Increased Sclerostin Levels after Further Ablation of Remnant Estrogen by Aromatase Inhibitors
Article information
Abstract
Background
Sclerostin is a secreted Wnt inhibitor produced almost exclusively by osteocytes, which inhibits bone formation. Aromatase inhibitors (AIs), which reduce the conversion of steroids to estrogen, are used to treat endocrine-responsive breast cancer. As AIs lower estrogen levels, they increase bone turnover and lower bone mass. We analyzed changes in serum sclerostin levels in Korean women with breast cancer who were treated with an AI.
Methods
We included postmenopausal women with endocrine-responsive breast cancer (n=90; mean age, 57.7 years) treated with an AI, and compared them to healthy premenopausal women (n=36; mean age, 28.0 years). The subjects were randomly assigned to take either 5 mg alendronate with 0.5 µg calcitriol (n=46), or placebo (n=44) for 6 months.
Results
Postmenopausal women with breast cancer had significantly higher sclerostin levels compared to those in premenopausal women (27.8±13.6 pmol/L vs. 23.1±4.8 pmol/L, P<0.05). Baseline sclerostin levels positively correlated with either lumbar spine or total hip bone mineral density only in postmenopausal women (r=0.218 and r=0.233; P<0.05, respectively). Serum sclerostin levels increased by 39.9%±10.2% 6 months after AI use in postmenopausal women; however, no difference was observed between the alendronate and placebo groups (39.9%±10.2% vs. 55.9%±9.13%, P>0.05).
Conclusion
Serum sclerostin levels increased with absolute deficiency of residual estrogens in postmenopausal women with endocrine-responsive breast cancer who underwent AI therapy with concurrent bone loss.
INTRODUCTION
Bone remodeling is a tightly regulated process leading to balanced resorption and formation of skeletal tissue with the coordinated action of osteocytes [1,2]. Sclerostin is a glycoprotein secreted by osteocytes and a potent Wnt inhibitor. Sclerostin travels through osteocyte canaliculi to the bone surface where it binds to the coreceptors lipoprotein receptor-related protein (LRP) 5 and LRP 6; thus, it prevents colocalization with Frizzled and Wnt proteins, and reduces osteoblastogenesis and bone formation [3]. The clinical relevance of sclerostin in bone metabolism was initially recognized in patients with sclerosteosis or van Buchem's disease with sclerostin mutations, resulting in excessive bone formation leading to osteosclerosis [4].
Overexpression of normal human SOST alleles (the sclerostin gene) in mice results in osteopenia [5]. Mirza et al. [6] compared 20 postmenopausal and 20 premenopausal women and found that the former had significantly higher serum sclerostin levels. Estrogen replacement and intermittent parathyroid hormone (PTH) therapy in postmenopausal women lead to a significant reduction in sclerostin levels [7,8]. Several studies have revealed the molecular mechanism of decreased sclerostin production by estrogens. Estrogens bind to estrogen receptors and interact with the Wnt/β-catenin signaling pathway, which downregulates osteocyte production of sclerostin via estrogen receptor-induced prostaglandin E2 [9].
Aromatase inhibitors (AIs) are used to treat endocrine-responsive breast cancer by lowering remnant 17β-estradiol (E2) or estrione levels to prevent cancer recurrence [10]. This is the preferred adjuvant therapy for postmenopausal women with estrogen receptor/progesterone receptor-positive breast cancer, because it reduces breast cancer recurrence up to 50% compared to tamoxifen [11,12,13]. AIs affect bone metabolism by increasing bone turnover rates and accelerating bone loss, leading to increased fracture risk [2,14,15,16]. Bisphosphonates are the preferred treatment for AI-induced bone loss [2], and calcitriol, an active metabolite of vitamin D, in combination with an AI is effective for inhibiting tumors [10]. In a previous study [2], we reported that the combination of alendronate (5 mg) and calcitriol (0.5 µg) (Maxmarvil, Yuyu Co., Seoul, Korea) was quite beneficial for preventing the bone loss that occurs with AI administration in patients with endocrine-responsive breast cancer.
Here, we analyzed serum sclerostin levels in patients with endocrine-responsive breast cancer treated with an AI to assess the changes in serum sclerostin levels following treatment with alendronate or placebo.
METHODS
Study design and subjects
This study was a randomized, placebo-controlled, double-blind study performed at a single center. A cross-sectional comparison of sclerostin was performed between premenopausal and postmenopausal women. Changes in sclerostin levels were analyzed in 90 endocrine-responsive breast cancer patients during new AI administration over 24 weeks. Forty-four patients received 5 mg alendronate with 0.5 µg calcitriol, calcium, and vitamin D, and the remaining 46 patients received only calcium and vitamin D [2].
