Accelerated and Exacerbated Effects of High Dietary Fat on Neuronal Damage Induced by Transient Cerebral Ischemia in the Gerbil Septum
Article information
Abstract
Background
Obesity induced by high-fat diet (HFD) is one of the most widespread metabolic disorders in current society. However, there has been little research regarding the effects of HFD-induced obesity in the septa of animal models of cerebral ischemia. Therefore, in the present study, we investigated septal effects of HFD on neuronal damage and gliosis induced by transient cerebral ischemia.
Methods
Body weight, blood glucose levels and serum lipid profiles levels were measured both in the normal diet (ND) and HFD-group. We also investigated the effects of ND and HFD on neuronal damage and gliosis in the septum after transient cerebral ischemia using immunohistochemistry.
Results
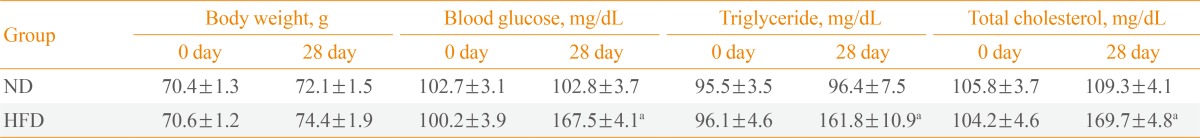
The levels of blood glucose, serum triglyceride, and total cholesterol were significantly increased in the HFD-fed gerbils compared with the ND-fed gerbils, although body weight was not significantly changed after HFD feeding. In the ND-fed gerbils, ischemia-induced neuronal damage was found in the septohippocampal nucleus (SHN) of the septum 7 days after ischemia. In the HFD-fed gerbils, ischemia-induced neuronal damage in the SHN was much more severe compared with that of the ND-fed gerbils 4 and 7 days after ischemia. In addition, we found that ischemia-induced glial activation including astrocytes and microglia was accelerated and exacerbated in the HFD-fed gerbils compared with that in the ND-fed gerbils.
Conclusion
These results indicate that HFD can lead to much more severe effects in ischemia-induced neuronal damage/death in the septum after ischemia-reperfusion, and that it may be associated with accelerated change in glial activation.
INTRODUCTION
Obesity, which is commonly induced by high-fat diet (HFD), is one of the most widespread metabolic disorders in current society [1]. Obesity is associated with low-grade inflammation that is characterized by the production of inflammatory cytokines [2]. In addition, obesity is also related to changes in brain morphology, deficits in learning and memory, and cognitive impairment [3]. On the other hand, HFD contributes to the elevation of blood pressure and increases the risk of stroke and coronary artery disease [4]. A ketogenic diet containing high fat, low-carbohydrate and adequate protein have effects on cognitive function and glial activation [5]. A HFD can induce changes in oxidative stress, inflammation, apoptosis, and neuronal regeneration in some brain regions such as the hypothalamus [6].
The septum or septal area is a limbic structure in the middle anteroventral region of the telencephalon, and it is involved in the regulation of several autonomic, learning-related, and behavioral functions [7,8]. The septum is composed of medium-size neurons that are classified into the medial, lateral, and posterior groups [9,10,11]. It is well known that the septal nuclei contribute to the medial forebrain bundle and receive reciprocal connections from the hippocampus, amygdala, hypothalamus, midbrain, and thalamus [8,12].
Transient cerebral ischemia induced by disruption of the blood supply to the entire brain or a large part of the brain can result in tissue deprivation of oxygen and glucose and may result in permanent brain damage. This ischemia leads to extensive neuronal degeneration and/or death in some brain regions, such as the hippocampus, striatum, and cerebral cortex. Recently, some studies on transient cerebral ischemia have shown that some harmful factors, such as psychological stress and/or obesity, can lead to stroke or affect its outcome [13,14,15]. Some investigators have focused on the effects of HFD on ischemic neuronal damage [16,17]. HFD-induced obesity leads to exacerbation of ischemic injury in the brains of some rodent models of ischemic stroke [18,19]. Park et al. [20] recently reported that feeding gerbils a HFD postischemia induced exacerbated neuronal death in the hippocampal CA1 region. However, there has been little study regarding the effects of HFD in the septa of animal models of cerebral ischemia. Therefore, in the present study, we investigated effects of HFD on neuronal damage and gliosis in the septum induced by 5 minutes of transient cerebral ischemia in the gerbil, which is a good model for long-term observation of transient cerebral ischemia and its effects [21].
