Efficacy and Safety of the Novel Dipeptidyl Peptidase-4 Inhibitor Gemigliptin in the Management of Type 2 Diabetes: A Meta-Analysis
Article information
Abstract
Background
No meta-analysis has holistically analysed and summarised the efficacy and safety of gemigliptin in type 2 diabetes. The meta-analysis addresses this knowledge gap.
Methods
Electronic databases were searched for randomised controlled trials (RCTs) involving diabetes patients receiving gemigliptin in the intervention arm and placebo/active comparator in the control arm. The primary outcome was change in haemoglobin A1c (HbA1c). The secondary outcomes were alterations in glucose, glycaemic targets, lipids, insulin resistance, and adverse events.
Results
Data from 10 RCTs involving 1,792 patients were analysed. Four had an active control group (ACG), with metformin/dapagliflozin/sitagliptin/glimepiride as the active comparator; six had a passive control group (PCG), with placebo/rosuvastatin as controls. HbA1c reduction by gemigliptin at 24 weeks was comparable to ACG (mean difference [MD], 0.09%; 95% confidence interval [CI], −0.06 to 0.23; P=0.24; I2=0%; moderate certainty of evidence [MCE]), but superior to PCG (MD, −0.91%; 95% CI, −1.18 to −0.63); P<0.01; I2=89%; high certainty of evidence [HCE]). Gemigliptin was superior to PCG regarding achieving HbA1c <7% (12 weeks: odds ratio [OR], 5.91; 95% CI, 1.34 to 26.08; P=0.02; I2=74%; 24 weeks: OR, 4.48; 95% CI, 2.09 to 9.60; P<0.01; I2=69%; HCE). Gemigliptin was comparable to ACG regarding achieving HbA1c <7% after 24 weeks (OR, 0.92; 95% CI, 0.52 to 1.63; P=0.77; I2=66%; MCE). Adverse events were similar between the gemigliptin and control groups (risk ratio [RR], 1.06; 95% CI, 0.82 to 1.36; P=0.66; I2=35%; HCE). The gemigliptin group did not have increased hypoglycaemia (RR, 1.19; 95% CI, 0.62 to 2.28; P=0.61; I2=19%; HCE).
Conclusion
Gemigliptin has good glycaemic efficacy and is well-tolerated over 6 months of use.
INTRODUCTION
Gemigliptin is a potent, selective, and competitive dipeptidyl peptidase-4 inhibitor (DPP4i), approved for clinical use in more than 11 different countries across the globe, including South Korea, India, and several Central American and South American countries [1]. It is being been evaluated for clinical use in Germany, China, Russia, and Thailand [1]. Gemigliptin has been shown to exert more potent DPP-4 inhibition for 24 hours than sitagliptin (the flagship molecule of the DPP4i class) at the same dose in rats, dogs, and monkeys [2]. Gemigliptin has more than 23,000 times higher selectivity for the DPP4 enzyme than all other DPP enzymes including DPP8, DPP9, and fibroblast activation protein-alpha [3]. Gemigliptin binds to the S1, S2, and S2 extensive subsites of the DPP4 enzyme; specifically, the interaction of the active part of the S2 extensive subsite with CF3 groups on gemigliptin, a pyrimidine-piperidine derivative, explains the increased selectivity and potency of gemigliptin compared to other DPP4i drugs [1,2]. In humans, gemigliptin has 63.4% and 27.1% excretion from the urine and faeces respectively, highlighting a balanced excretion pattern; hence, it is likely to be safe in patients with mild to moderate renal or hepatic disease [3]. Gemigliptin has been demonstrated not to require dose adjustment in people on renal replacement therapy [4], and has been found to be safe in mild to moderate hepatic insufficiency [5]. Several studies have documented the glycaemic efficacy of gemigliptin, either alone or in combination with metformin, other oral anti-diabetes medications, and insulin in the management of type 2 diabetes mellitus (T2DM) [6]. More than 10 randomised controlled trials (RCTs) have been published on the use of gemigliptin in different clinical scenarios of T2DM from different countries across the globe [6]. However, to date, no meta-analysis has holistically analysed and summarised the clinical efficacy and safety of this novel DPP4i. Hence, the aim of this meta-analysis was to evaluate the efficacy and safety of gemigliptin in the management of T2DM.
METHODS
Methodology
The meta-analysis was carried out according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [7]. The predefined protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number, CRD42020190625). All RCTs published till May 2020 were considered for this meta-analysis. This meta-analysis has been reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), the completed checklist of which can be found at the end of the manuscript [7]. Since ethical approval was already given for the individual studies included in the meta-analysis, no separate approval was required for this study.
The PICOS criteria were used to screen and select the studies for this meta-analysis, with the patients (P) being people living with T2DM; the intervention (I) being the use of gemigliptin for managing T2DM; the control (C) being patients either on placebo or any other approved medication for managing T2DM; and the outcomes (O) being impact on haemoglobin A1c (HbA1c), fasting plasma glucose (FPG), post-prandial glucose (PPG), and any adverse effects noted. Only patients with T2DM were considered for this meta-analysis. Patients with other forms of diabetes were excluded. Studies were only included in this meta-analysis if they had at least two treatment arms/groups, with one of the groups having patients with T2DM on gemigliptin either alone or a part of standard diabetes treatment regimen and the other arm/group receiving either placebo or any other diabetes medication in place of gemigliptin, either along with or as a part of standard diabetes treatment regimen. These criteria were chosen to reflect the goal of this meta-analysis to evaluate the efficacy and safety of gemigliptin in T2DM.
The primary outcome was changes in HbA1c. The secondary outcomes of this study were alterations in FPG, PPG, percentage of patients achieving HbA1c <7% and 6.5% at the end of the study, adverse events reported, hypoglycaemia, serum lipid parameters, insulin resistance parameters, inflammatory markers, and glycaemic variability parameters. Glycaemic outcomes were analysed based on whether the control group received an active comparator (any other anti-diabetes/blood glucose lowering medication), labelled here as the active control group (ACG), or a placebo/any other non-diabetes medication, labelled as the passive control group (PCG).
