Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(3); 2022 > Article
-
Original ArticleThyroid Clinical Outcomes of Repeated Radioactive Iodine Therapy for Graves’ Disease
 Keypoint
Keypoint
This study investigated the clinical outcomes of repeated radioactive iodine (RAI) therapy for Graves’ disease. Patients who underwent RAI therapy as second-line therapy after failure of antithyroid drug treatment between 2001 and 2015 were reviewed. The remission rate of the second RAI therapy was significantly higher than that of the first RAI therapy or long-term antithyroid drug treatment. The response to the second RAI therapy was more rapid than the response to the first RAI therapy. -
Min Joo Kim1,2,3
 , Sun Wook Cho1,2
, Sun Wook Cho1,2 , Ye An Kim2,4, Hoon Sung Choi5, Young Joo Park1,2,6, Do Joon Park1,2, Bo Youn Cho1,2,7
, Ye An Kim2,4, Hoon Sung Choi5, Young Joo Park1,2,6, Do Joon Park1,2, Bo Youn Cho1,2,7 -
Endocrinology and Metabolism 2022;37(3):524-532.
DOI: https://doi.org/10.3803/EnM.2022.1418
Published online: June 16, 2022
1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
3Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea
4Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, Korea
5Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea
6Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
7Thyroid Center, Chung-Ang University Hospital, Seoul, Korea
- Corresponding author: Sun Wook Cho. Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-4761, Fax: +82-2-762-2292, E-mail: swchomd@snu.ac.kr
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Radioactive iodine (RAI) therapy is a successful therapeutic modality for Graves’ disease. However, RAI therapy can fail, and RAI therapy after antithyroid drugs (ATDs) has a lower remission rate. Therefore, many patients require repeated RAI therapy. This study investigated the clinical outcomes of repeated RAI therapy for Graves’ disease.
-
Methods
- Patients who underwent RAI therapy as second-line therapy after failure of ATD treatment between 2001 and 2015 were reviewed. Remission was defined as hypothyroid or euthyroid status without ATD, and with or without levothyroxine at 12 months after RAI therapy.
-
Results
- The 1-year remission rate after 2nd RAI therapy (66%, 152/230) is significantly higher than that after 1st RAI therapy (48%, 393/815) or long-term ATD treatment after 1st RAI therapy failure (42%). The clinical response to 2nd RAI therapy was more rapid. The median time intervals from the 2nd RAI therapy to ATD discontinuation (1.3 months) and to the start of levothyroxine replacement (2.5 months) were significantly shorter than those for the 1st RAI therapy. A smaller goiter size, a longer time interval between the 1st and 2nd RAI therapies, and a longer ATD discontinuation period predicted remission after the 2nd RAI therapy. Finally, in 78 patients who failed the 2nd RAI therapy, the mean ATD dosage significantly reduced 5.1 mg over 12 months.
-
Conclusion
- Repeated RAI therapy can be a good therapeutic option, especially in patients with smaller goiters and those who are more responsive to the 1st RAI therapy.
- Graves’ disease (GD), a common autoimmune endocrinopathy, causes hyperthyroidism, which is characterized by excess production and secretion of thyroid hormones by the thyroid gland. The global prevalence of GD is approximately 0.5% [1,2], and its incidence is approximately 0.5–1.0 per 1,000 person-years [2,3]. GD is treated in three ways: antithyroid drugs (ATDs), radioactive iodine (RAI) therapy with iodine-131 (131I), and thyroidectomy. Each of these three treatments has advantages and disadvantages, and none of them can be considered optimal in absolute terms [4,5]. ATD therapy is commonly used as the initial treatment of choice for GD, because it is the only treatment aiming to restore normal thyroid function [6]. Although the preferred initial treatment varies across countries and regions [7], an ATD is administered initially in 97% to 98% of Asian patients and 16% to 41% of American patients [3,7-10]. Recently, ATD as an initial treatment in United States has increased to almost 60% [11]. However, a low remission rate (40% to 50%) and a high recurrence rate are major limitations of ATD treatment [10]. Therefore, many patients do not achieve remission after ATD use and require a second-line treatment such as RAI therapy, surgery, or long-term ATD. Many patients in whom ATD treatment fails select RAI therapy because surgery is invasive and poses a risk of surgical complications, such as hypoparathyroidism and recurrent laryngeal nerve damage, whereas RAI therapy is less invasive and has fewer complications and lower costs. Previous studies reported that 46% to 77% of patients in whom ATD treatment failed selected RAI therapy [10,12].
- The success rate of initial RAI therapy was reported to be 74% to 81% in a previous randomized controlled study [13], which is higher than that of ATD therapy. Nonetheless, the response rate of RAI is still limited, especially in patients with previous ATD use [14,15]. For this reason, some patients (6% to 10% of GD patients according to previous research) require repeated RAI therapy [10,16,17]. However, little is known about the clinical outcomes of additional rounds of RAI therapy. Therefore, in the present study, we investigated the clinical implications of repeated RAI therapy.
INTRODUCTION
- Subject
- In this retrospective study, GD patients who underwent RAI therapy as second-line therapy after the failure of ATD treatment at a single tertiary referral hospital between 2001 and 2015 were enrolled [18]. The diagnosis of GD was based on a clinical examination, typical alterations of the thyroid function test (high free thyroxine [T4] and/or high total triiodothyronine and low thyrotropin [TSH]), and the presence of TSH receptor (TSHR) antibody and/or diffusely increased thyroid uptake of 99m-Tc on radionuclide scintigraphy. Among 10,986 GD patients, 1,319 (12%) patients underwent RAI therapy were screened. Patients who started their initial RAI therapy before 2001 (n=115), who underwent thyroidectomy (n=114) before or after RAI therapy for reasons other than GD, who received RAI therapy as an initial treatment (n=5), or who had a follow-up duration of less than 1 year (n=122) were excluded. In addition, patients who received RAI therapy due to severe adverse events of ATDs such as agranulocytosis and toxic hepatitis (n=148) were excluded because their characteristics were significantly different from those of patients with ATD failure (Supplemental Table S1). Finally, 815 patients were enrolled in the analysis of the clinical outcomes of repeated RAI therapy for GD. The study was approved by the Institutional Review Board committee of Seoul National University Hospital (No. 1410-097-619). Informed consent was waived due to a retrospective nature of our study. Information regarding age, sex, the time of diagnosis, goiter size, ATD use history, and laboratory test results (TSH, free T4, and TSHR antibody) was obtained retrospectively by reviewing patients’ electronic medical records. In our hospital, the goiter sized was evaluated and recorded at the first visit for GD. The goiter size was assessed by the endocrinologists through physical examination (palpation).
