Current Guidelines for Management of Medullary Thyroid Carcinoma
Article information
Abstract
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor originating from the parafollicular cells. The diagnostic and therapeutic strategies for the condition are different from those used for well-differentiated thyroid cancer. Since the 2015 American Thyroid Association guidelines for the diagnosis and treatment of MTC, the latest, including the National Comprehensive Cancer Network and European Association for Medical Oncology guidelines have been updated to reflect several recent advances in the management of MTC. Advances in molecular diagnosis and postoperative risk stratification systems have led to individualized treatment and follow-up strategies. Multi-kinase inhibitors, such as vandetanib and cabozantinib, can prolong disease progression-free survival with favorable adverse effects. In addition, potent selective rearranged during transfection (RET) inhibitors (selpercatinib and pralsetinib) have shown a promising efficacy in recent clinical trials. This review summarizes the management of MTC in recent guidelines focused on sporadic MTC.
INTRODUCTION
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor that can produce calcitonin from parafollicular cells. Thus, both the diagnostic and therapeutic strategies for MTC differed from those used for well-differentiated thyroid cancer (DTC) derived from follicular cells. MTC accounts for 0.6% of all thyroid cancers in Korea and 1% to 2% in the United States [1,2]. MTC occurs in a sporadic form and is associated with multiple endocrine neoplasia (MEN) type 2 as a hereditary form, accounting for 75% and 25%, respectively [1].
Sporadic MTC is usually diagnosed as advanced disease in many patients because the most common presentation is a solitary thyroid nodule without any symptoms. The diagnosis of sporadic MTC is mostly based on fine-needle aspiration (FNA) biopsy and immunohistochemical staining for calcitonin [1]. However, preoperative diagnosis of MTC is difficult because of the presentation of a solitary thyroid nodule and its rare incidence. Therefore, MTC is sometimes diagnosed after thyroidectomy for indeterminate or suspicious malignant FNA results [1].
Complete surgical resection of the thyroid tumor and any locoregional metastases is the only curative option for locoregional MTC. Therefore, early diagnosis and detection of MTC are very important for prognosis [3]. Treatment options for recurrent or metastatic MTC include surgical resection, external beam radiation therapy (EBRT), and directed local therapies or systemic therapies [1,4]. However, the best management is still controversial in patients with recurrent MTC and distant metastases, because many patients with metastatic disease have indolent disease courses for a long time [1,4]. Management guidelines for MTC have been published and updated internationally by several societies. Guidelines from the American Thyroid Association (ATA) were updated in 2015 [1]. Subsequently, treatment guidelines for MTC have been updated to reflect the results of recent research and clinical trials. The European Association for Medical Oncology (ESMO) clinical practice guidelines also provide updated recommendations on the diagnosis and treatment of MTC [4]. The Japan Associations of Endocrine Surgeons (JAES) has revised the clinical practice guidelines for thyroid tumors including MTC [5]. Lastly, the National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology were updated (version 3.2020; February 2, 2021) [6]. This review summarizes the management of MTC in recent guidelines focused on sporadic MTC.
PREOPERATIVE EVALUATION
Serum calcitonin and carcinoembryonic antigen (CEA) in MTC are important diagnostic, prognostic, and predictive biomarkers because of their direct relationship with the C-cell mass [7]. If MTC is confirmed on FNA, thorough neck ultrasonography (US) should be performed to identify neck lymph node (LN) metastasis. In patients with extensive neck disease, serum calcitonin levels greater than 500 pg/mL, signs of distant metastasis, contrast-enhanced (CE) computed tomography (CT) of the neck and chest, three-phase CE liver protocol CT or CE magnetic resonance imaging (MRI) of the liver, and bone scan and axial MRI are considered [1]. However, 2-[18F]-fluoro-2-deoxy-D-glucose (FDG)-positron emission tomography (PET/CT) or 18F-dihydroxyphenyl-alanine (F-DOPA)-PET/CT is not recommended because they are less sensitive in detecting metastases [1]. NCCN guidelines also recommend that CE CT of the neck and chest, 3 phase liver protocol CT, liver MRI, bone scan and/or skeletal MRI are indicated based on a high burden of disease, calcitonin above 400 pg/mL, or elevated CEA levels [6]. If available, gallium-68-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid -octreotate (DOTATATE) PET/CT can be optionally considered in such patients because of the improved localization of MTC [6,8]. The ESMO guidelines recommend if F-DOPA-PET/CT were available in MTC patients with high serum calcitonin levels (≥500 pg/mL) [4].