Search method for identification of studies
The electronic databases of MEDLINE (via PubMed), Embase (via Ovid SP), Cochrane Central Register of Controlled Trials (CENTRAL) (for trials only), ctri.nic.in, clinicaltrials.gov, Global Health, and Google scholar were searched using a Boolean search strategy: (gemigliptin) AND (diabetes).
Data extraction and study selection
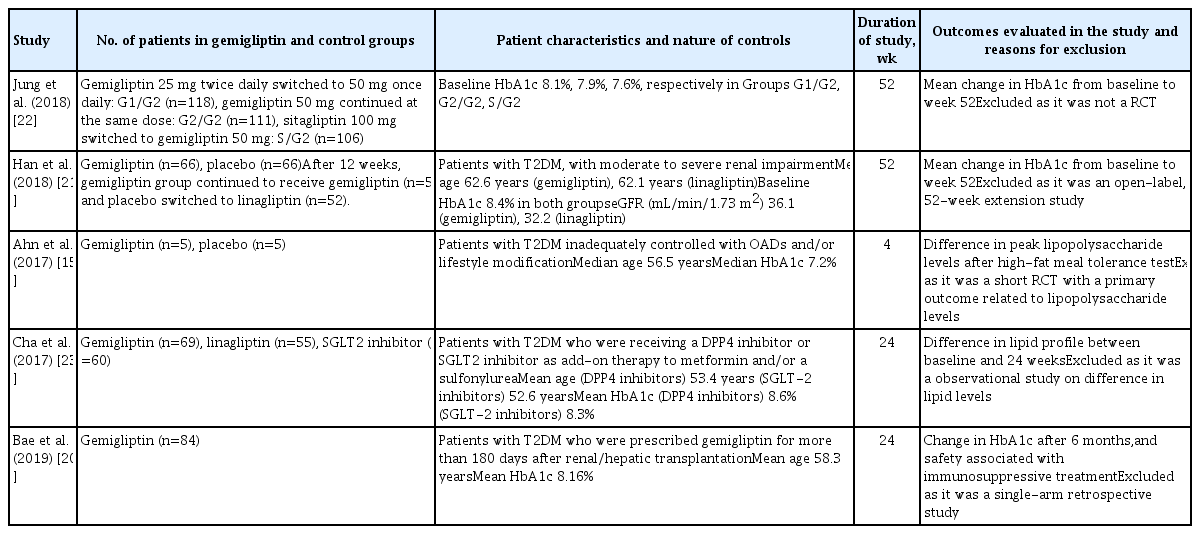
Data extraction was carried out independently by two authors using standard data extraction forms. In cases where multiple publications from a single study group were found, the results were grouped and relevant data from each report were used in the analyses. Data on the primary and secondary outcomes as described above were extracted. Patient characteristics (including demographic information and comorbidities) from the different studies included and excluded from the analysis were noted in tabular form (Tables 1, 2). All disagreements were resolved by the third and fourth authors.

Characteristics of Patients in the Randomised Controlled Trials Evaluated in this Meta-Analysis on the Use of Gemigliptin in Type 2 Diabetes Mellitus
Assessment of risk of bias in included studies
Three authors independently assessed the risk of bias using the risk of bias assessment tool in Review Manager (RevMan) version 5.3 (The Cochrane Collaboration, Oxford, UK, 2014) software. The following points were taken into consideration: whether there was adequate sequence generation (selection bias), whether the allocation was adequately concealed (selection bias), whether knowledge of the allocated interventions was adequately prevented during the study, whether participants and personnel were appropriately blinded (performance bias), whether the outcome assessors were blinded (detection bias), whether incomplete outcome data were adequately addressed (attrition bias), whether the report of the study was free of suggestion of selective outcome reporting (reporting bias), and whether the study was apparently free of other problems that could put it at risk of bias. Any disagreements were resolved by the fourth author.
Measures of treatment effect
For continuous variables, the outcomes were expressed as mean differences (MDs). Conventional units were used for the analysis, and all results reported in SI units were converted to conventional units. For dichotomous outcomes (treatment success) results were expressed as risk ratios (RRs) with 95% confidence intervals (CIs). For adverse events, results were expressed as post-treatment absolute risk differences. RevMan version 5.3 was used to compare the MDs of the primary and secondary outcomes between the gemigliptin and the control groups of the included studies.
Dealing with missing data
Any additional information required from the original authors was requested by e-mail, and any relevant information thus obtained was included in the meta-analysis. Evaluations of important numerical data, such as screened and randomised people, as well as the intention-to-treat, as-treated, and per-protocol populations, were carefully performed. Attrition rates (e.g., drop-outs, patients lost to follow-up, and withdrawals) were investigated.
Assessment of heterogeneity
Heterogeneity was initially assessed by studying the forest plot generated for the primary and secondary outcomes of this study. Subsequently, heterogeneity was analysed using the chi-square test on N-1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test [8]. The interpretation of I2 values is as follows: 0% to 40%: heterogeneity may not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; and 75% to 100%: considerable heterogeneity. The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of the evidence for heterogeneity (e.g., P value from the chi-square test, or the CI for I2) [8].
Grading of the results
Overall grading of the evidence (certainty of the evidence) related to each of the primary and secondary outcomes of the meta-analysis was done using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach [9]. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias [9]. The GRADEpro Guideline Development Tool software (McMaster University and Evidence Prime Inc., Hamilton, ON, Canada, 2015) was used to create the summary of findings table in this meta-analysis (Table 3). Certainty of evidence was graded into four categories, namely “high” (there is a high level of confidence that the true effect lies close to that of the estimated effect), “moderate” (there is moderate confidence in the estimated effect: the true effect is likely to be close to the estimated effect, but there is a possibility that it is substantially different), “low” (there is limited confidence in the estimated effect: the true effect might be substantially different from the estimated effect) and, “very low” (there is very little confidence in the estimated effect: the true effect is likely to be substantially different from the estimated effect) [9].
