. Some pattern recognition receptors (PRRs), a type of toll-like receptors (TLRs), were found to be engaged in the initiation of an innate immune response in the recognition of pathogens followed by immune defense through intracrine activation of vitamin D [45]. To date, evidence has revealed the interlink between vitamin D metabolizing enzymes (CYP27B1) and VDR in innate immune cells such as macrophages and neutrophils [44,45]. Furthermore, vitamin D has been identified as playing a prominent role in the induction of the immune response via TLRs by the cathelicidin antimicrobial peptide (CAMP), nucleotide-binding oligomerization domain-containing protein 2 (NOD2), hepcidin antimicrobial protein (HAMP), and β-defensin 4 (DEFB4) genes, which play a protective role in various infective diseases [46]. The clinical examination of monocyte-derived dendritic cells from individuals treated with a high dose of vitamin D revealed a lower susceptibility towards dengue virus 2 (DENV-2) infection than those who received a low dose; this effect was mediated by strengthening the immune system via TLR expression [47,48]. This finding implies that supplementation with a sufficient amount of vitamin D promotes immunity. However, further studies focusing on the disease progression and fixation of dosage regimens are needed.
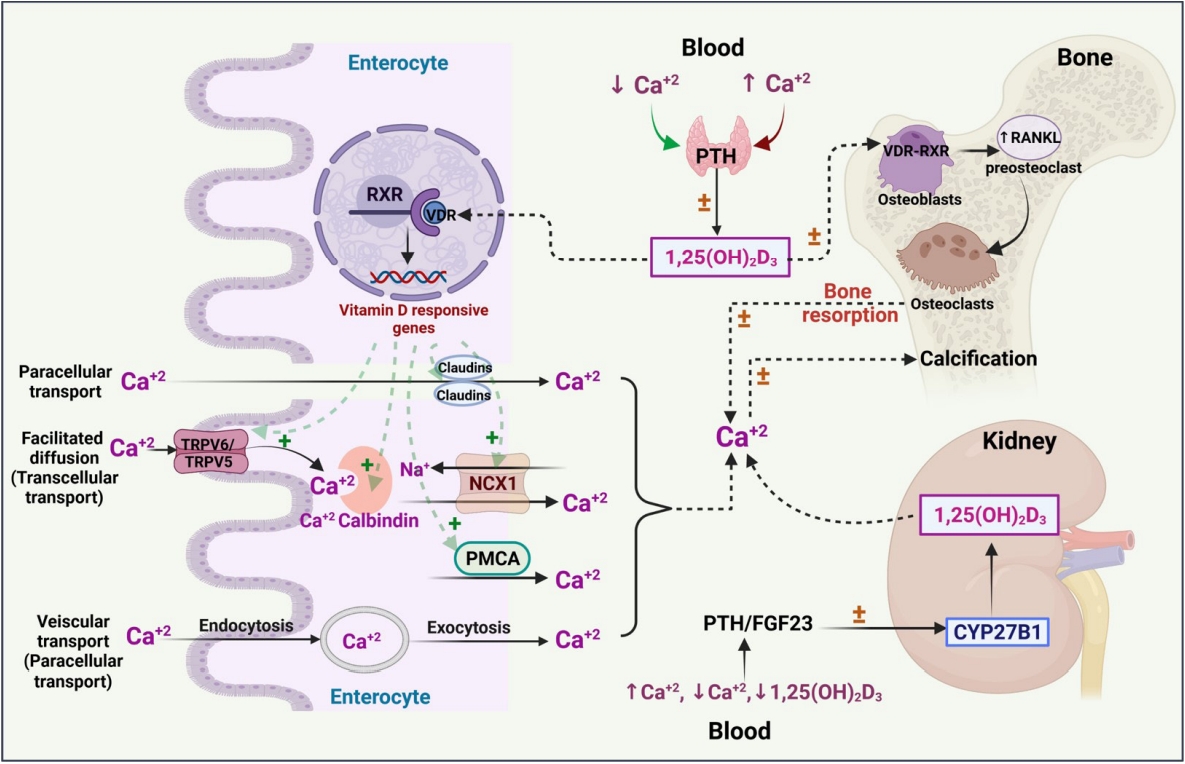
The molecular interplay between vitamin D and other PRRs, such as RIG-I-like receptors (RLRs), Nod-like receptors (NLRs), AIM2-like receptors (ALRs), C-type lectin receptors (CLRs), and intracellular DNA sensors such as cyclic GMP-AMP synthase (cGAS), needs to be studied in various pathological conditions to broaden the use of vitamin D supplementation and its therapeutic value (Fig. 3).
