Articles
- Page Path
- HOME > Endocrinol Metab > Volume 31(2); 2016 > Article
-
Review ArticleCardiovascular Effects of Glucagon-Like Peptide-1 Receptor Agonists
- Yu Mi Kang, Chang Hee Jung
-
Endocrinology and Metabolism 2016;31(2):258-274.
DOI: https://doi.org/10.3803/EnM.2016.31.2.258
Published online: April 25, 2016
Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding author: Chang Hee Jung. Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea. Tel: +82-2-3010-1418, Fax: +82-2-3010-6962, chjung0204@gmail.com
Copyright © 2016 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Glucagon-like peptide-1 (GLP-1) is a member of the proglucagon incretin family, and GLP-1 receptor agonists (RAs) have been introduced as a new class of antidiabetic medications in the past decade. The benefits of GLP-1 RAs are derived from their pleiotropic effects, which include glucose-dependent insulin secretion, suppressed glucagon secretion, and reduced appetite. Moreover, GLP-1 RAs also exert beneficial roles on multiple organ systems in which the GLP-1 receptors exist, including the cardiovascular system. Cardiovascular effects of GLP-1 RAs have been of great interest since the burden from cardiovascular diseases (CVD) has been unbearably increasing in a diabetic population worldwide, despite strict glycemic control and advanced therapeutic techniques to treat CVD. Preclinical studies have already demonstrated the beneficial effects of GLP-1 on myocardium and vascular endothelium, and many clinical studies evaluating changes in surrogate markers of CVD have suggested potential benefits from the use of GLP-1 RAs. Data from numerous clinical trials primarily evaluating the antihyperglycemic effects of multiple GLP-1 RAs have also revealed that changes in most CVD risk markers reported as secondary outcomes have been in favor of GLP-1 RAs treatment. However, to date, there is only one randomized clinical trial of GLP-1 RAs (the ELIXA study) evaluating major cardiovascular events as their primary outcomes, and in this study, a neutral cardiovascular effect of lixisenatide was observed in high-risk diabetic subjects. Therefore, the results of ongoing CVD outcome trials with the use of GLP-1 RAs should be awaited to elucidate the translation of benefits previously seen in CVD risk marker studies into large clinical trials with primary cardiovascular outcomes.
- Because CVD is the leading cause of death in patients with type 2 diabetes (T2D), T2D is often referred as a 'cardiovascular disease (CVD)' [1]. Despite a significant improvement in the treatment of T2D and CVD over the past few decades, reducing CVD in patients with T2D solely with an optimal glycemic control has been challenging [2]. Therefore, current treatment guidelines recommend maintenance of an optimal weight and stringent management of lipid levels in addition to glycemic control [3]. Clearly, there has been a need for a new class of antidiabetic agents with advantages over currently marketed drugs that can lower glucose via mechanisms resulting in either neutral or protective effects on the cardiovascular system.
- In the last 10 to 15 years, discoveries on certain gut peptides that influence insulin secretion related to meal ingestion, namely incretins, have opened up a new paradigm in the treatment of diabetes [4]. The levels of incretins including glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic hormone rise in response to a meal, and they are rapidly degraded by the enzyme dipeptidyl peptidase 4 (DPP-4) [5]. Accordingly, incretin-based therapies, such as DPP-4 inhibitors and GLP-1 receptor agonists (GLP-1 RAs) that are resistant to degradation by DPP-4, have been introduced in the market within the past decade to overcome and extend the short half-life of the incretin effect.
- GLP-1 RAs exert an antihyperglycemic effect by enhancing pancreatic β-cell–mediated glucose-dependent insulin secretion, suppressing glucagon secretion, delaying gastric emptying, and reducing food intake in patients with T2D [6]. The key beneficial features of GLP-1 RAs are weight loss and a relatively low risk of hypoglycemia compared with other antidiabetic agents [7]. Moreover, additional roles for GLP-1 have been revealed since the discovery of an extrapancreatic distribution of GLP-1 receptors in multiple organs such as the gastrointestinal tract, lung, heart, liver, and kidneys [58910], and evidence shows that administration of GLP-1 or GLP-1 RAs result in beneficial effects in these organs, indeed [11].
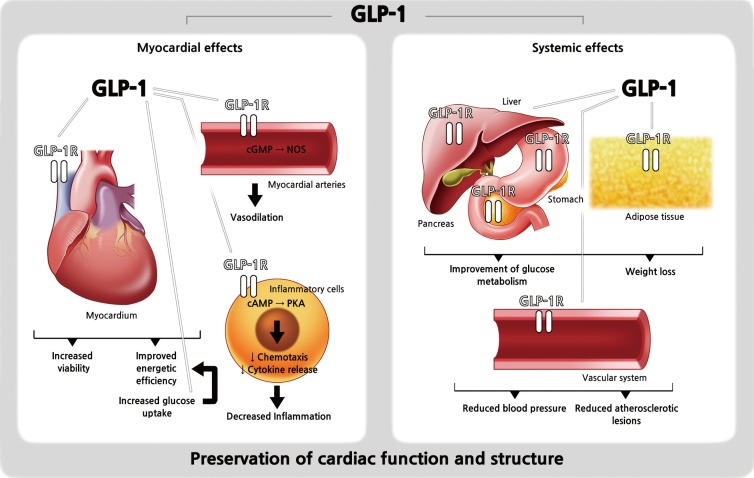
- Of particular interest, the discovery of GLP-1 receptor expression in the myocardium and in the vascular endothelium suggested possible roles of GLP-1 in the cardiovascular system and multiple preclinical studies support cardioprotective effects of native GLP-1 (Fig. 1) [1213141516171819202122232425]. For example, Gros et al. [16] found that the lack of GLP-1 receptors resulted in impaired left ventricular (LV) contractility and a decreased resting heart rate (HR) in GLP-1 receptor knockout mouse models. Also, Nikolaidis et al. [18] showed that intravenous infusion of GLP-1 for 48 hours in animal models with induced dilated cardiomyopathy significantly improved LV contractility, stroke volume, and cardiac output. Furthermore, many studies of ischemia/reperfusion animal models consistently revealed beneficial effects of GLP-1 on post-ischemia recovery and myocardial viability [13172224]. Regarding the effect of GLP-1 on vascular endothelium, numerous in vitro studies and animal studies showed either nitric oxide-dependent or -independent vasodilative effects [121415192021]. Lastly, in hypertension-prone animal models, GLP-1 infusion exerted its diuretic and natriuretic effects and resulted in a reduction of hypertension and albuminuria development, and an improved endothelial function [23]. These beneficial preclinical roles of GLP-1 in the cardiovascular system might translate to the clinical setting. Therefore, attempts have been made to elucidate the cardioprotective effects of GLP-1 RAs in humans.