The diagnosis of breast cancer and indication for adjuvant chemotherapy were made according to the guidelines of the National Comprehensive Cancer Network [10]. Patients who met the inclusion criteria were reviewed from March 2010 to March 2011. Postmenopausal women who were taking an AI after breast cancer surgery were included, and the definitions of menopause in these patients were as follows: (1) prior bilateral oophorectomy; (2) amenorrhea for ≥12 months in the absence of tamoxifen, toremifene, or ovarian suppression; and (3) follicle-stimulating hormone and E2 levels in the postmenopausal range.
Thirty-six healthy premenopausal women were recruited by advertisement and served as controls. All subjects were informed of the nature of the study, and consent was obtained from each participant. This protocol was approved by the Institutional Review Board of Severance Hospital.
Biochemical measurements
Blood samples were collected in the morning after an overnight fast. Routine serum chemistry determinations including calcium and phosphate were performed by standard automated techniques. Bone turnover markers were measured using the following methods: osteocalcin (OCN; by enzyme-linked immunosorbent assay [ELISA], CIS Bio International, Gif-sur-Yvette, France; intra-assay coefficient of variation [CV], <2.0%; interassay CV, <5.0%), C-telopeptide of type I collagen (CTx; Osteomark, Ostex International, Seattle, WA, USA; intra-assay CV, <5.8%; interassay CV, <5.9%), intact PTH (by immunoradiometric assay, Biosource, Nivelles Belgium; intra-assay CV, 2.7%; interassay CV, <3.5%), and 25-hydroxyvitamin D (25[OH]D by D3-radioimmunoassay-coated tube, Biosource; intra-assay CV, 11.0%; interassay CV, 12.5%).
Serum sclerostin concentrations were measured using a human sclerostin ELISA kit (Biomedica Co., Wien, Austria) according to the manufacturer's instructions. Intraassay and interassay CVs were 4% to 6% and 5% to 7%, respectively. Sclerostin measurements are reported in pmol/L, and the lower limit of detection was <8.9 pmol/L. Measurements were taken twice at intervals of 24 weeks in postmenopausal women with endocrine-responsive breast cancer. Sclerostin was measured only at baseline in the premenopausal control group.
Assessment of bone mineral density
Bone mineral density (BMD) at the lumbar spine L1 to L4, femoral neck, and total hip was measured in all patients by dual-energy X-ray absorptiometry (Hologic Delphi A version 12.6, Hologic, Waltham, MA, USA). The measurements were performed at baseline and after 24 weeks of treatment.
Statistical analysis
Differences in continuous variables between the premenopausal and postmenopausal endocrine-responsive breast cancer groups were determined using Student t test. The correlation between continuous variables was analyzed by Pearson correlation analysis. Student t test and the paired t test were used to compare sclerostin levels and bone turnover markers in the postmenopausal breast cancer group. A P<0.05 was considered statistically significant. All analyses were performed using IBM SPSS version 18.0 (IBM Co., Armonk, NY, USA).
RESULTS
Baseline clinical and biochemical characteristics
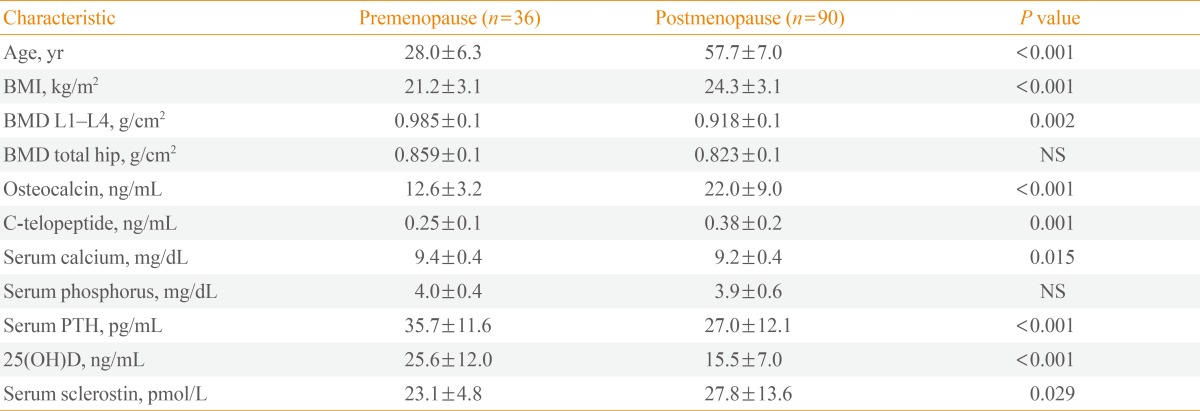
This study included 90 postmenopausal women who were diagnosed with endocrine-responsive breast cancer and 36 healthy premenopausal control women. The postmenopausal women had a mean age of 57.7 years, and the premenopausal women had a mean age of 28.0 years (Table 1). Among the 90 postmenopausal women, nine (10%) were diagnosed with osteoporosis.
