Asian Conference on Tumor Ablation Guidelines for Adrenal Tumor Ablation
Article information
Abstract
Thermal ablation is a good alternative treatment in patients who are unable to undergo adrenalectomy. Even though the Asian Conference on Tumor Ablation (ACTA) has been held for many years, adrenal ablation guidelines have not been established. No guidelines for adrenal ablation are established in American and European countries, either. The aim of this review was to introduce the first version of ACTA guidelines for adrenal tumor ablation.
INTRODUCTION
Most adrenal tumors are benign incidentalomas which do not need treatment in patients without a history of extra-adrenal malignancy. Surgery is the treatment of choice in patients with a functioning or malignant tumor because adrenal insufficiency is rare following unilateral adrenalectomy.
Since image-guided thermal ablation was introduced to clinical practice, this minimally invasive treatment has been applied for adrenal glands later compared to other organs such as liver, kidney, and lung. Moreover, many kinds of ablation guidelines for these extra-adrenal organs have been established and updated for years. However, we only have several review articles that deal with adrenal ablation, but there is much overlap in terms of their content [1–6]. They have mainly focused on technical points only for those who are already used to interventional radiology.
For these reasons, appropriate guidelines are necessary not only for experts but also beginners who want to know how to prepare or perform adrenal ablation. Although the Asian Conference on Tumor Ablation (ACTA) has been held for 6 years, we do not have official guidelines for adrenal ablation. The aim of this review was to introduce the first version of ACTA guidelines for adrenal tumor ablation.
METHODS
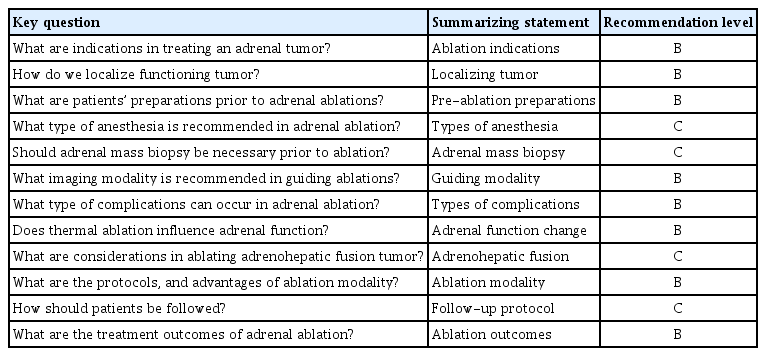
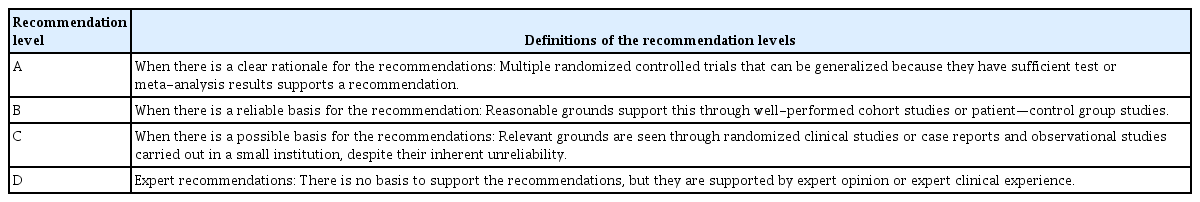
Four experts, who were working as interventional radiologists from four Asian countries including Korea, Japan, Taiwan, and Singapore, developed key questions about preparations prior to ablation, ablation procedures, and post-ablation management. They were the key members of guideline committee in ACTA. Each guideline was made by reaching their agreement via consensus after many relevant studies were reviewed. The recommendation levels were graded as A, B, C, and D by the following considerations: numbers of randomized controlled trials, meta-analyses, cohort studies, case–control studies, expert opinions, and case reports. These were summarized in Table 1 and the recommendation levels were defined in Table 2 [7].
INDICATIONS TO ADRENAL ABLATIONS
Adrenal masses are comprised of primary and secondary adrenal tumors. Primary adrenal tumors are comprised of non-functioning and functioning masses. Secondary adrenal tumor is an adrenal metastasis, which is the most common malignant mass in the adrenal gland [8,9].