METHODS
Experimental animals and diets
Male Mongolian gerbils (Meriones unguiculatus) were obtained from the Experimental Animal Center, Hallym University, Chuncheon, Korea. Gerbils were used at 6 months (B.W., 65 to 75 g) of age. The animals were housed in a conventional state under adequate temperature (23℃) and humidity (60%) control with a 12-hour light/12-hour dark cycle and provided with free access to food and water. All experimental procedures were in accordance with the National Institutes of Health guidelines and ARRIVE guidelines for the care and use of laboratory animals. The animal protocol used in present study was reviewed and approved based on ethical procedures and scientific care by the Kangwon National University-Institutional Animal Care and Use Committee. All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used. The animals were fed commercially available rodent diet, consisted of different fat concentrations as follows: normal diet (ND; D12450B, 10% kcal% fat, 20% kcal% protein, 70% kcal% carbohydrate, Research Diets, New Brunswick, NJ, USA), HFD (D12492, 60% kcal% fat, 20% kcal% protein, 20% kcal% carbohydrate, Research Diets), for 28 days. Finally, the animals were divided into four experimental groups: (1) ND-fed sham-operated group (ND-sham-group); (2) ND-fed ischemia-operated group (ND-ischemia-group); (3) HFD-fed sham-operated group (HFD-sham-group); and (4) HFD-fed ischemia-operated group (HFD-ischemia-group). On day 28, body weight was determined, and blood glucose and serum lipid profiles were measured.
Analysis of glucose levels and lipid profiles
On day 28 after feeding ND or HFD, blood samples were collected from each animal by orbital puncture, and blood glucose levels were analyzed using a blood glucose monitor (Ascensia Elite XL Blood Glucose Meter, Bayer, Toronto, ON, Canada). In addition, serum was separated from the blood by centrifugation and kept at -80℃ until analyzed. Triglyceride and total cholesterol in the serum was measured using an enzymatic method with commercial kits (Asan Pharmaceutical Co., Seoul, Korea).
Induction of transient cerebral ischemia
One day after blood sampling, transient cerebral ischemia was induced. The animals were anesthetized with a mixture of 2.5% isoflurane (Baxtor, Deerfield, IL, USA) in 33% oxygen and 67% nitrous oxide. Bilateral common carotid arteries were isolated and occluded using nontraumatic aneurysm clips. The complete interruption of blood flow was confirmed by observing the central artery in retinae using an ophthalmoscope. After 5 minutes of occlusion, the aneurysm clips were removed from the common carotid arteries. The body (rectal) temperature under free-regulating or normothermic (37℃±0.5℃) conditions was monitored with a rectal temperature probe (TR-100, Fine Science Tools, Foster City, CA, USA) and maintained using a thermometric blanket before, during and after the surgery until the animals completely recovered from anesthesia. Thereafter, animals were kept on the thermal incubator (Mirae Medical Industry, Seoul, Korea) to maintain body temperature until they were euthanized. Sham-operated animals were subjected to the same surgical procedures except that the common carotid arteries were not occluded.
Tissue processing for histology
For the histological analysis, animals were anesthetized with 40 mg/kg sodium pentobarbital and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (pH 7.4). The brains were removed and postfixed in the same fixative for 6 hours. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter, frozen tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30-µm coronal sections, and they were then collected into six-well plates containing PBS.
