Comparison of Thyroglobulin Measurements Using Three Different Immunoassay Kits: A BRAMHS Tg-Plus RIA Kit, a BRAMHS hTg Sensitive Kryptor Kit, and a Beckman Coulter ACCESS Immunoassay Kit
Article information
Abstract
Background
Second-generation thyroglobulin immunometric assays (Tg-IMAs) have been developed with improved sensitivity. Our aim was to compare the diagnostic value of Tg-IMA measurements using a Kryptor (BRAHMS AG) kit (Tg-K) and an ACCESS (Beckman Coulter) kit (Tg-A) with that of the first-generation Tg measurement using a Tg-plus (BRAHMS AG) kit (Tg+).
Methods
We enrolled 82 differentiated thyroid cancer patients who underwent total thyroidectomy with radioactive iodine remnant ablation and who underwent diagnostic whole body scan using recombinant human thyroid stimulating hormone (rhTSH). The Tg+, Tg-K, and Tg-A were measured before rhTSH administration during levothyroxine treatment (suppressed Tg) from the same sample. Serum Tg+ was measured after rhTSH stimulation (stimulated Tg).
Results
Suppressed Tg+ was more significantly correlated with suppressed Tg-K (R2=0.919, P<0.001) than with suppressed Tg-A (R2=0.536, P<0.001). The optimal cut-off values of suppressed Tg+, Tg-K, and Tg-A for predicting stimulated Tg+ of 1 ng/mL were 0.3, 0.2, and 0.2 ng/mL, respectively. The sensitivity, specificity, and accuracy of suppressed Tg+ were 67%, 100%, and 90%, respectively; those of suppressed Tg-K were 83%, 90%, and 88%; those of suppressed Tg-A were 96%, 82%, and 87%, respectively. The positive predictive and negative predictive values of Tg+ were 100% and 87%, respectively; those of Tg-K were 79% and 92%; and those of Tg-A were 73% and 98%.
Conclusion
We could not clearly demonstrate which kit had better diagnostic performance after comparison of first-generation Tg measurements with Tg-IMA measurements. Also, there were kit-to-kit variations between Tg-IMA kits. Suppressed Tg measured by Tg-IMA was insufficient to completely substitute for a stimulated Tg measurement.
INTRODUCTION
Differentiated thyroid cancer (DTC) has a favorable prognosis with an 85% 10-year survival rate after primary treatment [1]. DTC patients need lifelong monitoring for recurrence of disease because it can occur at any time during follow-up periods [23]. Serum thyroglobulin (Tg) is an useful biochemical tumor marker for detecting persistent or recurrent DTC [45]. Because the source of Tg is both normal remnant thyroid tissue and residual cancer tissue, thyroid stimulating hormone (TSH) influences the interpretation of serum Tg concentration. Serum Tg values during TSH suppression therapy (suppressed Tg) are not sensitive enough to detect small amounts of thyroid tissue or small changes in thyroid tissue. Serum Tg measurements during TSH stimulation (stimulated Tg) should be performed to maximize the diagnostic sensitivity with a negative predictive value (NPV) of 99% in absence of anti-thyroglobulin antibody (TgAb) [67].
Stimulated Tg should be evaluated 4 weeks after cessation of thyroid hormone or 48 to 72 hours after recombinant human TSH (rhTSH) intramuscular injection [8910]. Cessation of thyroid hormone treatments may induce severe hypothyroidism, and rhTSH injections may induce high cost or inconvenience due to frequent clinic visits [911].
Second-generation Tg immunometric assays (Tg-IMAs) with improved functional sensitivity (less than 0.1 ng/mL) have been newly developed and are now commercially available [1213]. These assays show high NPVs, so it has been reported that they might substitute for stimulated Tg measurements in DTC patients with low recurrence risk [31415].