Publication bias was assessed using funnel plots, which specifically target small study bias, in which small studies tend to show larger estimates of effects and greater variability than larger studies [9]. The presence of one or more of the smaller studies outside the inverted funnel plot was interpreted as evidence for the presence of significant publication bias [10].
Data synthesis
Data were pooled using a random-effect model for the analysis of primary and secondary outcomes. The outcomes were expressed as 95% CIs. Forest plots were plotted with the left side of the graph favouring gemigliptin and the right side of the graph favouring the control using RevMan 5.3 software. P values <0.05 were considered to indicate statistical significance.
RESULTS
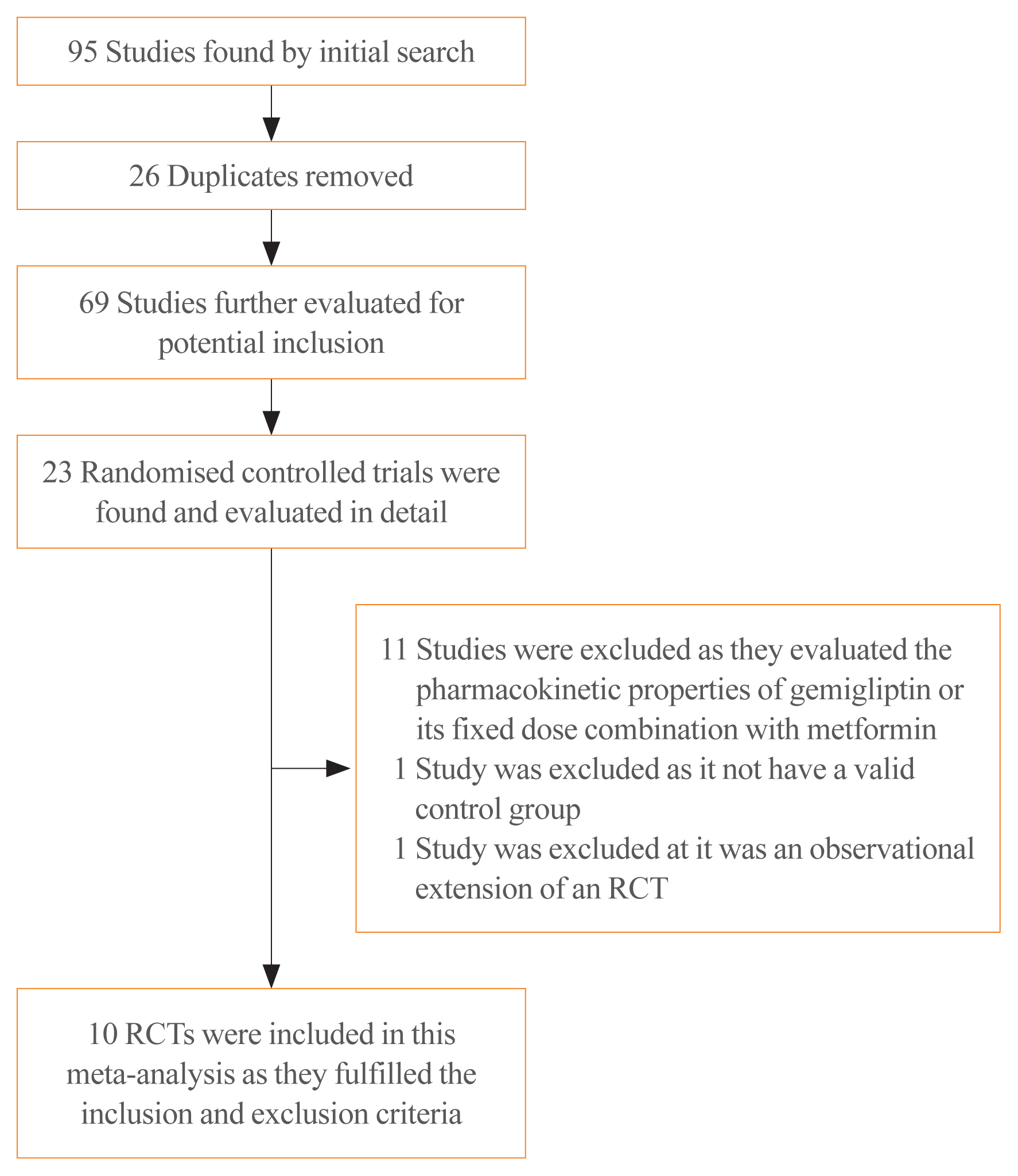
A total of 95 articles were found through the initial search (Fig. 1). After screening of the titles and abstracts, followed by screening of the full texts, the search was reduced to 23 studies, which were evaluated in detail for inclusion in this meta-analysis (Fig. 1). Ten RCTs in people with T2DM that fulfilled all the inclusion criteria were analysed in this meta-analysis [11–20]. Thirteen studies were excluded as they either evaluated the pharmacokinetic and pharmacodynamic properties of gemigliptin, did not have a valid control group, or were observational extensions of RCTs.
Of the 10 RCTs included in this meta-analysis, a subgroup analysis was done based on the nature of the control group. Four studies that had an anti-diabetes medication in the control group were analysed as the ACG. The active controls in the studies by Kwak et al. [11], Lim et al. [12], and Rhee et al. [13] were dapagliflozin (10 mg/day), metformin (2 g/day), and sitagliptin (100 mg/day), respectively. The study by Park et al. [14] had two ACGs, one of them receiving sitagliptin (100 mg/day) and the other receiving glimepiride (2 mg/day). Hence, the results of that study were analysed separately; the findings comparing the outcomes of gemigliptin with sitagliptin are designated as “Park a” in the forest plots, and the outcomes of gemigliptin compared to glimepiride are presented as “Park b” [14]. The remaining six studies, which included placebos or any other medications without blood glucose-lowering properties in the control group (e.g., anti-lipid medications) were analysed as the PCG. The PCG included the studies by Ahn et al. [15], Cho et al. [16], Rhee et al. [17], Yang et al. [18], and Yoon et al. [19], which had placebos in the control arm, and the study by Bae et al. [20], which had an anti-lipid medication (rosuvastatin 20 mg/day) in the control arm.
