Recovery of Adrenal Function in Patients with Glucocorticoids Induced Secondary Adrenal Insufficiency
Article information
Abstract
Background
The chronic use of glucocorticoids (GC) suppresses function of the hypothalamic-pituitary-adrenal axis and often results in secondary adrenal insufficiency (AI). The present study aimed to determine the recovery rate of adrenal function in patients with secondary AI within 1 to 2 years and to assess the factors predictive of adrenal function recovery.
Methods
This was a retrospective observational study that enrolled patients diagnosed with GC-induced secondary AI between 2007 and 2013. AI was defined by peak serum cortisol levels <18 µg/dL during a standard-dose short synacthen test (SST). A follow-up SST was performed after 1 to 2 years, and responders were defined as those with adrenocorticotropic hormone (ACTH)-stimulated peak serum cortisol levels ≥18 µg/dL.
Results
Of the total 34 patients diagnosed with GC-induced secondary AI at first, 20 patients (58.8%) recovered normal adrenal function by the time of the follow-up SST (median follow-up period, 16.5 months). Although the baseline serum ACTH and cortisol levels at the first SST did not differ between responders and non-responders, the incremental cortisol response during the first SST was higher in responders than that of non-responders (7.88 vs. 3.56, P<0.01). Additionally, higher cortisol increments during the first SST were an independent predictive factor of the adrenal function recovery (odds ratio, 1.58; 95% confidence interval, 1.02 to 2.46; P<0.05).
Conclusion
In the present study, adrenal function recovery was achieved frequently in patients with GC-induced secondary AI within 1 to 2 years. Additionally, an incremental cortisol response at the first SST may be an important predictive factor of adrenal function recovery.
INTRODUCTION
Glucocorticoids (GC) have been widely used since 1940s to treat inflammatory, autoimmune, and malignant disorders. The prevalence of oral GC use among the general population varies according to age, sex, and country, as well as the year of the survey was conducted; the observed incidence rates have been reported as 0.5% to 0.9% in the United Kingdom [12], 0.7% in Northeast Iceland [3], and 1.2% in the United States [4]. Although the exact prevalence of GC use in the general Korean population has yet to be determined, it may be similar to even higher than the rates of other countries due to the high chance that patients are being administered factitious GC in regions that do not separate dispensaries from medical practices [56].
Despite their beneficial effects, the chronic and/or high-dose exposure to GC is associated with a number of adverse events including osteoporosis, Cushingoid appearance with weight gain, diabetes, cardiovascular disease, and dyslipidemia, among others [7]. Furthermore, chronic exposure to GC can inhibit the function of the hypothalamic-pituitary-adrenal (HPA) axis via negative feedback, which may result in adrenal insufficiency (AI) following the cessation of GC treatment or in critically ill patients [89]. As a result, prolonged GC treatment is considered to be the most common cause of secondary AI [10]. Therefore, it is important to detect AI during its early stages to initiate appropriate treatment, particularly for patients with a history of chronic GC treatment.
The clinical symptoms associated with AI are elusive and nonspecific, but often include nausea, vomiting, abdominal pain, shock, and even organ failures. Because these symptoms are nonspecific, it is difficult to differentiate AI from other medical conditions and it is important to perform biochemical stimulation tests to detect AI. The standard-dose (250 µg) short synacthen test (SST) has high sensitivity and specificity and is considered to be a safe and reliable method for the evaluation of adrenal function [111213].
Many studies have reported that patients with secondary AI exhibit recovery of HPA axis function within several months (up to 1 year or more) [141516], but no studies have investigated the predictive biochemical cut-off values associated with this recovery. Therefore, the present study aimed to determine the recovery rate of adrenal function in patients with secondary AI within 1 to 2 years, and to assess the factors predictive of adrenal function recovery during the follow-up.