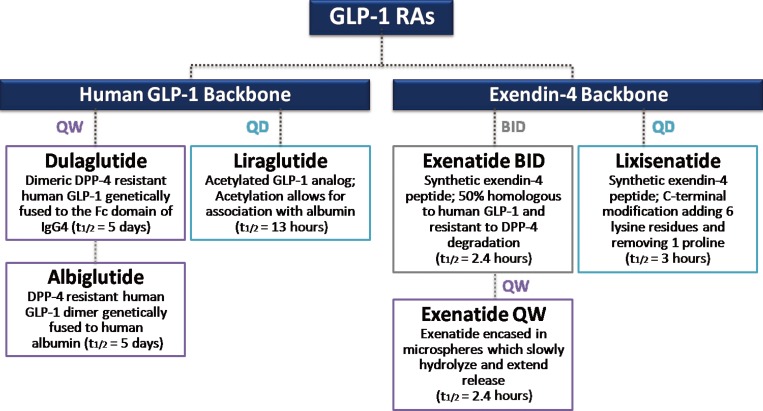
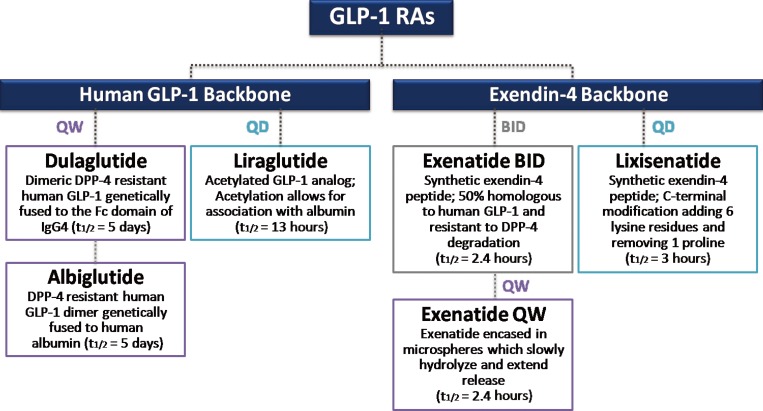
- Numerous GLP-1RAs are currently available on the market, and each agent possesses a unique biochemical profile, depending the alterations made to its backbone, human GLP-1, or exendin-4 (Fig. 2) [26]. Exenatide and liraglutide were released onto the market relatively early, whereas newer agents, such as lixisenatide, dulaglutide, and albiglutide, were approved more recently (Table 1). Accordingly, large clinical trials on the safety and efficacy of these earlier GLP-1 RAs are available. However, concerns have recently emerged about the long-term cardiovascular safety of antidiabetic medications, and U.S. Food and Drug Administration published guidelines to assess the cardiovascular risk of novel antidiabetic drugs [27]. Therefore, the role of GLP-1 RAs specifically in the cardiovascular system has been of particular interest in the past several years, and multiple clinical trials have examined the cardiovascular effects and safety of GLP-1 RAs (Table 1). Most trials are still ongoing and only one prospective randomized clinical trial with a primary outcome of major cardiovascular events in patients with T2D has reported its results [28]. Therefore, this present review examines the cardiovascular effects of the GLP-1 RAs in T2D by revisiting the results of clinical studies evaluating CVD surrogate markers and cardiovascular events.
INTRODUCTION
- Studies using surrogate markers of CVD risks
- Hypertension affects almost three-quarters of T2D patients [29] and is a powerful independent predictor of CVD mortality [30]. Accordingly, better control of cardiovascular risk factors such as blood pressure (BP) in patients with T2D is required to achieve favorable cardiovascular safety goals. Indeed, GLP-1 RAs have consistently showed BP-lowering effects in human subjects, although the exact mechanisms are not fully understood [31323334]. For example, Wang et al. [34] showed that treatment with the GLP-1 RAs exenatide and liraglutide reduced both systolic and diastolic BP by 1 to 5 mm Hg in patients with T2D, compared with some other anti-diabetic drugs, including insulin, glimepiride, and placebo.
- For most GLP-1 trials, HR is included as a secondary safety endpoint, and the results have been inconsistent. Nonetheless, the effect of GLP-1 RAs on HR should not be overlooked, because studies have shown that an elevated HR was potentially associated with a higher cardiovascular risk [353637]. Although the subjects were non-diabetic, one meaningful study observed acute changes of cardiovascular risk markers such as HR and BP upon intravenous administration of GLP-1 RA [38]. In this study, Smits et al. [38] performed automated oscillometric BP measurements and a finger photoplethysmography during the intravenous administration of placebo (saline 0.9%), exenatide, or a combination of exenatide and the nitric oxide-synthase inhibitor (L-NMMA) in 10 healthy overweight males. As a result, exenatide increased HR by a mean maximum of 6.8 beats per minute (bpm), as well as systolic BP by 9.8 mm Hg [38]. Simultaneously, sympathetic nervous system activity measured by HR variability and the rate-pressure-product were also elevated, and increases in HR, systolic BP and sympathetic nervous system activity were still observed even with a concomitant L-NMMA-infusion [38]. On the other hand, Mendis et al. [39] recruited healthy male subjects and found that HR was elevated by 8.2 in the exenatide group compared with the placebo group. The exenatide group in this study, however, showed a significant increase in the mean cardiac output by 1.2 L/min, a reduced total peripheral resistance by the mean of 120 dyn·s/cm–5, and a significantly reduced urinary sodium: creatinine ratio without a significant change in the BP [39]. Therefore, the authors postulated that the GLP-1-induced vasodilation might reduce total peripheral resistance, leading to a reflex tachycardia [39]. They also suggested that the natriuretic effect of GLP-1 RAs they have seen in their study might be one of the mechanisms behind the BP-lowering effect of GLP-1 RAs, although the BP reduction was not significant in their study [39]. Nonetheless, the exact mechanisms underlying the GLP-1 RA-associated HR acceleration are still unclear and these preliminary study results involving healthy male subjects cannot be over-generalized to a diabetic population using GLP-1 RAs. However, the results clearly suggest the need for further investigation of the effect of GLP-1 RAs on HR and BP and their long-term consequences.