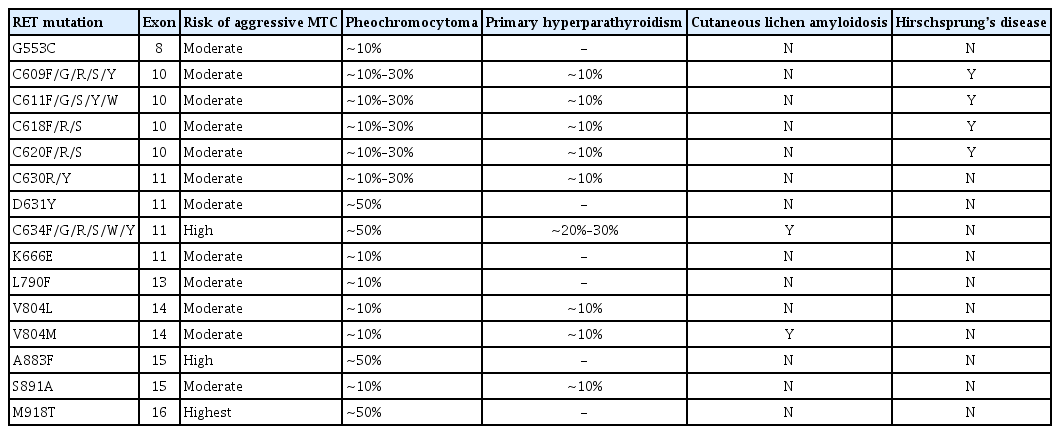
Analysis of germline rearranged during transfection (RET) mutations may be performed perioperatively. RET mutations are detected in all inherited MTC and present in 6% to 10% of apparent sporadic MTC [1]. Therefore, genetic testing for RET proto-oncogene mutations is recommended for all patients with clinically apparent sporadic MTC. It is also recommended for screening children and adults with hereditary MTC in known relatives [1,4]. Specific germline RET mutations in inherited MTC are closely associated with genotype–phenotype affecting the onset time of disease and tumor aggressiveness (Table 1) [1]. It is well known that RET M918T somatic mutation is associated with more aggressive features such as distant metastasis than wild-type MTC [9]. However, the ATA guidelines suggest that it is not standard practice to analyze RET M918T somatic mutations in patients with sporadic MTC [1]. The 2019 ESMO guidelines optionally recommended that somatic RET mutation testing may be needed for individual targeted therapy if selective RET inhibitor treatment is planned in patients with advanced MTCs [4].
Preoperative evaluation of coexisting pheochromocytoma is very important to prevent hypertensive crisis during surgery. Therefore, pheochromocytoma and primary hyperparathyroidism should be excluded in all patients with either an unknown RET mutation status or a germline RET mutation [1]. Biochemical evaluation, including serum calcium and plasma fractionated metanephrines, should be performed. If a pheochromocytoma coexists, it should be treated with alpha-blocker and removed by laparoscopic or retroperitoneal adrenalectomy before thyroid surgery to prevent hypertensive crisis [1,4,6].
SURGICAL MANAGEMENT
As mentioned previously, complete surgical resection of the thyroid mass and locoregional metastasis is the only curative option for locoregional MTC. Total thyroidectomy is the preferred surgical approach because bilateral or multifocal disease is occurred in all patients with inherited MTC and approximately 10% of sporadic MTC [1,4]. Total thyroidectomy and dissection of cervical LN compartments is a standard approach for sporadic or inherited MTC according to preoperative serum calcitonin values, US findings, and intraoperative proven LN metastasis [1,4–6]. However, there is a controversial issue in performing lateral neck dissection (ND) in patients without evidence of LN metastasis on preoperative US. Ipsilateral ND is generally performed due to intraoperative evidence of central LN involvement. More aggressive prophylactic lateral ND can lead to postoperative morbidity without obvious survival benefits [1].
