Programmed Cell Death-Ligand 1 (PD-L1) gene Single Nucleotide Polymorphism in Graves’ Disease and Hashimoto’s Thyroiditis in Korean Patients
Article information
Abstract
Background
Programmed cell death-ligand 1 (PD-L1) has an important role in regulating immune reactions by binding to programmed death 1 (PD-1) on immune cells, which could prevent the exacerbation of autoimmune thyroid disease (AITD). The aim of this study was to evaluate the association of PD-L1 polymorphism with AITD, including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT).
Methods
A total of 189 GD patients, 234 HT patients, and 846 healthy age- and sex-matched controls were enrolled in this study. We analyzed PD-L1 single nucleotide polymorphism (SNP) (rs822339) and investigated the associations with clinical disease course and outcome.
Results
Genotype frequency at the PD-L1 marker RS822339 in GD (P=0.219) and HT (P=0.764) patients did not differ from that among healthy controls. In patients with GD, the A/G or G/G genotype group demonstrated higher TBII titer (20.6±20.5 vs. 28.0±25.8, P=0.044) and longer treatment duration (39.0±40.4 months vs. 62.4±65.0 months, P=0.003) compared to the A/A genotype group. Among patients in whom anti-thyroid peroxidase (TPO) antibody was measured after treatment of GD, post-treatment anti-TPO positivity was higher in the A/G or G/G genotype group compared to the A/A genotype group (48.1% vs. 69.9%, P=0.045). Among patients with HT, there was no significant difference of anti-TPO antibody positivity (79.4% vs. 68.6%, P=0.121), anti-thyroglobulin antibody positivity (80.9% vs. 84.7%, P=0.661), or development to overt hypothyroidism (68.0% vs. 71.1%, P=0.632) between the A/A genotype group and the A/G or G/G genotype group.
Conclusion
The genotype frequency of PD-L1 (rs822339) is not different in patients with AITD compared with healthy controls. The intact PD-1/PD-L1 pathway in GD and HT might be important to maintain chronicity of AITD by protecting immune tolerance. However, the PD-L1 SNP could be associated with difficulty in achieving remission in patients with GD, which may be helpful to predict the possibility of longer treatment. Further studies are required to investigate the complex immune tolerance system in patients with AITD.
INTRODUCTION
Autoimmune thyroid disease (AITD), including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT), is the most common organ-specific autoimmune disease [1]. Studies of genetic and environmental factors related to AITD have progressed, but genetic-environmental interactions and their pathogenesis are still unclear [2,3]. Activated immune responses are modulated by multi-step regulating factors, and previous studies have demonstrated that the programmed death 1 (PD-1)/PD-ligand 1 (PD-L1) axis is an important modulating point for autoimmunity [4,5]. The PD-1/PD-L1 axis contributes to maintaining immune tolerance by down-regulation of T-cell immune responses and cytokine production [6]. PD-1 gene polymorphisms have been associated with autoimmune diseases, such as type 1 diabetes mellitus and systemic lupus erythematous [7,8], but no associations were observed in Addison’s disease and GD [9]. Conversely, PD-L1 gene single nucleotide polymorphisms (SNP) have been related to GD [10].
Since immunotherapy with immune checkpoint inhibitors (ICPi) was introduced, and has been more frequently used for cancer treatment, numerous immune-related adverse effects have been reported [11]. Among ICPi-induced endocrinopathies, thyroid immune-related adverse events (irAEs) have been the most common, and progression to hypothyroidism through rapid inflammatory change has been observed in patients with ICPi-induced thyroiditis, which differs from the generally chronic course of HT [3,12]. Reduction of immune tolerance by gene polymorphisms of PD-1/PD-L1 could cause fast-paced thyroiditis in patients treated with ICPi [13]. However, the relationship between PD-1/PD-L1 polymorphisms and the clinical course of AITD is not well established. Only one Japanese study showed an association of PD-L1 gene polymorphisms and GD development [14]. The aim of this study was to assess a PD-L1 SNP (rs822339) in patients with GD and HT compared to normal controls and evaluate the association of the PD-L1 SNP with the clinical course of AITD.