- Protocols for RAI therapy
- Prior to RAI, ATDs were stopped for more than 7 days and iodine-rich food such as seaweed was avoided for 2 weeks according to the guidelines [4,9]. 131I was administered to patients at a fixed dose of 15 mCi (555 MBq). Thyroid radioactive iodine uptake (RAIU) was measured at 48 hours after oral administration of 131I. ATD was restarted 3 days later, if needed.
- Definition of remission after RAI therapy
- Remission was defined as (1) hypothyroid or euthyroid status without using an ATD or (2) a requirement for levothyroxine (LT4) at 12 months after RAI therapy. Otherwise, the disease was considered to be persistent.
- Statistical analysis
- Data were presented as the mean±standard deviation. To compare the characteristics of patients according to remission status, categorical and continuous variables were analyzed using the chi-square test or the Student t test. Kaplan-Meier curves for the discontinuation of ATD and the initiation of LT4 were plotted, and the curves of the 1st and 2nd RAI therapies were compared using the log-rank test. To investigate predictive factors for remission, univariate/multivariate logistic regression analysis was performed. P values <0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS version 23.0 for Windows (IBM Corp., Armonk, NY, USA).
METHODS
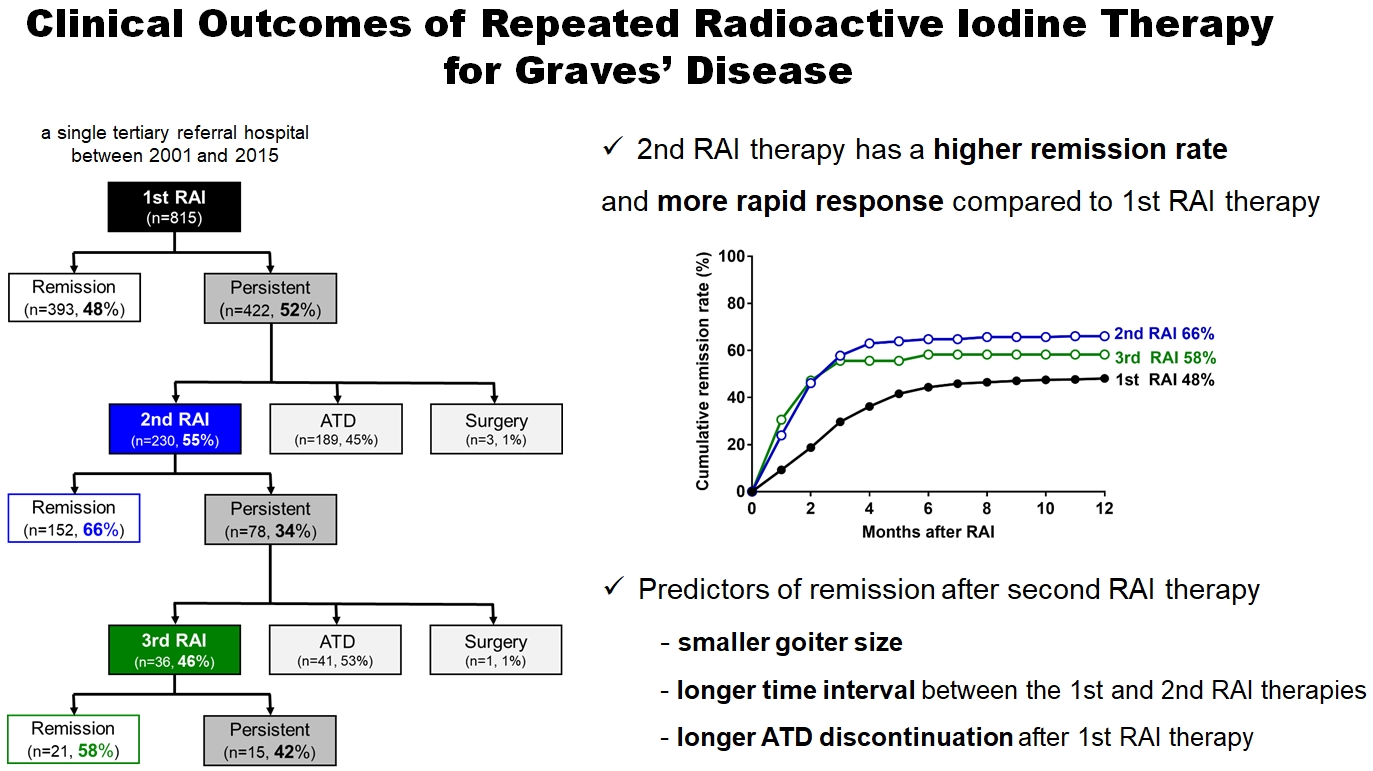
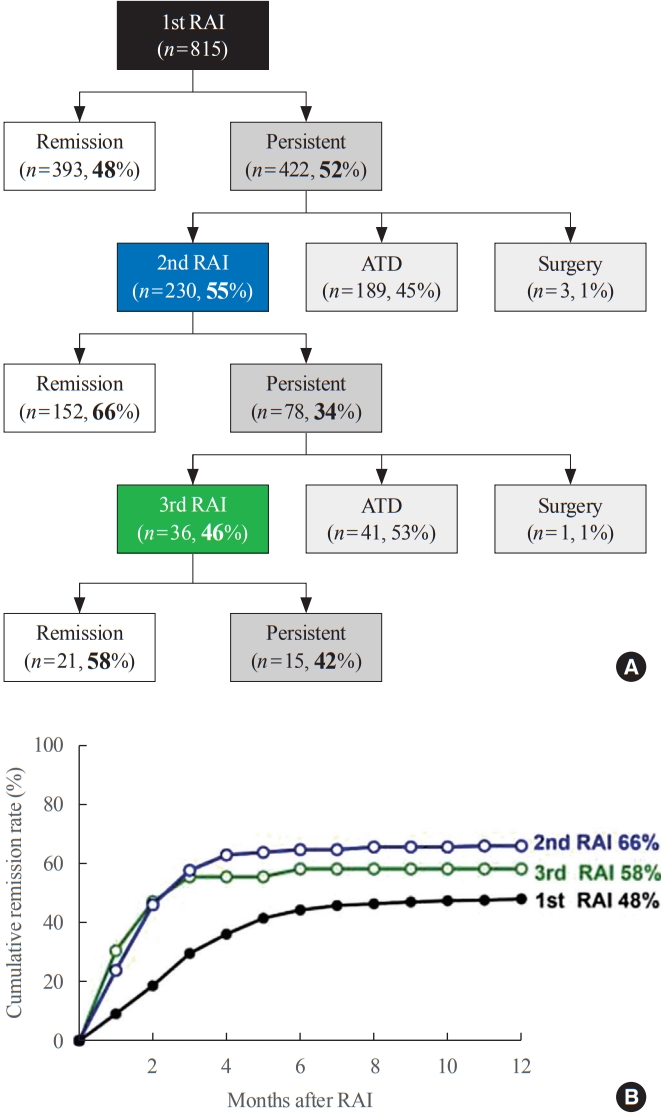
- Higher remission rate of the second RAI therapy
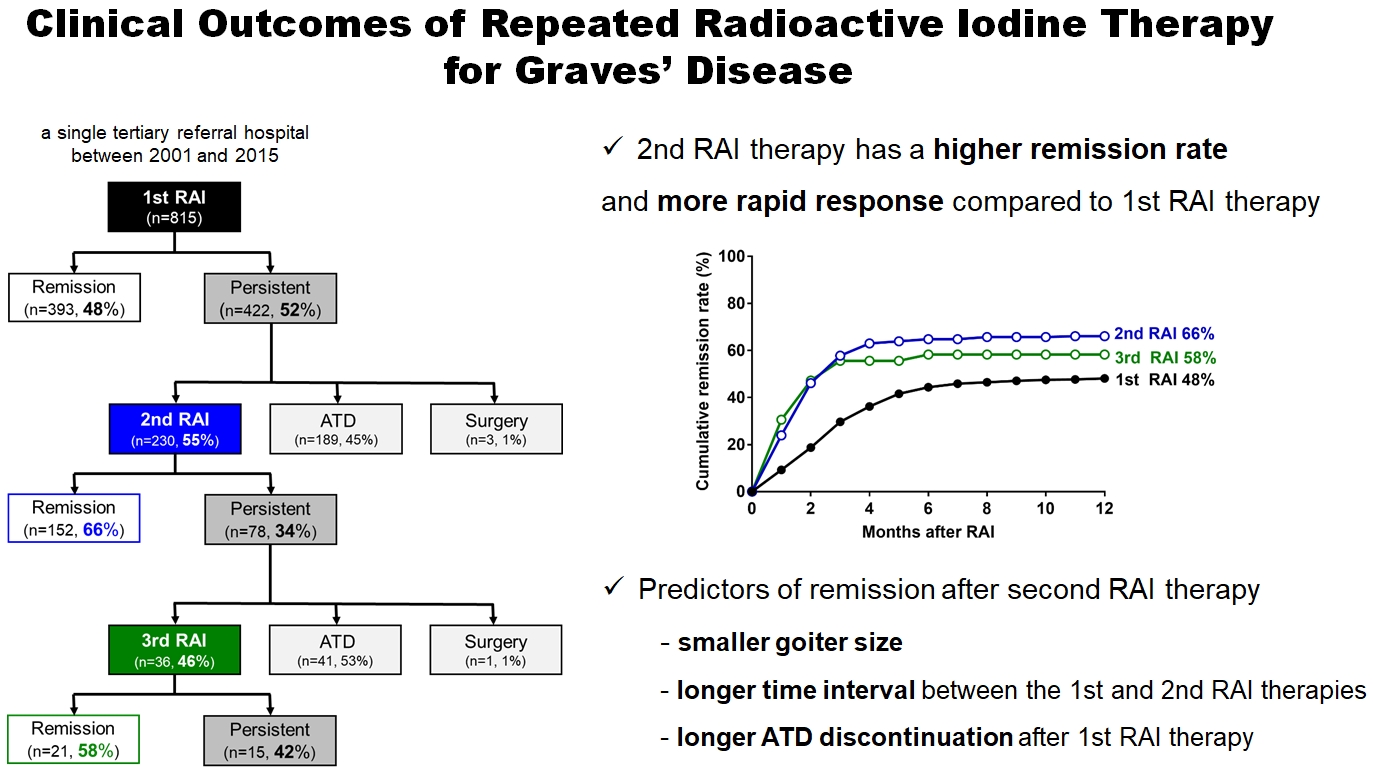
- In total, 815 patients underwent RAI therapy as second-line therapy after ATD failure. Their mean age was 41±13 years and 67% were women. The 1-year remission rate was 48% (393/815). Among the 422 (52%) patients who did not achieve remission, 230 patients underwent 2nd RAI therapy, with a median interval between the 1st and 2nd RAI treatments of 6 months (range, 3 to 78) (Fig. 1A). The remission rate of the 2nd RAI therapy was 66% (152/230), which was significantly higher than that of the 1st RAI therapy (P<0.001) (Fig. 1B). Some patients with persistent GD after 1st RAI therapy received longterm ATD treatment. Delayed response was observed, and the remission was achieved in 42% (81/189) of patients (Supplemental Table S1). The remission rate of long-term ATD treatment was significantly lower than the 1-year remission rate of 2nd RAI therapy (P<0.001), and it was achieved at a median of 35 months (range, 25 to 53). After the 2nd RAI therapy, 78 (34%) patients did not achieve remission, and of them, 36 patients underwent a 3rd RAI therapy, of whom 61% (21/36) achieved remission within 1 year (Fig. 1A).