Lumbar spine and total hip BMD were lower in postmenopausal women than in premenopausal women (0.92±0.14 g/cm2 vs. 0.99±0.01 g/cm2; 0.82±0.11 g/cm2 vs. 0.86±0.10 g/cm2; postmenopausal vs. premenopausal women; P<0.05, respectively) (Table 1). OCN levels were 74.6% higher and CTx was 52.0% higher in postmenopausal women than in premenopausal women (P<0.001, respectively) (Table 1). Baseline serum sclerostin levels were 20.3% higher in the postmenopausal group compared to those in the premenopausal group (P<0.05) (Table 1).
Correlation between sclerostin and bone metabolism
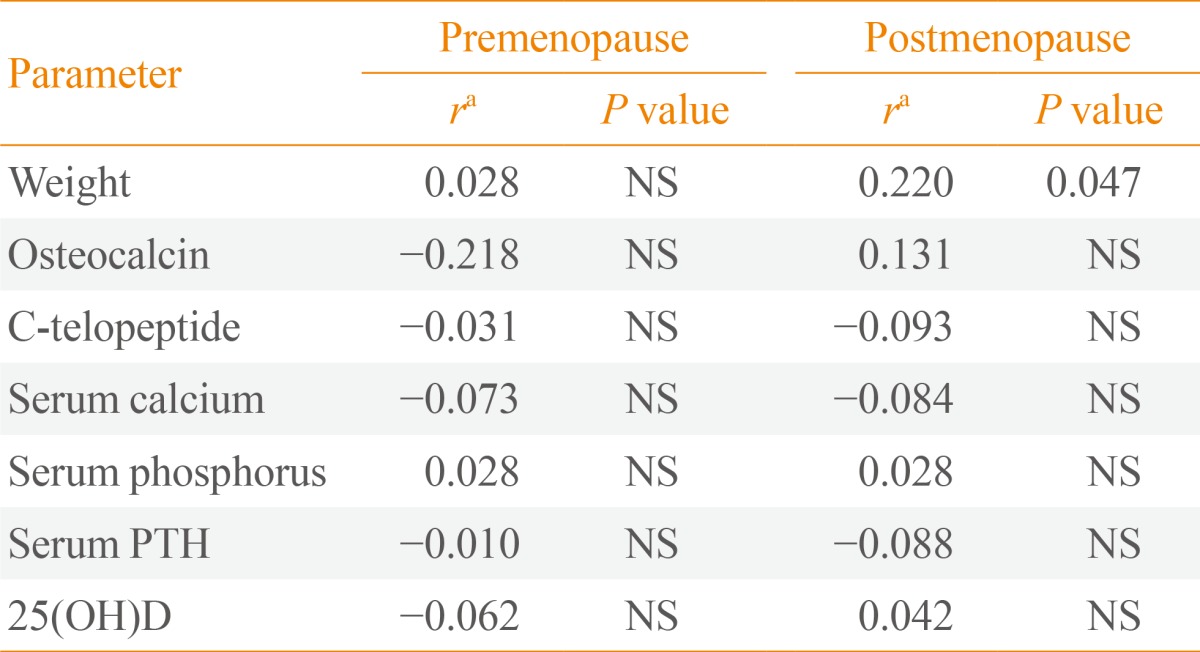
Table 2, Fig. 1 show the relationships between sclerostin and various parameters according to Pearson correlation analysis. Serum baseline sclerostin level positively correlated with lumbar spine and total hip BMD (r=0.218 and r=0.233; P<0.05, respectively) (Fig. 1), but only in the postmenopausal group. No significant correlations were observed between bone turnover markers such as CTx and OCN and serum sclerostin (Table 2). A negative correlation was observed between PTH and sclerostin in postmenopausal women (r=-0.074), but was not significant. Serum calcium, phosphorus, and 25(OH)D had no relevant relationships with sclerostin levels.