The aim of the current study was to compare the diagnostic value of Tg-IMA measurements using a BRAMHS hTg sensitive Kryptor (BRAHMS AG, Henningsdorf, Germany) kit (Tg-K) and a Beckman Coulter ACCESS immunoassay (Beckman Coulter, Brea, CA, USA) kit (Tg-A) with the first-generation Tg measurements using a BRAMHS Tg-plus RIA (BRAHMS AG, Henningsdorf, Germany) kit (Tg+). Additionally, we assessed whether suppressed Tg-K or suppressed Tg-A measurements can obviate the need for stimulated Tg measurement.
METHODS
Patients
This study included a total of 82 DTC patients who underwent total thyroidectomy with radioactive iodine remnant ablation and who underwent diagnostic whole body scan using rhTSH between March 2015 and November 2015 at a single center. Serum suppressed Tg and TgAb were measured using the same sample from each patient during levothyroxine suppression therapy by three different immunoassay kits: Tg+, TgAb+; Tg-K, TgAb-K; Tg-A, TgAb-A. Stimulated Tg+ was measured 24 hours after the second injection of 0.9 mg rhTSH, and all study samples had TSH values higher than 30 µU/mL. One patient with missing Tg-A was excluded in the final Tg-A analysis. Our study protocol was approved by the Institutional Review Board at Asan Medical Center, Seoul, Korea.
Serum Tg and TgAb measurement
Tg+ was measured using the BRAMHS Tg-plus RIA, a first-generation immunoassay kit. The functional sensitivity of Tg-plus kit was 0.2 ng/mL, and the analytical sensitivity was 0.08 ng/mL. The coefficients of variation (CV) within- and between assays were 1.5% to 5.6% and 2.2% to 9.9%, respectively. TgAb+ measurements were performed using the BRAHMS anti-Tg RIA kit (BRAHMS AG, Henningsdorf, Germany) with within- and between assay CVs of 2.0% to 7.5% and 3.1% to 5.5%, respectively. The status of TgAb+ was defined as positive when the value of TgAb+ was higher than 60 U/mL.
Tg-K was measured by Tg-IMA using the BRAMHS hTg sensitive Kryptor kit, with a functional sensitivity of 0.15 ng/mL and an analytical sensitivity of 0.09 ng/mL. The CV of Tg-K was 4.5% at low concentrations and 2.8% at high concentrations. TgAb-K was measured using a BRAHMS anti-Tgn Kryptor kit (BRAHMS AG). The CV of TgAb-K was 6.0% at low concentrations and 4.2% at high concentrations. TgAb-K positivity was defined when the value of TgAb-K was higher than 33 U/mL.
Tg-A was measured using the Beckman Coulter ACCESS immunoassay kit, with functional and analytical sensitivities of 0.1 and 0.01 ng/mL, respectively. Within- and between Tg-A CVs were 1.3% to 2.7% and 1.7% to 4.9%, respectively. Serum TgAb was measured using a Beckman Access Antibody II Calibrators (Beckman Coulter, Brea, CA, USA) and was named TgAb-A. TgAb-A positivity was defined when the value of TgAb-A was higher than 4 U/mL.
Statistical analysis
Categorical variables are presented as number and percentage. The chi-squared test was used for comparison of categorical variables between groups. The linear regression model was used to analyze associations among the values of suppressed Tg of the three different immunoassay kits and to analyze associations between suppressed Tg and stimulated Tg from the samples with Tg values higher than the analytical sensitivity of each kit. Receiver operating characteristics (ROC) curve analysis was performed to evaluate the optimal cut-off values of suppressed Tg+, Tg-K, and Tg-A for predicting a stimulated Tg+ of 1.0 ng/mL. Sensitivity, specificity, and accuracy were defined as 'true positive/(true positive+false negative),' 'true negative/(true negative+false positive),' and '(true positive+true negative)/total,' respectively. Positive predictive value (PPV) and NPV were defined as 'true positive/(true positive+false positive)' and 'true negative/(true negative+false negative),' respectively. Only P values less than 0.05 were considered statistically significant. Statistical analysis was conducted using R version 3.10 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
Of the 82 patients enrolled in our study, 24 (29%) were male. The mean age of the study patients was 51.7 years, and the mean primary tumor size was 1.69 cm. According to the TNM (tumor, lymph node, metastasis) classification system of the 7th edition of the American Joint Committee on Cancer and the Union for International Cancer Control, except for three patients whose histologic findings were unknown, 15 patients (19%) were N0, 34 (43%) were N1a, and 30 (38%) were N1b; 59 (75%) had extrathyroidal extension. Distant metastasis was detected in three patients at the time of initial diagnosis.