Open or laparoscopic adrenalectomy is the conventional treatment for symptomatic adenomas or metastasis with good outcomes. This surgical treatment is associated with relatively long hospital stay and necessitates the requisite surgical expertise [8,10]. This in-turn drives the demand and growth of minimally invasive options, such as percutaneous thermal ablation of adenomas and adrenal metastases [1,11–15]. At present, functioning tumors or adrenal metastasis are main indications to thermal ablation in patients who are poor surgical candidates (Figs. 1, 2).

A recurrent pheochromocytoma in a 35-year-old man with von-Hippel-Lindau disease. (A) Contrast-enhanced axial computed tomography image shows a recurrent pheochromocytoma (white arrow) in the residual left adrenal gland. The patient underwent right total adrenalectomy and left partial adrenalectomy due to recurrent pheochromocytomas. An asterisk indicates the left lung intervening between the pheochromocytoma and the skin. (B) Left pheochromocytoma is safely targeted with a radiofrequency electrode (black arrow) with the patient positioned left side down, resulting in ipsilateral collapse of the left lung to avoid pneumothorax.
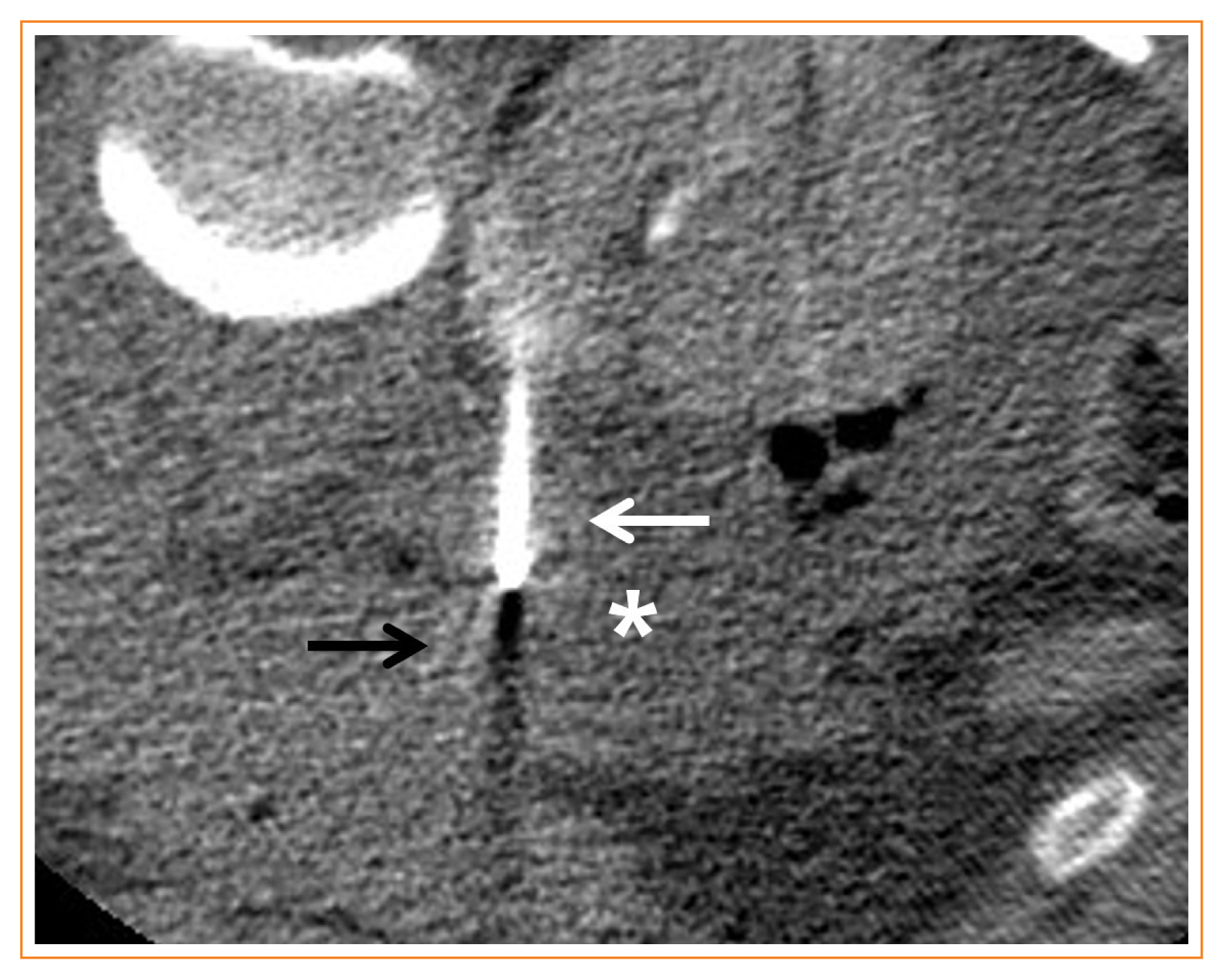
Hydro-dissection in a 36-year-old female with von-Hippel-Lindau disease. Axial computed tomography image shows 5% dextrose water (asterisk) which is instilled for hydro-dissection to avoid thermal damage to the pancreas body (black arrow). A white arrow indicates a radiofrequency electrode targeting a recurrent left pheochromocytoma.