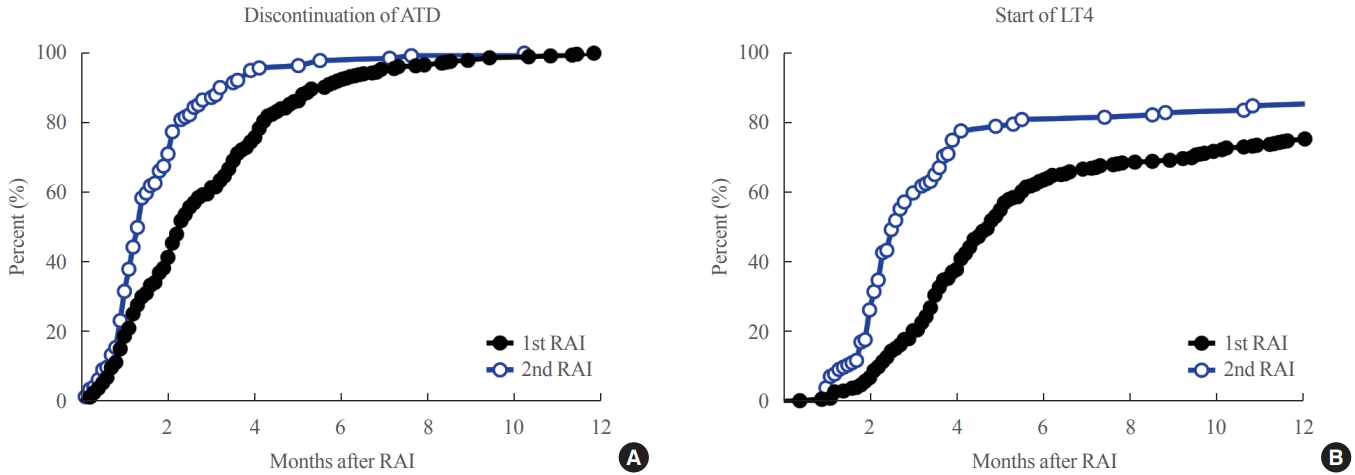
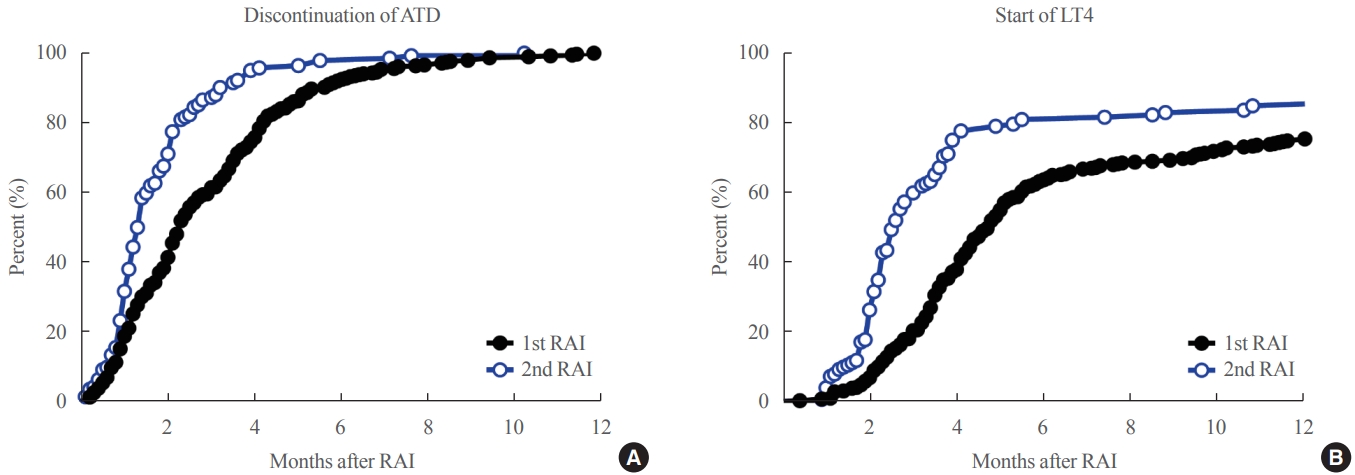
- Rapid response to RAI in the second RAI therapy
- To clarify the optimal time for assessing the clinical outcomes of each round of RAI therapy, the intervals until ATD discontinuation and the start of LT4 replacement were explored. Compared to the 1st RAI therapy, the 2nd RAI therapy showed significantly shorter intervals until ATD discontinuation (median 1.3 months [0 to 10.2] vs. 2.3 months [0 to 11.8], P<0.001) and the start of LT4 replacement (median 2.5 months [0.9 to 76.1] vs. 4.4 months [0.4 to 11.8], P=0.001). Kaplan-Meier curves showed that 80% of patients who achieved remission discontinued ATD within 4.2 and 2.3 months after 1st and 2nd RAI therapies, respectively (Fig. 2A). Additionally, 80% of those who achieved remission started LT4 within 17.0 and 5.5 months after the 1st and 2nd RAI therapies, respectively (Fig. 2B). The time to discontinue ATD (1.7 months) or start LT4 after the 3rd RAI therapy (3.5 months) was similar to those after the 2nd RAI therapy.
