Articles
- Page Path
- HOME > Endocrinol Metab > Volume 37(3); 2022 > Article
-
Original ArticleCalcium & Bone Metabolism Real-World Safety and Effectiveness of Denosumab in Patients with Osteoporosis: A Prospective, Observational Study in South Korea
 Keypoint
Keypoint
This post-marketing surveillance study aimed to investigate the safety and effectiveness of denosumab in Korean real-world clinical practice, including 3,221 patients. The mean study period was 350.0 days. In this study, the safety and effectiveness of denosumab in Korean patients with osteoporosis were comparable with those in the Korean randomized controlled trial of denosumab, with no new safety findings. -
Yumie Rhee1
 , Dong-Gune Chang2, Jeonghoon Ha3, Sooa Kim4, Yusun Lee4, Euna Jo4, Jung-Min Koh5
, Dong-Gune Chang2, Jeonghoon Ha3, Sooa Kim4, Yusun Lee4, Euna Jo4, Jung-Min Koh5
-
Endocrinology and Metabolism 2022;37(3):497-505.
DOI: https://doi.org/10.3803/EnM.2022.1427
Published online: June 3, 2022
1Department of Internal Medicine, Severance Hospital, Endocrine Research Institute, Yonsei University College of Medicine, Seoul, Korea
2Department of Orthopedic Surgery, Inje University Sanggye Paik Hospital, College of Medicine, Inje University, Seoul, Korea
3Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
4Amgen Korea Ltd., Seoul, Korea
5Division of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding author: Jung-Min Koh. Division of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-3247, Fax: +82-2-3010-6962, E-mail: jmkoh@amc.seoul.kr
Copyright © 2022 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The efficacy and safety of denosumab have been established in a phase 3, randomized, placebo-controlled trial in Korean postmenopausal women with osteoporosis. This postmarketing surveillance study was aimed to investigate the safety and effectiveness of denosumab in Korean real-world clinical practice.
-
Methods
- Patients with osteoporosis who had received denosumab per the Korean approved indications in the postmarketing setting between September 2014 and September 2019 were enrolled. The primary endpoint was the incidence of adverse events (AEs) and adverse drug reactions (ADRs). The secondary endpoint was the percent change from baseline in bone mineral density (BMD) of the lumbar spine, total hip, and femoral neck.
-
Results
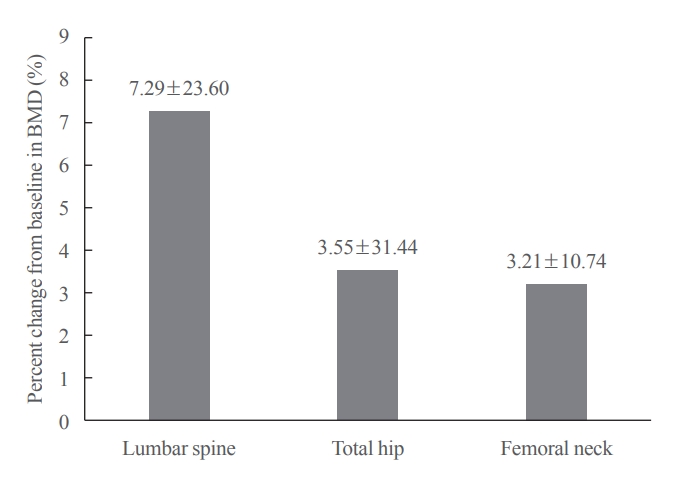
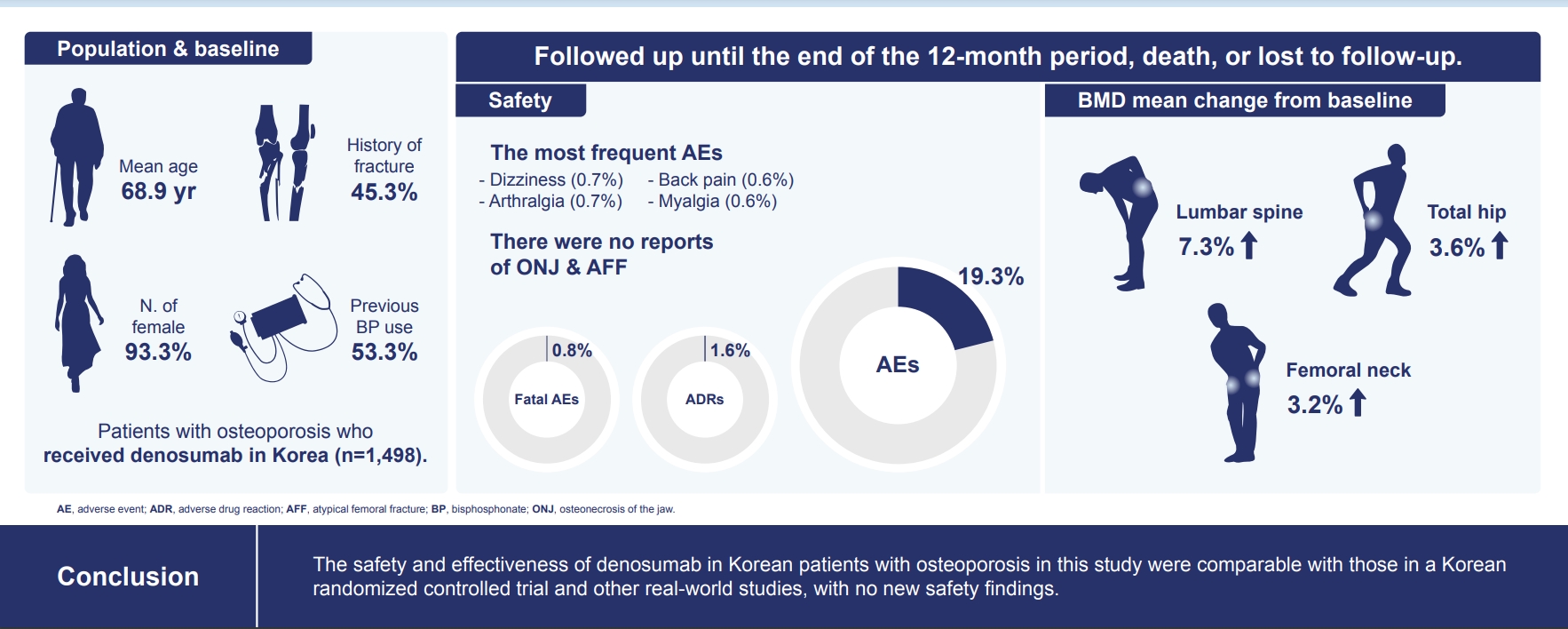
- Of the 3,221 patients enrolled, 3,185 were included in the safety analysis set; 2,973 (93.3%) were female, and the mean±standard deviation (SD) age was 68.9±9.9 years. The mean±SD study period was 350.0±71.4 days. AEs, fatal AEs, and ADRs occurred in 19.3%, 0.8%, and 1.6%, respectively. The most frequent AEs, occurring in >0.5% of patients, were dizziness (0.7%), arthralgia (0.7%), back pain (0.6%), and myalgia (0.6%). Hypocalcemia occurred in 0.3% of patients. There were no cases of osteonecrosis of the jaw and atypical femoral fracture. Mean±SD percent change from baseline in BMD of the lumbar spine, total hip, and femoral neck was 7.3%±23.6%, 3.6%±31.4%, and 3.2%±10.7%, respectively.
-
Conclusion
- The safety and effectiveness of denosumab in Korean patients with osteoporosis in this study were comparable with those in the Korean randomized controlled trial, with no new safety findings.
- Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and susceptibility to fracture [1]. Risk factors for osteoporosis include women aged ≥65 years, men aged ≥75 years, low body mass index (<18.5 kg/m2), North America and Asia region, family history of hip fracture, history of falls, low calcium and vitamin D intake, smoking, alcohol consumption, androgen deprivation therapy (ADT), aromatase inhibitor therapy (AIT), and long-term use of glucocorticoids [2-4]. Data from a Korea National Health and Nutrition Examination Survey (KNHANES; 2008 to 2011) showed that 22.4% of Korean adults aged ≥50 years had osteoporosis and 47.9% had osteopenia [5]. Another KNHANES reported that the prevalence of osteoporosis in Korean women and men aged ≥50 years was 38.0% and 7.3%, respectively [6].
- Denosumab, a fully human monoclonal antibody administered subcutaneously once every 6 months (Q6M), reduces bone resorption and increases bone mineral density (BMD) by selectively targeting the receptor activator of nuclear factor kappa B ligand (RANKL), which is crucial for osteoclast differentiation, activation, and survival [7]. In the international, randomized, placebo controlled Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, denosumab significantly reduced the risk of new radiographic vertebral fracture, nonvertebral fracture, and hip fracture versus placebo in postmenopausal women with osteoporosis after 3 years of treatment [8]. Denosumab was well tolerated; there were no significant differences in the incidence of adverse events (AEs), serious AEs (SAEs), or discontinuation of study treatment because of AEs between patients treated with denosumab and those treated with placebo [8]. In an open-label extension (OLE) study of the FREEDOM trial, patients treated with denosumab for 3 years in the randomized controlled trial (RCT) continued taking denosumab for an additional 7 years (long-term group; total exposure to denosumab, up to 10 years) and those treated with placebo in the RCT received denosumab for up to 7 years (crossover group) [9]. Compared with a 3-year treatment period in the RCT, long-term treatment with denosumab resulted in a continuous increase in BMD, low fracture incidence, and low rates of AEs [9].
- In a phase 3 RCT in Korean postmenopausal women with osteoporosis, a significant improvement in the mean percent change from baseline in BMD of the lumbar spine at month 6 was observed in patients treated with denosumab compared with those treated with placebo, and the efficacy increased at month 12 (mean percent change in lumbar spine BMD from baseline to month 12: 5.6%; 95% confidence interval [CI], 4.6% to 6.6%]) [10]. The AE profile was similar to that observed in denosumab trials conducted in other ethnic populations [10].
- Denosumab was first approved in Korea in September 2014 for the treatment of postmenopausal women with osteoporosis, increasing bone mass in men with osteoporosis, treatment of bone loss in men receiving ADT for nonmetastatic prostate cancer, and treatment of bone loss in women receiving adjuvant AIT for breast cancer. In April 2019, denosumab was approved for the treatment of glucocorticoid-induced osteoporosis [11]. Since October 2017, denosumab is reimbursed twice a year under the Korean insurance plan for patients who have a T-score <−2.5 and insurance benefits are allowed six times for 3 years for patients who have been diagnosed with osteoporotic fracture on radiographic examination [12]. The 2020 Endocrine Society Guidelines recommend denosumab as one of the initial treatment options for postmenopausal women with osteoporosis who are at a high risk of osteoporotic fractures [13].
- This postmarketing drug surveillance (PMS) study was conducted as a requirement of the Ministry of Food and Drug Safety, Republic of Korea, for products for which a marketing authorization application was submitted before July 1, 2015, to investigate the safety and effectiveness of denosumab administered in Korean real world clinical practice to patients with osteoporosis.
INTRODUCTION
- Study objectives
- The primary objective was to evaluate the incidence rates of AEs, SAEs, and adverse drug reactions (ADRs) among patients receiving denosumab in a postmarketing setting. Secondary objectives were to determine the effectiveness of denosumab by examining the change from baseline in BMD of the lumbar spine, total hip, and femoral neck (if available) and to describe the characteristics (e.g., demographics, medical history) of patients receiving denosumab in the postmarketing setting.
- Study design
- This was a prospective, observational, single arm study conducted between September 2014 and September 2019 in patients being treated with denosumab at 36 centers across Korea. Patients were followed up from the time of administration of the first dose of denosumab until the end of the 12-month period, death, or being lost to follow-up (e.g., patients transferred to another clinic), whichever occurred first. Eligible patients received a single dose of denosumab 60 mg during their initial visit or on day 1 (which could be the same day as screening) and returned for follow-up visits at the discretion of the investigator based on their course of treatment. Since the recommended dose of denosumab is 60 mg Q6M, patients who continued treatment had up to two follow-up visits during the 12-month follow-up period. The protocol was approved by the Institutional Review Board at each study site (representatively, no. 2017-0516 of the Asan Medical Center Ethics Review Committee), and the study was conducted in accordance with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Declaration of Helsinki. All enrolled patients provided informed consent to participate in the study.
- Patients
- Eligible patients included those who had received denosumab for the approved indications in the postmarketing setting in Korea, were willing to provide access to their previous and future medical information and had consented to participate in the study. Patients were excluded if they had hypocalcemia, were pregnant, or had known hypersensitivity to denosumab or any of its components.
- Treatment
- Denosumab is formulated as a subcutaneous injection and administered at a dose of 60 mg Q6M for bone loss indications, in accordance with the Korean prescribing information [14]. A single subcutaneous injection of denosumab 60 mg Q6M was administered in the upper arm, upper thigh, or abdomen. If a dose was missed, the injection was administered as soon as the patient was available. Daily intake of calcium 1,000 mg and vitamin D ≥400 IU was recommended to all patients.
- Assessments
- Reports of AEs (including their seriousness and causal link to denosumab) were collected throughout the study period and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1 [15]. An AE was defined as any untoward medical occurrence in a patient administered denosumab irrespective of a causal relationship with denosumab. An ADR was defined as any untoward medical occurrence in a patient administered denosumab in which there was a causal relationship between the occurrence of the event and treatment with denosumab as judged by the investigators. An SAE was defined as any AE that was fatal, life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability/incapacity, caused a congenital anomaly or birth defect, or was a significant medical hazard. Events of vertebral compression fracture and vertebral fracture were pooled and reported as vertebral compression fracture. There were no independent adjudication committees for osteonecrosis of the jaw (ONJ) and atypical femoral fracture (AFF). Effectiveness of denosumab was assessed by measuring the percent change from baseline in BMD of the lumbar spine, total hip, and femoral neck at 12 months. BMD was measured using dual-energy X-ray absorptiometry (DXA), per the study site’s clinical standard of measurement.
- Statistical analysis
- The target sample size was ≥3,000 patients, which was considered large enough to rule out an AE incidence of >0.1% with 95% CI if no AE was observed and to detect the known severe side effects of denosumab in ≥1 patient. The safety analysis set included enrolled patients who received ≥1 dose of denosumab and were followed up for AEs and excluded patients who did not receive denosumab during the study period and/or those with off label use. The effectiveness analysis set included patients from the safety analysis set for whom effectiveness (BMD measured at baseline and at least one subsequent time point at the same site) was evaluated. Descriptive analysis summarized categorical values by number and percentage. Continuous outcomes were summarized by the number of nonmissing values and mean±standard deviation (SD). Missing BMD data were not imputed. To identify the factors associated with an increased risk of AEs, a stepwise multivariate analysis was performed, and results expressed as odds ratios (ORs) and 95% CIs. The effects of prior bisphosphonate (BP) use, renal impairment, and hepatic impairment on the percent change from baseline in BMD of the lumbar spine, total hip, and femoral neck were determined by univariate analysis.
METHODS
- Patient disposition
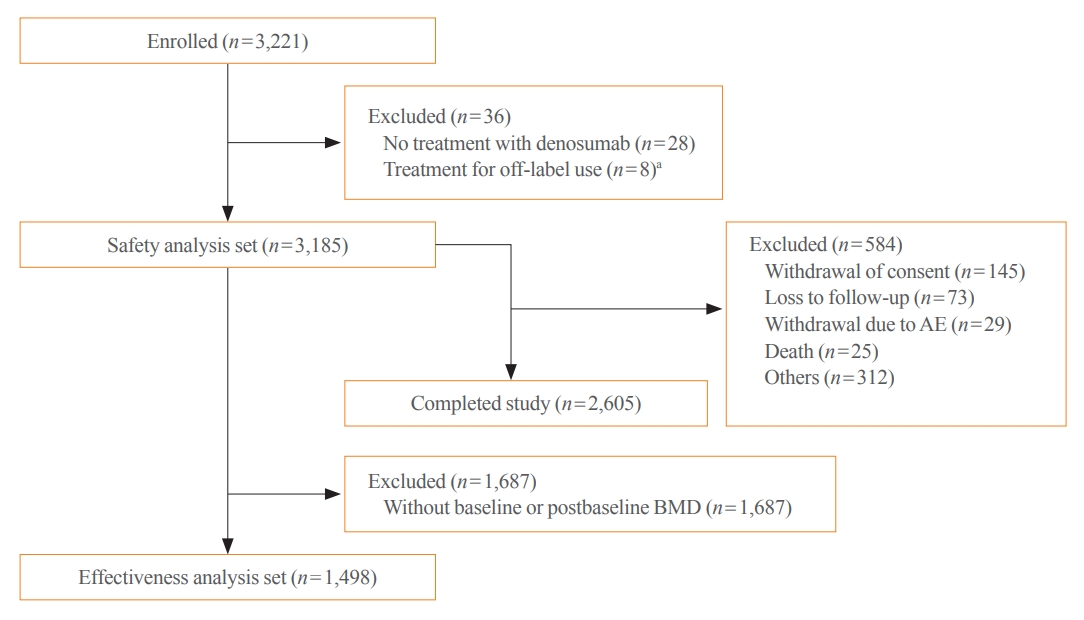
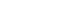
- Overall, 3,221 patients were enrolled, of whom 3,185 were included in the safety analysis set (Fig. 1). Thirty-six enrolled patients were excluded from the safety analysis set for not receiving denosumab treatment (n=28) and for off-label use of denosumab (n=8). A total of 2,605 (80.9%) patients completed the study. An additional 1,687 patients were excluded from the effectiveness analysis set due to the nonavailability of baseline or postbaseline BMD data and 1,498 patients were assessed for effectiveness.
- Demographics and baseline clinical characteristics
- Of the 3,185 Korean patients included in the safety analysis set, 2,973 (93.3%) were female, and the mean±SD age was 68.9±9.9 years (Table 1). In total, 1,413 of 3,116 (45.3%) patients had a history of fracture, with prevalent vertebral fracture being the most common (30.1%). Postmenopausal osteoporosis (91.9%) was the most common cause of osteoporosis, followed by male osteoporosis (6.6%), bone loss due to AIT (1.4%), and bone loss due to ADT (0.06%). A total of 35.4% had an osteoporosis duration of ≥1 to <5 years, and 73.4% had a history of prior or current use of osteoporosis medications. Among the 1,498 patients in the efficacy analysis set, anatomical location osteoporosis rates were as follows: 68.5% in lumbar spine; 23.0% in total hip. Mean±SD BMD T-scores for the lumbar spine, total hip, and femoral neck were −2.8±0.97, −1.9±0.90, and −2.4±0.87, respectively.
- Treatment exposure
- Overall, 2,062 (64.7%) patients received three doses of denosumab, and the mean±SD study period was 350.0±71.4 days.
- Safety
- Overall, 1,057 AEs occurred in 613 (19.3%) patients (Table 2). Most (618 [58.5%]) AEs were mild, whereas 305 (28.9%) AEs were moderate, and 133 (12.6%) AEs were severe. Overall, 71.7% of AEs resolved, 22.4% were ongoing, 3.3% resolved with sequelae, 2.5% were fatal, and 0.1% were unknown. AEs occurring in 36 (1.1%) patients led to the discontinuation of denosumab. The most frequent AEs, occurring in >0.5% of patients, were dizziness (0.7%), arthralgia (0.7%), back pain (0.6%), and myalgia (0.6%). A total of 227 (7.1%) patients experienced SAEs, with the most common SAEs being infections and infestations (1.4%), neoplasms (0.6%), and cardiac disorders (0.5%). Twenty-six (0.8%) patients experienced fatal AEs.
- ADRs were reported in 50 (1.6%) patients, with myalgia (0.3%), pain (0.3%), and hypocalcemia (0.3%) being the most commonly reported ADRs. Serious ADRs were reported in three (0.09%) patients, which included pneumonia in two (0.06%) patients and vertebral compression fracture in one (0.03%) patient; pneumonia was considered to be related by the investigators.
- Hypocalcemia was reported in 10 (0.3%) patients. Although calcium blood test was not recommended to be performed regularly, it was performed and reported if the physicians determined that hypocalcemia has occurred. Of those, eight (0.3%) patients did not have renal impairment at baseline and two (0.06%) patients had chronic kidney disease stage 3. Seventeen (0.6%) patients had undergone tooth extraction within 30 days before the day 1 visit (first dosing), and 30 (1.1%) patients had received a dental implant within 3 months preceding the day 1 visit. Despite these risk factors, there were no suspected reports of ONJ. One patient had undergone tooth extraction and received a dental implant 2 months after receiving the first dose of denosumab and continued treatment up to the end of the study, i.e., received three doses of denosumab. There were no reported AEs of AFF. Overall, 49 SAEs of infections and infestations occurred in 44 (1.4%) patients, of which two events, occurring in two (0.06%) patients, were considered serious ADRs. Common SAEs of infections and infestations included pneumonia in 10 (0.3%) patients (of which two were serious ADRs in two [0.06%] patients), influenza in six (0.2%) patients, and urinary tract infection in four (0.1%) patients. Eleven events of vertebral compression fracture were reported in nine patients. Eight patients experienced vertebral compression fracture during denosumab treatment as an AE, while one patient without a history of fracture experienced a vertebral compression fracture >6 months after the administration of the last dose of denosumab; follow-up data for antiosteoporotic treatment were not captured for this patient. Vertebral compression fracture in one (0.03%) patient as considered to be a serious ADR.
- Stepwise logistic regression analysis revealed that the incidence of AEs was higher in patients aged ≥75 years versus those aged <65 years (OR, 1.4; 95% CI, 1.1 to 1.8; P=0.0020) and in those with versus without the presence of medical history (OR, 1.9; 95% CI, 1.3 to 2.9; P=0.0023).
- Effectiveness
- Mean±SD percent changes from baseline in BMD of the lumbar spine, total hip, and femoral neck were 7.3%±23.6%, 3.6%±31.4%, and 3.2%±10.7%, respectively (Fig. 2). On univariate analysis, the percent change from baseline in BMD at all measured sites did not differ between patients with and those without prior BP use (Table 3).
RESULTS
AEs
AEs of special interest
Post hoc analysis
Change in BMD of the lumbar spine, total hip, and femoral neck
- Osteoporosis is a chronic condition necessitating long-term and, sometimes, lifelong treatment [16]. The fear of rare side effects and concerns about long-term effectiveness are two of the most important reasons for the undertreatment of patients with osteoporosis [17]. Furthermore, the stringent eligibility criteria followed by RCTs such as FREEDOM limit the availability of safety and efficacy data of antiosteoporotic treatments in diverse populations, necessitating real-world evidence post-drug approval that can provide salient insights among a broader population in a clinical setting. Hence, we present the results from this large, observational real-world study establishing the safety and effectiveness of denosumab in patients with osteoporosis in Korea.
- Compared with the 6-month incidence of AEs and SAEs in the Korean RCT, the incidence of AEs was lower (19.3% vs. 55.0%) but that of SAEs was higher (7.1% vs. 3.0%) in this real world study [10]. One-year data from FREEDOM OLE revealed the incidence of all AEs, SAEs, and malignancies to be 188.5, 11.8, and 1.8 yearly exposure adjusted patient incidence of AEs per 100 patient-years for long-term denosumab-treated patients, respectively [9]. Overall, these results suggest that denosumab was well tolerated by Korean patients with osteoporosis. Dizziness, arthralgia, myalgia, and back pain were some of the most frequent AEs reported in this study. Cardiac disorders were reported as an SAE in 0.5% of patients in this study. The findings of a meta analysis of RCTs of denosumab in patients with osteoporosis or osteopenia found that cardiac disorders were unlikely to be a consequence of denosumab use [18]. The investigators of this study also considered the relationship between denosumab use and cardiac disorders to be unlikely. Hypocalcemia as an ADR was reported in 0.3% of Korean patients with osteoporosis in this study versus 3.9% in the Japanese PMS study [14]. In this study, hypocalcemia was observed in patients with normal kidney function at baseline. In case of symptomatic hypocalcemia, clinical AEs reported by one patient were vertigo and dizziness. After the development of hypocalcemia, one patient required hospital admission for intravenous calcium supplementation. Eight patients were managed with initiating or increasing calcium supplementation as outpatients. Two patients discontinued denosumab due to hypocalcemia. In a retrospective cohort study in which Korean women were treated with a subcutaneous injection of denosumab (60 mg Q6M), approximately 8.2% of patients developed hypocalcemia [19]. Furthermore, an adjusted multiple regression model indicated that patients with low baseline albumin corrected calcium level and estimated glomerular filtration rate (lower than stage 3b) were significantly associated with an increased likelihood of developing hypocalcemia following treatment with denosumab [19].
- Denosumab discontinuation results in a complete and rapid reversal of its effects on BMD and bone turnover markers, predisposing denosumab-treated patients to an increased risk of fracture [20]. In patients previously treated with denosumab who discontinue treatment, there is a rebound in bone turnover with an increase in vertebral fractures to the level observed in untreated patients [21]. A post hoc analysis of FREEDOM and its 10-year OLE study revealed that among patients who received ≥2 injections of denosumab Q6M, the risk of multiple vertebral fractures following cessation of denosumab was higher compared with that in patients who stopped placebo [22]. In this study, vertebral compression fracture was reported as an AE in nine patients and as a serious ADR in one patient, with one patient developing a fracture >6 months after the administration of the last dose of denosumab. As this study observed patients only for 1 year, this vertebral fracture could not be confirmed to be related to the discontinuation of denosumab. The Health Insurance Committee of the Korean Endocrine Society proposes that in patients with low or moderate risk for fractures after denosumab therapy, BPs or selective estrogen receptor modulator or hormone therapy must be used for 1 to 2 years along with vitamin D and calcium [23]. Patients at high or very high risk for fractures should continue on denosumab or switch to another therapy [23].
- A total of 73.4% of patients in this study were current or previous users of medications to manage osteoporosis, indicating that most patients were not treatment-naïve. Despite enrolling patients pretreated with antiosteoporotic medications in this study compared with the Korean RCT, the mean percent change from baseline in BMD was comparable with that observed at month 12 in the Korean RCT which enrolled only treatment-naïve patients (lumbar spine, 7.3% vs. 5.6%) [10]. This suggests that denosumab treatment in Korean patients with osteoporosis is effective in a real-world clinical setting.
- The percent change from baseline in BMD of the lumbar spine, total hip, and femoral neck in this study was independent of BP use. However, no requirement of a wash out period for patients with previous BP use is one of the limitations. The long-term residual effect of BP is likely to prevent bone resorption and, hence, preclude a BMD decrease. On the other hand, BP use has a blunting effect on the BMD increases when patients are transitioned to other therapies such as denosumab.
- One strength of this PMS study is that it is the largest real-world study in Korea published to date that demonstrated the safety and effectiveness of denosumab in patients with osteoporosis. The study enrolled patients with male osteoporosis and those who developed osteoporosis following ADT and AIT, thereby establishing the safety and effectiveness of denosumab in indications other than postmenopausal osteoporosis, which was investigated in an RCT. However, this study has some limitations. Since the primary endpoint of this study was safety, the investigators used their discretion to collect data on treatment effectiveness. Thus, the baseline and postbaseline BMD were not evaluated in all patients; therefore, many patients were excluded in the effectiveness analysis set. Patients with glucocorticoid-induced osteoporosis were not enrolled as denosumab was not approved for this indication in Korea at the time of this study and, therefore, could not be investigated. Furthermore, fracture data were captured as a safety endpoint; therefore, the association between increase in BMD and reduction in the risk of fractures could not be determined. In terms of effectiveness analysis, one of the limitations of this study is that different types of DXA scan equipment were used in each center. Lack of cross-calibration procedures for DXA scanners at different facilities reduced sensitivity to detect significant change when comparing BMD among different centers. It is also possible that patients with secondary osteoporosis were not excluded. There was also a lack of data on bone turnover markers. Future studies should be focused on addressing these gaps as well as studying the effects of denosumab on treatment adherence and quality of life of patients with osteoporosis.
- In conclusion, the safety and effectiveness of denosumab in Korean patients in this PMS study were similar to those in the Korean RCT and other real-world studies, with no new safety findings. Approximately 60.0% of AEs were mild, approximately 70.0% of AEs had resolved, and events of ONJ and AFF were not reported in this study. An improvement from baseline in BMD of the lumbar spine, total hip, and femoral neck was observed and was independent of prior BP use. Denosumab was well tolerated and showed a persistent increase in percent change in BMD in Korean patients with osteoporosis.
DISCUSSION
-
Acknowledgements
- The authors thank all the investigators and patients who participated in this study. Medical writing support was provided by Sarayu Pai, PhD, and Annirudha Chillar, MD, PhD, of Cactus Life Sciences (part of Cactus Communications).
-
CONFLICTS OF INTEREST
Sooa Kim, YuSun Lee, and Euna Jo are employees of Amgen Korea. This study was sponsored by Amgen Inc.
-
AUTHOR CONTRIBUTIONS
Conception or design: Y.R., D.G.C., J.H., S.K., J.M.K. Acquisition, analysis, or interpretation of data: Y.R., D.G.C., J.H., S.K., Y.L., E.J., J.M.K. Drafting the work or revising: Y.R., D.G.C., J.H., S.K., Y.L., E.J., J.M.K. Final approval of the manuscript: Y.R., D.G.C., J.H., S.K., Y.L., E.J., J.M.K.
Article information
| Variable | Safety analysis set (n=3,185) |
|---|---|
| Sex | |
| Female | 2,973 (93.3) |
| Male | 212 (6.7) |
| Age, yr | 68.9±9.9 |
| <65 | 1,120 (35.2) |
| 65–74 | 1,010 (31.7) |
| ≥75 | 1,055 (33.1) |
| BMI, kg/m2 | 22.5±3.3 |
| History of fracturea | 1,413 (45.3) |
| Prevalent vertebral fracture | 939 (30.1) |
| Prevalent hip fracture | 236 (7.6) |
| Prevalent other fracture | 454 (14.6) |
| Diagnosis | |
| Postmenopausal osteoporosis | 2,926 (91.9) |
| Male osteoporosis | 211 (6.6) |
| Bone loss due to AIT | 46 (1.4) |
| Bone loss due to ADTb | 2 (0.06) |
| Duration of osteoporosis, yr | |
| <1 | 874 (27.4) |
| ≥1 to <5 | 1,127 (35.4) |
| ≥5 to <10 | 661 (20.8) |
| ≥10 | 468 (14.7) |
| Unknown | 55 (1.7) |
| History of medication use for osteoporosisa | |
| Never usedc | 780 (25.0) |
| Prior use or current use | 2,288 (73.4) |
| Unknownd | 48 (1.5) |
| History of BP usea | |
| Not used | 1,277 (40.1) |
| Previously used | 1,699 (53.3) |
| Currently in use | 46 (1.4) |
| Unknown | 94 (3.0) |
| BMD T-scoree | |
| Lumbar spine | −2.8±0.97 |
| Total hip | −1.9±0.90 |
| Femoral neck | −2.4±0.87 |
Values are expressed as number (%) or mean±standard deviation.