Patients should be selected based on a multi-disciplinary team approach of interventional radiologists, surgical and medical oncologists, radiation oncologists, and endocrinologists. Preprocedural multiphasic computed tomography (CT) or magnetic resonance imaging (MRI) is required and laboratory tests including blood count, clotting profile, and renal panel should be performed. Functioning adenomas can be excluded based on history, physical examination, and the relevant biochemical work-up.
CONSIDERATIONS PRIOR TO ABLATION
Localizing functioning tumors
Multiple adrenal masses are not uncommon in patients with primary hyperaldosteronism resulting from adrenocortical adenoma or hyperplasia. In this clinical setting, adrenal venous sample is essential to localize a functioning mass prior to ablation [16,17]. Otherwise, a non-functioning mass can be ablated and additional ablation procedure performed as necessary. Even if a single mass is detected on CT, adrenal venous sampling cannot be omitted because the tumor can be a non-functioning lesion [18]. Another reason is that an aldosterone-producing adenoma or hyperplasia can be too small to be seen on CT [18].
Great care should be taken to interpret the results of adrenal venous sampling. First of all, adrenal vein sampling should be checked to confirm it was adequate using adrenocorticotropic hormone (ACTH) stimulation. Both aldosterone and cortisol should be increased in both adrenal veins 10-fold higher than their values in the peripheral veins [19]. One adrenal aldosterone/cortisol ratio should be increased four-fold greater than the other one [19].
Another issue that we need to be concerned with is that the right adrenal vein is more difficult to sample compared with the left adrenal vein [20,21]. It is frequently small or invisible on contrast-enhanced CT images. Additionally, it may drain into the right hepatic vein, resulting in underestimating the level of aldosterone because systematic venous flow is mixed with the right adrenal venous flow. Recent investigations show how to localize primary hyperaldosteronism if right adrenal vein sampling is inadequate [21,22].
Adenoma is one of the common etiology of bilateral adrenal incidentaomas. However, ACTH-independent cortisol-producing adrenal tumors with overt Cushing symptoms uncommonly involve bilateral glands [23–25]. Although primary adrenal hyperplasia may excrete cortisol excessively, it is not common in Cushing syndrome [23,26–28]. Therefore, adrenal vein sampling is not a routine procedure for tumor localization in Cushing syndrome unless bilateral hyperplasia is suspected in the differential diagnosis [29].
Pheochromocytomas can occur bilaterally in hereditary diseases [30,31], but all of these tumors produce catecholamines and show characteristic imaging features [32,33]. Therefore, adrenal vein sampling is not necessary to localize a functioning tumor in case of bilateral pheochromocytomas.
Preparations for ablations
Patients should fast for 8 hours prior to adrenal ablation, a procedure in which general anesthesia is necessary. Emptying the stomach is essential to prevent aspiration during ablation procedures. Laboratory tests should include complete blood count, liver function test, electrolyte analysis, pulmonary function test, urinalysis, electrocardiogram, and chest simple radiography. Importantly, platelet count and international normalized ratio should be checked to detect bleeding tendency and coagulopathy. The number of platelets should be more than 50,000 and the international normalized ratio should be less than 1.5. If bleeding time or coagulation factors can be corrected prior to ablation, these are not absolute contraindications. Fever is another contraindication to adrenal ablation. The cause of fever should be identified and if fever is successfully treated, adrenal ablation can be performed.