METHODS
Study subjects
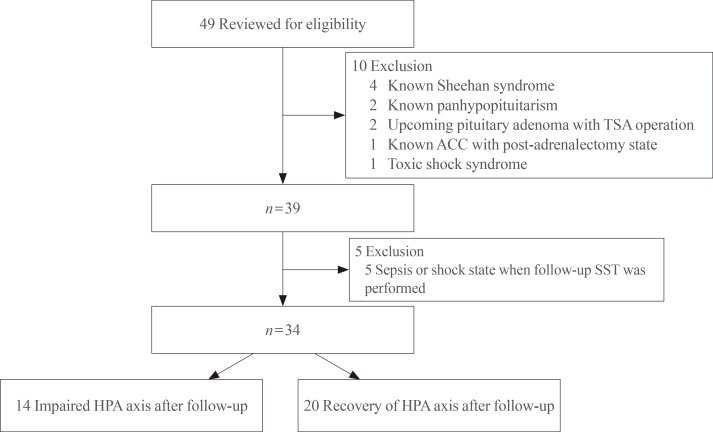
The present study conducted a retrospective screening of all patients who attended Gyeongsang National University Hospital and underwent a SST between July 2007 and December 2013. Patients who were diagnosed with AI at the first SST and had a follow-up SST within 1 to 2 years were enrolled. Forty-nine patients fulfilled these criteria but 10 patients were excluded due to previous history of being diagnosed with panhypopituitarism or Sheehan syndrome (n=6), pituitary adenoma (n=2), toxic shock syndrome, and post-adrenalectomy-induced AI (n=1). Additionally, five patients were also excluded from the present study because the follow-up SST was performed under conditions of sepsis or shock (Fig. 1).
Anthropometric and laboratory measurements
The personal information of the enrolled patients including age, sex, and body measurement data, such as height, weight, and laboratory results were verified by a review of medical chart records. The body mass index (BMI) of each patient was calculated by dividing the weight in kilograms by the square of the height in meters. Patients who had any appearance of truncal obesity, a rounded face, supraclavicular fullness, and/or easy bruising with skin atrophy upon physical examination were regarded as having Cushingoid features. The medical chart review also revealed the reasons that each patient was administered GC.
Blood samples were obtained by venipuncture at a single central certified laboratory at Gyeongsang National University Hospital. Serum albumin levels (normal range, 3.5 to 5.2 g/dL) were measured by a bromocresol green test using a Roche modular DP analyzer (Roche Diagnostics, Basel, Switzerland), and serum cortisol (normal range, 5 to 25 µg/dL at morning, 5 to 15 µg/dL at afternoon, and 0 to 10 µg/dL at night) and adrenocorticotropic hormone (ACTH; normal range, 5 to 60 pg/mL) levels were measured by an electrochemiluminescence immunoassay (Roche, Manheim, Germany). The intra- and interassay variabilities of the cortisol and ACTH kits used in the present study were less than 3.0% and 6.0%, respectively, and the sensitivity values were 0.018 µg/dL and 0.220 pmol/L, respectively.
Standard-dose SST
A SST was performed to evaluate the adrenal function of the patients. The test was conducted with the patients in a supine position after the cessation of GC administration for at least 48 hours. Blood samples were collected prior to intravenous administration of tetracosactide (250 µg, Synacthen, Dalim BioTech, Seoul, Korea) and then 30 and 60 minutes after administration to determine serum cortisol levels. A peak stimulated serum cortisol level ≥18 µg/dL within 60 minutes was considered a normal adrenal response regardless of basal cortisol level. A patient was considered a responder if their follow-up SST showed an ACTH-stimulated peak serum cortisol levels ≥18 µg/dL.
Statistical methods
With regard to the baseline characteristics of the patients, continuous variables are presented as medians and interquartile ranges, and categorical variables are presented as numbers and percentages. The comparison between responders and non-responders were performed using the Mann-Whitney U test. A logistic regression analysis was conducted to estimate the predictive factors associated with HPA axis recovery after the follow-up, and a multivariate logistic regression analysis was conducted to further evaluate the predictive factors after adjusting for confounding factors. The first model (model 1) was adjusted for sex and age, and the second model (model 2) was adjusted for age, sex, serum albumin, BMI, peak cortisol concentration, basal ACTH levels, and model 3 was adjusted for age, sex, serum albumin, BMI, basal cortisol concentration, basal ACTH levels. A receiver operating characteristic (ROC) curve was employed to assess the ability of cortisol increments at the first SST to predict HPA axis recovery. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) and two-tailed P≤0.05 were considered to indicate statistical significance.
RESULTS
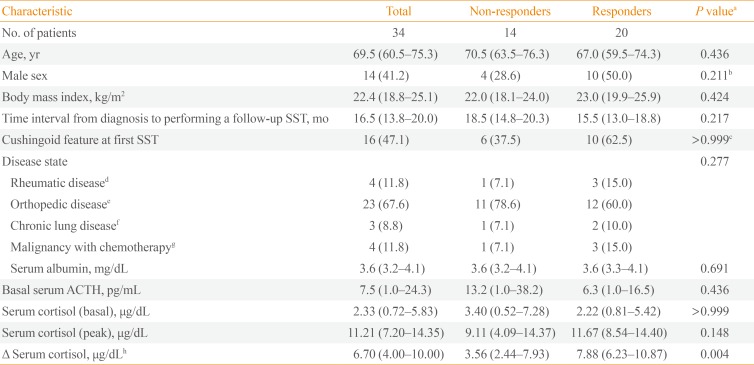
This retrospective observational study analyzed 34 consecutive patients who were diagnosed with GC-induced secondary AI; the median age was 69.5 years, and 14 of the patients (41.2%) were male. Of these 34 patients, 20 (58.8%) recovered to normal adrenal function within 1 to 2 years (the median follow-up period, 16.5 months), 16 patients (47.1%) had Cushingoid features at the initial diagnosis. GC treatment was administered for rheumatologic reasons in four patients, orthopedic reasons in 23 patients, pulmonological reasons in three patients, and malignant disorders in four patients (Table 1). All patients were prescribed physiological doses of prednisolone after being diagnosed with secondary AI (median 5 mg/day with minimal 2.5 mg/day and maximum 10 mg/day). But unfortunately, it was not possible to evaluate the cumulative dose, duration of GC treatment, or the patients' compliance to the GC therapy before and after AI diagnosis, because many patients (n=25) had a history of taking factitious steroids from regions without separation of dispensary from medical practice as well as this retrospective observational study obtained data only from medical charts.
There were no significant differences between the responder and non-responder groups in terms of anthropometric and biochemical parameters, including basal serum ACTH and cortisol levels, and peak serum cortisol levels at the first SST. However, the degree of cortisol increments at the first SST (delta cortisol, which was defined as the peak cortisol level at either 30 or 60 minutes subtracted by the baseline) was higher in the responders (on average, 7.88 vs. 3.56, P=0.004) (Table 1).
The factors predictive of adrenal function recovery after 1 to 2 years of follow-up were also evaluated, and it was revealed that only delta cortisol was predictive of HPA axis recovery (odds ratio [OR], 1.48; 95% confidence intervals [CI], 1.10 to 1.97; P<0.01). This association remained significant even after adjusting for age, sex, serum albumin levels, BMI, and basal serum cortisol and ACTH levels (in model 3: OR, 1.57; 95% CI, 1.02 to 2.46; P=0.041) (Table 2). The area under the ROC curve (AUC) was used to assess the ability of the biochemical values to predict the HPA axis recovery after the first SST. Although neither the baseline serum cortisol (AUC, 0.50; P>0.999) nor peak cortisol (AUC, 0.65; P=0.142) levels were predictive of the recovery of adrenal function, the delta cortisol levels were predictive (AUC, 0.79; 95% CI, 0.62 to 0.96; P=0.005). The cut-off values of the serum delta cortisol levels for HPA axis recovery were 8.15 µg/dL (a sensitivity of 50% with specificity of 79%), and 8.99 µg/dL (a sensitivity of 40% with specificity of 86%), respectively (Fig. 2).