Partial Correlations between Sclerostin and Various Parameters in Premenopausal and Postmenopausal Women Adjusted for Body Mass Index
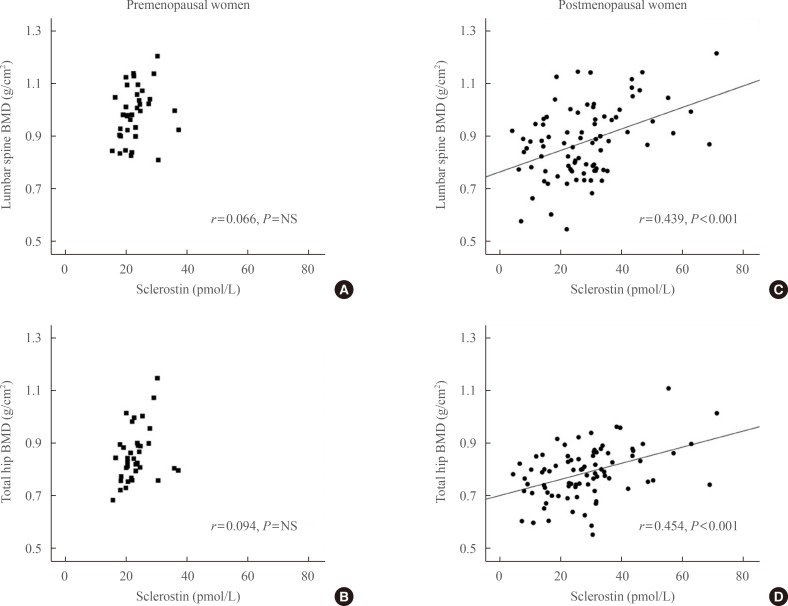
Partial correlations between sclerostin, lumbar spine bone mineral density (BMD), and total hip BMD after adjusting for body mass index. (A, B) Premenopausal women. (C, D) Postmenopausal women. Circulating sclerostin positively correlated with lumbar spine and total hip BMD only in postmenopausal women (C, r=0.439, P<0.001; D, r=0.454, P<0.001, respectively). The age-adjusted correlation between BMD and sclerostin remained positive (data not shown). NS, not significant.
Biochemical changes after AI treatment with or without alendronate
We further divided the postmenopausal patients into two subgroups based on medications: 5 mg alendronate and calcitriol in one group, and calcium and vitamin D only in the other group. Baseline characteristics were presented in a previous report [2]. Baseline features, such as the period after menopause, biochemical parameters, and baseline BMD at all sites were similar between the two groups. Serum OCN and CTx levels increased significantly in both groups after 24 weeks of AI administration, but increased lesser in the alendronate-treated group by 29.0% and 72.4%, respectively [2].
We also analyzed percent changes in sclerostin levels from baseline to 24 weeks after AI use and found a significant increase (48.1%±6.9%, P<0.05) in all postmenopausal women. Sclerostin levels in the placebo group increased significantly after 24 weeks of AI administration (55.9%±9.13%, P< 0.001). Less of an increase was observed in patients treated with alendronate (39.9%±10.2%, P<0.001) (Fig. 2), but this was not significantly different from the increase in the placebo group.
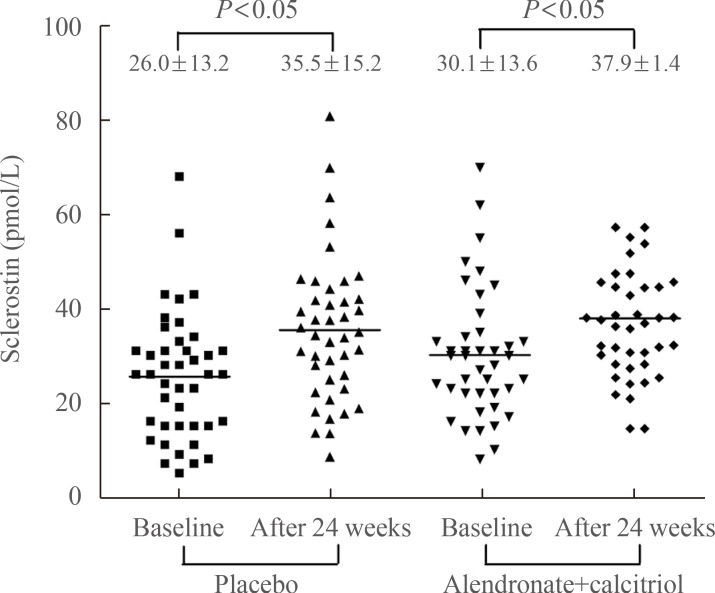
Comparison of changes in sclerostin. Sclerostin was measured at baseline and after 24 weeks of aromatase inhibitor administration. We compared changes in sclerostin in the placebo and alendronate with calcitriol groups, and showed a significant increase in sclerostin levels in both groups (placebo group, P<0.05; alendronate with calcitriol group, P<0.05, by paired t test). However, the changes in sclerostin levels in the groups were not significantly different (P=not significant).