BMI, body mass index; AIT, aromatase inhibitor therapy; ADT, androgen deprivation therapy; BP, bisphosphonate; BMD, bone mineral density.
a Included in patients for whom information on previous medication use was available (n=3,116);
b One patient was incorrectly reported to be female;
c Defined “never used” in the history of osteoporosis medication as answered “never use” of BP and other medication in the medical history;
d Defined “unknown” as answered “unknown” of BP and other medication in the medical history;
e BMD data were analyzed in patients in the effectiveness analysis set for whom baseline and postbaseline BMD data were available (lumbar spine, n=1,423; total hip, n=1,222; femoral neck, n=1,362).
| Variable |
Safety analysis set (n=3,185) |
|
|---|---|---|
| No. of patients (%) | No. of events | |
| AEs | 613 (19.3) | 1,057 |
| AEs leading to the discontinuation of denosumab | 36 (1.1) | 40 |
| SAEs | 227 (7.1) | 295 |
| Infections and infestationsa | 44 (1.4) | 49 |
| Neoplasmsb | 18 (0.6) | 19 |
| Cardiac disorders | 16 (0.5) | 18 |
| Fatal AEsc | 26 (0.8) | 26 |
| Most frequent AEs (>0.5%) | ||
| Dizziness | 22 (0.7) | 25 |
| Arthralgia | 21 (0.7) | 21 |
| Back pain | 20 (0.6) | 21 |
| Myalgia | 19 (0.6) | 19 |
| Pneumonia | 17 (0.5) | 17 |
| Headache | 16 (0.5) | 17 |
| AEs of special interest | ||
| Fracture | 40 (1.3) | 42 |
| Musculoskeletal pain | 28 (0.9) | 28 |
| Hypersensitivity | 20 (0.6) | 20 |
| Hypocalcemia | 10 (0.3) | 11 |
| Hyperparathyroidism tertiary | 1 (0.03) | 1 |
| Fracture nonunion (delayed healing) | 1 (0.03) | 1 |
| ADRs | 50 (1.6) | 62 |
| Most frequent ADRs | ||
| Myalgia | 10 (0.3) | 10 |
| Paind | 9 (0.3) | 9 |
| Hypocalcemia | 9 (0.3) | 10 |
| Serious ADRs | 3 (0.09) | 4 |
| Pneumonia | 2 (0.06) | 2 |
| Vertebral compression fracture | 1 (0.03) | 2 |
AE, adverse event; ADR, adverse drug reaction; SAE, serious adverse event.
a Aspiration pneumonia was excluded;
b All benign tumors were excluded (e.g., uterine leiomyoma, thymoma);
c Other fatal AEs included sepsis in two patients and bacterial sepsis, pneumonia, septic shock, gastric cancer, Hodgkin’s disease, lung neoplasm malignant, metastatic gastric cancer, cardiac arrest, cardiac failure, Still’s disease, cerebral hemorrhage, chronic obstructive pulmonary disease, and thrombosis in one patient each;
d Includes general pains and shoulder and knee pains.
| Variable |
Percent change in BMD, % |
P valuea | ||
|---|---|---|---|---|
| No. | Mean±SD | LS mean±SE | ||
| Lumbar spine | ||||
| Prior use of BP | ||||
| Yes | 842 | 6.9±18.6 | 7.0±0.8 | 0.55 |
| No | 581 | 7.9±29.4 | 7.7±1.0 | |
| Total hip | ||||
| Prior use of BP | ||||
| Yes | 734 | 3.9±40.0 | 3.9±1.2 | 0.61 |
| No | 488 | 3.0±8.2 | 3.0±1.4 | |
| Femoral neck | ||||
| Prior use of BP | ||||
| Yes | 814 | 3.5±10.4 | 3.4±0.4 | 0.36 |
| No | 548 | 2.8±11.3 | 2.9±0.4 | |
- 1. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646–50.ArticlePubMed
- 2. Harris JR, Korolchuk VI. Biochemistry and cell biology of ageing: Part II clinical science. Subcellular Biochemistry; Singapore: Springer; 2019. Chapter 16, Osteoporosis and the ageing skeleton. p. 453–76.