Types of anesthesia
All adrenal ablations should be performed under general anesthesia with intra-arterial blood pressure (BP) monitoring (e.g., radial arterial line), as BP fluctuations due to hypertensive crisis, and potential dysrhythmias during ablation that require active hemodynamic management, need to be anticipated [1,11–15]. Therefore, conscious sedation is not recommended for adrenal ablation because vital signs and patient symptoms are not well-controlled.
For functioning adenomas and pheochromocytomas, pre-procedure α-adrenergic and/or β-adrenergic blockade, in consultation with an endocrinologist is recommended. Agents typically used consist of combinations of α-adrenergic inhibition, β-adrenergic inhibition, and inhibition of catecholamine synthesis [15]. A pre-procedure target BP of 120/80 mm Hg is the usual accepted threshold.
Adrenal metastases on the other hand do not require routine pre-medication with adrenergic blockers. Instead, active intra-operative management of raised BP using direct acting vasodilators and short-acting α-adrenergic antagonists for all adrenal ablations is advocated [1,11–15].
Adrenal biopsy
Percutaneous biopsy is not necessary in patients with benign functioning masses prior to adrenal ablations unlike with renal, lung, or thyroid ablations because hormonal analysis alone can replace the invasive procedure to confirm the histologic diagnoses [2,6]. It is mandatory in patients with extra-adrenal malignancy to determine if an adrenal mass is a metastatic tumor. Percutaneous biopsy should not be performed on the same day as adrenal ablation because the histologic diagnosis takes a couple of days. Accordingly, this procedure can avoid unnecessary ablation of a benign adrenal mass.
Conscious sedation and local anesthesia are just enough to reduce pain during the biopsy procedure. General anesthesia is not necessary when doing an adrenal biopsy alone. The best imaging modality to guide biopsy procedures especially for targeting a small adrenal mass is CT. Ultrasound (US) can be used to target a large adrenal mass which is visible sonographically. Intra-arterial BP monitoring is not necessary because vital signs are usually stable.
Choosing guiding modality
For guiding adrenal ablation, CT is preferred to MRI and US despite radiation exposure because interventional radiologists are familiar with CT-guided procedures (Figs. 1, 2). MRI provides better soft tissue contrast than CT or US. However, MRI-compatible radiofrequency ablation (RFA) or microwave ablation (MWA) is not generally commercially available. US is not a good modality for monitoring ablation area because of its poor sonic window that results in under-treatment or serious complications.
COMPLICATIONS
Hypertensive crisis
Hypertensive crisis can be a severe complication in adrenal ablation. This is defined as an increase in systolic BP more than 180 mm Hg or diastolic pressure more than 120 mm Hg due to massive catecholamine release from the adrenal gland [34–36]. This is observed shortly after initiation of hyperthermal ablation of RFA and MWA, whereas hypertensive crisis can occur during the thawing cycle in cryoablation [11,34,37–40]. This incidence ranges from 0% to 62.5% as reported in previous studies [11,13,37–45]. Although adrenal ablations were successfully performed under local anesthesia in several studies, general anesthesia is strongly recommended to address possible BP surges immediately [2,40]. Premedications for preventing procedural life-threatening hypertension include α- and β-blockers [2,39, 40,46]. A hypertensive crisis can develop even after preoperative preparation [39]. Careful patient monitoring is required, and sodium nitroprusside, nitroglycerin, α- and β-blockers, and calcium-channel blockers can be used during the procedure [11,13,35–39,41–44]. Arrhythmias including tachycardia and premature ventricular contraction accompanied by hypertensive crisis have been reported [38,40].
Other complications
Bleeding is a potential complication for percutaneous ablation [37,43]. Transarterial embolization prior to ablation can be useful for preventing hemorrhagic complications [42,47]. Tests of hemostasis including platelet count and coagulation factors results should be within normal limits [2,4,48]. A system for emergent embolization should be maintained, because transarterial embolization is needed for severe hemorrhagic complications [43].
Thermal damage to surrounding critical organs or structures can occur. Appropriate patient position and hydro dissection can contribute to safe treatment (Figs. 1, 2) [4,41,43,44,49].