NeuN immunohistochemistry and Fluoro-Jade B staining
To examine the delayed neuronal death in the septal region after ischemia-reperfusion, the animals (n=7 at each time) were used at sham, 2, 4, and 7 days after ischemia-reperfusion. NeuN immunohistochemistry and Fluoro-Jade B (F-J B) histofluorescence staining were performed according to our previous method [22]. In brief, the sections were incubated with diluted mouse anti-NeuN (1:1,000, Chemicon, Temecula, CA, USA) and subsequently exposed to biotinylated goat antimouse immunoglobulin G and streptavidin peroxidase complex (1:200, Vector, Burlingame, CA, USA). They were visualized by staining with 3,3'-diaminobenzidine (Sigma, St. Louis, MO, USA) in 0.1 M Tris-HCl buffer (pH 7.2).
For F-J B staining, the sections were first immersed in a solution containing 1% sodium hydroxide in 80% alcohol and then in 70% alcohol. They were transferred to a solution of 0.06% potassium permanganate and then to a 0.0004% F-J B (Histochem, Jefferson, AR, USA) staining solution. The sections were examined using an epifluorescent microscope (Carl Zeiss, Göttingen, Germany) with blue (450 to 490 nm) excitation light and a barrier filter.
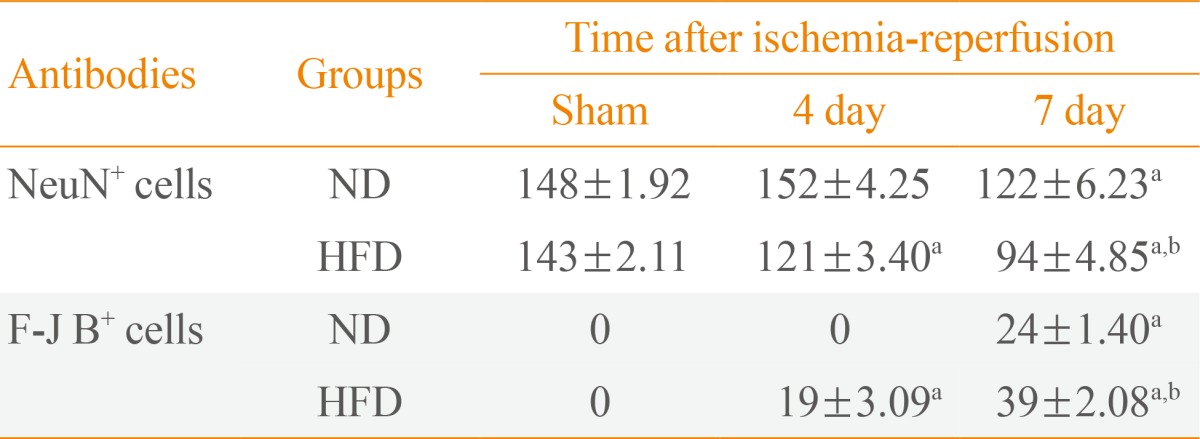
To evaluate the effects of ND and HFD against ischemic damage, NeuN-immunoreactive (+) neurons and F-J B positive (+) cells were counted in a 250×250 µm square applied approximately at the septal region using an image analyzing system (Optimas 6.5, CyberMetrics, Scottsdale, AZ, USA). The studied tissue sections were selected at 120-µm intervals, and cell counts were obtained by averaging the counts from each animal.
Immunohistochemistry for glial fibrillary acidic protein and ionized calcium-binding adapter molecule 1
To examine the gliosis, the animals (n=7 at each time) were used at sham, 2, 4, and 7 days after ischemia-reperfusion, and immunohistochemistry for gliosis was performed according to our previous method [23]. In brief, immunohistochemical staining for rabbit antiglial fibrillary acidic protein (GFAP; 1:800, Chemicon) for astrocytes and rabbit anti-ionized calcium-binding adapter molecule 1 (Iba-1; 1:800, Chemicon) was performed according to the above-mentioned protocol. A negative control test was also carried out using preimmune serum instead of primary antibody in order to establish the specificity of the immunostaining. The negative control test resulted in the absence of immunoreactivity in all structures.