METHODS
Subjects
Patients who were diagnosed with AITD, including GD and HT, at Chonnam National University Hwasun Hospital between April 2013 and January 2015 were included. The diagnostic criteria for GD were biochemical evidence of hyperthyroidism with serum anti-thyrotropin receptor antibody (thyrotropin binding inhibiting immunoglobulin [TBII]) and/or increased diffuse 123I uptake on 99Tc-pertechnetate radionuclide scan or presence of Graves’ ophthalmopathy [15]. The diagnosis of HT was based on the enlargement of the thyroid gland, typical ultrasonographic features [16], positivity of either anti-thyroid peroxidase (TPO) or anti-thyroglobulin (Tg), and/or biochemical hypothyroidism. Patients with a history of thyroid cancer (n=16) were excluded. The control subjects were healthy and were matched to patients in a 2:1 ratio by age (within a 3-year interval) and sex and were from the same geographic areas. Healthy controls did not have a thyroid goiter or personal or family histories of AITD. In total, 189 GD patients, 234 HT patients, and 846 healthy controls were enrolled. After the diagnosis of GD, patients were treated with anti-thyroid drugs such as methimazole, carbimazole, and propylthiouracil. Follow-up was performed every 1 to 3 months with measurement of thyroid function status and TBII. Among patients with HT, patients with normal thyroid function were followed up every 6 to 12 months, and in patients who had overt hypothyroidism, synthyroxine replacement therapy was started. Informed consent for study participation was obtained from all cases and controls. The study was approved by the Institutional Review Board of the Chonnam National University Hwasun Hospital in Chonnam, the Republic of Korea (IRB No. CNUHH-2015-081).
SNP: PD-L1 (rs822339)
Genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s protocol. Genotyping to analyze polymorphisms was performed using high-resolution melting (HRM) analysis. The forward polymerase chain reaction (PCR) primer was (5′-CCCCATTTTAGCAAATGTGAC-3′) and the reverse was (5′-CATGAGTCATCATCCTCTTGC-3′). HRM genotyping was performed in 10-μL reaction volumes with 200 nM PCR primer, 1 μM Syto 9 fluorescent dye (Invitrogen, Carlsbad, CA, USA), 0.5 U f-Taq polymerase (Solgent, Daejeon, Korea), and 40 ng of genomic DNA, using a Rotor-Gene 6000 high-resolution melter (Corbett Research, Sydney, Australia). The cycling conditions included an initial 5-minute hold at 95°C, followed by 40 cycles at 95°C for 5 seconds, 56°C for 30 seconds, 72°C for 20 seconds, and melting increasing from 72°C to 83°C at 0.1°C per second.
Laboratory test
Thyroid function tests, including thyroid-stimulating hormone, free thyroxine (T4), and triiodothyronine, were measured by electrochemiluminescence methods (Roche Cobas e601 automatic immunoassay, Roche Diagnostics, Basel, Switzerland). Thyroid anti-TPO and anti-Tg were measured using electrochemiluminescence (Roche Cobas e601 automatic immunoassay). A TPO antibody titer >34 IU/mL and a Tg antibody titer >115 IU/mL were defined as elevated autoantibody levels.
Statistical analysis
Data were expressed as mean±standard deviation/error or median (interquartile range) or number (%). Continuous variables were analyzed using Student’s t test and categorical variables were analyzed using the chi-square test. Hardy-Weinberg equilibrium was also performed using chi-square test. In subgroup analysis for GD patients treated with only anti-thyroid medications, two adjusted multiple linear regression analysis models were used to evaluate an independent association of clinical factors with PD-L1 SNP (rs822339). Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, and TBII titer. After adopting model 1 model 2, the association between treatment duration and PD-L1 SNP (rs822339) was compared by analysis of covariance (ANCOVA). All statistical analyses were performed using SPSS statistics version 25 (IBM, Armonk, NY, USA), and a P<0.05 was considered statistically significant.