Physiological activities of hormonally active vitamin D. (A) The role of vitamin D in innate immunity. The entry of pathogens by pattern recognition receptors (PRRs) promotes the induction of vitamin D receptor (VDR) and forms 1,25(OH)2D3. The active form of vitamin D (1,25(OH)2D3) stimulates the expression of various genes, including cathelicidin antimicrobial peptide (CAMP), nucleotide-binding oligomerization domain-containing protein 2 (NOD2), hepcidin antimicrobial protein (HAMP), and β-defensin 4 (DEFB4), which aid in pathogen killing. It also blocks pathogen entry by inhibiting toll-like receptors 2/4 (TLR2/4), regulates the tight junctions of epithelial cells, promotes various activities (e.g., generation of reactive oxygen species [ROS], chemotaxis, and phagocytosis), and inhibits interleukins and maturation of dendritic cell migration/maturation, thereby controlling inflammation and the development of autoimmunity [42,47]. (B) The development of adaptive immunity by activating B-cells and regulating the activities of different kinds of T-cells [42,47]. (C) The physiological effects of 1,25(OH)2D3 on cancerous cells, pancreatic β-cells, and the cardiovascular system. 1,25(OH)2D3 also inhibits the imbalance of angiotensin-converting enzyme-II (ACE-II) and angiotensin-II (Ang-II) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which prevents pulmonary vasoconstriction and also improves the ability of the immune system to fight against the virus [81]. VDRE, vitamin D responsive elements; IL, interleukin; TGFβ, transforming growth factor beta; CCR10, chemokine receptor; Treg, regulatory T cells; TH, helper T cells; CVD, cardiovascular disease.
Cancer
Vitamin D has notable clinical implications for more than 10 different kinds of cancers—most notably, the most prevalent cancers such as breast, prostate, and colorectal cancers, and to a smaller extent for other types of cancer [49]. First, the in vitro tumor suppression by the active metabolites of vitamin D3 was said found to be dose-dependent and revealed that the presence of related receptors on tumor cells manifested physiological effects [50]. The aggregated data from recent reviews and meta-analyses revealed that vitamin D affects all stages of tumor development [51].
Vitamin D has also been found to play a role in cell proliferation by acting on p21 and p27 gene expression, promoting apoptosis and autophagy, regulating angiogenesis, stimulating antioxidants, and exhibiting anti-inflammatory properties through attenuated gene expression of multiple inflammatory mediators (interleukin [IL]-12, IL-2, tumor necrosis factor-alpha, and interferon gamma), as well as regulating various inflammatory pathways such as (nuclear factor kappa-light-chain-enhancer of activated B cells [NF-κB], mitogen-activated protein kinase [MAPK], and cyclooxygenase-2 [COX-2] signaling) [49]. These findings collectively emphasize the effect of 1,25(OH)2D3 on malignancies [49,52,53]. Moreover, vitamin D enhanced the chemotherapeutic properties of anticancer drugs like irinotecan, which is highly metabolized by CYP3A4 [54]. Vitamin D promotes miR-627 expression, which eventually controls the CYP3A4-mediated metabolic conversion of irinotecan, escalating its antitumor effects. However, an in vitro study on human liver microsomes also revealed the ability of CYP3A4 to catalyze other active forms of vitamin D and forms 20(OH)D3 and other respective metabolites, which may also have a physiological effect on cancer cell proliferation (Fig. 3) [54].
THE CYP3A4 GENE AND ITS POLYMORPHISMS
CYP metabolizing enzymes are widely recognized for their key role in the metabolism of many drugs as well as endogenous substances. Among all the variant types of CYP enzymes, research studies on human recombinant microsomal enzymes showed that CYP3A4 is highly abundant in the human liver and intestine, and has also been identified in the prostate, breast, gut, and colon. CYP3A4 accounts for 30% of CYP enzymes with septuplet dominance in hydroxylase activity [55].