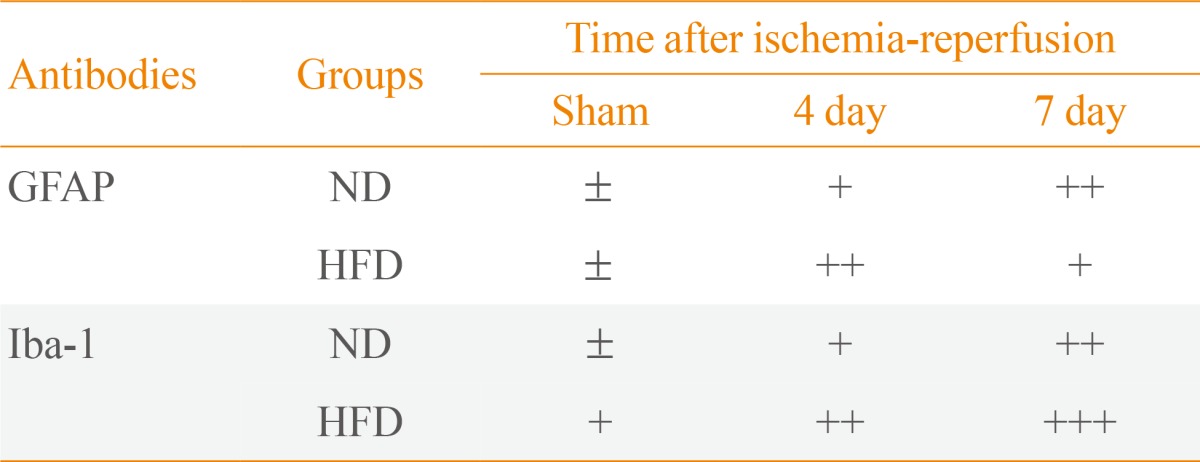
Digital images of the septal region were captured with an AxioM1 light microscope (Carl Zeiss) equipped with a digital camera (Axiocam, Carl Zeiss) connected to a PC monitor. Semiquantification of the immunostaining intensities was evaluated with digital image analysis software (MetaMorph 4.01, Universal Imaging Corp., Downingtown, PA, USA). The level of immunoreactivity was scaled as -, ±, +, or ++, representing no staining (gray scale value, ≥200), weakly positive (gray scale value, 150 to 199), moderate (gray scale value, 100 to 149), or strong (gray scale value, ≤99), respectively.
Statistical analysis
The data shown here represent the mean±SEM. Differences in the means among the groups were statistically analyzed by one-way analysis of variance with a post hoc Bonferroni's multiple comparison test in order to elucidate ischemia-related differences among experimental groups. Statistical significance was considered at P<0.05.
RESULTS
Body weight, blood glucose levels, and serum lipid profiles levels
The changes in body weight, blood glucose, serum triglyceride, and total cholesterol of the ND- and HFD-groups were measured on day 28 after feeding. No difference in body weight gain was noted between the ND- and HFD-groups. However, the levels of blood glucose, serum triglyceride, and total cholesterol were markedly increased in the HFD-group compared with those in the ND-group (Table 1).
Neuroprotective effects
NeuN+ neurons
NeuN+ neurons were well distributed throughout the septum of the ND- and HFD-sham-group (Fig. 1A, D). In the septohippocampal nucleus (SHN) of the septum of the ND-ischemia-group 4 days after ischemia-reperfusion, the number of NeuN+ neurons was not significantly different compared with those in the ND-sham-group (Table 2, Fig 1B). However, the number of NeuN+ neurons was decreased in the SHN of the septum of the ND-ischemia-groups 7 days after ischemia-reperfusion (Table 2, Fig. 1C). In the HFD-ischemia-groups, the number of NeuN+ neurons was decreased in the SHN 4 and 7 days after ischemia-reperfusion (Table 2, Fig. 1E, F).

(A-F) NeuN immunohistochemistry in the septohippocampal nucleus (SHN) of the septum in the normal diet (ND)- and high-fat diet (HFD)-groups at sham, 4 and 7 days after ischemia-reperfusion. A loss of NeuN+ neurons (asterisks) is detected 4 and 7 days after ischemia-reperfusion in the HFD-ischemia-group. Scale bar=50 µm.
