Effects of Vildagliptin or Pioglitazone on Glycemic Variability and Oxidative Stress in Patients with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy: A 16-Week, Randomised, Open Label, Pilot Study
Article information
Abstract
Background
Glycemic variability is associated with the development of diabetic complications through the activation of oxidative stress. This study aimed to evaluate the effects of a dipeptidyl peptidase 4 inhibitor, vildagliptin, or a thiazolidinedione, pioglitazone, on glycemic variability and oxidative stress in patients with type 2 diabetes.
Methods
In this open label, randomised, active-controlled, pilot trial, individuals who were inadequately controlled with metformin monotherapy were assigned to either vildagliptin (50 mg twice daily, n=17) or pioglitazone (15 mg once daily, n=14) treatment groups for 16 weeks. Glycemic variability was assessed by calculating the mean amplitude of glycemic excursions (MAGE), which was obtained from continuous glucose monitoring. Urinary 8-iso prostaglandin F2α, serum oxidised low density lipoprotein, and high-sensitivity C-reactive protein were used as markers of oxidative stress or inflammation.
Results
Both vildagliptin and pioglitazone significantly reduced glycated hemoglobin and mean plasma glucose levels during the 16-week treatment. Vildagliptin also significantly reduced the MAGE (from 93.8±38.0 to 70.8±19.2 mg/dL, P=0.046), and mean standard deviation of 24 hours glucose (from 38±17.3 to 27.7±6.9, P=0.026); however, pioglitazone did not, although the magnitude of decline was similar in both groups. Markers of oxidative stress or inflammation including urinary 8-iso prostaglandin F2α did not change after treatment in both groups.
Conclusion
In this 16-week treatment trial, vildagliptin, but not pioglitazone, reduced glycemic variability in individuals with type 2 diabetes who was inadequately controlled with metformin monotherapy, although a reduction of oxidative stress markers was not observed.
INTRODUCTION
Type 2 diabetes is a chronic metabolic disorder characterised by dysregulated insulin action and hyperglycemia. Lowering serum glucose levels is a mainstay of managing type 2 diabetes in order to reduce the risk of micro- and macrovascular complications [1]. Glycated hemoglobin (HbA1c), time-averaged mean glycemia, is a well-established surrogate marker of long-term glycemia [2]. Most clinical trials of type 2 diabetes, therefore, have focused on lowering serum levels of HbA1c. However, emerging evidence suggests that glycemic variability could contribute to the development of diabetes-related vascular complications, additionally or independently beyond HbA1c [34]. Several studies have indicated that glucose fluctuation is more closely associated with oxidative stress or inflammation than mean glycemia, which has been proposed as a putative mediator of diabetes complications [56].
On the basis of this evidence, several trials have examined the role of various glucose-lowering agents in the reduction in glycemic variability. For example, acarbose was shown to reduce glucose excursion, measured by mean amplitude of glycemic excursions (MAGE), as well as mean serum glucose in individuals with diabetes [78]. Vildagliptin also reduced MAGE, and was associated with a reduction of markers of oxidative stress or inflammation [69]. On the other hand, the efficacy of agents other than α-glucosidase inhibitor or dipeptidyl peptidase 4 (DPP-4) inhibitor has not been investigated in terms of glycemic variability. Research evidence, however, indicates that pioglitazone, a thiazolidinedione, also possibly contributed to reducing glycemic variability. Pioglitazone reduced glycemic excursion and had a favorable impact on oxidative stress compared to glibenclamide [10].
Therefore, this study aimed to investigate the effectiveness of different glucose lowering agents, vildagliptin and pioglitazone, on glycemic variability and oxidative stress in patients with type 2 diabetes.
METHODS
Study design and population
A prospective, randomised, open-label, parallel-group, pilot study was performed from September 2008 through January 2015 at the Korea University Anam Hospital and Konkuk University Hospital, Seoul, Korea. The Institutional Review Boards of both Korea University Anam Hospital and Konkuk University Hospital approved the protocol. The trial was registered at ClinicalTrials.gov (NCT01339143).