- Table 2 summarizes the cardiovascular risk-relevant outcomes of large clinical trials such as the Diabetes therapy Utilization: Researching changes in A1c, weight and other factors Through Intervention with exenatide Once weekly (DURATION) trials of exenatide once-weekly, the Liraglutide Effect and Action in Diabetes (LEAD) studies of liraglutide, the Assessment of Weekly Administration of LY2189265 in Diabetes-1 (AWARD) studies of dulaglutide, the HARMONY studies of albiglutide, and the GetGoal studies of lixisenatide. Most clinical trials on the efficacy of GLP-1 RAs found a reduced systolic BP, and this beneficial effect was somewhat more apparent in studies of liraglutide and exenatide (Table 2). Although data on HR were not provided in most studies of twice-daily exenatide, HR elevation was generally more apparent in the GLP-1 RA treatment arms, compared with the comparator arms (Table 2). Although these changes in HR upon GLP-1 RA administration were subtle and the underlying mechanisms are unclear, further studies are necessary to elucidate the long-term effects of GLP-1 RAs primarily on HR and other related variables to clarify whether the elevated HR can be regarded as a transient reflex mechanism or whether it might in fact lead to poor cardiovascular outcomes in a diabetic population.
- Several studies have primarily evaluated the changes in cardiovascular risk biomarkers upon the use of GLP-1 RAs. Courreges et al. [40] evaluated the effects of liraglutide on cardiovascular risk biomarkers such as adiponectin, leptin, high-sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), plasminogen activator inhibitor 1 (PAI-1), and B-type natriuretic peptide (BNP). Of these parameters, PAI-1 and BNP levels were significantly decreased following a 14-week treatment with liraglutide (–29% for PAI-1 with 1.25 mg liraglutide and –38% for hs-CRP with 1.90 mg liraglutide) versus placebo [40]. Because the level of PAI-1 is correlated with atherosclerosis formation [41] and is markedly elevated in T2D patients with CVD compared with healthy or T2D patients without CVD [42], this result implies more fibrinolytic thereby less atherogenic processes. In this study, a dose-dependent reduction of hs-CRP levels was seen, albeit nonsignificant [40]. Fourteen weeks of liraglutide treatment in 59 overweight or obese T2D patients whose glycemic control was inadequate (hemoglobin A1c >7% with metformin and/or sulfonylurea) at the initiation period resulted in a nonsignificant decrease in pro-inflammatory factors such as TNF-α and IL-6 [43]. These decreased pro-inflammatory markers suggest liraglutide might attenuate inflammatory cascade which leads to the development of atherosclerosis [44].
- In addition, several cardiovascular marker studies were conducted also with the exenatide treatment [4546]. Gurkan et al. [45] enrolled 34 insulin and incretin-naïve T2D patients on metformin and randomized subjects to exenatide or insulin glargine treatment arms. After 26 weeks, the levels of hs-CRP and endothelin-1 decreased (27.5% and 18.75%, respectively) in the exenatide arm whereas the levels of fibrinogen, monocyte chemoattractant protein-1, leptin and endothelin-1 increased in the insulin glargine arm (13.4%, 30.2%, 47.5%, and 80%, respectively) [45]. Blonde et al. [47] observed that 82 weeks of adjunctive exenatide administration in 314 T2D patients with sulfonylurea and/or metformin resulted in reduced triglyceride concentrations (–0.43 mmol/L) and elevated high density lipoprotein cholesterol concentrations (0.12 mmol/L). Other parameters such as total cholesterol, low density lipoprotein cholesterol, and apolipoprotein B showed trends toward improvement, albeit nonsignificant [47].
- Likewise, multiple large clinical studies with various GLP-1 RAs have shown a more favorable trend in lipid profiles with GLP-1 RAs, although these were not evaluated as the primary outcomes (Table 2). Moreover, some trials revealed improvements in cardiovascular risk markers such as BNP and CRP. For instance, Bunck et al. [48] and Derosa et al. [49] observed significant reduction in hs-CRP with twice-daily exenatide, and DURATION-2, DURATION-3, DURATION-3 extension, and DURATION-5 studies observed considerable reductions in inflammatory markers such as hs-CRP or CRP (Table 2) [484950515253545556575859606162636465666768697071727374757677787980818283848586]. Notably, the 65% reduction in hs-CRP levels observed in one study was statistically significant, independent of body fat mass and body weight [48]. These results imply that the anti-inflammatory and potentially anti-atherosclerotic effects of GLP-1 RAs are not attributable to improved adiposity, which is often associated with a low-grade inflammation [87].
- Carotid artery wall thickening is one of the earliest detectable manifestations of CVD, and is significantly correlated with future CVD events [88]. Intima-media thickness (IMT) measured at a common carotid artery level is widely used as an effective, non-invasive surrogate measure of atherosclerosis [89]. A few longitudinal studies evaluated the anti-atherosclerotic effects of GLP-1 RAs using carotid IMT data [9091]. Hopkins et al. [90] enrolled 11 severely obese T2D patients, treated them with either exenatide twice-daily (nine patients) or liraglutide (two patients) for 6 months, and compared the carotid IMT before and after the GLP-1 RA treatment. However, there was no change in carotid IMT with GLP-1 RA treatment despite an improved glycemic profile and a significant adipose tissue reduction [90]. This result might be attributable to the small number of patients and the long duration and severity of T2D, as well as the presence of comorbidities. In contrast, an 8-month prospective pilot study in which 64 T2D patients with no prior CVD history were treated with liraglutide revealed that the carotid IMT decreased from 1.19±0.47 to 0.94±0.21 mm (P<0.01) [91]. This study was the first to show a beneficial effect of GLP-1 RAs on carotid IMT in human subjects. Although there are many ongoing large CVD outcome trials of GLP-1 RA treatment (Table 1), more simultaneous investigation of the causal relationship between GLP-1 RA administration and useful surrogate measures of CVD, including carotid IMT, would help us understand the potential mechanisms through which GLP-1 RAs may result in favorable cardiovascular outcomes in patients with T2D.
- In 14 patients with coronary artery disease and good LV function, intravenous GLP-1 infusion improved global and regional wall LV function on dobutamine stress echocardiography compared with control [92]. Similarly, Lonborg et al. [9394] found that intravenous infusion of GLP-RA exenatide prior to revascularization in patients with ST-segment elevation myocardial infarction (MI) reduced reperfusion injury and final infarct size, compared with placebo treatment and Woo et al. [95] obtained similar results with subcutaneous injection of exenatide.
- With subcutaneous liraglutide treatment, Chen et al. [96] observed a mild improvement in LV ejection fraction in ST-segment elevation MI patients who were not necessarily diabetic. On the other hand, in a retrospective study with acute MI patients who were diabetic, Nozue et al. [97] observed that liraglutide use was an independent negative predictor of LV mass index, suggesting liraglutide might prevent the progression of post-MI LV remodeling. Taken together, the results of numerous small studies equivocally suggest that GLP-1 RA treatment in coronary artery disease patients would be beneficial, regardless of the presence of diabetes. However, more large-scale clinical trial data are needed for a clear causal relationship to be established.