The details of the studies included in this meta-analysis are shown in Table 1. The studies that were evaluated but excluded are summarized in Table 2 [15,20–23]. The study by Han et al. [21] was excluded as it was an open observational extension of an RCT wherein the placebo was replaced with linagliptin.
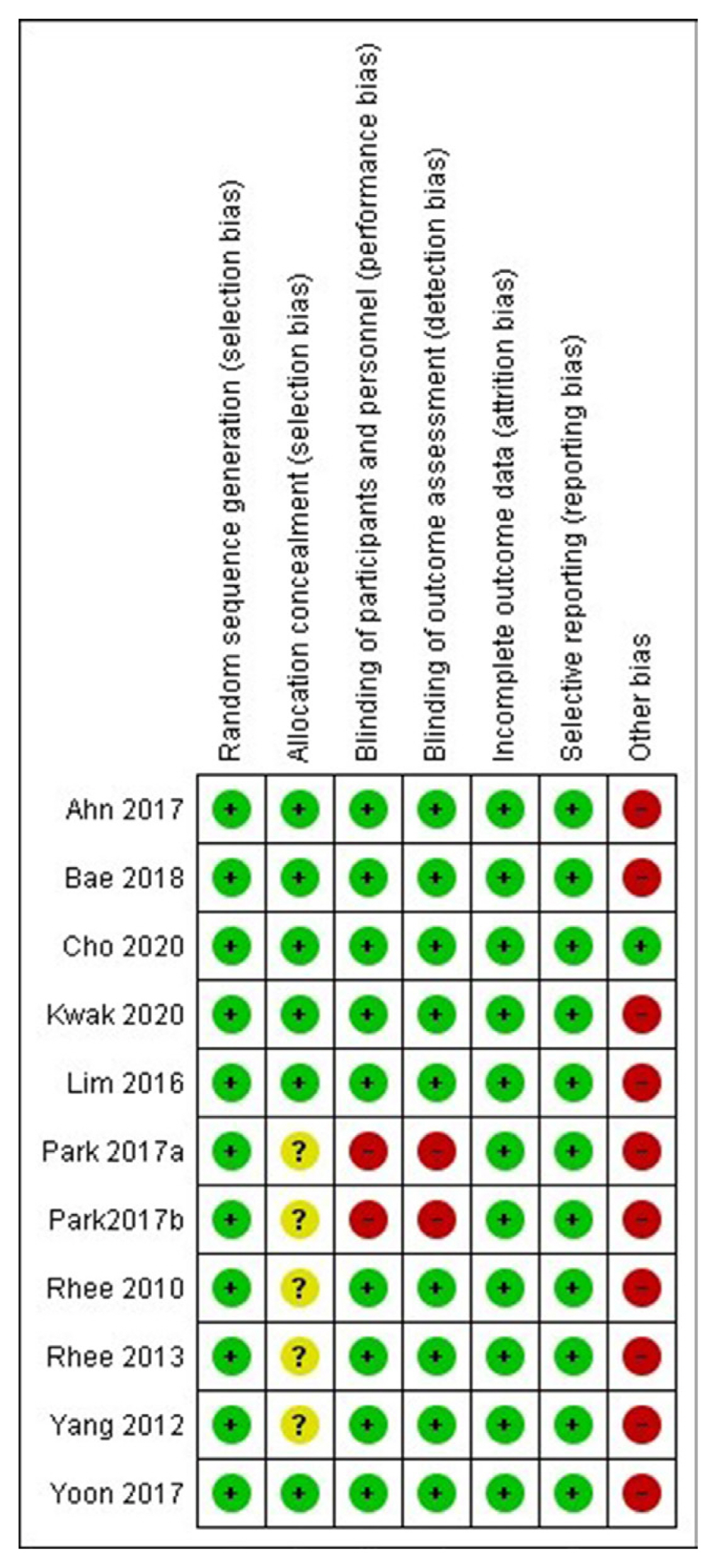
Risk of bias in the included studies
The summaries of risk of bias of the 10 studies included in the meta-analysis are elaborated in Figs. 2, 3, Supplemental Table S1. Random sequence generation, attrition bias, and reporting bias were judged to be at a low risk of bias in all 10 studies (100%). Allocation concealment bias (selection bias) was judged to be at a low risk in six studies (60%). Performance bias (blinding of participants and investigators) and detection bias (blinding of outcome assessors) were judged to be at a low risk of bias in eight studies (80%). The source of funding (especially pharmaceutical), presence of authors from pharmaceutical organizations, and conflicts of interests were scrutinised under the category of “other bias,” which was judged to be at a low risk in only one out of 10 studies (10%) (Figs. 2, 3).

Risk of bias graph presenting the review authors’ judgements about each risk of bias item shown as percentages across all included studies.
Effects of gemigliptin on outcomes
HbA1c
Data from four studies involving 359 people with T2DM were analysed to identify the impact of gemigliptin on HbA1c after 12 weeks of treatment. Individuals receiving gemigliptin (50 mg/day) had a significantly greater reduction of HbA1c than those in the ACG (dapagliflozin 10 mg/day, sitagliptin 100 mg/day, or glimepiride 2 mg/day) (MD, −0.30%; 95% CI, −0.35 to −0.25; P<0.01; I2=0% [low heterogeneity]) (Supplemental Fig. S1A) as well as the PCS (MD, −1.06%; 95% CI, −1.3 to −0.81; P<0.01; I2=2% [low heterogeneity]) (Supplemental Fig. S2A).