Pneumothorax can develop if an ablation needle is inserted during a trans-pulmonary approach with an incidence of 2% to 25% [12,13,41,43]. Positioning the patient ablation-side down is preferred to avoid pneumothorax (Fig. 1) [2]. For the treatment of developing pneumothorax, needle aspiration or chest tube placement is required [13,41].
Adrenal insufficiency is a rare complication after adrenal ablation because more than 90% destruction is needed to cause the insufficiency [13,38,43,50]. Endocrinology consultation is recommended before adrenal ablation for the patient who has received adrenalectomy on the contralateral side or who will undergo bilateral adrenal ablation [38,43,50].
Abscess formation [51], bradycardia [44], hypovolemic shock [39], and pain [11,38,39,41,44] have also been reported as rare complications.
INFLUENCE ON ADRENAL FUNCTION
Adrenal insufficiency is rare even though viable adrenal tissue is left as low as 10%. Therefore, adrenal ablation of a unilateral adrenal tumor does not influence adrenal function if the other gland is normal [43,52,53]. Theoretically, selective ablation of adrenal tumors preserves more normal adrenal tissue than adrenalectomy. For this reason, adrenal ablation may provide a longer adrenal insufficiency-free survival period in hereditary recurrent adrenal tumors compared to adrenalectomy (Figs. 1, 2) [54,55].
If a cortisol-producing adenoma is treated with adrenal ablation as well as adrenalectomy, steroid replacement is necessary because cortisol production is suppressed in the residual adrenal tissue due to long-term suppression of ACTH in Cushing syndrome [41,56].
ADRENOHEPATIC FUSION
Adrenohepatic fusion (AHF) is defined as the histological mixture of the right adrenocortical and hepatic tissues and adrenocortical cells and hepatocytes co-exist [57–60]. Theoretically, all kinds of adrenal or hepatic tumors may occur in the AHF. Therefore, tumor margin should be sufficiently treated with thermal ablation to avoid residual or recurrent tumor in the liver [19,20]. Unresected adrenal tumor may be left after adrenalectomy due to AHF. This residual tumor can be treated with thermal ablation for salvage treatment [19].
ABLATION MODALITIES
Radiofrequency ablation
RFA is the most widely used hyperthermal ablation technique for solid tumors including both benign and malignant adrenal tumors [13,37,41–45]. RFA induces thermal damage by delivering electrical energy into target tissue resulting in coagulation necrosis [61].
Electrical current travels between the needle and grounding pads on the skin in a monopolar system or among needles in a multipolar system [13,49,61–63]. Tissue temperature rising is generated by resistive heating by a high frequency electrical current of 375 to 500 kHz [61]. A tissue temperature exceeding 60°C can kill tumor cells immediately [63].
Among various ablation techniques, RFA is characterized by long-term use, a well-established safety profile, and widespread availability [2,4,51]. Compared with the hyperthermal ablation technique of MWA, the RFA needle is a smaller gauge, but tissue temperature increase is slower, ablation time is longer, and ablation volume with a single needle is smaller [2,38,43]. Precise evaluation of the ablated area in the monitoring image is difficult in RFA compared with cryoablation [64,65]. The heat sink effect is another weak point of RFA, therefore transarterial embolization can be performed prior to RFA to reinforce the treatment effect [42,66,67].
Cryoablation
Cryoablation is a minimally invasive technique which causes cell death by freezing. This ablation causes direct cell injury based on two biophysical changes [68]: osmotic dehydration of the cells [69] and the formation of intracellular ice. The predominance of one type of injury mechanism over the other depends on the cooling rate, the end temperature, the time tissue is held at the minimum temperature and the thawing rate [70].
The main advantage of cryoablation for adrenal tumor over other heat-based ablation techniques is clear visualization of the ablation zone under CT or magnetic resonance guidance. Unwanted injury to adjacent vulnerable structures can be avoided and ablation completeness can be assured. Also, cryoablation allows applying multiple probes simultaneously and thus has greater flexibility to create various isotherm shapes.
Hypertensive crisis has been reported during adrenal cryoablation [46,52], which usually occurs during the active thaw cycle. This may be due to a large release of catecholamines from lysed cells during active thawing.