- Predictors of remission after second RAI therapy
- For the 1st RAI therapy (n=815), univariable logistic regression analysis showed that female sex, a shorter time interval after diagnosis, a smaller goiter size, a smaller dosage of ATD, a higher pre-RAI TSH level, and a lower pre-RAI TSHR antibody level were associated with a higher likelihood of remission (Table 1). However, in the multivariable logistic regression analysis, only a shorter time (≤4 years) from diagnosis to the 1st RAI therapy and smaller goiter size (<90 g) were significant predictors of remission (Table 1).
- Next, for the 2nd RAI therapy, the characteristics of 152 patients who achieved remission 1 year after the 2nd RAI therapy were compared with 78 patients with persistent GD even after the 2nd RAI therapy (Supplemental Table S2). Patients with remission had a longer time interval between the 1st and 2nd RAI therapies (12±12 months vs. 8±7 months, P=0.002) and smaller goiter size than those with persistent GD (Supplemental Table S2). To determine whether the response to the 1st RAI therapy predicted remission after the 2nd RAI therapy, the dosage and discontinuation of ATD were investigated for 6 months after the 1st RAI therapy. Patients with remission were more likely to have discontinued ATD treatment for over 2 months (36% vs. 21%, P=0.023) and to have used a lower ATD dosage (12±7 mg vs. 14±7 mg, P=0.023). Most patients (89%) used methimazole and 9% used propylthiouracil, and types of ATD were not different between remission and persistent group (89% vs. 90%, P=0.807). The remission group had a higher pre-RAI TSH level (1.58±8.43 μIU/mL vs. 0.10±0.25 μIU/mL, P=0.036) and a lower pre-RAI free T4 level (2.25±1.25 ng/dL vs. 2.89±2.14 ng/dL, P=0.016) (Supplemental Table S2). In the univariable logistic regression analysis, a longer time interval (≥12 months) between the 1st and 2nd RAI therapies, smaller goiter size (<90 g), ATD discontinuation (≥2 months), and a higher TSH level (≥0.1 μIU/mL) were significantly associated with remission (Table 2). However, older age (>60 years) was associated with a higher likelihood of persistence. Multivariable logistic regression analysis showed that age, time interval between consecutive RAI therapies, goiter size, and ATD discontinuation were significant predictors of remission after the 2nd RAI therapy (Table 2).
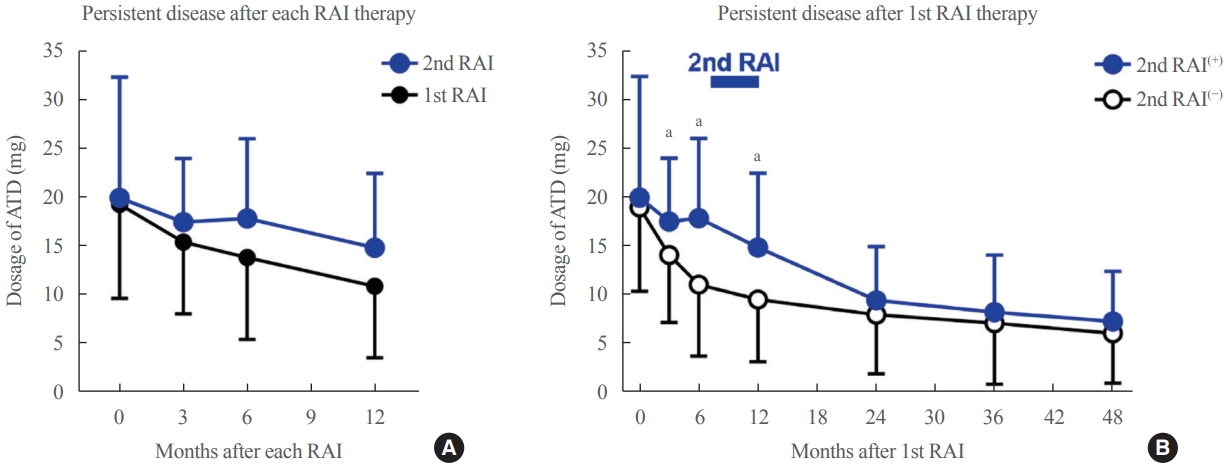
- ATD dose reduction effect of second RAI therapy in patients with persistent GD
- Even if RAI therapy fails and GD persists, RAI therapy can reduce the dosage of ATD. In patients with persistent disease after RAI therapy, 1st RAI therapy and 2nd RAI therapy significantly reduced 8.4 and 5.1 mg of ATD over 12 months, respectively (both P<0.01) (Fig. 3A). Next, we compared the clinical outcomes of two groups that failed 1st RAI therapy: 189 patients who maintained ATD treatment without 2nd RAI therapy (2nd RAI(−) group) and 78 patients who received 2nd RAI therapy but failed (2nd RAI(+) group). After 1st RAI therapy, a higher dosage of ATD was required in the 2nd RAI(+) group than in the 2nd RAI(−) group (18±8 mg vs. 11±7 mg, P<0.001) (Fig. 3B). However, the 2nd RAI therapy reduced the dosage of ATD in the 2nd RAI(+) group to a comparable level to that of the 2nd RAI(−) group (Fig. 3B).
RESULTS
- In the present study, the 2nd RAI therapy showed a higher remission rate (66%) and a shorter time to remission, mostly within 4 months, compared with the 1st RAI therapy. Even in patients who did not achieve remission after the 2nd RAI therapy, the 2nd RAI therapy led to ATD dose reduction. Therefore, repeated RAI therapy can be an effective therapeutic choice in patients with persistent GD after the 1st RAI therapy, especially in those with a small goiter size, a long time interval from the 1st RAI therapy, and a history of ATD discontinuation after the 1st RAI therapy.
- The remission rate after the 1st RAI therapy in this study was 48%, which is lower than the remission rate of 74% to 81% in a previous randomized controlled study [13]. This may be because RAI therapy was performed as a second-line treatment. A meta-analysis showed that ATD use before RAI therapy had 1.48 to 2.05-fold higher risk of RAI treatment failure [14,15]. Park et al. [19] also reported that the remission rate of RAI therapy as a second-line treatment was 63%. Previous epidemiological studies with higher proportion of RAI therapy as a second-line treatment have tended to report worse remission rates than other studies (56%–79% vs. 83%–88%) [20-23].