The CYP3A family mediates the biotransformation activity, which is usually accomplished via the activation of pregnane X receptor (PXR), constitutive androstane receptor (CAR), glucocorticoid receptor (GR), and VDR. However, the gene expression of CYP3A4 is particularly induced by the translocation of PXR from the cytosol to the nucleus, where it undergoes heterodimerization with RXR. In vivo research studies on transgenic PXR mice also revealed the crucial role of PXR in CYP3A4 gene induction and function [56]. To date, 6,574 single-nucleotide polymorphisms (SNPs) have been found in human CYP3A4, as reported in the National Center for Biotechnology Information (NCBI) SNP database (http://www.ncbi.nlm.nih.gov/snp).
In the last few decades, genetic and environmental factors have been identified as playing a prominent role in disease progression [57]. Most importantly, CYP enzymes have shown greater interindividual variability, leading to therapeutic failure and disease progression (Table 1). Researchers have expressed optimism regarding genetic factors, such as polymorphisms, due to their ability to alter the activity of enzymatic genes, which in turn are responsible for various metabolic activities pathways [58]. Genetic variability refers to differences in the metabolic rate of enzymes among individuals, and it has been established that many of the CYP enzymes, including CYP3A4, are highly polymorphic [56]. Hence, particular attention should be paid to focusing on metabolic discrimination among variant individuals in various disease conditions. Furthermore, the effect of the loss or gain of function in CYP3A4 polymorphic alleles (labeled “CYPs” in https://www.pharmvar.org/) and their impact on corresponding drug substrates need to be monitored (Table 1) [59-64].
CYP3A4 POLYMORPHISMS IN VARIOUS PATHOLOGICAL CONDITIONS: CLINICAL PERSPECTIVES
Vitamin D deficiency/insufficiency and calcium homeostasis
Vitamin D is well known for its activity as a steroid, and its deficiency and insufficiency have become major global concerns in recent years [65]. Vitamin D deficiency results in the progression of various pathologies, including vitamin D-dependent rickets and osteoporosis, which lead to social disability and discomfort. Genetic factors have recently been recognized as a major cause of many diseases, including vitamin D deficiency [66].
Recent research revealed that patients with rickets had a decline in serum vitamin D metabolites due to CYP3A4 mutations; in particular, the CYP3A4 I301T mutation showed greater activity for the inactivation of vitamin D metabolites, reducing active vitamin D levels [67]. As a result, it was demonstrated that indiscriminate changes in CYP3A4 can lead to worsening pathological conditions. Likewise, an oligogenic study on healthy female Koreans revealed that subjects with the CYP3A4*18 genotype displayed low BMD due to a gain in function of CYP3A4 enzyme activity. As CYP3A4 is highly important for metabolizing enzymes of estrogens, mutations in CYP3A4 lead to rapid clearance of estrogens. In contrast, midazolam (an in vivo probe drug generally used for phenotyping of CYP3A4 activity) showed poor catalytic activity. This shows that the CYP3A4 mutation was two-faced, resulting in poor catalytic activity towards drugs (midazolam) and rapid catalytic activity towards endogenous substances (estrogen metabolism) [59]. This highlights the need to perform phenotyping of enzymes to better understand the pharmacokinetics of drugs.
Another clinical study on middle-aged and elderly Chinese in Singapore also revealed a relationship between serum metabolites of vitamin D and genetic alterations in vitamin D-metabolizing enzymes, including CYP3A4. The researchers found that individuals with the CYP3A4 rs2242480TT variant showed a loss of function of CYP3A4, which promoted increased serum concentrations of 1,25(OH)2D3 [68]. However, a study on Russian children and adolescents revealed that genetic polymorphisms of various vitamin D-metabolizing enzymes, including CYP3A4, did not affect serum concentrations of active vitamin D, which instead depended on various exogenous (seasonal changes, lifestyle conditions), and endogenous factors (age, sex) [60]. In a similar fashion, a study on an Uyghur population focused on determining possible CYP3A4 polymorphic alleles and found 21 genetic polymorphs, of which CYP3A4*8 and CYP3A4*3 showed a loss of enzymatic activity, but the effect on serum concentrations of active vitamin D was not explored [61]. Therefore, it is necessary to study the genetic divergence of major metabolizing enzymes with variants among diverse populations. This collective knowledge of polymorphic CYP alleles and their effects on vitamin D and other CYP3A4 substrates will aid in the development of better and safer therapeutic strategies for the management of various associated disease conditions (Table 2) [59-61,69-101].