Microwave ablation
MWA uses frequencies from 900 to 2,450 MHz which is between infrared radiation and radiowaves [71]. MWA induces agitation of water molecules, resulting in cell death by means of coagulation necrosis. The electrical charge of water molecules flips back and forth 2–5×109 times per second according to the frequency of the microwave energy [72].
MWA is a more recent ablation modality compared to other ablation techniques. It offers higher tissue temperature, larger ablation volume, faster ablation times, and less heat-sink effect compared to RFA [72–76]. Recently, MWA has been used to treat adrenal benign and metastatic tumors [77–82] as well as liver, kidney, and lung tumors. The outcomes of this ablation technique have been reported to be excellent although long-term follow-up data are not still available [77–82].
FOLLOW-UP PROTOCOL
Imaging follow-up may be necessary in case of functional adrenal tumors. For pheochromocytoma or paraganglioma, the levels of catecholamines may be normal when they are small in size. Accordingly, regular check-up of hormones alone may result in missing recurrent pheochromocytoma following thermal ablation. For another reason, regular imaging examination may be necessary because it is not uncommon that adrenal masses are incompletely treated with thermal ablation. However, further investigation will be necessary to determine the necessity or follow-up interval of post-ablation imaging examination.
Adrenal hyperplastic nodules can show early wash in and out of contrast material as if they were adenomas [83]. Moreover, pheochromocytoma can manifest as a distant metastasis and thus catecholamine levels should be regularly measured even if follow-up imaging examination is negative. Radio-isotope scans is useful to detect metastatic pheochromocytomas [84,85].
Non-functioning malignant adrenal tumors need to be followed up with regular imaging examination to determine if there is local tumor progression [2,43]. CT is most commonly used to determine if there is local tumor progression or distant metastasis. PET-CT may be necessary for staging work up in recurrent cases.
TREATMENT OUTCOMES
At present, long-term data detailing the outcomes of adrenal ablation remain scarce, consisting mainly of retrospective case series with no prospective trials. This is confounded by the mixture of both primary and metastatic disease of different origins in most series, and the use of different ablation techniques [1,11–15,86]. Nevertheless, the ability to achieve complete ablation and technical success is high, and complete ablation is between 92% and 96% can be expected to have a low complication rate [1,11–15,86]. This is a significant improvement from earlier series that showed a 79% technical success rate [13] for clinical outcomes associated with hormonally active adrenal tumors such as aldosteronoma, cortisol-secreting adenoma, and pheochromocytoma [6,87–92]. The majority of the current data is centered around RFA, with ablation of aldosteronomas being more commonly performed than the ablation of cortisol-secreting adenomas or pheochromocytomas [87–92]. Short- and long-term resolution of biochemistry can range from between 90% to 100% (median, 100%) [6,87–92], and normalization of aldosterone, renin, and aldosterone to renin ratio can be achieved in almost all cases of aldosteronoma [87–90,92]. However, persistent hypertension can occur despite normalization of hormones [87,88,90,92].
Outcomes for ablation of metastatic adrenal lesions on the other hand are not as good compared to benign adrenal tumors [13,43,44,93]. The local control rate ranges from 77% to 80% (median, 78%), and outcome is dependent on the size of the metastatic deposits, which are typically larger than their benign counterpart, ranging from 3.3 to 4.0 cm [2,13,43,44,93]. For this reason, cryoablation or MWA with the ability to produce a larger ablation zone than RFA is preferred. Overall survival remains poor due to the advanced oncological stage of metastatic disease, even in the context of oligometastatic adrenal metastasis [2,13,43,44,93]. Ablation of malignant primary adrenal tumors, such as adrenocortical carcinomas is rare, as adrenalectomy is the current standard of care.
CONCLUSIONS
The first version of ACTA guidelines will be useful for both experts and beginners who are tasked with performing adrenal ablation because they must be aware of knowledge from basic principles to technical tips. Prior to adrenal ablation, re-reading these guidelines may improve treatment outcomes. Achieving good clinical outcomes also requires a multi-disciplinary approach, in which endocrinologists, surgeons, pathologists, and anesthesiologists as well as radiologists need to participate to discuss how to triage or treat adrenal tumors.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We appreciate Dr. Jung Hwan Baek, and Dr. Hyun Chul Rhim helping many things to initiate adrenal ablation guidelines.