- Few studies have investigated the clinical outcomes of the 2nd RAI therapy separately. A strength of this study is that a large number of GD patients who received repeated RAI therapies were recruited, and the treatment response of 2nd RAI therapy was compared with that of 1st RAI therapy. The remission rate of the 2nd RAI therapy (66%) was significantly higher than that of the 1st RAI therapy (48%) or long-term ATD treatment (42%); thus, repeated RAI therapy can be a good therapeutic strategy for patients who fail the 1st RAI therapy. Although RAI therapy is effective for GD, RAI has some concerns such as the risk of exacerbating Graves’ ophthalmopathy and a potential radiation hazard [4,24,25]. Therefore, it is necessary to individualize which treatment to choose. Next, the time from RAI therapy to achieving remission was shorter after 2nd RAI therapy than after the 1st RAI therapy, suggesting that the clinical outcomes of repeated RAI therapy can be assessed more promptly. The American Thyroid Association guideline recommends considering repeated RAI if GD persists after 6 months following the 1st RAI therapy [4]. Indeed, the cumulative remission rates plateaued after 6 months following the 1st RAI therapy in this study. Meanwhile, remission was achieved earlier in 2nd RAI therapies, as approximately 80% of patients were expected to stop ATD within 2.3 months. Therefore, the treatment response of repeated RAI therapy needs to be assessed before 4 months, and an earlier plan for LT4 replacement should be followed to maintain the quality of life of those patients [26].
- Many previous researchers have made efforts to predict the success of the 1st RAI therapy. A recent meta-analysis reported that male sex, a longer time from GD diagnosis to RAI therapy (>6 months), previous ATD use, a higher free T4 level, a higher 24-hour RAIU (≥60%), and a larger thyroid volume (>35 mL) increased the risk of RAI therapy failure [15]. In this study, a longer time from GD diagnosis to RAI therapy and a larger goiter size were also significantly associated with failure of the 1st RAI therapy. In both the 1st and 2nd RAI therapies, goiter size assessed by physical examination was a strong predictor of remission. It suggested that the assessment of goiter size through physical examination can make a sufficiently significant prediction for the success of RAI therapy. However, the predictors of the 1st and 2nd RAI therapies were not always the same. The time from diagnosis to RAI therapy, a significant predictor of remission after the 1st RAI therapy, did not predict remission after the 2nd RAI therapy. Instead, a long interval between the 1st and 2nd RAI therapies (>12 months) was associated with an increased likelihood of remission (odds ratio, 3.82) (Table 2). Stabilizing GD using an ATD before 2nd RAI therapy may be beneficial for achieving remission. Even if the initial ATD treatment fails, the remission may be achieved in the second or third ATD treatment [27]. Therefore, the long-term use of ATD before 2nd RAI therapy may improve the GD itself and contribute to the success of the 2nd RAI therapy. In addition, a good response to the 1st RAI therapy, allowing the ATD to be discontinued for over 2 months, was associated with remission (odds ratio, 2.86) (Table 2). In other words, patients who were more responsive to the 1st RAI therapy were more likely to achieve remission after the 2nd RAI therapy. In this study, pre-RAI TSH was not associated with the remission in multivariable logistic regression analysis. Some patients (n=16) showed higher TSH than normal range just before RAI. In these patients, as GD worsened, ATD dose was increased, and TSH became higher than normal range. However, even if pre-RAI TSH levels of these patients were corrected, pre-RAI TSH level was not associated with the remission (odds ratio, 1.84; 95% confidence interval, 0.82 to 4.13; P=0.140).
- This study has the limitations inherent to retrospective studies. The initial thyroid hormone or anti-TSHR antibody levels at the time of GD diagnosis could not be collected and analyzed, because of the nature of a tertiary referral hospital, although previous studies have reported that free T4 or TSHR antibody levels at the time of diagnosis predicted remission [15,21,28]. Instead, the levels of free T4, TSH, and TSHR antibody just before RAI therapy were used in the analysis, but they did not predict remission after RAI therapy. In addition, the RAI dose was not calculated based on the size of the thyroid and its ability to absorb 131I, but a fixed dose (15 mCi) was used empirically according to the doctor’s judgment. Therefore, it would be difficult to evaluate the effect of the RAI dose on remission. However, the RAI dose was not associated with the success of RAI therapy in previous studies [15].
- In conclusion, repeated RAI therapy can be a good therapeutic option in patients with persistent GD after the 1st RAI therapy, especially in patients who have a small goiter size and show a good response to the 1st RAI therapy. LT4 replacement therapy should be prepared earlier after repeated RAI therapy than after the 1st RAI therapy (i.e., within 4 months).
DISCUSSION
Supplementary Information
Supplemental Table S1.
Supplemental Table S2.
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: S.W.C., Y.J.P. Acquisition, analysis, or interpretation of data: M.J.K., S.W.C., Y.A.K., H.S.C., Y.J.P., D.J.P., B.Y.C. Drafting the work or revising: M.J.K., S.W.C., Y.J.P. Final approval of the manuscript: M.J.K., S.W.C., Y.A.K., H.S.C., Y.J.P., D.J.P., B.Y.C.