Prophylactic lateral ND for MTC without evidence of neck metastasis and distant metastasis could not achieve a consensus agreement in the ATA guidelines committee, but they recommend neither for nor against prophylactic lateral ND being considered based on serum calcitonin levels [1]. Total thyroidectomy, central LN dissection, and the dissection involved lateral neck compartments (levels II–V) should be performed in restricted disease of the neck [1,4,6]. Contralateral ND should be considered if the basal serum calcitonin level is greater than 200 pg/mL when the ipsilateral lateral neck LN is positive but the contralateral neck compartment is negative on preoperative imaging according to ATA and ESMO guidelines [1,4]. The ESMO guidelines generally recommended total thyroidectomy with bilateral central ND and ipsilateral ND at least at levels IIA, III, and IV even if serum calcitonin level is 50 to 200 pg/mL in patients with negative neck US [4]. There is no need to perform prophylactic central ND in small intrathyroidal MTC with a preoperative calcitonin level <20 pg/mL because of no risk of LN metastasis [1,4,10].
According to the NCCN guidelines, total thyroidectomy is recommended, and ND can be considered for unilateral thyroid disease with less than 1 cm. Total thyroidectomy and bilateral central ND are indicated in all MTCs with bilateral thyroid disease or tumor size ≥1 cm. More extensive lateral ND (levels II–V) is considered in patients with MEN2A and familial MTC in cases of tumors greater than 1 cm or central LN metastasis [6]. Even if the MTC is locally advanced or metastatic, total thyroidectomy with involved LN compartments dissection is recommended to preserve the function of speech, swallowing, and the parathyroid gland [1]. It is very important that individualized surgical decisions should be made according to life expectancy, underlying comorbidities, and the patient’s wishes.
POSTOPERATIVE MANAGEMENT
If MTC is postoperatively diagnosed after lobectomy, completion thyroidectomy is not routinely performed in patients without a germline RET mutation. However, completion thyroidectomy is indicated in sporadic MTC with detectable postoperative serum calcitonin and abnormal neck US [1,4]. In cases of inadequate lateral ND at the initial surgery, compartment-oriented lateral ND should be performed if the preoperative serum calcitonin level is less than 1,000 pg/mL and five or fewer metastatic LNs are removed at the initial operation based on an expert opinion of the ATA guidelines [1].
The eighth edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system for thyroid cancer was published in late 2016. There were some major changes, including a new definition of T3 classification (removal of minor extrathyroidal extension from T3 disease) and an increase in the cutoff age (55 years) for high risk in DTC. The AJCC/UICC tumor node metastasis (TNM) staging system is also recommended for MTC [11]. However, a TNM staging system based on DTC cannot be applied uniformly to the MTC. The eighth AJCC/UICC staging system for MTC does not seems to be the best in differentiating mortality risk among stage groups and upstaging many patients to stage IV [12]. Therefore, the NCCN guidelines do not use TNM stages as the initial determinant of management because of the lack of other important prognostic factors, such as age of patients at diagnosis, especially in inherited MTC and postoperative serum calcitonin levels [6].
Postoperative management depends on postoperative serum calcitonin and CEA levels to detect residual diseases (Fig. 1). Because serum calcitonin slowly decreases in MTC patients, the nadir of calcitonin level cannot be reached for several months. Therefore, the timing of measurement of serum calcitonin level after surgery is crucial [13]. ATA guidelines recommend that serum calcitonin and CEA levels should be measured 3 months postoperatively [1]. The ESMO and NCCN guidelines also recommend that they be measured 30 to 60 days and 2 or 3 months after surgery, respectively [4,6]. Postoperative disease monitoring depends on serum calcitonin and CEA levels in the ATA and NCCN guidelines, and ESMO guidelines suggest dynamic risk stratification (Fig. 1) [4]. As dynamic risk stratification has been used with clinical values in DTC, dynamic risk stratification for MTC was developed to modify initial risk estimation based on the response to therapy and biological tumor behavior, including change in serum calcitonin and CEA levels, and it has provided more useful real-time prognostic information than initial TNM staging [14–16].