- Cardiovascular outcome studies
- Although multiple clinical trials evaluating cardiovascular events associated with GLP-1 RA use as the primary outcome are currently ongoing (Table 1), abundant non-primary outcome data are available from many clinical trials on the efficacy of GLP-1 RAs marketed earlier, such as exenatide (DURATION studies) and liraglutide (LEAD studies). In addition, some studies on recently marketed GLP-1 RAs such as dulaglutide (AWARD studies), albiglutide (HARMONY studies), and lixisenatide (GetGoal studies) included cardiovascular parameters as a part of their non-primary endpoints (Table 2). Furthermore, we could get some information on the effect of GLP-1 RAs on cardiovascular outcomes through a few retrospective studies published in the literature [289899100101]. Best et al. [98], for instance, led a retrospective study of 39,275 patients with T2D on exenatide twice-daily and 381,218 patients on other glucose-lowering therapies. In this study, twice-daily exenatide treatment was associated with a lower risk of CVD events including MI, ischemic stroke, or coronary revascularization procedure (hazard ratio [HR], 0.81; 95% confidence interval [CI], 0.68 to 0.95; P=0.01) and hospitalizations (HR, 0.88; 95% CI, 0.79 to 0.98; P=0.02) than treatment with other glucose-lowering therapies [98]. It is worth noting that this cardioprotective effect of exenatide outweighed the fact that a greater proportion of patients on exenatide treatment possessed CVD risk factors such as prior ischemic heart disease, obesity, hyperlipidemia, and hypertension [98]. Monami et al. [100] reported a meta-analysis of 33 published and unpublished trials that evaluated the effect of GLP-1 RAs and had information on major adverse cardiovascular events (MACE), mortality, and cardiovascular risk factors. Although the difference in the incidence of MACE between GLP-1 RA and comparators failed to show statistical significance (Mantel-Haenzel odds ratio, 0.78; 95% CI, 0.54 to 1.13; P=0.18), GLP-1 RA were associated with a significant reduction in the incidence of MACE compared with placebo and pioglitazone, with a non-significant trend towards reduction in DPP-4 inhibitor-controlled studies [100]. There was no significant effect of GLP-1 RAs on mortality, although a nonsignificant favorable trend was observed in comparisons with placebo [100]. A retrospective longitudinal analysis of ambulatory care data with a median follow-up of 3.5 years showed that twice-daily exenatide use with or without insulin was associated with significantly fewer cardiovascular events than insulin treatment [101]. In addition, a meta-analysis of the HARMONY studies of albiglutide revealed that the risk of cardiovascular events was not significantly higher in subjects on albiglutide than in subjects on all other comparators including placebo and oral antidiabetic drugs such as glimepiride, insulin glargine, insulin lispro, liraglutide, pioglitazone, and sitagliptin [99].
- Of the numerous prospective randomized clinical trials evaluating the influence of GLP-1 RA use on cardiovascular outcomes (Table 1), only the ELIXA study of lixisenatide [28] has thus far published its results. In the ELIXA study, 6,068 patients with T2D who had an MI or who had been hospitalized for unstable angina within the previous 180 days were randomized to receive lixisenatide or placebo in addition to baseline treatment. In this study, with a median follow-up of 25 months, the primary composite end point including cardiovascular death, MI, stroke, and hospitalization for unstable angina occurred in 406 patients (13.4%) in the lixisenatide group and in 399 (13.2%) in the placebo group (HR, 1.02; 95% CI, 0.89 to 1.17) [28]. In addition, the results of the ELIXA study answered the question of cardiovascular safety related to heart failure by displaying no significant between-group differences in the rate of hospitalization for heart failure (HR, 0.96; 95% CI, 0.75 to 1.23) or the rate of death (HR, 0.94; 95% CI, 0.78 to 1.13) in the lixisenatide group [28].
- Although the analysis revealed the non-inferiority of lixisenatide to placebo (P<0.001), it did not display superiority (P=0.81) [28]. The lack of superiority is probably attributable to the short follow-up period and high CVD risk in the enrolled subjects. However, there was no clear explanation for the neutral cardiovascular profile of lixisenatide, rather than the expected cardioprotective effect. Upcoming CVD outcome trials, such as the EXSCEL study of exenatide weekly, the LEADER study of liraglutide and the REWIND study of dulaglutide might provide further insight in this matter (Table 1).
CARDIOVASCULAR EFFECTS OF GLP-1 RAs IN HUMAN STUDIES
Blood pressure and heart rate
Circulating biomarkers and lipid profiles
Carotid intima-media thickness
LV function and reperfusion injury in patients with coronary artery disease
Retrospective studies on cardiovascular outcomes
A prospective randomized clinical trial with primary cardiovascular outcome: the ELIXA study
- Preclinical and preliminary clinical evidence has consistently revealed potential cardiovascular benefits from the administration of GLP-1 RAs. Likewise, the results of large clinical trials employing cardiovascular surrogate markers as their secondary outcomes also imply the potential beneficial effects of GLP-1 RAs in the cardiovascular system in human subjects. Although the ELIXA study—the only CVD outcome trial of GLP-1 RAs published to date—reported a neutral cardiovascular effect of lixisenatide, the potential cardiovascular benefits of GLP-1 RAs should not be overlooked, because high-risk subjects with established CVD were recruited in this study to delineate its cardiovascular safety within a short-term period. As depicted in Table 1, multiple large-scale clinical trials are ongoing to clarify the cardiovascular efficacy and safety of GLP-1 RAs and the results have been awaited with bated breath. The accumulation of the results of these ongoing cardiovascular outcome trials should be able to answer questions about cardiovascular benefits of GLP-1 RAs that were strongly suggested by previous preclinical and clinical studies and their possibility to lower the excessive cardiovascular burden in patients with T2D.