Article information
-
Acknowledgements
- This study was funded by the Ministry of Health and Welfare through the Korea Health Industry Development Institute, Korea Health Technology R&D Project (HI14C1277).
| Variable |
Univariable |
Multivariablea |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at RAI therapy, yr | ||||
| 20–39 | 1.36 (0.81–2.29) | 0.245 | ||
| 40–59 | 1 (reference) | |||
| ≥60 | 1.00 (0.60–1.78) | 0.988 | ||
| Sex | ||||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 1.35 (1.01–1.81) | 0.043 | 1.09 (0.73–1.64) | 0.680 |
| Time from diagnosis to 1st RAI therapy, yr | ||||
| ≤1 | 1.09 (0.77–1.55) | 0.621 | 2.08 (1.25–3.47) | 0.005 |
| 2–4 | 1.40 (1.01–1.95) | 0.044 | 1.68 (1.06–2.66) | 0.028 |
| ≥5 | 1 (reference) | |||
| Goiter size, ga | ||||
| <60 | 8.06 (4.70–13.83) | <0.001 | 9.07 (4.74–17.39) | <0.001 |
| 60–90 | 3.36 (1.90–5.93) | <0.001 | 3.77 (1.99–7.16) | <0.001 |
| ≥90 | 1 (reference) | 1 (reference) | ||
| Mean dosage of ATD, mga | ||||
| ≤10 | 1.92 (1.34–2.76) | <0.001 | 0.81 (0.47–1.40) | 0.442 |
| 10–20 | 1.65 (1.19–2.28) | 0.003 | 1.03 (0.66–1.60) | 0.889 |
| >20 | 1 (reference) | 1 (reference) | ||
| Pre-RAI TSH, μIU/mLa | ||||
| <0.1 | 1 (reference) | |||
| ≥0.1 | 1.51 (1.13–2.03) | 0.006 | 0.89 (0.56–1.40) | 0.607 |
| Pre-RAI free T4, ng/dLa | 1 (reference) | |||
| ≤1.8 | 1.18 (0.89–1.57) | 0.239 | ||
| >1.8 | 1 (reference) | |||
| Pre-RAI TSHR antibody, %a | ||||
| ≤30 | 2.58 (1.71–3.89) | <0.001 | 1.68 (1.00–2.82) | 0.050 |
| 30–60 | 1.73 (1.19–2.49) | 0.004 | 1.24 (0.80–1.91) | 0.335 |
| >60 | 1 (reference) | 1 (reference) | ||
| 48-hr RAIU, % | ||||
| ≤50 | 0.925 (0.627–1.367) | 0.925 | ||
| 51–75 | 0.785 (0.574–1.076) | 0.785 | ||
| ≥75 | 1 (reference) | |||
| Variable |
Univariable |
Multivariablea |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age at 2nd RAI therapy, yr | ||||
| 20–39 | 0.79 (0.44–1.42) | 0.435 | 1.38 (0.64–2.96) | 0.410 |
| 40–59 | 1 (reference) | 1 (reference) | ||
| ≥60 | 0.34 (0.13–0.90) | 0.030 | 0.13 (0.03–0.56) | 0.006 |
| Sex | ||||
| Male | 1 (reference) | |||
| Female | 1.12 (0.65–1.94) | 0.688 | ||
| Time interval between 1st and 2nd RAI therapies, mo | ||||
| <6 | 1 (reference) | 1 (reference) | ||
| 6–11 | 1.79 (0.97–3.30) | 0.062 | 1.98 (0.94–4.19) | 0.074 |
| ≥12 | 3.17 (1.39–7.26) | 0.006 | 3.82 (1.15–12.69) | 0.029 |
| Goiter size, ga | ||||
| <60 | 2.21 (1.08–4.53) | 0.030 | 2.52 (1.09–5.84) | 0.031 |
| 60–90 | 3.91 (1.81–8.43) | 0.001 | 3.30 (1.38–7.86) | 0.007 |
| ≥90 | 1 (reference) | 1 (reference) | ||
| ATD discontinuation, mob | ||||
| <2 | 1 (reference) | 1 (reference) | ||
| ≥2 | 2.14 (1.12–4.06) | 0.021 | 2.86 (1.08–7.55) | 0.034 |
| Mean dosage of ATD, mgb | ||||
| ≤10 | 2.59 (1.11–6.08) | 0.028 | 1.15 (0.33–3.95) | 0.829 |
| 10–20 | 1.16 (0.52–2.57) | 0.719 | 0.72 (0.26–1.96) | 0.515 |
| >20 | 1 (reference) | 1 (reference) | ||
| Pre-RAI TSH, μIU/mLa | ||||
| <0.1 | 1 (reference) | 1 (reference) | ||
| ≥0.1 | 2.30 (1.07–4.92) | 0.033 | 2.48 (0.84–7.33) | 0.099 |
| Pre-RAI free T4, ng/dLa | ||||
| ≤1.8 | 1.27 (0.71–2.27) | 0.417 | ||
| >1.8 | 1 (reference) | |||
| Pre-RAI TSHR antibody, %a | ||||
| ≤30 | 1.49 (0.53–4.17) | 0.446 | ||
| 30–60 | 1.72 (0.76–3.88) | 0.190 | ||
| >60 | 1 (reference) | |||
| 48-hr RAIU, % | ||||
| ≤50 | 1.08 (0.42–2.78) | 0.869 | ||
| 51–75 | 0.78 (0.31–1.96) | 0.593 | ||
| ≥75 | 1 (reference) | |||
- 1. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–99.ArticlePubMed
- 2. Garmendia Madariaga A, Santos Palacios S, Guillen-Grima F, Galofre JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab 2014;99:923–31.ArticlePubMed
- 3. Kornelius E, Yang YS, Huang CN, Wang YH, Lo SC, Lai YR, et al. The trends of hyperthyroidism treatment in Taiwan: a nationwide population-based study. Endocr Pract 2018;24:573–9.ArticlePubMed
- 4. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016;26:1343–421.ArticlePubMed
- 5. Smith TJ, Hegedus L. Graves’ disease. N Engl J Med 2016;375:1552–65.ArticlePubMed
- 6. Wiersinga WM. Graves’ disease: can it be cured? Endocrinol Metab (Seoul) 2019;34:29–38.ArticlePubMedPMCPDF
- 7. Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab 2012;97:4549–58.ArticlePubMed
- 8. Seo GH, Kim SW, Chung JH. Incidence & prevalence of hyperthyroidism and preference for therapeutic modalities in Korea. J Korean Thyroid Assoc 2013;6:56–63.Article
- 9. Yi KH, Moon JH, Kim IJ, Bom HS, Lee J, Chung WY, et al. The diagnosis and management of hyperthyroidism consensus: report of the Korean Thyroid Association. J Korean Thyroid Assoc 2013;6:1–11.Article