RESULTS
Association between PD-L1 SNP (rs822339) and AITD
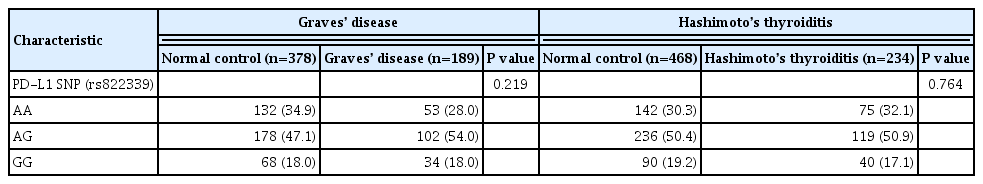
The genotype frequencies were not significantly deviated from Hardy-Weinberg equilibrium in the control group (P=0.554 for GD control and P=0.647 for HT control). Compared with age- and sex-matched healthy controls, alleles at PD-L1 markers (rs822339) were not associated with GD (P=0.219). Additionally, there was no significant difference in allele or genotype frequency of the PD-L1 SNP (rs822339) between patients with HT and healthy controls (P=0.764) (Table 1).
Clinical characteristics according to PD-L1 SNP in patients with GD
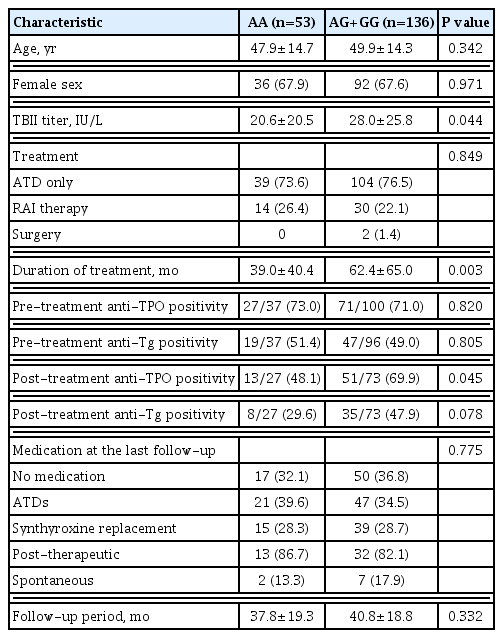
There was no difference of age (47.9±14.7 years vs. 49.9±14.3 years, P=0.342) or sex (female, 67.9% vs. 67.6%, P=0.971) between the A/A genotype group and A/G or G/G genotype group. The A/G or G/G genotypes of rs822339 were associated with high TBII titer compared to the A/A genotype (20.6±20.5 vs. 28.0±25.8, P=0.044). The treatment strategy (P=0.849) and medication status at the last follow-up (P=0.775) with more than a mean of 37.8 months were not different between the two groups. Two patients in the A/A genotype group and seven patients in the A/G or G/G genotype group converted to hypothyroidism spontaneously. Treatment duration in the A/G or G/G genotype group was longer than in the A/A genotype group (39.0±40.4 months vs. 62.4±65.0 months, P=0.003). Among patients in whom anti-TPO antibody was measured after treatment of GD, post-treatment anti-TPO positivity was higher in the A/G or G/G genotype group compared to the A/A genotype group (48.1% vs. 69.9%, P=0.045) (Table 2). In subgroup analysis to evaluate associations between treatment duration and PD-L1 SNP (rs822339) among GD patients who treated with only anti-thyroid medications, multiple linear regression analyses were performed (Supplemental Table S1). Before adjusting for confounding factors, treatment duration in the A/G or G/G genotype group was longer compared to A/A genotype group (P=0.005). We adopted two multivariable models for analyses. Longer treatment duration in the A/G or G/G genotype group were observed after adjusting for age and sex (model 1, P=0.022) and the statistical significances remained after adjusting for additional TBII titer (model 2, P=0.001).
Clinical characteristics according to PD-L1 SNP in patients with HT
There was no significant difference regarding age (52.5±11.6 vs. 52.9±12.4, P=0.831) or sex (female, 85.3% vs. 85.5%, P= 0.967). Also, anti-TPO antibody (79.4% vs. 68.6%, P=0.121) and anti-Tg antibody (80.9% vs. 84.7%, P=0.661) positivity did not differ between the A/A genotype group and A/G or G/G genotype group at the time of diagnosis. At the last follow-up with more than a mean of 38.7 months, development of overt hypothyroidism requiring synthyroxine replacement treatment in the A/G or G/G genotype groups did not differ from that in the A/A genotype group (68.0% vs. 71.1%, P=0.632) (Table 3).