CONCLUSIONS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Article information
- 1. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999;100:1134–1146. ArticlePubMed
- 2. Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care 2013;36(Suppl 2):S259–S263. ArticlePubMedPMC
- 3. American Diabetes Association.8. Cardiovascular disease and risk management. Diabetes Care 2016;39(Suppl 1):S60–S71. ArticlePubMed
- 4. Doggrell SA. After 10 years of clinical trials with liraglutide in diabetes, what do we know about its effects on clinical cardiovascular outcomes? Rev Recent Clin Trials 2015;10:68–77. ArticlePubMed
- 5. Fava S. Glucagon-like peptide 1 and the cardiovascular system. Curr Diabetes Rev 2014;10:302–310. ArticlePubMed
- 6. Saraiva FK, Sposito AC. Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc Diabetol 2014;13:142ArticlePubMedPMCPDF
- 7. Lorber D. GLP-1 receptor agonists: effects on cardiovascular risk reduction. Cardiovasc Ther 2013;31:238–249. ArticlePubMed
- 8. Amato A, Baldassano S, Liotta R, Serio R, Mule F. Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle. J Endocrinol 2014;221:29–37. ArticlePubMed
- 9. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996;137:2968–2978. ArticlePubMedPDF
- 10. Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010;51:1584–1592. ArticlePubMedPMC
- 11. Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, et al. Myocardial AKT: the omnipresent nexus. Physiol Rev 2011;91:1023–1070. ArticlePubMedPMC
- 12. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340–2350. ArticlePubMed
- 13. Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 2005;54:146–151. ArticlePubMed
- 14. Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7-36)amide and amylin on the pulmonary circulation of the rat. Regul Pept 2001;102:81–86. ArticlePubMed
- 15. Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 2008;478:136–142. ArticlePubMed
- 16. Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, et al. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology 2003;144:2242–2252. ArticlePubMedPDF
- 17. Huisamen B, Genade S, Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7-36) amide in the rat heart and their role in protection against ischaemia. Cardiovasc J Afr 2008;19:77–83. PubMedPMC
- 18. Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 2004;110:955–961. ArticlePubMed
- 19. Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept 2005;125:173–177. ArticlePubMed
- 20. Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215. ArticlePubMed
- 21. Richter G, Feddersen O, Wagner U, Barth P, Goke R, Goke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol 1993;265(4 Pt 1):L374–L381. ArticlePubMed
- 22. Xie Y, Wang SX, Sha WW, Zhou X, Wang WL, Han LP, et al. Effects and mechanism of glucagon-like peptide-1 on injury of rats cardiomyocytes induced by hypoxia-reoxygenation. Chin Med J (Engl) 2008;121:2134–2138. ArticlePubMed
- 23. Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 2003;21:1125–1135. ArticlePubMed
- 24. Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 2006;317:1106–1113. ArticlePubMed
- 25. Ravassa S, Zudaire A, Diez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res 2012;94:316–323. ArticlePubMedPDF
- 26. Kuritzky L, Umpierrez G, Ekoe JM, Mancillas-Adame L, Lando LF. Safety and efficacy of dulaglutide, a once weekly GLP-1 receptor agonist, for the management of type 2 diabetes. Postgrad Med 2014;126:60–72. ArticlePubMed
- 27. Regulatory watch: FDA issues guidance for cardiovascular risk assessment of novel antidiabetic agents. Nat Rev Drug Discov 2009;8:99ArticlePubMed
- 28. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257. ArticlePubMed
- 29. Primatesta P, Poulter NR. Improvement in hypertension management in England: results from the Health Survey for England 2003. J Hypertens 2006;24:1187–1192. ArticlePubMed
- 30. Kannel WB, Higgins M. Smoking and hypertension as predictors of cardiovascular risk in population studies. J Hypertens Suppl 1990;8:S3–S8.
- 31. Katout M, Zhu H, Rutsky J, Shah P, Brook RD, Zhong J, et al. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens 2014;27:130–139. ArticlePubMedPDF
- 32. Robinson LE, Holt TA, Rees K, Randeva HS, O'Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013;3:e001986.ArticlePubMedPMC
- 33. Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res Clin Pract 2015;110:26–37. ArticlePubMed
- 34. Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab 2013;15:737–749. ArticlePubMed
- 35. Cook S, Togni M, Schaub MC, Wenaweser P, Hess OM. High heart rate: a cardiovascular risk factor? Eur Heart J 2006;27:2387–2393. ArticlePubMed
- 36. Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J 2010;159:612–619.e3. ArticlePubMed
- 37. Perret-Guillaume C, Joly L, Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog Cardiovasc Dis 2009;52:6–10. ArticlePubMed
- 38. Smits MM, Muskiet MH, Tonneijck L, Hoekstra T, Kramer MH, Diamant M, et al. Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol 2016;81:613–620. ArticlePubMedPMC
- 39. Mendis B, Simpson E, MacDonald I, Mansell P. Investigation of the haemodynamic effects of exenatide in healthy male subjects. Br J Clin Pharmacol 2012;74:437–444. ArticlePubMedPMC
- 40. Courreges JP, Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med 2008;25:1129–1131. ArticlePubMedPMC
- 41. Lalic K, Jotic A, Rajkovic N, Singh S, Stosic L, Popovic L, et al. Altered daytime fluctuation pattern of plasminogen activator inhibitor 1 in type 2 diabetes patients with coronary artery disease: a strong association with persistently elevated plasma insulin, increased insulin resistance, and abdominal obesity. Int J Endocrinol 2015;2015:390185PubMedPMC
- 42. Wang G, Gao S, Su N, Xu J, Fu D. Plasma adiponectin levels inversely correlate to clinical parameters in type 2 diabetes mellitus patients with macrovascular diseases. Ann Clin Lab Sci 2015;45:287–291. PubMed
- 43. Diaz-Soto G, de Luis DA, Conde-Vicente R, Izaola-Jauregui O, Ramos C, Romero E. Beneficial effects of liraglutide on adipocytokines, insulin sensitivity parameters and cardiovascular risk biomarkers in patients with type 2 diabetes: a prospective study. Diabetes Res Clin Pract 2014;104:92–96. ArticlePubMed
- 44. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y, Ali F. Atherosclerotic cardiovascular disease: a review of initiators and protective factors. Inflammopharmacology 2016;24:1–10. ArticlePubMedPDF
- 45. Gurkan E, Tarkun I, Sahin T, Cetinarslan B, Canturk Z. Evaluation of exenatide versus insulin glargine for the impact on endothelial functions and cardiovascular risk markers. Diabetes Res Clin Pract 2014;106:567–575. ArticlePubMed
- 46. Plutzky J, Garber A, Falahati A, Toft AD, Poulter NR. Reductions in lipids and CV risk markers in patients with type 2 diabetes treated with liraglutide: a meta-analysis. Can J Diabetes 2009;33:209–210. Article
- 47. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006;8:436–447. ArticlePubMed
- 48. Bunck MC, Diamant M, Eliasson B, Corner A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 2010;33:1734–1737. ArticlePubMedPMC
- 49. Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Ragonesi PD, Querci F, et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther 2010;12:233–240. ArticlePubMed
- 50. Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431–439. ArticlePubMed
- 51. Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:1301–1310. ArticlePubMed
- 52. Diamant M, Van Gaal L, Stranks S, Guerci B, MacConell L, Haber H, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care 2012;35:683–689. ArticlePubMedPMC