Eligible participants were >18 years of age, and diagnosed with type 2 diabetes using American Diabetes Association criteria. All participants had been treated with metformin monotherapy at a stable dose (≥1,000 mg per day) for at least 1 month before randomisation, and had HbA1c of ≥7%, and <10%. Exclusion criteria were type 1 diabetes, uncontrolled hyperglycemia (HbA1c ≥10%), uncontrolled hypertension (systolic blood pressure >160 mm Hg, or diastolic blood pressure >100 mm Hg), symptomatic heart failure, and severe hepatic dysfunction (alanine aminotransferase or aspartate aminotransferase levels more than three times the normal upper limit). Those who had been taking medications affecting oxidative stress markers including a 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor, angiotensin-converting enzyme inhibitor, or angiotensin II receptor blocker were not excluded, but were to continue the medications without dose adjustment during the study period. All the individuals provided written informed consent at randomisation.
Of 49 individuals screened, a total of 31 individuals who met the inclusion and exclusion criteria underwent randomisation. Seventeen individuals were assigned to the vildagliptin (50 mg twice daily) group, and 14 individuals to the pioglitazone (15 mg once daily) group. Among them, 25 individuals (n=14 vildagliptin, n=11 pioglitazone) completed the study and were included in the analysis (Fig. 1). At baseline, anthropometric and laboratory measurements were taken after an overnight fast. Height, body weight, and waist circumference were measured, and body mass index (BMI) was calculated. Blood was drawn for biochemical analyses including fasting plasma glucose (FPG), HbA1c, total cholesterol, triglyceride, high density lipoprotein cholesterol, low density lipoprotein (LDL) cholesterol, liver enzymes, and creatinine. C-peptide and insulin were also measured using radioimmunoassay (GammaPro, Seyoung NDC Ltd., Seoul, Korea). A continuous glucose monitoring (CGM) system for measurement of glycemic variability was applied to all participants at baseline and at the final visit. Urine and serum measurements of oxidative stress and inflammatory markers were also taken at the same time.
The primary outcome was a change in glycemic variability measured by MAGE from baseline to week 16 in each treatment arm. Secondary outcomes were changes in oxidative stress and inflammatory markers, FPG, and HbA1c from baseline to week 16.
Continuous subcutaneous glucose monitoring and assessment of glycemic variability
All individuals underwent continuous subcutaneous glucose monitoring (Medtronic, Minneapolis, MN, USA) for 3 consecutive days at the first and last visits. The sensor was inserted on day 1 and removed on day 3 at both visits. Subcutaneous interstitial glucose levels were measured every 5 minutes and automatically stored in the software program. On day 2, a standardised meal was provided to all participants, and venous sampling for FPG was performed for calibration.
The MAGE was measured in a standardised manner proposed by Service et al. [11]. MAGE is defined as the average height of glucose excursions (peak to nadir, or nadir to peak) that exceeds one standard deviation (SD) for a day. A numerical value of SD was used provided by 24 hours CGM data as an additional marker of glycemic variability [12].
Oxidative stress and inflammatory markers
For the measurement of urinary 8-iso prostaglandin F2α (8-iso PGF2α), three consecutive first voided urine samples during applying CMG were used. Samples were measured by Spetramax 190 enzyme-linked immunosorbent assay (ELISA) kit (Cell Biolabs, Seoul, Korea). The intra-assay coefficient of variation was 5.3%. Commercially available ELISAs were also used to measure plasma oxidised LDL (Mercodia Co., Uppsala, Sweden), and high-sensitivity C-reactive protein (hsCRP) (Roche Diagnostics, Indianapolis, IN, USA). The intra-assay coefficient of variation was 7.8% for oxidised LDL, and 9.1% for hsCRP.