F-J B+ cells
We found no F-J B+ cells, the degenerating cells in the SHN (Fig. 2), at sham or 4 days in the ND-ischemia-group (Fig. 2A, B). Seven days after ischemia-reperfusion in the ND-ischemia-group, many F-J B+ cells were detected in the SHN of the septum (Table 2, Fig. 2C). In the HFD-sham-group, F-J B+ cells were minimally detected in the SHN (Fig. 2D). However, many F-J B+ cells were detected in the SHN of the HFD-ischemia-group 4 and 7 days after ischemia-reperfusion, with many more cells present 7 days after ischemia-reperfusion than at 4 days after ischemia-reperfusion (Table 2, Fig. 2E, F).
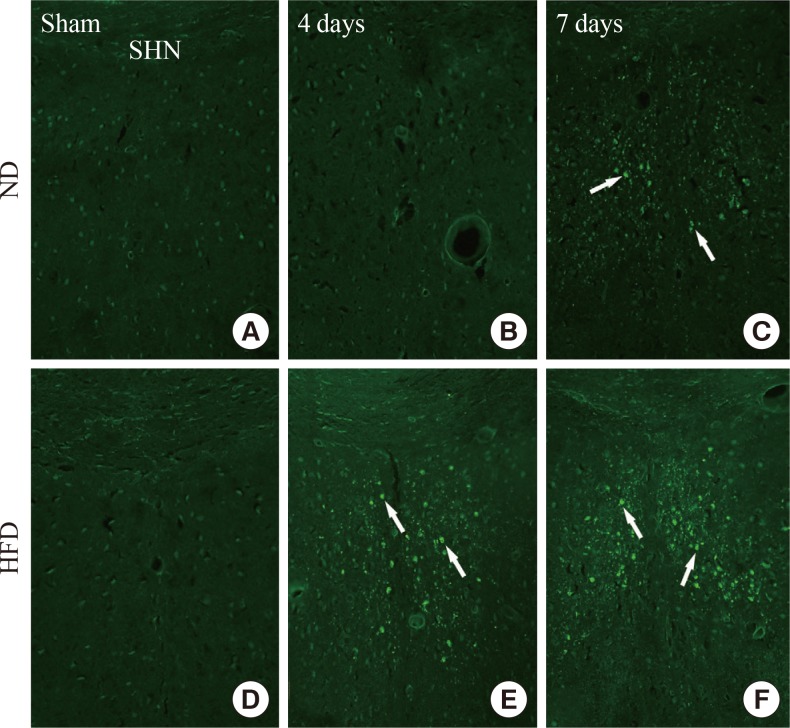
(A-F) Fluoro-Jade B (F-J B) histofluorescence in the septohippocampal nucleus (SHN) of the normal diet (ND)- and high-fat diet (HFD)-groups at sham, 4 and 7 days after ischemia-reperfusion. F-J B+ cells (arrows) are detected in the SHN 4 days after ischemia-reperfusion in the HFD-ischemia-groups as well as 7 days after ischemia-reperfusion in both groups. Scale bar=50 µm.
Glial activation
Astrocytes
In the ND-sham-group, resting GFAP+ astrocytes were observed in the SHN (Fig. 3A). In the ND-ischemia-group, GFAP immunoreactivity was increased, and many activated GFAP+ astrocytes were observed in the SHN 4 days after ischemia-reperfusion (Table 2, Fig. 3B). Seven days after ischemia-reperfusion, more severe GFAP immunoreactivity was found in the SHN (Table 3, Fig. 3C). In the HFD-sham-group, GFAP+ astrocytes were similar to those in the ND-sham-group (Fig. 3D). However, GFAP immunoreactivity in the SHN of the HFD-ischemia-group was much higher than that of the ND-ischemia-group 4 and 7 days after ischemia-reperfusion (Table 3, Fig. 3E, F).