Data from six studies involving 1,412 people with T2DM were analysed to determine the impact of gemigliptin on HbA1c after 24 weeks of treatment. Individuals receiving gemigliptin (50 mg/day) had a comparable reduction of HbA1c to those in the ACG (metformin 2 g/day or sitagliptin 100 mg/day) (MD, 0.09%; 95% CI, −0.06 to 0.23; P=0.24; I2=0% [low heterogeneity]; moderate certainty of evidence [MCE]) (Fig. 4A) after 24 weeks of therapy. Patients receiving gemigliptin had a significantly greater HbA1c reduction than those in the PCS (MD, −0.91%; 95% CI, −1.18 to −0.63; P<0.01; I2=89% [considerable heterogeneity]; high certainty of evidence [HCE]) (Fig. 5A), after 24 weeks of therapy.
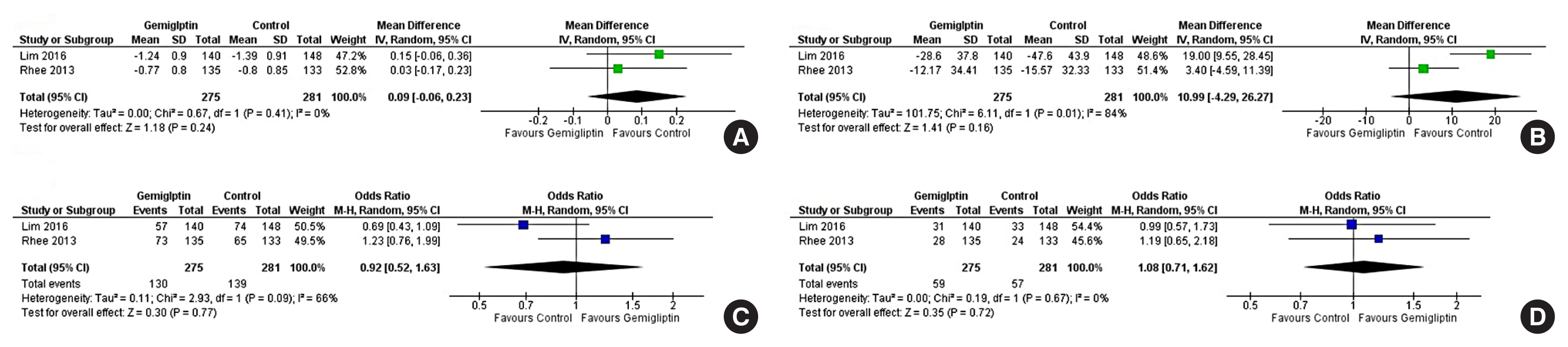
Forest plot highlighting the impact of gemigliptin as compared to the active control group after 24 weeks of therapy on (A) haemoglobin A1c (HbA1c), (B) fasting glucose, (C) the percent of people achieving HbA1c <7%, (D) the percent of people achieving HbA1c less than 6.5%. SD, standard deviation; IV, inverse variance; CI, confidence interval; M-H, Mantel-Haenszel.
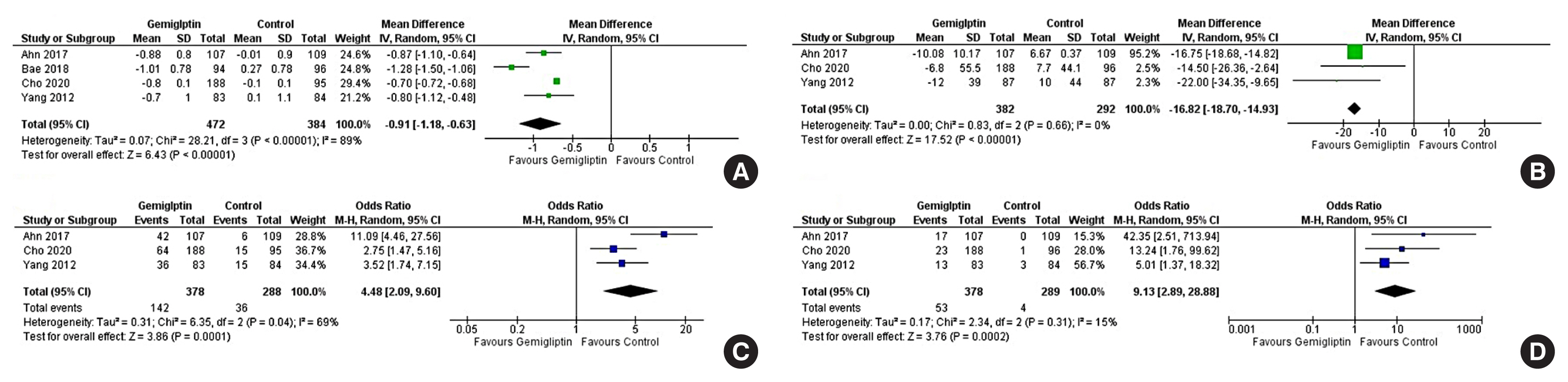
Forest plot highlighting the impact of gemigliptin as compared to the passive control group after 24 weeks of therapy on (A) haemoglobin A1c (HbA1c), (B) fasting glucose, (C) the percent of people achieving HbA1c <7%, (D) the percent of people achieving HbA1c less than 6.5%. SD, standard deviation; IV, inverse variance; CI, confidence interval; M-H, Mantel-Haenszel.
Fasting glucose
Data from four studies involving 359 people with T2DM were analysed to determine the impact of gemigliptin on FPG after 12 weeks of treatment. Individuals receiving gemigliptin (50 mg/day) had a comparable reduction of FPG to those in the ACG (dapagliflozin 10 mg/day, sitagliptin 100 mg/day, or glimepiride 2 mg/day) (MD, −0.13 mg/dL; 95% CI, −1.84 to 1.57; P=0.88; I2=0% [low heterogeneity]) (Supplemental Fig. S2B), but a significantly greater reduction than those in the PCG (MD, −29.08 mg/dL; 95% CI, −32.32 to −25.85; P<0.01; I2=0% [low heterogeneity]) (Fig. 5B) after 12 weeks of therapy.