DISCUSSION
We showed higher serum sclerostin levels in postmenopausal women compared to those in premenopausal women. Sclerostin was positively correlated with BMD in postmenopausal women at all sites. In addition, sclerostin levels increased further after use of an AI which is a treatment for breast cancer that ablates the remnant estrogen, but were not affected by the use of alendronate.
Sclerostin, encoded by the SOST gene, is a major Wnt antagonist and a potent inhibitor of bone formation [4]. Previous animal studies have shown that overexpression of the SOST gene causes osteopenia [5]. Moreover, production of sclerostin by osteocytes is dramatically reduced by mechanical loading in rodents [17,18,19]. Thus, sclerostin may be a potential specific marker for bone metabolism, but the clinical implications are not completely understood. Modder et al. [20] showed that sclerostin levels increase with age and are higher in men compared to women. Polyzos et al. [21] reported that serum sclerostin is positively correlated with lumbar spine BMD and T-score. Arasu et al. [22] also found a positive correlation between sclerostin and BMD. A positive correlation was observed between serum sclerostin and spine and total hip BMD by Garnero et al. [23], which was in accordance with our data. They also found negative correlations between sclerostin and CTx, serum intact N-terminal propeptide of type I collagen, and intact PTH. However, the risk of hip fractures is higher in patients with a higher serum sclerostin level [22]. A strong association was observed between increased sclerostin levels and osteoporosis-related fracture risk in postmenopausal women [24]. The paradoxical positive correlation between sclerostin and BMD suggests that serum sclerostin may reflect the number of osteocytes rather than individual cell activity or individual bone remodeling units [25].
The possible regulators of circulating sclerostin levels are known as such; increasing the level by mechanical unloading, immobilization, male sex, aging, and decreasing by mechanical loading, exercise and PTH [17,18,19,20,21,22,23,24,26]. Among them, PTH is probably one of the most important regulators of sclerostin secretion in postmenopausal women [23]. Sclerostin is also affected by estrogens. It is well established that estrogen deficiency increases serum sclerostin levels and is associated with bone loss [27,28,29]. Modder et al. [7] reported that 4 weeks of estrogen treatment in postmenopausal women decreased serum sclerostin levels. Endogenous estrogen and testosterone production were ablated in 59 elderly men by administering gonadotropin-releasing hormone, after which these patients were given physiological levels of testosterone and estrogen. As a result, estrogen, but not testosterone, prevented an increase in sclerostin following induction of sex steroid deficiency [7]. Consistent with previous studies, our study also revealed a negative relationship between estrogen and sclerostin. The possible mechanism of how estrogen suppresses sclerostin production is that estrogen interacts with the Wnt/β-catenin signaling pathway, a main pathway of sclerostin action, by binding to the estrogen receptor via several factors such as prostaglandin E2 [9,25].
In this study, subjects with endocrine-responsive breast cancer who were treated with an AI showed reduced remnant estrogens and bone mass [14,15,16], and showed elevated sclerostin levels. As mentioned above, estrogens and sclerostin have an inverse correlation [27,28,29]. In addition to this concept, our results revealed that further suppression of remnant estrogens sensitively affected serum sclerostin levels. Thus, we suggest that sclerostin may be a surrogate marker for quantitative and sensitive changes in estrogens and bone remodeling.
Polyzos et al. [21] reported that serum sclerostin increases significantly after 6 months of risedronate use. However, Chung et al. [30] found that circulating sclerostin levels were suppressed by raloxifene, but not by bisphosphonates. They suggested that sclerostin may mediate the action of estrogens on bone metabolism independently of the anti-resorptive effects. Our results revealed a significant increase in sclerostin levels after 24 weeks of AI administration, with a slightly blunted increase during alendronate treatment, but without statistical significance.
This study had some limitations. We demonstrated differences in bone markers, BMD, and serum sclerostin levels between premenopausal and postmenopausal women. However, the results are difficult to generalize because of the small number of subjects.
In conclusion, our data show that serum sclerostin levels positively correlated with BMD at all sites, and that serum sclerostin increased according to the level of estrogen deficiency in postmenopausal women, and was further increased by AI treatment. Additional experimental and clinical studies in a larger population and with other drugs that correlate with sclerostin are needed.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.