Statistical analysis
For data summaries, continuous variables were summarised using the mean±SD. Categorical variables were summarised using frequency counts and percentages. The baseline characteristics were compared between groups using Mann-Whitney test for continuous variables, and Fisher exact test for categorical variables. For the analyses of the primary and secondary outcomes, a Wilcoxon signed rank test was performed. Statistical significance was assessed based on a two-sided 5% level of significance, and all statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline characteristics of participants in both the vildagliptin (n=14) and pioglitazone (n=11) groups are presented in Table 1. The mean age was 56.4±11.1 years, and the mean BMI was 26.2±3.4 kg/m2 in all participants. All clinical and anthropometric variables were comparable between groups. Baseline HbA1c (7.2% vs. 7.4%), insulin, and C-peptide levels were also not different between study groups. Markers of glycemic variability including MAGE, SD of 24 hours glucose, and the difference between maximum and minimum glucose levels (Δglucose, max-min) were measured via CGM during 3 consecutive days. Their mean values were similar in both groups.
During the 16-week treatment period, both treatments significantly lowered mean HbA1c (7.2% to 6.4%, P=0.001 in the vildagliptin group; 7.4% to 6.7%, P=0.005 in the pioglitazone group), and 24-hour mean glucose (160.9 to 122.9 mg/dL, P=0.001 and 145.0 to 125.9 mg/dL, P=0.012, respectively) (Table 2). Only vildagliptin treatment, however, significantly reduced markers of glycemic variability including MAGE (P=0.046), SD of 24 hours glucose (P=0.026), and Δglucose, max-min (P=0.036) (Table 2, Fig. 2). Although it was not significant, the magnitude of decline of MAGE in the pioglitazone group was similar to that in the vildagliptin group; hence, each individual's change of MAGE was assessed in both groups (Fig. 3). A more consistent decline of MAGE was found in the vildagliptin group than in the pioglitazone group. In the vildagliptin group, the MAGE value was increased in only two of 14 individuals, but four of 11 individuals in pioglitazone group.

Fasting Plasma Glucose, HbA1c, and Markers of Glycemic Variability before and after Treatments (16 Weeks) in Vildagliptin and Pioglitazone Group

Changes of (A) mean amplitude of glycemic excursion (MAGE) and (B) standard deviation (SD) before and after 16 weeks of treatment in the vildagliptin and pioglitazone groups. aP<0.05.
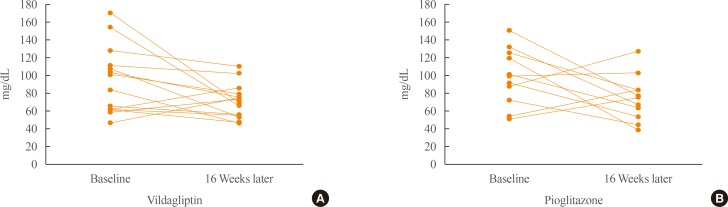
Changes of mean amplitude of glycemic excursion before and after 16 weeks of treatment in the (A) vildagliptin and (B) pioglitazone groups on individual levels.
Finally, oxidative stress markers were measured, but both treatments did not change urinary 8-iso PGF2α (Table 3). Oxidised LDL and hsCRP were also not changed after either treatment.
DISCUSSION
In this 16-week randomised controlled trial, vildagliptin, but not pioglitazone, reduced glycemic variability in individuals with type 2 diabetes inadequately controlled with metformin. Vildagliptin significantly reduced markers of glycemic variability including MAGE, SD of 24 hours glucose measured by CGM, as well as HbA1c, and FPG. The attenuated glucose excursion in a glucose-dependent manner with DPP-4 inhibition may contribute to the reduction of glycemic variability markers. Pioglitazone also was effective in lowering HbA1c, but did not reduce glycemic variability.