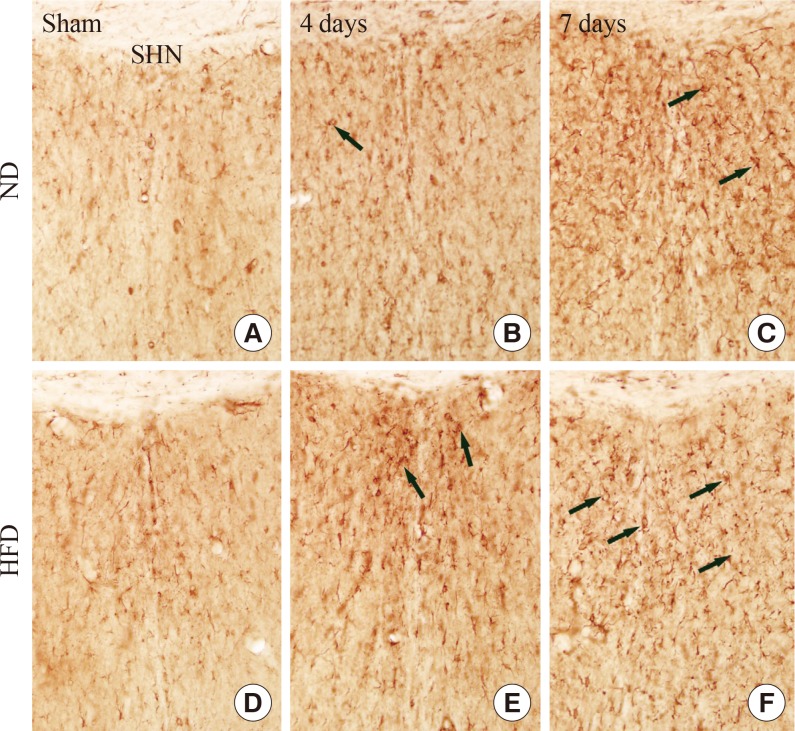
(A-F) Glial fibrillary acidic protein (GFAP) immunohistochemistry in the septohippocampal nucleus (SHN) of the normal diet (ND)- and high-fat diet (HFD)-groups at sham, 4 and 7 days after ischemia-reperfusion. In the HFD-ischemia-group, GFAP immunoreactivity (arrows) is increased compared to that in the ND-ischemia-group 7 days after ischemia-reperfusion. Scale bar=50 µm.
Microglia
Resting Iba-1+ microglia were found in the SHN in the ND-sham-group (Fig. 4A). In the ND-ischemia-group, Iba-1 immunoreactivity was increased, and many hypertrophied and activated Iba-1+ microglia were observed 4 and 7 days after ischemia-reperfusion (Table 2, Fig. 4B, C). In the HFD-sham-group, Iba-1+ microglia were more hypertrophied than in the ND-sham-group (Table 2, Fig. 4D). In the HFD-ischemia-group, Iba-1 immunoreactivity in the SHN was much higher than that of the ND-ischemia-group 4 and 7 days after ischemia-reperfusion, respectively (Table 2, Fig. 4E, F); Iba-1+ microglia were highly aggregated in the SHN 7 days after ischemia-reperfusion.
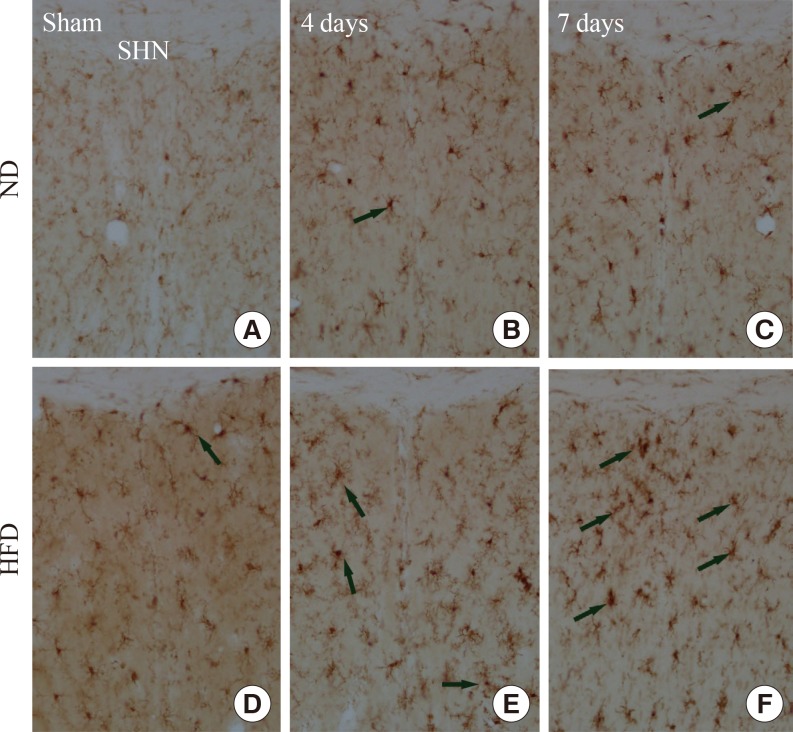
(A-F) Iba-1 immunohistochemistry in the septohippocampal nucleus (SHN) of the normal diet (ND)- and high-fat diet (HFD)-groups at sham, 4 and 7 days after ischemia-reperfusion. In the HFD-ischemia-group, Iba-1 immunoreactivity (arrows) is increased compared to that in the ND-ischemia-group at all times. Scale bar=50 µm.
DISCUSSION
In the present study, we observed changes in physiological agents in ND- and HFD-fed gerbils and found that the levels of blood glucose, serum triglyceride, and total cholesterol were significantly increased on day 28 after feeding; however, no significant difference was found in body weight between ND- and HFD-fed gerbils. This result is consistent with a previous study that showed that body weight gain was not affected by HFD in gerbils, although HFD resulted in higher hepatic vitamin A stores and lower hepatic β-carotene stores [24]. We are aware that the result regarding body weight is inconsistent with other studies, which showed that HFD induced obesity and increased body weight in experimental animals [1,20]. These differences may be due to the ages of the animal models and/or methods used in their experiments.
The septum is an integral part of the limbic system and connects the bilateral hippocampus [12]. In our previous study, we reported that the degree of neuronal damage/death in the septum was distinctively different according to some conditions such as duration time of transient cerebral ischemia [25]. In our present study, we examined the negative effects of HFD on neuronal damage in the septum after 5 minutes of transient cerebral ischemia. Our result showed that HFD chronologically exacerbated and accelerated ischemia-induced neuronal death in the SHN of the septum after transient cerebral ischemia. Some previous studies have shown that diet-induced obesity caused cerebral vessel remodeling with increased stiffness, which increases ischemic damage after middle cerebral artery occlusion in mice and rats [18,19]. It has also been reported that HFD exacerbated ischemic damage and spatial learning following traumatic brain injury [26]. In addition, post-treatment HFD exacerbated neuronal damage in the hippocampal CA1 region following 8 minutes of transient cerebral ischemia in the gerbil [20].
Glial cells including microglia and astrocytes become activated after ischemic injury and release pro- and anti-inflammatory mediators and reactive oxygen species, which are important factors in ischemic damage [27]. In our present study, we observed that microglial cells in the SHN of the HFD-sham-group was more hypertrophied and activated compared those in the ND-sham-group. This result is supported by a previous study that showed that high fat-induced inflammation in adipose tissue was evident by macrophage infiltration and proinflammatory gene expression [28]. Furthermore, in the HFD-ischemia-group, GFAP immunoreactivity in astrocytes was much more severe than that of the ND-ischemia-group. Especially, the aggregation of hypertrophied microglia was observed in the SHN of the HFD-ischemia-group 4 and 7 days after ischemia-reperfusion. It is well known that glial activation leads to neuronal death after ischemic damage through excitotoxic mechanisms [29,30]. Therefore, the obvious increase in glial activation in the HFD-ischemia-group may be closely associated with detrimental effects on ischemia-induced neuronal damage/death.
In brief, our present results indicate that HFD leads to accelerated and exacerbated effects on ischemia-induced neuronal damage/death in the gerbil SHN region after transient cerebral ischemia. The effects of HFD may be associated with hyperactivation of glial cells after transient cerebral ischemia.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Seung Uk Lee for his technical help in this study. This work was supported by a 2013 Research Grant from Kangwon National University (No. 120131480).
Notes
No potential conflict of interest relevant to this article was reported.