- 3. Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int 2017;28:1531–42.ArticlePubMedPDF
- 4. Yoon DS, Lee YK, Ha YC, Kim HY. Inadequate dietary calcium and vitamin D intake in patients with osteoporotic fracture. J Bone Metab 2016;23:55–61.ArticlePubMedPMCPDF
- 5. Ahn SH, Park SM, Park SY, Yoo JI, Jung HS, Nho JH, et al. Osteoporosis and osteoporotic fracture fact sheet in Korea. J Bone Metab 2020;27:281–90.ArticlePubMedPMCPDF
- 6. Park EJ, Joo IW, Jang MJ, Kim YT, Oh K, Oh HJ. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2011. Yonsei Med J 2014;55:1049–57.ArticlePubMedPMC
- 7. Deeks ED. Denosumab: a review in postmenopausal osteoporosis. Drugs Aging 2018;35:163–73.ArticlePubMedPDF
- 8. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65.ArticlePubMed
- 9. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 Years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017;5:513–23.ArticlePubMed
- 10. Koh JM, Chung DJ, Chung YS, Kang MI, Kim IJ, Min YK, et al. Assessment of denosumab in Korean postmenopausal women with osteoporosis: randomized, double-blind, placebo-controlled trial with open-label extension. Yonsei Med J 2016;57:905–14.ArticlePubMedPMCPDF
- 11. AMGEN. Prolia® Pre-filled Syringe [Internet]. Seoul: AMGEN; 2022 [cited 2022 May 10]. Available from: https://www.amgen.co.kr/products/prolia.
- 12. Health Insurance Review & Assessment Service. [Pharmaceuticals] Notification No. 2019-57 Detailed information on the application standards and methods of medical care benefits [Internet]. Wonju: HIRA; 2019 [cited 2022 May 10]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=7268&pageIndex=145.
- 13. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab 2020;105:dgaa048.ArticlePubMedPDF
- 14. Tanaka S, Mizutani H, Tsuruya E, Fukuda R, Kuge K, Okubo N. Long-term safety and effectiveness of denosumab in Japanese patients with osteoporosis: 3-year post-marketing surveillance study. J Bone Miner Metab 2021;39:463–73.ArticlePubMedPDF
- 15. MedDRA. Introductory guide MedDRA version 22.1 [Internet]. Herndon: MedDRA; 2019 [cited 2022 May 10]. Available from: https://admin.meddra.org/sites/default/files/guidance/file/000354_intguide_22.1.pdf.
- 16. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet 2019;393:364–76.ArticlePubMed
- 17. Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol 2017;5:898–907.ArticlePubMedPMC
- 18. Lv F, Cai X, Yang W, Gao L, Chen L, Wu J, et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: systematic review and meta-analysis. Bone 2020;130:115121.ArticlePubMed
- 19. Kim KJ, Hong N, Lee S, Kim M, Rhee Y. A simple-to-use score for identifying individuals at high risk of denosumab-associated hypocalcemia in postmenopausal osteoporosis: a real-world cohort study. Calcif Tissue Int 2020;107:567–75.ArticlePubMedPDF
- 20. Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab 2021;106:264–81.ArticlePDF
- 21. Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ 2018;190:E485–6.ArticlePubMedPMC
- 22. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res 2018;33:190–8.ArticlePubMedPDF
- 23. Kim BK, Kim CH, Min YK. Preventing rebound-associated fractures after discontinuation of denosumab therapy: a position statement from the health insurance committee of the Korean Endocrine Society. Endocrinol Metab (Seoul) 2021;36:909–11.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations

- Prevalence of denosumab-induced hypocalcemia: a retrospective observational study of patients routinely monitored with ionized calcium post-injection
Anna Spångeus, Johan Rydetun, Mischa Woisetschläger
Osteoporosis International.2024; 35(1): 173. CrossRef - Cost-consequence analysis of continuous denosumab therapy for osteoporosis treatment in South Korea
Seungju Cha, Minjeong Sohn, Hyowon Yang, Eric J. Yeh, Ki-Hyun Baek, Jeonghoon Ha, Hyemin Ku
BMC Musculoskeletal Disorders.2024;[Epub] CrossRef - Denosumab and the Risk of Diabetes in Patients Treated for Osteoporosis
Huei-Kai Huang, Albert Tzu-Ming Chuang, Tzu-Chi Liao, Shih-Chieh Shao, Peter Pin-Sung Liu, Yu-Kang Tu, Edward Chia-Cheng Lai
JAMA Network Open.2024; 7(2): e2354734. CrossRef - Adverse Effects of Denosumab in Kidney Transplant Recipients: A 20-Year Retrospective Single-Center Observation Study in Central Taiwan
Tsung-Yin Tsai, Zi-Hong You, Shang-Feng Tsai, Ming-Ju Wu, Tung-Min Yu, Ya-Wen Chuang, Yung-Chieh Lin, Ya-Lian Deng, Chiann-Yi Hsu, Cheng-Hsu Chen
Transplantation Proceedings.2023; 55(4): 837. CrossRef - Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
Endocrinology and Metabolism.2023; 38(2): 260. CrossRef - Effect of Denosumab on Bone Density in Postmenopausal Osteoporosis: A Comparison with and without Calcium Supplementation in Patients on Standard Diets in Korea
Chaiho Jeong, Jinyoung Kim, Jeongmin Lee, Yejee Lim, Dong-Jun Lim, Ki-Hyun Baek, Jeonghoon Ha
Journal of Clinical Medicine.2023; 12(21): 6904. CrossRef - Denosumab
Reactions Weekly.2022; 1919(1): 221. CrossRef - Denosumab, an effective osteoporosis treatment option for men
Sung Hye Kong
The Korean Journal of Internal Medicine.2022; 37(5): 947. CrossRef

 KES
KES
 PubReader
PubReader ePub Link
ePub Link Cite
Cite