Since it has been suggested that glycemic variability contributes to the development of diabetic vascular complications in addition to long-term glycemia [413], some trials have examined the role of pharmacologic interventions in reducing glycemic variability in individuals with diabetes. Most of the trials focused on glucose-lowering medications which mainly acts to reduce postprandial hyperglycemia, such as the α-glucosidase inhibitor [8], DPP-4 inhibitor [6914], and rapid-acting insulin analogues [15]. In short-term clinical studies, the DPP-4 inhibitors, vildagliptin and sitagliptin, effectively reduced MAGE and other glycemic variability markers [914]. Notably, in a comparative study, vildagliptin was superior to sitagliptin in reducing glycemic variability, and was associated with reduction of oxidative stress, which was suggested as a possible link between glycemic variability and vascular complications [6]. The results from the present study are consistent with previous vildagliptin studies in terms of reducing glycemic variability. However, we did not observe the significant effects of vildagliptin on oxidative stress. This outcome was probably due to a small sample size, or a difference in oxidative stress markers. On the other hand, it is also possible that the reduction in glycemic variability does not necessarily lead to reduction of oxidative stress. In this study, overall decreasing trends of urinary 8-iso PGF2α were not observed after vildagliptin treatments in 14 individuals. In addition, the absence of a correlation between glycemic variability and oxidative stress has already been reported in previous several studies. For example, sitagliptin treatment for 4 weeks significantly reduced the MAGE; however, it was not associated with a reduction in oxidative stress markers [14]. In another study, there was no relationship between glucose variability and urinary 15(S)-8-iso PGF2α in individuals with type 1 diabetes [16]. Although it is well established that glucose fluctuation leads to the generation of oxidative stress and endothelial dysfunction, a condition that underlies development of diabetes complications [51718], additional clinical evidence is needed in order to demonstrate that interventions reducing glycemic variability would prevent oxidative stress and the development of further vascular damage.
We also evaluated the effects of a thiazolidinedione pioglitazone on glycemic variability. This is the first study to measure changes of MAGE after pioglitazone treatment. According to clinical evidence, pioglitazone effectively reduced postprandial glucose excursion and circulating levels of oxidative stress or proinflammatory markers in type 2 individuals with diabetes [1019]. However, whether those effects were accompanied by a reduction in glycemic variability was not clear. In the present study, we found that pioglitazone treatment during 16 weeks did not exert beneficial effects on either glycemic variability or oxidative stress, although it significantly decreased mean glycemia (HbA1c). Therefore, the finding a thiazolidinedione reduced glucose-induced oxidative stress via AMP-activated protein kinase-dependent mechanism seemed to be its own effect in response to hyperglycemia [20], not by preventing glucose fluctuation.
This study had the following limitations. First, the number of participants was small. We should not overlook the findings that absolute magnitude of decrease in mean value of MAGE was similar between pioglitazone and vildagliptin groups; however, statistically significant change was observed only in vildagliptin group. The interpretation of these findings might be limited by small sample size. Second, the study duration might be short assessing the full glucose-lowering effects of study drugs. So, a larger scale, longer-term study is still warranted to assess the role pharmacologic interventions in terms of glycemic variability. Third, the dose of pioglitazone was 15 mg per day, not 30 mg as used in previous studies, due to insurance criteria in Korea. It might produce smaller effect on glycemic control and variability than expected.
In conclusion, this study found that a 16-week treatment using vildagliptin, not pioglitazone, reduced glycemic variability in individuals with type 2 diabetes that was inadequately controlled with metformin monotherapy, although oxidative stress markers did not improve. The study identified that DPP-4 inhibitors might have a beneficial role in reducing glycemic variability beyond reducing mean glycemia in individuals with type 2 diabetes.
ACKNOWLEDGMENTS
This study was undertaken as an investigator-initiated research protocol and was funded by Novartis Korea. Dr. Sin Gon Kim also was partly supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI14C2750). We acknowledge the investigators and staff, as well as study participants.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.