- 10. Sundaresh V, Brito JP, Thapa P, Bahn RS, Stan MN. Comparative effectiveness of treatment choices for Graves’ hyperthyroidism: a historical cohort study. Thyroid 2017;27:497–505.ArticlePubMedPMC
- 11. Brito JP, Payne S, Singh Ospina N, Rodriguez-Gutierrez R, Maraka S, Sangaralingham LR, et al. Patterns of use, efficacy, and safety of treatment options for patients with Graves’ disease: a nationwide population-based study. Thyroid 2020;30:357–64.ArticlePubMed
- 12. Hussain YS, Hookham JC, Allahabadia A, Balasubramanian SP. Epidemiology, management and outcomes of Graves’ disease-real life data. Endocrine 2017;56:568–78.ArticlePubMedPMCPDF
- 13. Santos RB, Romaldini JH, Ward LS. A randomized controlled trial to evaluate the effectiveness of 2 regimens of fixed iodine (131I) doses for Graves disease treatment. Clin Nucl Med 2012;37:241–4.ArticlePubMed
- 14. Walter MA, Briel M, Christ-Crain M, Bonnema SJ, Connell J, Cooper DS, et al. Effects of antithyroid drugs on radioiodine treatment: systematic review and meta-analysis of randomised controlled trials. BMJ 2007;334:514.ArticlePubMedPMC
- 15. Shalaby M, Hadedeya D, Toraih EA, Razavi MA, Lee GS, Hussein MH, et al. Predictive factors of radioiodine therapy failure in Graves’ disease: a meta-analysis. Am J Surg 2022;223:287–96.ArticlePubMed
- 16. Wu VT, Lorenzen AW, Beck AC, Reid VJ, Sugg SL, Howe JR, et al. Comparative analysis of radioactive iodine versus thyroidectomy for definitive treatment of Graves disease. Surgery 2017;161:147–55.ArticlePubMedPMC
- 17. Wong KK, Shulkin BL, Gross MD, Avram AM. Efficacy of radioactive iodine treatment of graves’ hyperthyroidism using a single calculated 131I dose. Clin Diabetes Endocrinol 2018;4:20.ArticlePubMedPMCPDF
- 18. Kim MJ, Kim YA, Cho SW, Kim SJ, Lee KE, Park YJ, et al. Secular trends in ablation therapy for graves’ disease: an analysis of a 15-year experience at a tertiary hospital in South Korea. J Clin Med 2021;10:1629.ArticlePubMedPMC
- 19. Park H, Kim HI, Park J, Park SY, Kim TH, Chung JH, et al. The success rate of radioactive iodine therapy for Graves’ disease in iodine-replete area and affecting factors: a singlecenter study. Nucl Med Commun 2020;41:212–8.ArticlePubMed
- 20. Husseni MA. The incidence of hypothyroidism following the radioactive iodine treatment of Graves’ disease and the predictive factors influencing its development. World J Nucl Med 2016;15:30–7.ArticlePubMedPMC
- 21. Aung ET, Zammitt NN, Dover AR, Strachan M, Seckl JR, Gibb FW. Predicting outcomes and complications following radioiodine therapy in Graves’ thyrotoxicosis. Clin Endocrinol (Oxf) 2019;90:192–9.ArticlePubMedPDF
- 22. Fanning E, Inder WJ, Mackenzie E. Radioiodine treatment for graves’ disease: a 10-year Australian cohort study. BMC Endocr Disord 2018;18:94.ArticlePubMedPMCPDF
- 23. Liu M, Jing D, Hu J, Yin S. Predictive factors of outcomes in personalized radioactive iodine ((131)I) treatment for Graves’ disease. Am J Med Sci 2014;348:288–93.ArticlePubMed
- 24. Traisk F, Tallstedt L, Abraham-Nordling M, Andersson T, Berg G, Calissendorff J, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab 2009;94:3700–7.ArticlePubMed
- 25. Kim BW. Does radioactive iodine therapy for hyperthyroidism cause cancer? J Clin Endocrinol Metab 2022;107:e448–57.ArticlePubMedPDF
- 26. Taieb D, Bournaud C, Eberle MC, Catargi B, Schvartz C, Cavarec MB, et al. Quality of life, clinical outcomes and safety of early prophylactic levothyroxine administration in patients with Graves’ hyperthyroidism undergoing radioiodine therapy: a randomized controlled study. Eur J Endocrinol 2016;174:491–502.ArticlePubMed
- 27. Kim YA, Cho SW, Choi HS, Moon S, Moon JH, Kim KW, et al. The second antithyroid drug treatment is effective in relapsed Graves’ disease patients: a median 11-year followup study. Thyroid 2017;27:491–6.ArticlePubMed
- 28. Yang D, Xue J, Ma W, Liu F, Fan Y, Rong J, et al. Prognostic factor analysis in 325 patients with Graves’ disease treated with radioiodine therapy. Nucl Med Commun 2018;39:16–21.ArticlePubMedPMC
References
Figure & Data
References
Citations

- The Early Changes in Thyroid-Stimulating Immunoglobulin Bioassay over Anti-Thyroid Drug Treatment Could Predict Prognosis of Graves’ Disease
Jin Yu, Han-Sang Baek, Chaiho Jeong, Kwanhoon Jo, Jeongmin Lee, Jeonghoon Ha, Min Hee Kim, Jungmin Lee, Dong-Jun Lim
Endocrinology and Metabolism.2023; 38(3): 338. CrossRef - Effect of liver dysfunction on outcome of radioactive iodine therapy for Graves’ disease
Yuyang Ze, Fei Shao, Xuefeng Feng, Shanmei Shen, Yan Bi, Dalong Zhu, Xiaowen Zhang
BMC Endocrine Disorders.2022;[Epub] CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite