Testosterone Deficiency Associated with Poor Glycemic Control in Korean Male Diabetics
Article information
Abstract
Background
Recent studies have shown that men with diabetes have lower testosterone levels than healthy men. However, studies on the correlation between testosterone and diabetes are rare in Korea. We examined the relationship between testosterone deficiency and markers related to diabetes in adult Korean men.
Methods
A total 464 men with diabetes who visited an outpatient clinic at Ajou University Hospital and had serum total testosterone and serum insulin levels measured between January 2000 and September 2013 were selected. Blood samples were collected after the subjects had fasted overnight. We divided the participants into testosterone deficient and normal groups. Testosterone deficiency was defined as having a serum total testosterone level <3.5 ng/mL.
Results
Of 464 subjects, 34.9% had a testosterone deficiency. The mean levels of fasting plasma glucose (P=0.007) and glycated hemoglobin (HbA1c; P=0.038) were significantly higher in the testosterone deficiency group than in the normal group. To clarify the relationship between serum total testosterone level and fasting plasma glucose or HbA1c values, Pearson's correlation test was performed. Fasting plasma glucose levels (r=-0.142, P=0.002) and HbA1c values (r=-0.097, P=0.040) showed a significant negative correlation with serum testosterone levels in men with diabetes.
Conclusion
Major markers of diabetes that are associated with testosterone deficiency are fasting plasma glucose and HbA1c values. Poor glycemic control appears to be associated with testosterone deficiency in Korean men with diabetes.
INTRODUCTION
Cross-sectional epidemiologic studies have reported a relationship between male hypogonadism and diabetes [1,2]. A recent analysis of 1,292 men from Norfolk, England, found that low endogenous testosterone and sex hormone binding globulin levels are associated with glycemia, even below the threshold for diabetes in middle-aged and older men [1]. In a meta-analysis that included 28 cross-sectional studies, total testosterone was lower in men with diabetes compared with controls, and diabetes remained associated with lower total testosterone levels independent of age and body mass index (BMI) [2].
The role of insulin resistance could be one of the most important explanations of the relationship between male hypogonadism and diabetes. Previous studies have shown that hypogonadism is associated with upper abdominal adiposity and insulin resistance [3,4]. Treating systemic insulin resistance with rosiglitazone leads to only a modest increase in testosterone concentrations in men with type 2 diabetes [5], despite the restoration of normal testosterone concentrations. Moreover, results from the Massachusetts Male Aging Study suggest that low testosterone concentrations might play a role in the development of insulin resistance and subsequent type 2 diabetes [6].
Although some studies that have replaced testosterone found no effect on glycemic control in hypogonadal subjects with type 2 diabetes [7,8], many recent studies suggest that administration of testosterone improves insulin sensitivity and glucose homeostasis in subjects with type 2 diabetes and hypogonadism [9,10,11]. With these studies, it can be speculated that endogenous testosterone levels might influence glycemic control in subjects with type 2 diabetes. However, there are only a few studies on the relationship between endogenous testosterone levels and markers related to glycemic control in Korea [12,13].
Hence, we examined the prevalence of testosterone deficiency in subjects with type 2 diabetes and assessed the relationship between testosterone deficiency and markers related to diabetes to understand the association between testosterone and glycemic control in a Korean, diabetic population.
METHODS
Materials
A cross-sectional study was conducted among 464 diabetic men aged 20 years or older attending the outpatient clinic at Ajou University Hospital who had serum total testosterone and serum insulin levels measured between January 2000 and September 2013. We examined the subjects through a chart review and excluded those who had a pituitary or testicular disease, or were using testosterone, antiandrogen treatment, antifungal drugs, or steroidal agents that could affect serum testosterone levels.
Measurements
Height and body weight were measured by standard methods. BMI was calculated as weight divided by height squared (kg/m2). Blood pressure was measured after a period of 30 minutes rest. Blood samples were collected after the subjects had fasted overnight. Venous blood samples were collected for measuring serum total testosterone, fasting plasma glucose, glycated hemoglobin (HbA1c), serum insulin, connecting peptide (C-peptide), total cholesterol, triglyceride, high density lipoprotein cholesterol, blood urea nitrogen, and serum creatinine values. The homeostatic model assessment-insulin resistance (HOMA-IR) was calculated as fasting insulin concentration (µIU/L)×fasting glucose concentration (mg/dL)/405 [14]. The homeostatic model assessment of β-cell function (HOMA-β) was calculated as 360×fasting insulin concentration (µIU/L)/(fasting glucose concentration (mg/dL)-63) [14]. Total testosterone was measured by radioimmunoassay (Coat-A-Count, Siemens Healthcare Diagnostics, Los Angeles, CA, USA). The intra-assay coefficient of variation (CV) was 5%, for total testosterone concentrations of 4.00 ng/mL. The interassay CV was 6.7%, for total testosterone concentrations of 4.01 ng/mL.
Statistical analysis
Testosterone deficiency was defined as having a serum total testosterone level <3.5 ng/mL [15,16,17]. Continuous variables were expressed as mean±SD, and categorical variables were expressed as numbers (n) and percentage (%). The t test was performed for continuous variables to compare the testosterone deficiency group and the normal testosterone group. Pearson's correlation coefficients were calculated to evaluate the relationship between serum total testosterone levels and variables (fasting plasma glucose, HbA1c, BMI, HOMA-IR, and HOMA-β). Data processing and statistical analyses were performed using SPSS version 18.0 (IBM Co., Armonk, NY, USA). P<0.05 were considered statistically significant.
RESULTS
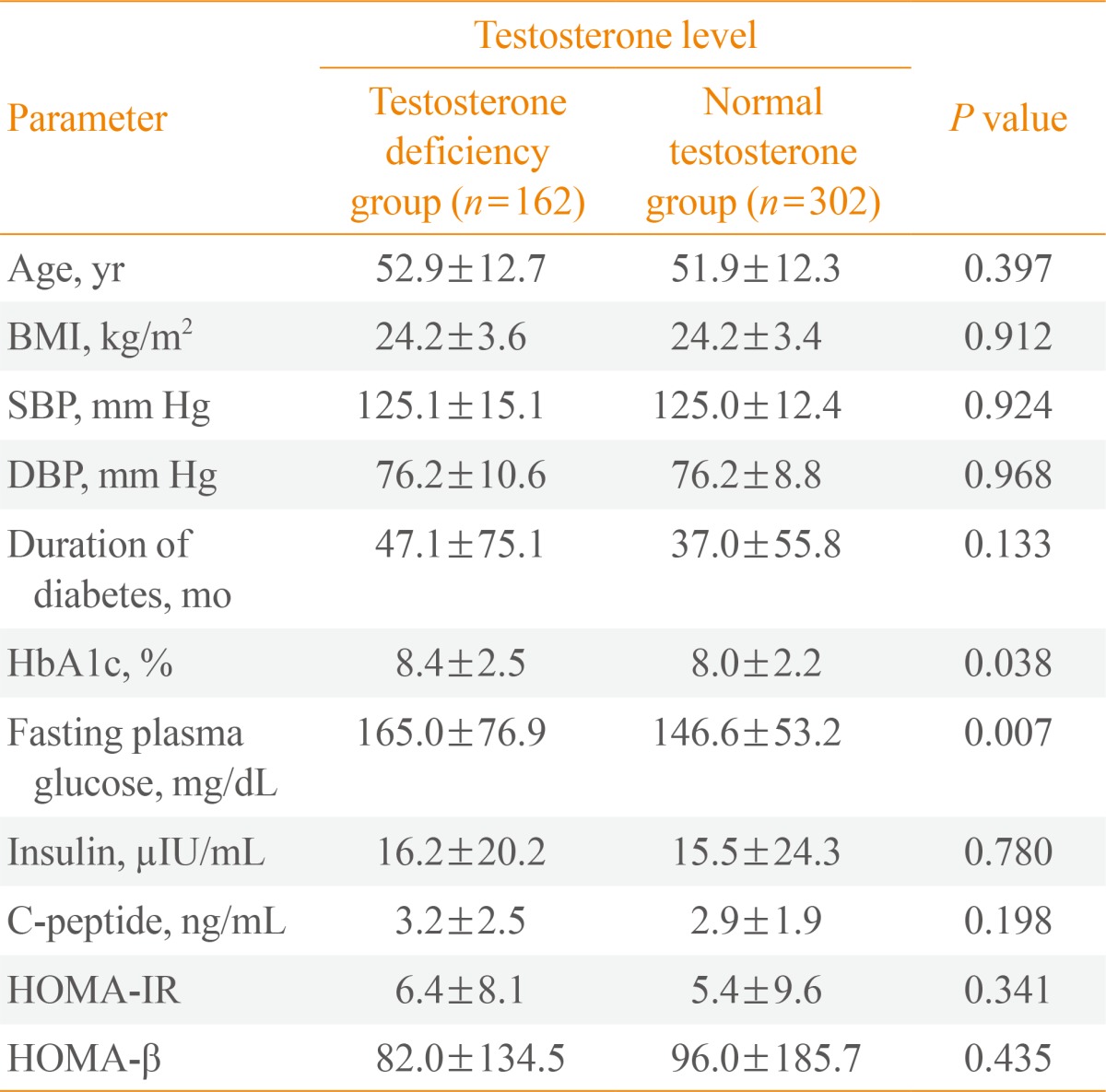
The baseline characteristics of the subjects are shown in Table 1. The mean age of the participants was 52.3 years (range, 20 to 85), and mean BMI, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were 24.2 kg/m2, 125.1, and 76.1 mm Hg, respectively. The mean duration of diabetes was 40.4 months. Mean levels of fasting plasma glucose, HbA1c, serum insulin, and C-peptide were 8.1%, 156.0 mg/dL, 15.7 µIU/mL, and 3.0 ng/mL, respectively. The mean level of total testosterone was 4.2 ng/mL.
In all, 34.9% of men had testosterone deficiency (162/464). The subjects were divided into two groups: a testosterone deficient group (total testosterone <3.5 ng/mL) and a normal group (total testosterone ≥3.5 ng/mL). The t test was performed to identify significantly different parameters between the two groups. We found that age, BMI, SBP, DBP, and the duration of diabetes were not significantly different between the two groups (P=0.397, P=0.912, P=0.924, P=0.968, and P=0.133). However, the parameters related to glycemic control-fasting plasma glucose and HbA1c-were significantly different between the two groups. Mean fasting plasma glucose levels in the testosterone deficient and normal groups were 165.0 and 146.6 mg/dL, respectively (P=0.007). The mean HbA1c levels in the testosterone deficient and normal groups were 8.4% and 8.0%, respectively (P=0.038). No significant differences were observed in other parameters between the testosterone deficient and normal groups (Table 2).
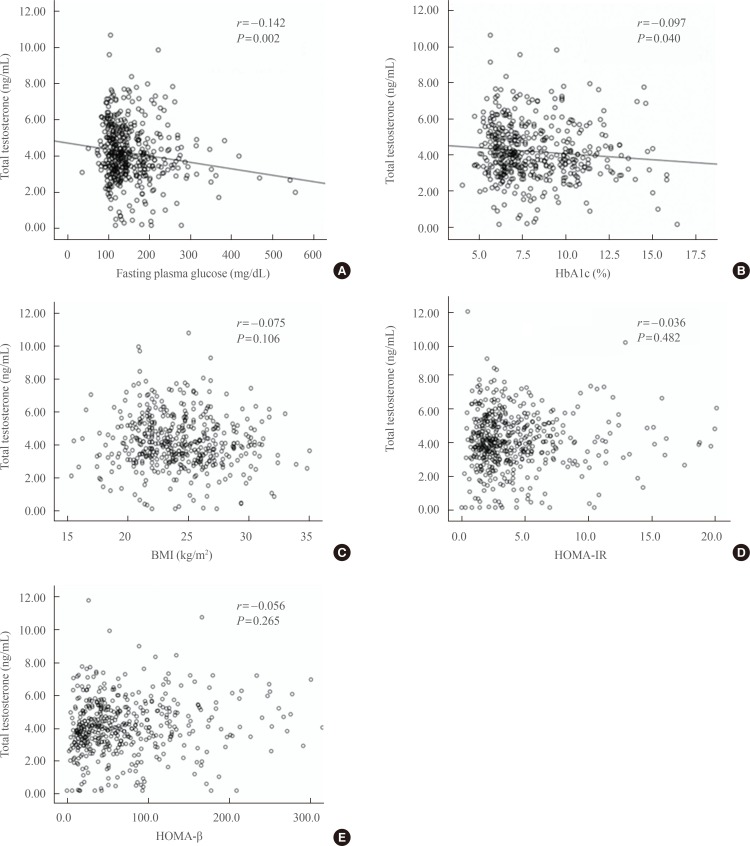
Subsequently, Pearson's correlation coefficients were calculated to evaluate the relationship between fasting plasma glucose, HbA1c, BMI, HOMA-IR, HOMA-β, and serum total testosterone values. As shown in Fig. 1, Pearson's coefficient correlation (r) between total testosterone and fasting plasma glucose levels was -0.142 and the P value was 0.002. The estimated regression relation was: testosterone=4.743-0.004×fasting plasma glucose (Fig. 1A). Thus, testosterone decreased 0.004 ng/mL with a 1 mg/dL increase in fasting plasma glucose. Pearson's coefficient correlation (r) between total testosterone and HbA1c levels was -0.097 and the P value was 0.040. The estimated regression relation was: testosterone=4.782-0.067×HbA1c (Fig. 1B). Thus, testosterone decreased 0.067 ng/mL as HbA1c levels increased 1%. However, BMI, HOMA-IR, and HOMA-β were not statistically correlated with serum total testosterone levels (P=0.106, P=0.482, and P=0.265) (Fig. 1C-E).
DISCUSSION
In this study, the prevalence of testosterone deficiency in diabetic men was 34.9%. The testosterone deficiency group showed significantly higher fasting plasma glucose and HbA1c levels compared to the normal group. Serum total testosterone levels were negatively correlated with fasting plasma glucose and HbA1c levels in Korean, diabetic men.
In previous studies, serum testosterone levels were shown to be negatively correlated with fasting plasma glucose levels, HbA1c values, and insulin sensitivity [4,18,19]. However, the debate continues. Corrales et al. [20] demonstrated that total testosterone levels are positively correlated with HbA1c levels, but not correlated with fasting plasma glucose, insulin, or C-peptide levels. Therefore, the relationship between total testosterone levels and markers of diabetes (fasting plasma glucose, HbA1c, serum insulin, C-peptide, HOMA-IR, and HOMA-β values) was evaluated in Korean, diabetic subjects.
In this study, fasting plasma glucose and HbA1c levels were significantly higher in the testosterone deficient group than in the normal group. However, we could not find any significant differences in parameters related to insulin resistance and insulin secretion. C-peptide and serum insulin levels are indicators of insulin secretion. HOMA-IR, which is a marker of insulin resistance, and HOMA-β, which is a marker of β-cell function, were calculated using fasting insulin levels. Therefore, testosterone levels might be associated with glycemic control, but are not associated with insulin resistance or insulin secretion.
Previous studies have demonstrated that insulin resistance is associated with low serum testosterone levels in men because testosterone affects insulin sensitivity by controlling the glycogen synthesis system, particularly in muscle [21,22,23]. Moreover, Heufelder et al. [24] suggested the beneficial effects of testosterone administration on insulin resistance in patients with diabetes. However in our study, no significant relationship was found between serum total testosterone and serum insulin, serum C-peptide, HOMA-IR, or HOMA-β values, which are directly related with serum insulin levels.
As an explanation for this discrepancy, it could be suggested that glycemic control might modulate testosterone levels. In another study, improved glycemic control using rosiglitazone increased testosterone levels in subjects with diabetes [5]. In addition, several studies evaluated the association of hypogonadotropic hypogonadism and diabetes [25,26,27,28]. George et al. [29] demonstrated that the pathophysiology of hypogonadism in men with type 2 diabetes is related to a hypothalamic neuropeptide named kisspeptin, which is down-regulated with hyperglycemia in animal studies [30]. In that study, kisspeptin administration increased luteinizing hormone (LH) pulse frequency and LH secretion, as a result enhancing endogenous testosterone secretion in hypotestosteronaemic men with type 2 diabetes.
The present study has several limitations. First, this study was cross sectional and a single hospital-based study. Thus, the results cannot be generalized to all patients with diabetes. Second, performing a single laboratory measurement of serum testosterone is a pitfall for making an erroneous diagnosis of testosterone deficiency; it has been reported that about 30% of such men may have normal testosterone levels on repeat measurements [31]. Third, we did not assess free testosterone and bioavailable testosterone. Some investigators have suggested that testosterone deficiency in patients with lower total testosterone levels should be confirmed by testing levels of free testosterone, which might correlate better with the biological activities of androgen than total testosterone. Fourth, we examined the duration of diabetes of the participants, but not a medication history of diabetes, which could affect glycemic control.
In conclusion, the prevalence of testosterone deficiency in men with diabetes was 34.9% in our study. Among markers related to diabetes, fasting plasma glucose and HbA1c levels-and not serum insulin, C-peptide, HOMA-IR, and HOMA-β-were associated with testosterone deficiency. Our findings suggest that poor glycemic control is associated with testosterone deficiency in Korean, male diabetics.
Notes
No potential conflict of interest relevant to this article was reported.