DISCUSSION
The PD-1/PD-L1 pathway is involved in maintaining immune intolerance and restraining autoimmune reactivity in patients with AITD [17,18]. In particular, PD-L1 expression in thyroid follicular cells and intra-thyroidal infiltrating lymphocytes might play a key role in the chronic progressive pattern of AITD [3]. The polymorphism of the PD-L1 gene could be critical for the development and progression of AITD. Two previous studies revealed an association of the PD-L1 gene SNP with GD in Japanese and United Kingdom cohorts [10,14], but no data exist on the association between the PD-L1 pathway and HT. Few data showed an unequivocal association of cytotoxic T-lymphocyte antigen-4 (CTLA-4) SNPs in patients with HT [19,20]. This study was the first to investigate a PD-L1 SNP (rs822339) in both GD and HT compared with age- and sex-matched healthy controls. No differences of the PD-L1 (rs822339) SNP in AITD compared with normal healthy controls were observed, which indicated that PD-L1 expression was not related to the initiation of AITD. After AITD is manifested by well-known genetic factors including the human leukocyte antigen (HLA)-DR family, protein tyrosine phosphatase non-receptor type 22 (PTPN22), and CTLA-4, the PD-1/PD-L1 pathway could restrain the autoimmune activity and maintain chronicity [21]. Recent two studies demonstrated PD-L1 SNPs (rs822339 and rs1411262) were associated with overall survival benefit in non-small cell lung cancer patients receiving nivolumab treatment by the analysis of seven PD-L1 SNPs which had an association with the prevalence of autoimmune disease and cancer [13,22]. Additionally, Funazo et al. [13] represented that patients who had A allele of rs822339 developed low free T4 level, which is related to survival benefit in patients with A/A genotype. Thyroid irAE after ICPi treatment represented mainly destructive thyroiditis pattern and some of them developed hypothyroidism (low T4). However, our study revealed no association between PD-L1 SNP (rs822339) and AITD in the clinical setting without PD-1/PD-L1 inhibitors. Clinically, ICPi induced thyroiditis and AITD have some differences. Thyroid irAEs occurred quickly; however, AITD showed generally indolent course of thyroiditis, so the patho-mechanism of ICPi induced thyroiditis and AITD could be different.
This study demonstrated that PD-L1 SNP was associated with higher TBII and post-treatment anti-TPO antibody. Additionally, GD patients with the PD-L1 SNP (rs822339) showed longer treatment duration compared to GD patients without the PD-L1 SNP. TBII had high sensitivity and specificity for the diagnosis of GD and was a possible predictor of relapse of GD [23–25]. In our study, higher TBII titer in GD patients with the PD-L1 SNP might be related to elevated disease activity through the dysregulation of immune activities. Positivity for anti-TPO and Tg autoantibodies was frequently observed in patients with GD during antigen spreading, even though a definitive patho-mechanism was not established [26]. One small study showed an increased anti-TPO titer was associated with relapse among GD patients with anti-TPO positivity at the time of diagnosis [27]. In a Japanese study, A/C SNP in the PD-L1 gene intron 4 position 8923 was related to difficulty in achieving remission of GD [14]. The overexpression of PD-1/PD-L1 in patients with GD, as shown in several previous studies, might reduce the severity of GD by suppressing excessively activated T-cell immunity via the PD-1/PD-L1 pathway [3,28]. In the current study, an immune reaction unbalanced by the PD-L1 SNP could prolong the treatment duration of GD by blocking the process of suppressing overactivated immunity.
However, PD-L1 SNP (rs822339) status was not related to clinical course and anti-thyroid autoantibodies of HT in our study. Only a few studies have shown an association of PD-1 positive follicular T helper cells and natural killer cells with the pathogenesis of HT [29,30]. Follicular T regulatory cells, which inhibit follicular T helper cells, were also increased in HT patients, but this was not associated with anti-TPO and Tg antibody titers [31]. Decreased number or dysfunction of regulatory T-cells was significantly correlated with the development of hypothyroidism in HT [32]. Apoptosis is important in the destruction of the thyroid in patients with HT, so the PD-1/PD-L1 pathway could have an important role through the inhibition of apoptotic inhibition, which could be complicated with a compensation process and is still not well-established.