Additional issues, including drug-induced vitamin D deficiency, have come into the spotlight in conjunction with drugs that induce the activity of vitamin D-metabolizing enzymes, such as CYP3A4. One such study found that long-term treatment with efavirenz induced genetically polymorphic CYP2B6 and CYP3A enzymes, which are usually involved in the biotransformation of vitamin D3, leading to intensified catalytic activity and a decline in vitamin D availability [62]. However, studies of interindividual variability, focusing on metabolizing enzymes, are recommended for dose adjustment and also to overcome possible comorbidities. Moreover, there are conditions such as idiopathic hypercalcemia (IIH), which occurs due to the inactivation of another principal enzyme CYP24A. As CYP24A is involved in the inactivation of vitamin D3, mutations in CYP24A often result in decreased enzyme activity, followed by increased 1,25-dihydroxyvitamin D3 levels, hypercalcemia, and hypercalciuria. In such instances, the activation of CYP3A4 by potent inducers such as rifampicin ameliorates IIH [102]. In this context, information about CYP3A4, which is highly heritable, and its metabolizing properties (e.g., whether an individual is a poor or rapid metabolizer) may collectively help in optimal dose selection.
Cancer
Multiple studies have reported data on the role of CYP3A4 polymorphisms in different types of cancers, such as prostate, breast, and lung cancer. Formerly, although more than 20 mutant CYP3A4 genetic phenotypes were observed, it was stated that the effect of genetic polymorphisms in CYP3A4 was unclear, and such polymorphisms were predicted to be infrequent [54]. More recently, exemplary data from a meta-analysis suggested that the CYP3A4*1B polymorphism may be associated with increased cancer risk among an African population [103]. Furthermore, CYP3A4*1B and CYP3A43*3 mutants were found to be associated with prostate cancer and tended to increase the risk of small cell lung cancer [63,69]. Furthermore, a comparative study on African-Americans and Caucasians revealed that the CYP3A43-Pro(340)Ala polymorphism was a risk factor for prostate cancer; this risk was mediated by androgen signaling, which promotes cancer cell proliferation [70,104]. In addition, as CYP3A family genes, including CYP3A43, are most apparently implied in cancer physiology, a study among African-American individuals and those of European ancestry revealed the important role of genetic polymorphisms of vitamin D metabolic enzymes with respect to the concentration of vitamin D metabolites, which might serve as a potential biomarker among polymorphic variants [105].
Moreover, a study of pharmacogenetic variations among individuals may also shed light on the therapeutic effects of anticancer drugs that are specifically metabolized by CYP3A4 [106]. However, a Western European study showed no significant interrelationship between CYP3A4*1B and CYP3A5*3 genetic polymorphisms and the activation of carcinogens in the digestive system, which led to an increased risk of digestive cancer. In such cases, it is necessary to investigate the effects of other loci that are on the verge of being elucidated on disease progression [71]. Furthermore, since CYP3A4 and CYP3A5 share a common substrate specificity, it was also stated that the individuals with CYP3A4 polymorphisms might also have mutations in CYP3A5 and vice versa; these double mutations would worsen the risk of unforeseen drug effects [107]. CYP3A4*1G is the variant that has received the most attention in the Chinese population, where it was found to be associated with breast cancer, and it has been shown to be linked to ovarian cancer in Polish women [72,73].
Chemotherapeutic failure has been frequently investigated in the last few decades. A prostate cancer prevention trial revealed that finasteride, an anticancer drug, showed altered concentrations due to the CYP3A4*1G polymorphism [74]. A recent study on ifosfamide treatment in children with solid embryonic tumors identified associations of drug treatment among CYP polymorph individuals, and found that detrimental therapeutic failure was associated with the rs2740574 polymorphism in CYP3A4 [108]. As CYP3A4 is not merely involved in vitamin D metabolism, its involvement in other aspects (including drug metabolism) needs to be meticulously examined to improve pharmacotherapy.
Other disease conditions and drugs
Vitamin D is also recognized for its effects on mediating both the innate and adaptive immune systems. Active vitamin D levels were associated with favorable outcomes of coronavirus disease 2019 (COVID-19), the recent pandemic infection [109]. As vitamin D is known for its anti-inflammatory properties and ability to mitigate virulence factors, it was affirmed that individuals with vitamin D deficiency are more vulnerable to being affected by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [110]. A recent pharmacogenomic study on individuals with rheumatoid arthritis receiving hydroxychloroquine was studied to elucidate the possible associations of the CYP3A4 and CYP2D6 polymorphisms with COVID-19 risk. These findings revealed that CYP3A4 polymorphic variants, especially CYP3A4*1B, were associated with less susceptibility to COVID-19 [111]. However, as CYP3A4 metabolizes drugs used to mitigate COVID-19, polymorphism-induced alterations in CYP activity may alter the effects of medications, which may be beneficial or harmful for disease progression.