- 53. Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010;375:2234–2243. ArticlePubMed
- 54. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47. ArticlePubMed
- 55. Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long-Acting insulin (HEELA) study. Diabetes Obes Metab 2009;11:1153–1162. ArticlePubMedPMC
- 56. DeFronzo RA, Triplitt C, Qu Y, Lewis MS, Maggs D, Glass LC. Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin. Diabetes Care 2010;33:951–957. ArticlePubMedPMC
- 57. Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250. ArticlePubMed
- 58. Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008;24:275–286. ArticlePubMed
- 59. Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008;30:1448–1460. ArticlePubMed
- 60. Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010;33:1255–1261. ArticlePubMedPMC
- 61. Macconell L, Pencek R, Li Y, Maggs D, Porter L. Exenatide once weekly: sustained improvement in glycemic control and cardiometabolic measures through 3 years. Diabetes Metab Syndr Obes 2013;6:31–41. PubMedPMC
- 62. Diamant M, Van Gaal L, Guerci B, Stranks S, Han J, Malloy J, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol 2014;2:464–473. ArticlePubMed
- 63. Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, Gonzalez JG, Chan M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind-study. Diabetes Care 2012;35:252–258. ArticlePubMedPMC
- 64. Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 2013;381:117–124. ArticlePubMed
- 65. Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009;26:268–278. ArticlePubMedPMC
- 66. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90. ArticlePubMedPMC
- 67. Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–481. ArticlePubMed
- 68. Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B, et al. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab 2011;13:348–356. ArticlePubMedPMC
- 69. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230. ArticlePubMedPMC
- 70. Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046–2055. ArticlePubMedPMCPDF
- 71. Buse JB, Sesti G, Schmidt WE, Montanya E, Chang CT, Xu Y, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care 2010;33:1300–1303. ArticlePubMedPMC
- 72. Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456. ArticlePubMed
- 73. Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract 2011;65:397–407. ArticlePubMedPMC
- 74. Rosenstock J, Raccah D, Koranyi L, Maffei L, Boka G, Miossec P, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 2013;36:2945–2951. ArticlePubMedPMC
- 75. Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J Diabetes Complications 2014;28:386–392. ArticlePubMed
- 76. Nauck MA, Stewart MW, Perkins C, Jones-Leone A, Yang F, Perry C, et al. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 2016;59:266–274. ArticlePubMedPDF
- 77. Ahren B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014;37:2141–2148. ArticlePubMed
- 78. Weissman PN, Carr MC, Ye J, Cirkel DT, Stewart M, Perry C, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia 2014;57:2475–2484. ArticlePubMedPDF
- 79. Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2014;2:289–297. ArticlePubMed
- 80. Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014;37:2159–2167. ArticlePubMed
- 81. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care 2015;38:2241–2249. ArticlePubMed
- 82. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014;37:2168–2176. ArticlePubMed
- 83. Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet 2015;385:2057–2066. ArticlePubMed
- 84. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care 2014;37:2149–2158. ArticlePubMedPMC
- 85. Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab 2015;17:849–858. ArticlePubMedPMC
- 86. Dungan KM, Povedano ST, Forst T, Gonzalez JG, Atisso C, Sealls W, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 2014;384:1349–1357. ArticlePubMed
- 87. van Greevenbroek MM, Schalkwijk CG, Stehouwer CD. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med 2013;71:174–187. PubMed
- 88. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011;300:H2–H12. ArticlePubMed
- 89. Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb 2016;23:18–31. ArticlePubMed
- 90. Hopkins ND, Cuthbertson DJ, Kemp GJ, Pugh C, Green DJ, Cable NT, et al. Effects of 6 months glucagon-like peptide-1 receptor agonist treatment on endothelial function in type 2 diabetes mellitus patients. Diabetes Obes Metab 2013;15:770–773. ArticlePubMed
- 91. Rizzo M, Chandalia M, Patti AM, Di Bartolo V, Rizvi AA, Montalto G, et al. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc Diabetol 2014;13:49ArticlePubMedPMC
- 92. Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart 2012;98:408–413. ArticlePubMed
- 93. Lonborg J, Kelbaek H, Vejlstrup N, Botker HE, Kim WY, Holmvang L, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv 2012;5:288–295. ArticlePubMed
- 94. Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012;33:1491–1499. ArticlePubMedPDF
- 95. Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, et al. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol 2013;33:2252–2260. ArticlePubMed
- 96. Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 2015;170:845–854. ArticlePubMed
- 97. Nozue T, Yamada M, Tsunoda T, Katoh H, Ito S, Iwaki T, et al. Effects of liraglutide, a glucagon-like peptide-1 analog, on left ventricular remodeling assessed by cardiac magnetic resonance imaging in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels 2015 8 21 [Epub]. ArticlePDF
- 98. Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care 2011;34:90–95. ArticlePubMed
- 99. Fisher M, Petrie MC, Ambery PD, Donaldson J, Ye J, McMurray JJ. Cardiovascular safety of albiglutide in the Harmony programme: a meta-analysis. Lancet Diabetes Endocrinol 2015;3:697–703. ArticlePubMed
- 100. Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014;16:38–47. ArticlePubMed
- 101. Paul SK, Klein K, Maggs D, Best JH. The association of the treatment with glucagon-like peptide-1 receptor agonist exenatide or insulin with cardiovascular outcomes in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol 2015;14:10ArticlePubMedPMCPDF
References
Schematic representation of the distribution of glucagon-like peptide-1 (GLP-1) receptors (GLP-1Rs) and the cardiovascular and systemic effects of GLP-1. Modified from Ravassa et al. [25], with permission from Oxford University Press. cAMP, cyclicadenosine monophosphate; cGMP, cyclic guanosine monophosphate; NOS, nitric oxide synthase; PKA, protein kinase A.
Classification of currently available glucagon-like peptide-1 receptor agonists (GLP-1 RAs) by structure and duration of action. Adapted from Kuritzky et al. [26], with permission from Taylor & Francis. QW, weekly; QD, daily; BID, twice a day; DPP-4, dipeptidyl peptidase 4; IgG4, immunoglobulin G4.
List of Clinical Trials Evaluating the Cardiovascular Efficacy and Safety of GLP-1 RAs
GLP-1 RA, glucagon-like peptide-1 receptor agonist; FDA, U.S. Food and Drug Administration; EMA, European Medicines Agency; CVD, cardiovascular disease; T2D, type 2 diabetes; BID, twice a day; QW, weekly; EXSCEL, exenatide study of cardiovascular event lowering trial; LEADER, liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results–a long-term evaluation; ELIXA, Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With Lixisenatide; REWIND, Researching Cardiovascular Events With a Weekly Incretin in Diabetes.
aClinicaltrials.gov identifier (https://www.clinicaltrials.gov).