Logistic Regression of Basal Serum ACTH and Cortisol, Peak Serum Cortisol, and Serum Cortisol Increment in Relation to Adrenal Function Recovery
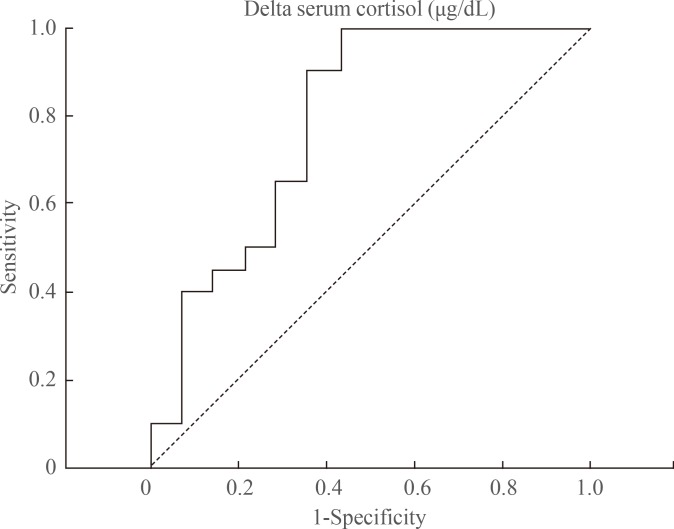
The area under the receiver operating characteristic curve to assess the ability of the serum cortisol increment (delta cortisol) to predict the hypothalamic-pituitary-adrenal axis recovery after the first short synacthen test. A peak cortisol cut-off of ≥8.15 µg/dL gives a sensitivity of 50% and a specificity of 79%, and that of ≥8.99 µg/dL gives a sensitivity of 40% and a specificity of 86% for predicting adrenal function recovery.
DISCUSSION
The present retrospective observational study was performed to estimate the percentage of patients who recovered from AI within 1 to 2 years, and to assess predictive baseline laboratory parameters to differentiate patients who will recover normal adrenal function during the follow-up from those who will not. In the present study, 20 secondary AI patients (58.8%) recovered normal adrenal function during the follow-up period (median 16.5 months), and a higher incremental cortisol response to ACTH stimulation at the first SST was an independent predictive factor of HPA axis recovery. To the best of our knowledge, the present study is the first to evaluate the recovery rate of adrenal function in GC-induced secondary AI in Korean patients and to assess predictive biochemical factors associated with HPA axis recovery.
Considering the risk of incident secondary AI associated with GC treatment, neither the duration nor the dose of GC treatment is a reliable predictor of GF-induced AI [1417]. Furthermore, there are no forms of administration or underlying diseases for which the risk of AI can be safely excluded [18]. Meanwhile, although it remains unknown exactly how long GC-induced suppression of the HPA axis will persist due to individual variations [14], many studies have demonstrated that it may take several months or even longer than 1 year [141516]. It has been shown that neither the total nor the highest dose of GC or the duration of GC treatment are indicators of HPA axis recovery in patients with secondary AI [19]. A recent study demonstrated that the probability of recovering adrenal function after treatment for endogenous Cushing syndrome is not correlated with the length or extent of hypercortisolism or the postoperative GC replacement doses, but rather with the underlying etiology of the syndrome [20]. In the present study, even though the patients were heterogeneous regarding the underlying cause of GC treatment, and it was not possible to ascertain the previous cumulative dose and duration of GC therapy, many of the patients (n=20, 58.8%) achieved normal adrenal function within 1 to 2 years, which is similar to the findings of previous studies [141516]. This suggests that patients diagnosed with GC-induced secondary AI due to any etiology or GC treatment may achieve normal adrenal function within 1 to 2 years. Thus, close monitoring of these patients is necessary to accurately assess and evaluate adrenal function in those undergoing GC replacement treatment.
On the other hand, Yo et al. [21] suggested that the basal morning cortisol levels can be a good initial assessment tool in predicting an adrenal response and the HPA axis with determining the need for dynamic test (a basal cortisol cut-off of >5 µg/dL gives a specificity of 95% and a sensitivity of 43% for predicting adrenal sufficiency). A prospective cohort study in patients with giant cell arteritis with long-term GC therapy demonstrated that 49% of patients had AI when performed by the SST after GC tapering, and recovery of adrenal function occurred in a mean time of 14 months, similar to that of our study [22]. In addition, the main predictive factors associated with an incidence of AI after GC treatment were total dose (>8.5 g), and duration of GC treatment (>19 months), as well as low basal cortisol concentration (<14 µg/dL).