Data from five studies involving 1,230 people with T2DM were analysed to evaluate the impact of gemigliptin on FPG after 24 weeks of treatment. Changes in FPG were not significantly different in patients receiving gemigliptin as compared to those in the ACG (metformin 2 g/day or sitagliptin 100 mg/day) (MD, 10.99 mg/dL; 95% CI, −4.29 to 26.27; P=0.16; I2=84% [considerable heterogeneity]; MCE) (Fig. 4B). Patients receiving gemigliptin had a significantly greater reduction in FPG than those in the PCG (MD, −16.82 mg/dL; 95% CI, −18.70 to −14.93; P<0.01; I2=0% [low heterogeneity]; HCE) (Fig. 5B) after 24 weeks of therapy.
Percentage of people achieving HbA1c <7%
Data from two studies (199 patients) and five studies (1,222 patients) were analysed to evaluate the impact of gemigliptin on attaining a glycaemic target of HbA1c <7% after 12 and 24 weeks of treatment, respectively. A significantly greater number of patients on gemigliptin achieved HbA1c <7% than those in the PCG after 12 weeks (odds ratio [OR], 5.91; 95% CI, 1.34 to 26.08); P=0.02; I2=74% [considerable heterogeneity]) (Supplemental Fig. S2C) and 24 weeks (OR, 4.48; 95% CI, 2.09 to 9.60; P<0.01; I2=69% [moderate heterogeneity]; HCE) (Fig. 5C) of therapy. Gemigliptin was comparable to the ACG (metformin or sitagliptin) regarding the percent of patients achieving HbA1c <7% after 24 weeks of therapy (OR, 0.92; 95% CI, 0.52 to 1.63; P=0.77; I2=66% [moderate heterogeneity]; MCE) (Fig. 4C). Similar data after 12 weeks of therapy were not available.
Percentage of people achieving HbA1c <6.5%
Data from one study (69 patients) and five studies (1,223 patients) were analysed to evaluate the impact of gemigliptin on attaining a glycaemic target of HbA1c <6.5% after 12 and 24 weeks of treatment, respectively. A greater number of patients on gemigliptin had HbA1c <6.5% than those in the PCG after 12 weeks (OR, 2.58; 95% CI, 0.61 to 10.97; P=0.20) (Supplemental Fig. S2D) and 24 weeks (OR, 9.13; 95% CI, 2.89 to 28.88; P<0.01; I2=15% [low heterogeneity]; HCE) (Fig. 5D) of treatment. Gemigliptin was non-inferior to the ACG (metformin or sitagliptin) regarding the percent of patients achieving HbA1c <6.5% after 24 weeks of therapy (OR, 1.08; 95% CI, 0.71 to 1.62; P=0.72; I2=0% [low heterogeneity]; HCE) (Fig. 4D). Similar data after 12 weeks of therapy were not available.
Safety
Data from 10 studies (1,792 patients) were analysed to evaluate the impact of gemigliptin on the occurrence of adverse events—both total adverse events (TAEs) and severe adverse events (SAEs)—over 12 to 24 weeks of treatment. The occurrence of TAEs was not statistically different in patients receiving gemigliptin as compared to the control group (RR, 1.06; 95% CI, 0.82 to 1.36; P=0.66; I2=35% [low heterogeneity]; HCE) (Fig. 6A). The most common adverse event noted across RCTs was upper respiratory manifestations, primarily nasopharyngitis. Other common mild adverse events were arthralgias, headache, cough, diarrhoea, hypertension, and urinary infections. The occurrence of all adverse events was comparable across study groups. The occurrence of SAEs was not statistically significantly different in patients receiving gemigliptin as compared to the control group (RR, 0.85; 95% CI, 0.50 to 1.45; P=0.56; I2=0% [low heterogeneity]; HCE) (Fig. 6B).

Forest plot highlighting the side effect profile of the use of gemigliptin as compared to controls focussing on (A) total adverse events, (B) severe adverse events, (C) total hypoglycaemic episodes, (D) death. M-H, Mantel-Haenszel; CI, confidence interval.
Data from nine studies (1,520 patients) were analysed to evaluate the occurrence of hypoglycaemia in patients receiving gemigliptin as compared to controls, over 12 to 24 weeks of treatment. Patients receiving gemigliptin did not have an increased occurrence of hypoglycaemia as compared to the controls (RR, 1.19; 95% CI, 0.62 to 2.28; P=0.61; I2=19% [low heterogeneity]; HCE) (Fig. 6C). The occurrence of symptomatic hypoglycaemia (RR, 1.27; 95% CI, 0.61 to 2.63; P=0.52; I2=11% [low heterogeneity]) (Supplemental Fig. S3A) and asymptomatic hypoglycaemia (RR, 1.13; 95% CI, 0.53 to 2.40; P=0.76; I2=0% [low heterogeneity]) (Supplemental Fig. S3B) was similar in patients receiving gemigliptin as compared to the controls.
Data from 10 studies (1,788 patients) were analysed to evaluate all-cause mortality/death over 12 to 24 weeks of therapy. A total of four deaths were reported across all 10 studies during the study period. Mortality was not significantly different among patients receiving gemigliptin as compared to controls (hazard ratio, 1.17; 95% CI, 0.11 to 12.19; P=0.89; I2=13% [low heterogeneity]; HCE) (Fig. 6D).