Summary of Clinical Trials in Which the Cardiovascular Effects of GLP-1 RAs Were Non-Primary Outcomes
| Study | Duration, wk | No. of patients | Background+Comparator | Intervention | ΔBW, kg | ΔHR, bpm | ΔSystolicBP, mm Hg | ΔCVD risk markers, mmol/L (for lipid profiles) |
|---|---|---|---|---|---|---|---|---|
| Exenatide BID | ||||||||
| Blevins et al. (2011) [51] (DURATION-5) | 24 | 252 | (None, 1 or combination of Met, SU, TZD)+exenatide QW | Exenatide 10 µg BID | –1.4 | 2.1a (4.1a for exenatide QW) | –1.2 | 0.07 LDL-C |
| 0.03 HDL-C | ||||||||
| 0.01 TG | ||||||||
| Bunck et al. (2010) [48] | 52 | 69 | Met+insulin glargine | –3.9a | b | b | –61% hs-CRPa | |
| Buse et al. (2009) [54] (LEAD-6) | 26 | 464 | (Met±SU)+liraglutide | –2.9 | 0.69a (+3.28a for liraglutide) | –2.0 | –0.40 LDL-C | |
| –0.05 HDL-C | ||||||||
| –0.23 TGc | ||||||||
| –0.10 FFAc | ||||||||
| –0.03 g/L ApoB | ||||||||
| Davies et al. (2009) [55] | 26 | 235 | 2 or 3 OADs+insulin glargine | –2.73a | b | –2.9 | –0.25 LDL-Ca | |
| 0.01 HDL-C | ||||||||
| –0.33 TG | ||||||||
| DeFronzo et al. (2010) [56] | 20 | 137 | Met+TZD | –2.8a | b | b | –0.05 LDL-Ca | |
| 0.02 HDL-C | ||||||||
| –0.34 TGa | ||||||||
| Derosa et al. (2010) [49] | 52 | 128 | Met+SU | –8.0a | b | b | ↓hs-CRP (VNR)a | |
| Drucker et al. (2008) [57] (DURATION-1) | 30 | 295 | (Met, SU, TZD or any two combined)+exenatide QW | –3.6 | b | –3.4 | +0.03 LDL-Cc | |
| –0.03 HDL-C | ||||||||
| –11% TG | ||||||||
| Klonoff et al. (2008) [58] | 3 years | 217 | (Met±SU)+placebo | –5.3a | b | –3.5a | –6% LDL-Ca | |
| 24% HDL-Ca | ||||||||
| –12% TG-Ca | ||||||||
| Moretto et al. (2008) [59] | 24 | 232 | None+placebo | –3.1a | b | –3.7a | –0.07 LDL-C | |
| 0.02 HDL-C | ||||||||
| Exenatide QW | ||||||||
| Drucker et al. (2008) [57] (DURATION-1) | 30 | 295 | (Met, SU, TZD, or any two combined)+exenatide BID | Exenatide QW | –3.7 | b | –4.7 | –0.13 LDL-Ca |
| –0.02 HDL-C | ||||||||
| –15% TG | ||||||||
| Buse et al. (2010) [60] (DURATION-1 extension) | +22 (52 in total) | 295 | (Met, SU, TZD, or any two combined)+exenatide BID or QW from DURATION-1 studies | –4.1 (QW)–4.5 (BID→QW) | b | –6.2a (QW)–3.8 (BID→QW) | –9.6 mg/dL TC | |
| –0.7 mg/dL HDL-C | ||||||||
| –3.4 mg/dL LDL-C | ||||||||
| –15% TG | ||||||||
| MacConell et al. (2013) [61] (DURATION-1 extension) | 3 years | 295 | (Met, SU, TZD, or any two combined)+exenatide BID→exenatide QW | –2.3a | b | –2.1 | –0.26 TC | |
| –0.18 LDL-C | ||||||||
| 0.03 HDL-C | ||||||||
| –12% TG | ||||||||
| Bergenstal et al. (2010) [50] (DURATION-2) | 26 | 491 | Met+(Sit or TZD) | –2.3a | b | ↓(VNR)a | ↑HDL-Ca | |
| –5% TG | ||||||||
| ↓hs-CRPa | ||||||||
| ↓BNPa | ||||||||
| Diamant et al. (2010) [53] (DURATION-3) | 26 | 456 | (Met±SU)+insulin glargine | –2.6a | 4.0a | –3.0a | –0.05 LDL-C | |
| 0.0 HDL-C | ||||||||
| –2.0 mg/dL hs-CRPa | ||||||||
| Diamant et al. (2012) [52] (DURATION-3 extension) | 84 | 456 | (Met±SU)+insulin glargine | –2.1a | 1.97a | –4.2a | –0.12 TC | |
| 0.95 TG | ||||||||
| –2.05 mg/dL hs-CRP | ||||||||
| Diamant et al. (2014) [62] (DURATION-3 extension) | 3 years | 456 | (Met±SU)+insulin glargine | –2.49a | 2 | –2 | –0.13 TCa | |
| –0.17 LDL-Ca | ||||||||
| 0.05 HDL-Ca | ||||||||
| 1.02 TG | ||||||||
| –2.00 mg/dL CRPa | ||||||||
| Russell-Jones et al. (2012) [63] (DURATION-4) | 26 | 820 | None+(Met, TZD or Sit) | –2.0 | 1.5 | –1.3 | No change in fasting serum lipids (VNR) | |
| Blevins et al. (2011) [51] (DURATION-5) | 24 | 252 | (None, 1, or combination: Met, SU, TZD)+exenatide BID | –2.3a | 4.1a (2.1a for exenatide BID) | –2.9a | –0.17 LDL-Ca | |
| 0.0 HDL-C | ||||||||
| 0.01 TG | ||||||||
| Buse et al. (2013) [64] (DURATION-6) | 26 | 911 | (Met, SU, Met+SU, or Met+TZD)+liraglutide | –2.68 | b | –2.48 (–3.45 for liraglutide) | –0.06 TC | |
| 0.02 HDL-C | ||||||||
| –0.05 LDL-C | ||||||||
| –2.19 nmol/L hs-CRP | ||||||||
| –4.45 ng/L BNP | ||||||||
| Liraglutide | ||||||||
| Marre et al. (2009) [65] (LEAD-1) | 26 | 1,041 | SU+(TZD or TZD placebo) | 1.2 mg/day | +0.3a | 2–4a | –2.6–2.8 | b |
| 1.8 mg/day | –0.2a | 2–4a | –2.6–2.8 | b | ||||
| Nauck et al. (2009) [66] (LEAD-2) | 26 | 1,091 | Met+(SU or placebo) | 1.2 mg/day | –2.6a | 2–3a | –2–3a | b |
| 1.8 mg/day | –2.8a | 2–3a | –2–3a | b | ||||