The peak serum cortisol levels during the SST were well correlated with the results of an insulin tolerance test, which is the gold standard for the detection of AI [2324]. Peak serum cortisol levels and the delta changes in cortisol levels after ACTH stimulation were once used as the diagnostic criteria for primary and secondary AI [2526], but peak cortisol levels (>18 µg/dL) are now accepted as the most appropriate diagnostic criteria to evaluate adrenal function due to the higher accuracy associated with their measurement [27], and because these levels are not affected by basal cortisol concentrations [24]. Meanwhile, in the case of critically ill patients, a delta cortisol level less than 9 µg/dL was used as the diagnostic cut-off value for critical illness-related corticosteroid insufficiency [28]. In the present study, not peak cortisol levels but delta cortisol levels during the first SST (defined as the peak cortisol level at either 30 or 60 minutes subtracted by the baseline) were positively associated with the possibility of achieving adrenal function recovery; this finding remained significant after adjusting for confounding factors. Using the ROC curve to predict the cut-off point for HPA axis recovery revealed that a serum delta cortisol level of 8.99 µg/dL showed a low sensitivity but a relatively high specificity (86%). This may suggest that the biochemical marker levels during the SST may be not solely predictive of HPA axis recovery, and that several confounding factors should be considered in clinical practice.
Total serum cortisol levels are known to be influenced by body composition, serum cortisol-binding globulin, and use of oral contraceptive pills [29]. This study reported that changes in the 30 minutes cortisol values are positively correlated with BMI in both gender. Meanwhile, 30 minutes total cortisol levels were independently associated with abdominal fat mass or waist circumference (but not with BMI) only in men. Additionally, cortisol-binding globulin levels, which are high in patients using oral contraceptive pills and low in patients with liver cirrhosis, are positively correlated with serum total cortisol levels. In the present study, neither delta cortisol levels (r=-0.017, P=0.939) nor 30-minute total cortisol levels (r=-0.014, P=0.949) were significantly associated with BMI (n=24). However, any drugs that could possibly affect the concentration of serum cortisol levels were not used, and patients with liver cirrhosis or nephrotic syndrome were excluded. The present study adjusted for the aforementioned confounding factors to assess the correlation more accurately between delta cortisol levels during the SST and the recovery of adrenal function; this relationship remained significant.
There are several limitations inherent to the present retrospective study that must be discussed. First, the cumulative dose and duration of GC treatment before and after the diagnosis of secondary AI were not evaluated in the present study. Reginal characteristics in which there are regions without separation of dispensary from medical practice in rural and mountain villages of west Gyeongsangnam-do province, were one of barriers to evaluate the exact duration or cumulative dose of GCs. Even though impaired adrenal function may be present regardless of dose of GC [30], and the cumulative dose and duration of GC treatment are not closely associated with the recovery of adrenal function [20], these factors should have been taken into consideration. Second, only a small number of patients from a single university hospital were included in the present study, and thus, selection bias could have influenced the results. Future studies should include larger general populations with multi-center, prospective designs. Third, we could not evaluate duration of SAI before being performed the first SST and there is a possibility that some patients were in the course of adrenal function recovery before the first SST. Despite these limitations, the present study demonstrated a good recovery rate of adrenal function within 1 to 2 years and is the first to demonstrate that delta cortisol levels are a predictive biochemical parameter associated with adrenal function recovery in patients with GC-induced secondary AI.
In summary, more than half of the patients with GC-induced secondary AI in the present study achieved normal adrenal function within 1 to 2 years of follow-up. Interestingly, serum cortisol increments during the SST may be an independent predictive factor of the recovery of adrenal function. These findings indicate that patients with GC-induced secondary AI should be monitored, and their biochemical profiles in response to the SST, including changes in cortisol increments should be assessed to predict accurately adrenal function recovery within 1 to 2 years. Additional well-controlled, prospective, large-population studies investigating GC-induced secondary AI are required to strengthen the observed correlation between delta cortisol levels and HPA axis recovery.
ACKNOWLEDGMENTS
We acknowledge the efforts of the data processing department in the hospital. We thanks to Dr. J.H. Byun for her help to analyze biochemical results.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.