Lipid parameters
Data from six studies (1,137 patients) were analysed to evaluate the impact of gemigliptin on serum triglycerides over 12 to 24 weeks of treatment. Patients receiving gemigliptin did not have significantly different serum triglyceride levels as compared to those in the control groups (MD, 9.47 mg/dL; 95% CI, −6.42 to 25.36; P=0.24; I2=87% [considerable heterogeneity]; MCE) (Supplemental Fig. S4A). Data from seven studies (1,271 patients) were analysed to evaluate the impact of gemigliptin on serum low-density lipoprotein cholesterol (LDL-C) over 12 to 24 weeks of treatment. Patients receiving gemigliptin did not have significantly different serum LDL-C levels as compared to those in the control groups (MD, 2.23 mg/dL; 95% CI, −10.04 to 14.51; P=0.72; I2=96% [considerable heterogeneity]; HCE) (Supplemental Fig. 4B). Data from six studies (1,145 patients) were analysed to evaluate the impact of gemigliptin on serum high-density lipoprotein cholesterol (HDL-C) levels over 12 to 24 weeks of treatment. Patients receiving gemigliptin did not have a significantly different change in HDL-C as compared to the controls (MD, −1.65 mg/dL; 95% CI, −3.59 to 0.28; P=0.09; I2=79% [low heterogeneity]; MCE) (Supplemental Fig. S4C). Data from six studies (1,085 patients) were analysed to evaluate the impact of gemigliptin on serum total cholesterol over 12 to 24 weeks of treatment. Patients receiving gemigliptin had significantly lower serum total cholesterol levels than the controls (MD, −5.55 mg/dL; 95% CI, −8.89 to −2.21; P<0.01; I2=27% [low heterogeneity]; MCE) (Supplemental Fig. S4D).
Body weight
Data from six studies (1,185 patients) were analysed to evaluate the impact of gemigliptin on body weight over 12 to 24 weeks of treatment. Patients receiving gemigliptin did not have a significantly different change in body weight after 12 to 24 weeks of therapy when compared to the PCS (MD, 0.84 kg; 95% CI, −0.37 to 2.06; P=0.17; I2=95% [considerable heterogeneity]; MCE) (Supplemental Fig. S5A).
Renal parameters
Data were analysed from a single study done in 130 patients with moderate to severe diabetic kidney disease evaluating the impact on the glomerular filtration rate (GFR) and the urinary albumin-creatinine ratio (ACR). There was a mild, but statistically significant greater, reduction in GFR in people receiving gemigliptin as compared to placebo (MD, −1.99 mL/min; 95% CI, −3.49 to −0.49; P<0.01). There was no significant difference in the urinary ACR in people receiving gemigliptin as compared to placebo (MD, −84.6 mg/g; 95% CI, −213.80 to 44.6; P=0.20).
Post-prandial blood glucose and glycaemic variability
Data from two studies (379 patients) were analysed to evaluate the impact of gemigliptin on 2-hour PPG over 12 to 24 weeks of treatment. Patients receiving gemigliptin (50 mg/day) had a significantly lower reduction in PPG than those in the ACG (receiving metformin 2 g/day or sitagliptin 100 mg/day) (MD, 19.76 mg/dL; 95% CI, 4.79 to 34.72; P=0.01; I2=0% [low heterogeneity]) (Supplemental Fig. S3C) after 24 weeks of therapy. Similar data after 12 weeks of therapy for the ACG were not available. Similar data were also not available for the PCG.
Data from three studies (153 patients) were analysed to evaluate the impact of treatment on the mean amplitude of glycaemic excursions (MAGE) over 12 to 24 weeks of treatment. Patients receiving gemigliptin had a significantly lower MAGE than those in the ACG (receiving dapagliflozin 10 mg/day, sitagliptin 100 mg/day, or glimepiride 2 mg/day) (MD, −15.90; 95% CI, −25.99 to −5.82; P<0.01; I2=51% [moderate heterogeneity]) (Supplemental Fig. S3D).
Insulin resistance and systematic inflammatory parameters
Data from seven studies (1,251 patients) were analysed to evaluate the impact of treatment on insulin resistance as estimated using homeostatic model of insulin resistance. Patients receiving gemigliptin did not have significantly different insulin resistance when compared to the controls (MD, −0.13; 95% CI, −0.53 to 0.28; P=0.54; I2=51% [moderate heterogeneity]) (Supplemental Fig. S5B). Data from five studies (833 patients) were analysed to evaluate the impact of treatment on estimated beta cell function (homeostatic model of insulin resistance-beta [HOMA-b]). Patients receiving gemigliptin did not have significantly different HOMA-b when compared to controls (MD, 7.74; 95% CI, −5.58 to 21.06; P=0.25; I2=88% [considerable heterogeneity]) (Supplemental Fig. S5C).
Data from three studies (443 patients) were analysed to evaluate the impact of treatment on systematic inflammation as estimated using high sensitivity C-reactive protein (hs-CRP) levels. Patients receiving gemigliptin had significantly lower hs-CRP levels than controls (MD, −0.35 mg/L; 95% CI, −0.62 to 0.07; P=0.01; I2=72% [considerable heterogeneity]) (Supplemental Fig. S5D).
The key summary of findings focussing on glycaemic outcomes after 24 weeks of therapy and the side effect profile is presented in Table 3. Funnel plots were used to evaluate the presence of publication bias, as shown in Supplemental Fig. S6.
DISCUSSION
This is the first meta-analysis to highlight the glycaemic efficacy; impact on lipid parameters, insulin resistance, and body weight; and tolerability, side effects, and hypoglycaemia profile of gemigliptin used for the management of diabetes, compared against a wide range of other established anti-diabetes medications and controls. The initial phase-2 RCTs established that the glycaemic efficacy of 50, 100, and 200 mg/day of gemigliptin was similar; the 50 mg/day dose had the maximum safety margin, leading to the use of this dose in clinical settings [17].
An important outcome of this meta-analysis was the good and comparable glycaemic efficacy of gemigliptin relative to other established anti-diabetes medications after 12 to 24 weeks of clinical use. Interestingly, gemigliptin use was associated with a greater HbA1c reduction than dapagliflozin (10 mg/day), sitagliptin (100 mg/day), or glimepiride (2 mg/day) after 12 weeks of use. This observation at 12 weeks can be partly explained by the use of a sub-maximal dose of glimepiride in the study (2 mg/day as compared to the maximal dose of 6 to 8 mg/day). However, after 24 weeks of use, the HbA1c reduction with gemigliptin 50 mg/day was similar to that of metformin (2 g/day) and sitagliptin (100 mg/day). The reductions in fasting glucose with gemigliptin were comparable to those with metformin, dapagliflozin, sitagliptin, and glimepiride over 12 to 24 weeks of clinical use. Gemigliptin was consistently superior to placebo with regards to reduction in HbA1c and FPG over 12 to 24 weeks of clinical use.