For instance, a multivariable case-control study showed a positive association of the CYP3A4 rs4646437 polymorphism with the development of hypertension in the Chinese population [112]. However, a cohort study of Han Chinese individuals showed that the CYP3A4 rs3735451, rs4646440, and rs4646437 polymorphisms were associated with less risk of ischemic stroke [113]. In a Chinese group of ischemic stroke patients, the CYP3A4*1G allele showed protective effects against clopidogrel resistance [114]. Another study on the CYP3A4*1G allelic variant showed a loss of function of CYP3A4 activity, which increased fentanyl concentrations in Chinese gynecologic patients with postoperative pain [115]. In addition, the same CYP3A4*1G showed different behavior—namely, a gain of CYP3A4 activity. This resulted in increased metabolic clearance of estrogens in female participants, which promoted the incidence of coronary heart disease [75]. This kind of dual activity needs to be further confirmed in large cohort studies. This also indicates that metabolizing enzymes not only alter the active metabolites of drugs, but also seem to modulate endogenous substances, with downstream effects.
Studies have found that vitamin D deficiency has a remarkable impact on type 2 diabetes mellitus (T2DM). Recent research has also clarified the interactions between CYP3A4 polymorphisms and T2DM, as individuals with CYP3A4 polymorphisms are at a higher risk of developing T2DM [116]. The underlying genetic associations with diseases need to be investigated for a better understanding of pathological conditions.
Despite of the use of conventional medicine alone, the approach of integrative medicine which is a combination of alternative medicine with conventional medicine has gained attention. Metabolic interactions should be taken into consideration in this context, since such interactions may result in unforeseen effects. A recent study on people from seven nationalities revealed the importance of studying the interethnic variability of CYP3A4 mutations through an analysis of their varying metabolic interactions with food and drugs [117].
CONCLUSIONS
Vitamin D seems to have significant beneficial effects in many pathological states. Many studies have established the potential of vitamin D to ameliorate various pathophysiological conditions, such as bone diseases, cancer, diabetes, and infectious diseases (e.g., SARS-CoV-2). Studies have also analyzed the use of vitamin D supplementation in therapeutic regimens to achieve beneficial effects. However, CYP metabolic enzymes are majorly responsible for the activation or inactivation of active vitamin D metabolites. Researchers have found that CYP enzymes are primarily responsible for maintaining vitamin D levels in the serum. Nonetheless, several factors such as age, climate, and sun exposure are directly involved in variation in vitamin D levels, and genetic and environmental factors are now among the matters of concern pertaining to this issue. In this context, the genetic polymorphisms of enzymes that participate in the vitamin D metabolic pathway have entered the spotlight due to their significant effect on clinical outcomes. As CYP3A4 is mainly involved in the inactivation of vitamin D metabolites, this review focused primarily on vitamin D, with an emphasis on various disease conditions and the effects of CYP3A4 genetic polymorphisms, especially SNPs, in disease conditions. Several studies have shown the impact of the CYP3A4 gene polymorphism on the progression of disease conditions. These effects result from an increase (gain of function) or decrease (loss of function) in the catalytic activity of CYP3A4. However, some research findings reported contradictory findings, with no observed effect of genetic factors on vitamin D levels. Thus, future research should attempt to uncover the wide interethnic and interindividual variability of CYP enzymatic polymorphisms, utilizing large amounts of data to identify rare and common mutants that may have a clinical impact on disease conditions. Further studies focusing on alterations in potential circulating vitamin D metabolites and their correlation with polymorphic variants may reveal potential biomarkers of genotypic variants. From a clinical standpoint, information on patients’ genotypic profiles could play a key role in personalized therapy in patients with vitamin D deficiency.
Studies have also shown that the therapeutic failure of drugs may be a consequence of CYP3A4 polymorphisms. Taken together, studies focusing on the pharmacogenetics of drug-metabolizing enzymes with the goal of understanding the comprehensive behavior of CYP enzymes among variant populations may play a decisive role in overcoming therapeutic failure and disease progression.
Acknowledgements
We thank Dr. Swapnil P Borse and Dipanka Tanu Sarmah for their valuable suggestions.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.