Serum TgAb using three different immunoassay kits
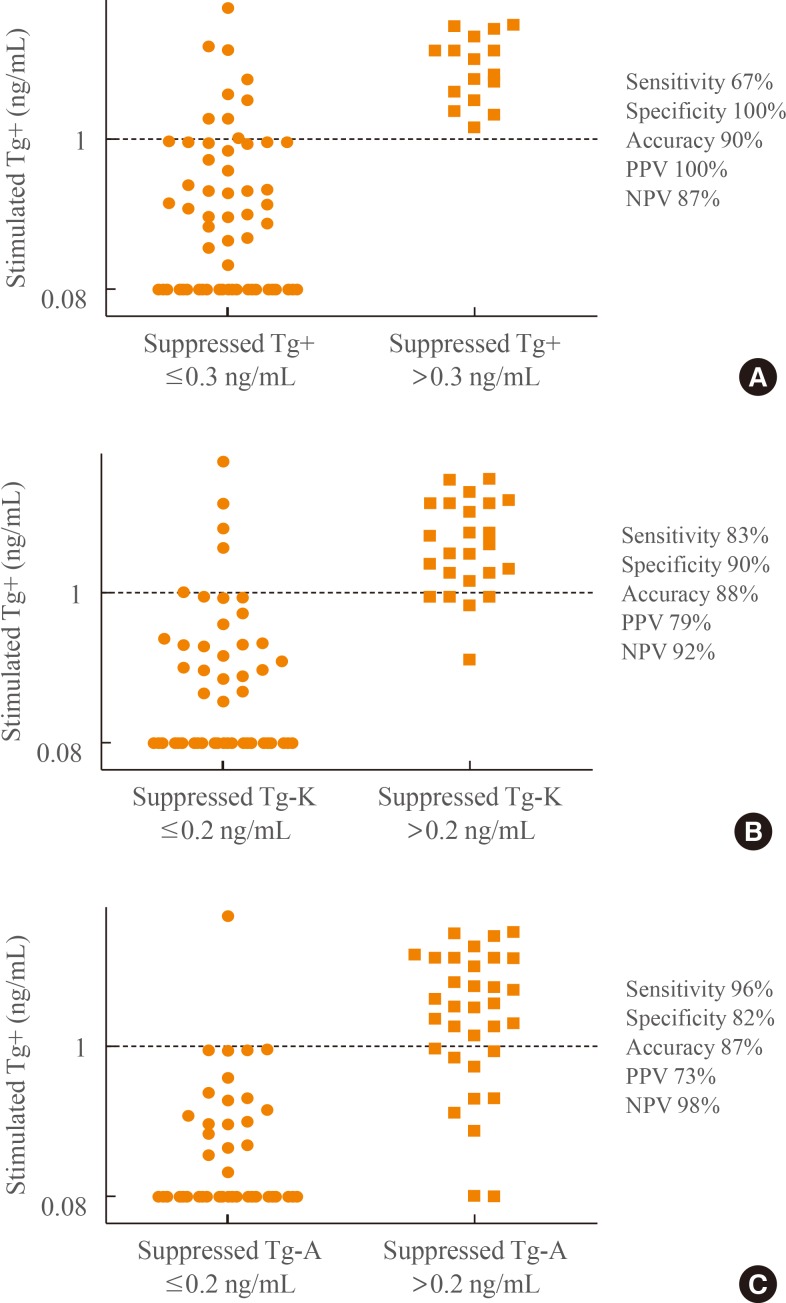
TgAb+, TgAb-K, and TgAb-A were positive in four (5%), eight (10%), and five (6%) of the 82 patients, respectively. All four TgAb+ positive patients were also positive for TgAb-K and TgAb-A. The values of TgAb+ for the four patients who were positive for TgAb-K but negative for TgAb+ were 25.7, 25.1, 58.8, and 17.1 U/mL. The value of TgAb+ for the one patient who was positive for TgAb-A but negative for TgAb+ was 40.9 U/mL (Table 1).
Comparison of suppressed Tg+ with suppressed Tg-K and suppressed Tg-A in TgAb negative patients
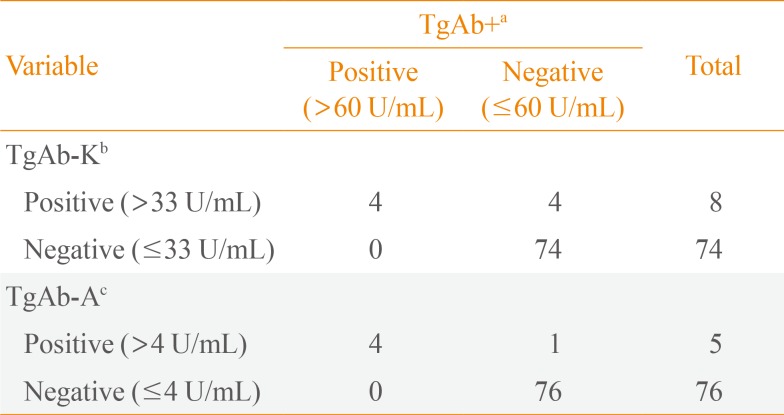
Suppressed Tg+ was highly correlated with suppressed Tg-K (R2=0.919, P<0.001). As suppressed Tg+ increased by 1 ng/mL, suppressed Tg-K increased by 0.93 ng/mL (Fig. 1A). Suppressed Tg+ was less correlated with suppressed Tg-A than with Tg-K and showed a linear relationship with a low concordance (R2=0.536, P<0.001) (Fig. 1B).
Concordance between (A) suppressed Tg+ and suppressed Tg-K and (B) suppressed Tg+ and suppressed Tg-A in anti-thyroglobulin antibody negative patients. Tg, thyroglobulin; Tg+, Tg level measured with the BRAHMS Tg-plus RIA kit (BRAHMS AG); Tg-K, Tg level measured with the BRAMHS hTg sensitive Kryptor kit (BRAHMS AG); Tg-A, Tg level measured with the Beckman Coulter ACCESS immunoassay kit (Beckman Coulter).
Comparison of stimulated Tg+ with suppressed Tgs from different kits in TgAb negative patients
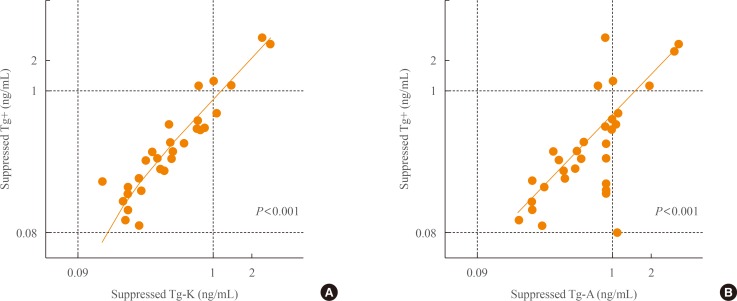
When we compared stimulated Tg+ with suppressed Tg+ in TgAb+ negative patients, there was a linear correlation (R2=0.316) (Fig. 2A). There was also a linear correlation between stimulated Tg+ and suppressed Tg-K in TgAb-K negative patients (R2=0.214) (Fig. 2B). However, stimulated Tg+ was not associated with suppressed Tg-A (Fig. 2C).
Concordance between stimulated Tg+ and (A) suppressed Tg+, (B) suppressed Tg-K, and (C) suppressed Tg-A as measured with each of the three different immunoassay kits in anti-thyroglobulin antibody negative patients. Tg, thyroglobulin; Tg+, Tg level measured with the BRAHMS Tg-plus RIA kit (BRAHMS AG); Tg-K, Tg level measured with the BRAMHS hTg sensitive Kryptor kit (BRAHMS AG); Tg-A, Tg level measured with the Beckman Coulter ACCESS immunoassay kit (Beckman Coulter).
Sensitivity, specificity, and accuracy of suppressed Tg+, Tg-K, and Tg-A in predicting positivity of stimulated Tg+
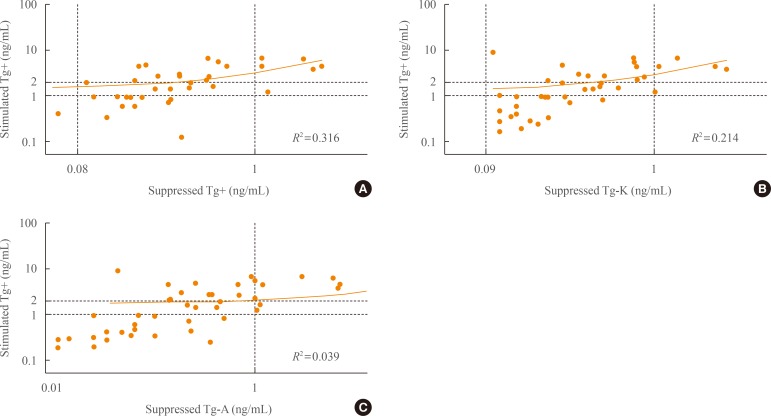
We evaluated the sensitivity, specificity, and accuracy of suppressed Tg according to immunoassay kits for predicting positivity of stimulated Tg. We performed ROC analysis to determine the optimal cut-off values of suppressed Tg+, Tg-K, and Tg-A for predicting a stimulated Tg+ of 1 ng/mL. The appropriate cut-off value of suppressed Tg+ was 0.3 ng/mL by ROC analysis, and the area under the curve (AUC) was 0.89 (P=0.001). The most reasonable cut-off value of suppressed Tg-K and Tg-A was 0.2 ng/mL (AUC=0.92, P<0.001; AUC=0.92, P<0.001, respectively).
When we set the cut-off value of suppressed Tg positivity at 0.3 ng/mL for Tg+ and at 0.2 ng/mL for Tg-K/Tg-A, the sensitivity, specificity, and accuracy of suppressed Tg+ for predicting a stimulated Tg over 1 ng/mL were 67% (16/24), 100% (54/54), and 90% (70/78), respectively (P<0.001) (Table 2, Fig. 3A). The sensitivity, specificity, and accuracy of suppressed Tg-K were 83% (19/23), 90% (46/51), and 88% (65/74) and those of suppressed Tg-A were 96% (24/25), 82% (42/51), and 87% (66/67), respectively (P<0.001 and P<0.001, respectively) (Table 2, Fig. 3B, C).

Comparison of Stimulated Tg+ with Suppressed Tg+, Suppressed Tg-K, and Suppressed Tg-A in TgAb Negative Patients
Sensitivity, specificity, accuracy, and positive predictive value (PPV) and negative predictive value (NPV) of (A) suppressed Tg+, (B) suppressed Tg-K, and (C) suppressed Tg-A in predicting positivity of stimulated Tg+. Tg, thyroglobulin; Tg+, Tg level measured with the BRAHMS Tg-plus RIA kit (BRAHMS AG); Tg-K, Tg level measured with the BRAMHS hTg sensitive Kryptor kit (BRAHMS AG); Tg-A, Tg level measured with the Beckman Coulter ACCESS immunoassay kit (Beckman Coulter).
Positive and negative predictive values of suppressed Tg+, Tg-K, and Tg-A in predicting positivity of stimulated Tg+
The PPVs of both Tg-K (79%, 19/24) and Tg-A (73%, 24/33) were lower than that of Tg+ (100%, 16/16). The NPV of Tg+ was 87% (54/62), that of Tg-K was 92% (46/50), and that of Tg-A was 98% (42/43) (Table 2, Fig. 3).
DISCUSSION
We compared the diagnostic value of a first-generation Tg measurement with two different Tg-IMA measurements and evaluated whether the suppressed Tg-IMA measurement obviates the need for stimulated Tg measurement. In summary, Tg-IMA measurements presented higher sensitivity and NPV than the first-generation Tg measurement. However, Tg-IMA measurements presented poorer specificity, PPV, and accuracy than the first-generation Tg measurement.
Suppressed Tg+ was significantly more correlated with suppressed Tg-K than with suppressed Tg-A. This discordance can be explained by the antigenic differences of the assay kits. Tg+ and Tg-K were measured with immunoassay kits from the same company (BRAHMS AG), while Tg-A was measured using an immunoassay kit from another company (Beckman Coulter). In previous studies, different Tg assays on the same serum displayed 2-fold differences in the numeric Tg values [1516171819]. The interassay variability can be explained by Tg molecular heterogeneity. Tg is a large (660 kDa), highly glycosylated dimeric protein that is heterogeneous with respect to differential thyroglobulin mRNA splicing, glycosylation, and degree of iodination. In addition, the processes involved in Tg maturation, dimerization, and molecular folding are complex and may become unregulated in thyroid tumor cells [34511132021]. These changes can lead to exposure or masking of epitopes and hence differences in Tg immunoreactivity. The different immunoassays, employing different epitopes, detect serum Tg isoforms with variable potency. This can result in variability in the measurement of different Tg isoforms in patient specimens and ultimately cause differences in Tg concentrations depending on the assay [2223]. In clinical practice, these kit-to-kit variations necessitate that Tg monitoring be performed using the same manufacturer kit [24].
In this study, we could not clearly demonstrate which kit had better diagnostic performance after comparison of first-generation Tg measurements with second-generation Tg-IMA measurements. When we set the cut-off value of suppressed Tg positivity at 0.3 ng/mL for Tg+ and at 0.2 ng/mL for Tg-K/Tg-A, the sensitivities of suppressed Tg-K and Tg-A for predicting a stimulated Tg over 1 ng/mL were higher than that of Tg+, but the specificity and accuracy were not higher than those of Tg+. Our results are consistent with the previous findings that the NPV of suppressed Tg measured using the Tg-IMA kit is higher than that measured using the first-generation Tg kit [2324]. However, the NPVs of Tg-K and Tg-A (92% and 98%, respectively) in this study were not higher than that of Tg-IMA measured in a recent meta-analysis (99%) [25]. Also this study included samples with stimulated Tg+ positive but suppressed Tg-K or Tg-A negative. Therefore, we are unable to state that suppressed Tg measured by Tg-IMA can completely substitute for a stimulated Tg measurement.
The present study has several limitations. First, we only enrolled patients who underwent follow-up at single institution. Second, we measured stimulated Tg using only the first-generation immunoassay. Third, we used the functional sensitivity of the manufacturer for each immunoassay kit. Fourth, this study only compared the values of serum Tg before and after TSH stimulation but could not confirm the presence of recurrence.
In conclusion, the sensitivity and NPV of suppressed Tg-K and Tg-A were higher than those of Tg+, whereas the specificity, accuracy, and PPV of suppressed Tg-K and Tg-A were lower than those of Tg+. Furthermore, there was great kit-to-kit variation between Tg-IMA kits. Suppressed Tg measured by Tg-IMA was insufficient to completely substitute for stimulated Tg measurements. Further studies are warranted to confirm the clinical utility of Tg-IMA measurement.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.