Hence, it was not surprising to note that the percentage of patients achieving HbA1c <7% and <6.5% over 12 to 24 weeks of clinical use of gemigliptin was comparable to that of all the different anti-diabetes medications used in the different RCTs in this meta-analysis, and superior to that from placebo. Open-label studies with follow-up data for up to 52 weeks demonstrated good glycaemic efficacy with gemigliptin use (mean HbA1c reduction, −1.06%) [13]. In another study involving T2DM patients with renal insufficiency that had open-label follow-up extending to 52 weeks of clinical use, the HbA1c reduction with gemigliptin as compared to linagliptin (relative to baseline) was −1.0% and −0.65%, respectively [21].
Serum LDL-C, HDL-C, and triglycerides were not significantly different in patients receiving gemigliptin as compared to the controls after 12 to 24 weeks of therapy. In fact, serum total cholesterol was significantly lower in patients receiving gemigliptin than in controls. These results need to be taken into account, as one of the studies had rosuvastatin as the comparative agent. Rosuvastatin is a very potent statin with very good cholesterol and triglyceride-reducing properties. Based on the above observations, it may be said that gemigliptin largely has a beneficial impact on the lipid profile through reductions in serum total cholesterol.
Gemigliptin use is associated with a significant reduction in glycaemic variability as compared to other diabetes medications, as evidenced by the significant reduction in MAGE. Low glycaemic variability is an added advantage, and represents the class effect of DPP4i [24]. No significant change in insulin resistance was noted with the use of gemigliptin. Gemigliptin was associated with a significantly greater reduction in hs-CRP levels, a measure of systemic inflammation, than the other diabetes medications. An improvement in glycaemic control in T2DM is inherently associated with a reduction in systemic inflammatory parameters such as hs-CRP. A previous meta-analysis showed that DPP4i use in clinical practice is associated with a reduction in hs-CRP levels, which is a class effect, and the magnitude of reduction in hs-CRP is similar to other anti-diabetes medications [25]. Hence, the greater reduction of hs-CRP with gemigliptin than with other anti-diabetes medications in our meta-analysis is a novel observation, which needs further evaluation and explanation.
The safety data of gemigliptin are reassuring based on this meta-analysis. Gemigliptin was weight-neutral, like other drugs in the DPP4i class. Gemigliptin was well tolerated across all 10 studies involving 1,792 patients. There were no increased risks of TAE, SAE, or hypoglycaemia. Yoon et al. [19] showed that gemigliptin can be safely used in patients with moderate to severe renal insufficiency, with good glycaemic efficacy and tolerability. In open-label, non-randomized studies involving patients with end-stage renal disease, less than 2.9% of gemigliptin was removed through dialysis, and the pharmacokinetic profiles of gemigliptin between dialysis and non-dialysis periods were similar, suggesting that gemigliptin does not need dose adjustment during dialysis [5]. However a mild reduction in the GFR was noted in our analysis, without any significant impact on the urinary ACR. Hence, although mechanistically gemigliptin is believed to be nephron-safe, further studies in larger cohorts of patients are needed across the spectrum of GFR to evaluate renal outcomes over long-term clinical use.
To conclude, this first meta-analysis on the efficacy and safety of gemigliptin in T2DM provides reassuring data on its favourable glycaemic efficacy and tolerability over a 6-month period of clinical use.
Supplementary Information
Risk of Bias Assessment Table
Forest plot highlighting the impact of gemigliptin as compared to the active control group after 12 weeks of therapy on (A) haemoglobin A1c, (B) fasting glucose. SD, standard deviation; IV, inverse variance; CI, confidence interval.
Forest plot highlighting the impact of gemigliptin as compared to the passive control group, after 12 weeks of therapy on (A) haemoglobin A1c (HbA1c), (B) fasting glucose, (C) the percent of people achieving HbA1c <7%, (D) the percent of people achieving HbA1c less than 6.5%. SD, standard deviation; IV, inverse variance; CI, confidence interval; M-H, Mantel-Haenszel.
Forest plot highlighting the side effect profile of the use of gemigliptin as compared to controls focussing on (A) symptomatic hypoglycaemia, (B) asymptomatic hypoglycaemia, (C) post-prandial blood glucose (at 24 weeks), (D) mean average glucose excursion. M-H, Mantel-Haenszel; CI, confidence interval; SD, standard deviation; IV, inverse variance.
Forest plot highlighting the impact of gemigliptin on serum lipid parameters (A) serum triglycerides, (B) serum total cholesterol, (C) low-density lipoprotein cholesterol, (D) high-density lipoprotein cholesterol. SD, standard deviation; IV, inverse variance; CI, confidence interval.
Forest plot highlighting the impact of gemigliptin on (A) body weight, (B) homeostatic model insulin resistance, (C) homeostatic model of estimated beta cell function, (D) high sensitivity C-reactive protein. SD, standard deviation; IV, inverse variance; CI, confidence interval.
Funnel plot of all the included studies in the meta-analysis (assessing the publication bias) of the main outcomes assessed (A) haemoglobin A1c (HbA1c; 24 weeks), (B) fasting glucose (24 weeks), (C) HbA1c <7% (24 weeks), (D) HbA1c <6.5% (24 weeks), (E) total adverse events, (F) severe adverse events, (G) total hypoglycaemic episodes, (H) body weight. SE, standard error; MD, mean difference; OR, odds ratio.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: D.D., M.S. Acquisition, analysis, or interpretation of data: D.D., A.A., R.S., D.K., M.S. Drafting the work or revising: D.D., A.A., I.M., D.K., M.S. Final approval of the manuscript: D.D., R.S., M.S.