Recently, immunotherapy has become a mainstream anti-cancer therapy, but the unleashed innate immune system has caused various immune-related adverse effects [33]. During ICPi therapy, endocrine organs were frequently attacked, and the thyroid gland was the most affected, mainly in the form of thyroiditis [34]. Also, the thyroid gland is the most common target organ of autoimmune disease [35]. Thyroiditis induced by ICPi and AITD have similarities and differences. Both might be triggered by the presence of anti-thyroid autoantibodies (anti-TPO and anti-Tg) as self-antigens via HLA-DR to T-cells, but anti-thyroid autoantibodies have frequently been observed in up to 18% of the general population, and immune tolerance prevented cytotoxic T-cell response [36]. In the current study, no difference of anti-thyroid autoantibodies according to PD-L1 SNP was observed. The progression to AITD might be prevalent in patients with a genetic propensity such as HLA-DR B1 [37]. The clinical course and disease activity after immune system activation were different between ICPi-induced thyroiditis and AITD. In patients with AITD, cytotoxic T-cell response was regulated by the innate immune system, and disease activity varied in pace and extent [38]. On the contrary, immune tolerance could not bear robust cytotoxic activity of ICPi-activated T-cells, which resulted in a rapid course of thyroiditis. The majority of cases of ICPi-induced thyroiditis might lead to irreversible hypothyroidism due to complete thyroid tissue destruction by an uncontrolled immune reaction [38,39]. Our study showed that the development of hypothyroidism was not associated with a PD-L1 SNP among patients with HT. It was assumed that a tighter and more complex immune regulation system might exist beyond the PD-L1 SNP to prevent thyroid tissue consumption by cytotoxic T-cells in patients with HT if a considerable proportion of the PD-1/PD-L1 pathway was not blocked by ICPi’s.
Our study has several limitations due to its retrospective design. Thyroid stimulating receptor antibody was not measured in patients with GD. Moreover, measurement of anti-TPO and anti-Tg autoantibodies was not performed in all patients with GD. This could cause difficulty in precise interpretation of the association between anti-thyroid autoantibody and PD-L1 SNP. Additionally, follow-up regularity was different in each patient, so we were unable to evaluate the recurrence-free time interval in GD patients. Our study had relatively small sample size to evaluate the association between clinical characteristics and AITD. So, we analyzed by dividing to two groups according to rs822339 SNP. Previous genome-wide association studies (GWAS) of AITD represented many susceptible loci for AITD [40]; however, PD-1/PD-L1 pathway related genetic susceptibility in patients with AITD was not fully analyzed. Recent world-widely used ICPi treatment for cancer patients reported high prevalence of thyroid irAEs, which is more frequently observed in patients treated by PD-1/PD-L1 inhibitors compared with those who treated with CTLA-4 antibodies. Therefore, future study using GWAS could provide more significant evidence of genetic susceptibility between PD-1/PD-L1 pathway and AITD.
In conclusion, the incidence of the PD-L1 (rs822339) SNP is not different in patients with AITD compared with healthy controls. Patients with AITD might have an intact PD-1/PD-L1 pathway, which is the basis for the chronicity of AITD. Meticulous follow-up is necessary in GD patients with the PD-L1 SNP due to the possibility of longer treatment. Further studies are required to investigate the complex immune tolerance system in patients with GD and HT.
Supplementary Information
Subgroup Analysis of Treatment Duration in GD Patients Who Are Treated with Only Anti-Thyroid Medication (n=143)
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: J.H.Y., H.K.K., H.C.K. Acquisition, analysis, or interpretation of data: J.H.Y., M.S., H.N.K. Drafting the work or revising: J.H.Y., W.C., J.Y.P., A.R.H., H.C.K. Final approval of the manuscript: H.K.K.
Acknowledgements
This work was supported by the Korean Endocrine Society New Faculty Research Award 2016.