Postoperative management in patients with medullary thyroid cancer (MTC) according to dynamic risk stratification in European Association for Medical Oncology (ESMO) guidelines. CEA, carcinoembryonic antigen; US, ultrasonography. aAmerican Thyroid Association (ATA) guidelines recommend that serum calcitonin and CEA should be measured after 3 months postoperatively; bOther imaging modalities including contrast-enhanced (CE) computed tomography (CT) of neck, chest, abdomen with liver protocol, and bone scan, axial magnetic resonance imaging (MRI) depending on the tumor stage, serum calcitonin (≥150 pg/mL) and CEA; cNegative other imaging modalities, consider [18F]-fluoro-2-deoxy-D-glucose (F18-FDG) positron emission tomography-CT or Gallium-68 DOTATATE or CE MRI with neck, chest, abdomen with liver protocol in National Comprehensive Cancer Network (NCCN) guidelines; dStable or progressive disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Postoperative persistent hypercalcitoninemia, short doubling times of calcitonin and CEA (<6 to 12 months), and rapid increase in CEA levels in a stable state of calcitonin level may be other important prognostic factors [1,4,6]. Codon analysis of RET proto-oncogene mutations is also useful for predicting prognosis because specific germline RET mutations predict tumor aggressiveness in hereditary MTC [1,4,6]. In addition, a tumor volume doubling time of less than 1 year in MTC patients with lung metastases is correlated with poorer overall survival [17].
Postoperative adjuvant EBRT to the neck and mediastinum should be considered in patients at high risk for airway obstruction and local recurrence, such as residual disease, extensive LN metastases, or extra-thyroidal extension as an expert opinion of ATA guidelines [1].
MANAGEMENT IN PERSISTENT OR RECURRENT MTC
Management options for recurrent or residual MTC include close observation for indolent disease, surgical resection of locoregional disease, EBRT, and local therapies, such as radiofrequency ablation, cryoablation, and embolization, or systemic therapies, such as conventional chemotherapy, kinase inhibitors, and immune checkpoint inhibitors for non-resectable diseases. Therefore, treatment approaches in these patients depend on various clinical factors, including presence of the symptoms, possibility of significant structural disease progression, disease localization, disease volume or burden, and location of the metastatic lesion [1,4,6].
Patients with biochemical incomplete responses without structural disease are followed-up with conservative surveillance [4,6]. Therapeutic interventions based on increased biochemical markers are not recommended. In cases of locoregional disease without distant metastasis, surgical resection is the primary treatment option with or without postoperative EBRT or intensity-modulated radiation therapy [4,6]. However, active surveillance can be an optimal management modality in many patients with asymptomatic small LN metastases because repeat operations are commonly not curative and can be associated with high surgical complications, such as adjacent nerve injury and permanent hypoparathyroidism [1].
Currently, most guidelines do not recommend the use of single or combinational chemotherapeutic regimens as first-line systemic therapy [1,4,6]. Systemic therapy as a first-line regimen, such as vandetanib or cabozantinib, should be considered in unresectable locoregional diseases and distant metastases with symptomatic or progressive MTCs by Response Evaluation Criteria in Solid Tumors (RECIST) [1,4,6,18]. Vandetanib and cabozantinib increased progression-free survival (PFS) in a phase III trial named the Zactima Efficacy in Thyroid Cancer Assessment (ZETA) trial [19] and Efficacy of XL184 (cabozantinib) in Advanced Medullary Thyroid Cancer (EXAM) trial [20], respectively (Table 2). Recent post hoc analysis from the ZETA trial also demonstrated that vandetanib treatment had significant prolonged median PFS (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.28 to 0.64; P<0.001) in patients with symptomatic and progressive diseases [21]. However, OS had not significant difference between vandetanib and placebo arm because of post-progression, unblinded, open-label treatment with vandetanib (HR, 1.08; 95% CI, 0.72 to 1.61; P=0.71) [21].

Summary of Phase 3 Clinical Trials of Vandetanib or Cabozantinib versus Placebo in Patients with Advanced Medullary Thyroid Cancer
Cabozantinib had a statistically non-significant increase in median OS compared with placebo (26.6 months for cabozantinib vs. 21.1 months for placebo: HR, 0.85; 95% CI, 0.64 to 1.12; P=0.24) [22]. However, cabozantinib treatment had a significant benefit in patients with RET M918T-positive MTC (median OS 44.3 months for cabozantinib vs. 18.9 months for placebo: HR, 0.60; 95% CI, 0.38 to 0.94; P=0.03) [22].
Several recent real-world multicenter studies and meta-analysis also showed that vandetanib and cabozantinib had significant clinical benefits for objective response rate (ORR) and PFS in advanced MTC [23–26]. We demonstrated that median PFS from the start of vandetanib was 25.9 months, but the median OS was not reached [23]. In addition, German real world multicenter study recently reported that the median OS from the initiation of vandetanib therapy was 53 months (95% CI, 43.7 to 62.3) and 24 months (95% CI, 5.9 to 42.1) for cabozantinib [24].
Unfortunately, there is no clear evidence as to which regimen between vandetanib and cabozantinib is better. Although cabozantinib seems to be effective as a second-line therapy [22], the choice of the first regimen among vandetanib and cabozantinib should be considered for potential toxicity in each patient. However, cabozantinib has not yet been approved for the treatment of MTC in South Korea.
The NCCN panels also recommend that other tyrosine kinase inhibitors (TKIs), including sunitinib, lenvatinib, sorafenib, and pazopanib are considered if clinical trials of vandetanib or cabozantinib are not available [6]. Among these kinase inhibitors, sunitinib and lenvatinib have good response rates [27–29]. If the disease progresses despite vandetanib or cabozantinib treatment, dacarbazine or combination chemotherapy can be administered [6,30]. Clinical studies for other novel multi-targeted TKIs, such as ponatinib (AP24534), anlotinib (AL3818), and TPX-0046, are ongoing in advanced or metastatic MTC [31–33].
The latest NCCN guidelines for MTC management introduced highly selective RET inhibitors, including selpercatinib (LOXO-292) and pralsetinib (BLU-667) for the first time [6]. They have higher potency and lower toxicity. Selpercatinib and pralsetinib can be considered for patients with a positive RET somatic mutation [6].
Selpercatinib is a highly selective small molecule RET kinase inhibitor that has very high potency to inhibit different RET alterations, both point mutations and fusions, including the RET V804M mutation responsible for other TKI resistance [34]. Selpercatinib (160 mg twice daily) showed remarkable and durable efficacy in MTC with or without prior vandetanib or cabozantinib treatment in phase 1/2 trials (LIBRETTO-001 trial) (Table 3) [34]. The ORR was 69% (95% CI, 55 to 81) and 1-year PFS was 82% (95% CI, 69 to 90) in patients with RET-mutant MTC who had previously been treated with vandetanib, cabozantinib, or both. The ORR was 73% (95% CI, 62 to 82) and 1-year PFS was 92% (95% CI, 82 to 97) in patients with RET-mutant MTC without previous treatment with vandetanib or cabozantinib. The most common severe adverse events (grade 3 or 4) were hypertension, increased liver enzymes, hyponatremia, and diarrhea [34]. Recently, the U.S. Food and Drug Administration approved the use of selpercatinib in patients with advanced or metastatic RET-mutant MTC [35]. A multicenter, randomized, open-label, phase 3 trial (LIBRETTO-531) is ongoing to compare selpercatinib to cabozantinib or vandetanib in patients without prior kinase inhibitor therapy in RET-mutant MTC (NCT04211337).

Summary of Clinical Trials of Selpercatinib and Pralsetinib versus Placebo in Patients with Advanced MTC with RET Alteration
On December 1, 2020, pralsetinib was approved for systemic therapy in advanced or metastatic RET mutation-positive MTC based on preliminary results of a phase 1/2 study (ARROW trial, NCT03037385). Pralsetinib therapy (400 mg orally once daily) had an ORR of 60% (95% CI, 46 to 74) in 55 patients with advanced or metastatic RET-mutant MTC who received prior therapy with vandetanib or cabozantinib. The ORR was 74% (95% CI, 49 to 91) in 22 patients with RET-mutant MTC who did not receive prior therapy of vandetanib or cabozantinib (Table 3). The most common serious laboratory abnormalities (grade 3 or 4, ≥2%) were leukopenia, anemia, decreased phosphate, hypocalcemia, hyponatremia, increased liver enzymes, thrombocytopenia, and increased alkaline phosphatase levels (NCT03037385) [36,37]. A multicenter, randomized, open-label, phase 3 trial (AcceleRET-MTC) has recently begun to compare pralsetinib with cabozantinib or vandetanib in patients without prior multi-kinase inhibitor therapy in RET-mutant MTC (NCT04760288). Other highly selective RET inhibitors, such as TPX-0046 and BOS172738 are also being tested in clinical trials [38,39].
Although the role of immunotherapy appears to be more promising in anaplastic thyroid cancer, to date, no immunotherapy has been approved for advanced MTC. It is well known that high tissue tumor mutational burden (TMB) could be a useful marker to recognize patients with recurrent or metastatic advanced solid tumors who will have a better therapeutic benefit from immune checkpoint inhibitors, such as pembrolizumab [40]. Therefore, the NCCN guidelines for MTC management suggest that pembrolizumab should be considered in patients with symptomatic, progressive MTC with high TMB (≥10 mutations/megabase) [6]. Pembrolizumab (NCT03072160, NCT-02721732), nivolumab (NCT03246958), and ipilimumab (NCT-03246958) are currently being evaluated in phase 2 trials for the treatment of recurrent or metastatic MTC [41].
Various other investigational therapeutic modalities, including immunotherapy, tumor vaccines [42,43], radio-immunotherapy using radiolabeled anti-CEA monoclonal antibodies [44, 45], and peptide receptor radionuclide therapy (PRRT) using 177Lu-labeled or 90Y-labeled somatostatin analogues [46,47], have been developed.
Treatment with yttrium-90–DOTA-[D-Phe1-Tyr3]-octreotide (90Y-DOTATOC) decreased serum calcitonin levels as a primary endpoint, and survival benefits in responders were reported in a phase 2 trial of metastatic MTC [47]. PRRT using 177Lu-octreotate could be a therapeutic option in MTC patients with a high tumor uptake on 111In-diethylenetriamine pentaacetic acid (DTPA)-octreotide scan and tumor somatostatin receptor type 2a (SSTR2a) receptor expression by immunohistochemistry [48]. The ESMO guidelines optionally recommend the use of radionuclide therapy in selected cases [4].
CONCLUSIONS
Since the ATA guidelines were published in 2015, treatment guidelines for MTC have been updated to reflect the results of recent research and novel conceptions. Extensive careful surgery is the only curative option for localized MTC. However, most repeated surgeries cannot improve the cure rate in patients with widespread locoregional or metastatic MTCs; thus, less aggressive management, including active surveillance, local therapy, and palliative therapy, should be considered first. Vandetanib or cabozantinib should be primarily considered in patients with non-resectable symptomatic, rapidly progressive MTC; they can extend PFS and provide long-term disease stabilization in some patients. However, complete responses by multi-kinase inhibitors are virtually uncommon and have many significant adverse effects due to off-target effects, which can lead to poor quality of life. Selpercatinib and pralsetinib have shown remarkable efficacy, including a few complete responses and favorable safety in phase 1/2 trials. Thus, molecular testing for germline or somatic RET mutations will be essential for finding an appropriate candidate who may benefit from these RET inhibitors. Pembrolizumab has some promising results in advanced MTC but limited clinical application.
Despite recent advances in the management of advanced MTC, various unmet needs still exist. Further studies are required to determine the mechanism of resistance to novel TKIs, selective RET inhibitors, and immune checkpoint inhibitors and to identify the appropriate combination regimens with various therapies. Ultimately, an evidence-based individualized approach based on molecular stratification seems to be the best option in precision medicine for the management of MTC.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a clinical research grant from Pusan National University Hospital in 2021.