| Garber et al. (2009) [67] (LEAD-3) | 52 | 746 | None+SU | 1.2 mg/day | –2.1a | 3.2a | –2.1 | b |
| 1.8 mg/day | –2.5a | 1.6 | –3.6a | b | ||||
| Garber et al. (2011) [68] (LEAD-3 extension) | 2 years | 746 | None+SU | 1.2 mg/day | –1.89a | 2.04 | –1.35 | b |
| 1.8 mg/day | –2.70a | 0.92 | –2.37 | b | ||||
| Zinman et al. (2009) [69] (LEAD-4) | 26 | 533 | (Met+TZD)+placebo | 1.2 mg/day | –1.0a | b | –6.7a | –0.21 TC |
| –0.28 LDL-Ca | ||||||||
| –0.03 HDL-C | ||||||||
| –0.38 TGa | ||||||||
| –0.03 FFAa | ||||||||
| 1.8 mg/day | –2.0a | b | –5.6a | –0.20 TC | ||||
| –0.23 LDL-C | ||||||||
| –0.04 HDL-C | ||||||||
| –0.32 TG | ||||||||
| –0.05 FFAa | ||||||||
| Russell-Jones et al. (2009) [70] (LEAD-5) | 26 | 581 | (Met+SU)+(insulin glargine or placebo) | 1.8 mg/day | –1.8a | b | –4.0a | b |
| Buse et al. (2009) [54] (LEAD-6) | 26 | 464 | (Met±SU)+exenatide | 1.8 mg/day | –3.2 | 3.28 (0.69 for exenatide BID) | –2.5 | –0.44 LDL-C |
| –0.04 HDL-C | ||||||||
| –0.41 TGa | ||||||||
| –0.17 FFAa | ||||||||
| –0.06 g/L ApoB | ||||||||
| Buse et al. (2010) [71] (LEAD-6 extension) | +14 (40 in total) | 376 | (Met±SU)+exenatideBID→liraglutide | 1.8 mg/day | –0.9a | b | –3.8a | b |
| Pratley et al. (2010) [72] | 26 | 658 | Met+Sit | 1.2 mg/day | –2.86a | 2.32 | –0.55 | –0.08 LDL-C |
| 0.00 HDL-C | ||||||||
| –0.19 TG | ||||||||
| –0.03 FFA | ||||||||
| –0.06 g/L ApoB | ||||||||
| 1.8 mg/day | –3.38a | 3.94 | –0.72 | –0.05 LDL-C | ||||
| 0.00 HDL-C | ||||||||
| –0.43 TG | ||||||||
| –0.07 FFA | ||||||||
| –0.07 g/L ApoB | ||||||||
| Pratley et al. (2011) [73] extension | +26 (52 in total) | 436 | Met+Sit | 1.2 mg/day | –2.78a | 1.72 | –0.37 | 0.09 LDL-C |
| –0.10 TG | ||||||||
| –0.07 FFA | ||||||||
| –0.03 g/L ApoB | ||||||||
| 1.8 mg/day | –3.68a | 3.09 | –2.55 | 0.09 LDL-C | ||||
| –0.32 TG | ||||||||
| –0.10 FFA | ||||||||
| –0.03 g/L ApoB | ||||||||
| Lixisenatide | ||||||||
| Rosenstock et al. (2013) [74] (GetGoal-X) | 24 | 634 | Met+exenatide BID | 20 µg QD | –2.8 | 0.1 | –2.9 | b |
| Rosenstock et al. (2014) [75] (GetGoal-S) | 24 | 859 | (SU±Met)+placebo | 20 µg QD | –1.76a | –0.1 | Slight decrease (VNR) | No relevant change in lipid levels (VNR) |
| Albiglutide | ||||||||
| Nauck et al. (2016) [76] (HARMONY 2) | 52 | 309 | None+placebo | 30 mg QW | –0.39 | 2.5 | –2.8 | –0.312 TC |
| –0.278 LDL-C | ||||||||
| 0.051 HDL-C | ||||||||
| –0.379 TG | ||||||||
| –0.038 FFA | ||||||||
| 50 mg QW | –0.86 | 0.8 | –1.3 | –0.101 TC | ||||
| –0.130 LDL-C | ||||||||
| 0.042 HDL-C | ||||||||
| 0.014 TG | ||||||||
| –0.007 FFA | ||||||||
| Ahren et al. (2014) [77] (HARMONY 3) | 104 | 1,049 | Met+(Sit, SU or placebo) | 30 mg QW | –1.21a | 1.3 | –1.0 | –0.07 TC |
| –0.03 LDL-C | ||||||||
| 0.04 HDL-C | ||||||||
| –0.20 TG | ||||||||
| Weissman et al. (2014) [78] (HARMONY 4) | 52 | 779 | (Met±SU)+insulin glargine | 30 mg QW | –1.06 | 1.0 | –1.4 | –2.1 mg/dL TC |
| –1.8 mg/dL LDL-C | ||||||||
| 2.1 mg/dL HDL-C | ||||||||
| –17.3 mg/dL TG | ||||||||
| Pratley et al. (2014) [79] (HARMONY 7) | 32 | 841 | (Met, TZD, SU, or any combination)+liraglutide 1.8 mg QD | 30 mg→ 50 mg QW at week 6 | –0.64 | 5.7 at week 4 (max) | <±1.0 | Investigator-assessed cardiovascular AE: 8.2% (forliraglutide: 10.5%) |
| Returned to baseline within study period | ||||||||
| Dulaglutide | ||||||||
| Wysham et al. (2014) [80] (AWARD-1) | 52 | 976 | (Met±TZD)+(exenatide BID or placebo→dulaglutide at week 26) | 0.75 mg/day | –0.20a | 1.56 (2.80a at week 26) | 1.62 (–0.36a at week 26) | –0.09 TC |
| –0.08 LDL-C | ||||||||
| 0.00 HDL-C | ||||||||
| 0.04 TG | ||||||||
| 1.5 mg/day | –1.30a | 1.68 (2.80a at week 26) | 0.83 (0.11a at week 26) | –0.12 TC | ||||
| –0.06 LDL-C | ||||||||
| 0.05 HDL-C | ||||||||
| –0.26 TGa | ||||||||
| Giorgino et al. (2015) [81] (AWARD-2) | 78 | 810 | (Met±SU)+insulin glargine | 0.75 mg/day | –1.33a | 0.61a | –0.59 | 0.03 TC |
| –0.02 LDL-C | ||||||||
| –0.02 HDL-C | ||||||||
| 0.03 TG | ||||||||
| 1.5 mg/day | –1.87a | 1.31a | –0.70 | 0.02 TC | ||||
| 0.00 LDL-C | ||||||||
| 0.00 HDL-C | ||||||||
| 0.05 TG | ||||||||
| Umpierrez et al. (2014) [82] (AWARD-3) | 52 | 807 | None+(Met+placebo) | 0.75 mg/day+oral placebo | –1.36 at week 26 (VNR for week 52) | 1.6 | –2.7 | –1% TCa |
| –2% LDL-Ca | ||||||||
| 2% HDL-C | ||||||||
| –1% TG | ||||||||
| 1.5 mg/day+oral placebo | –2.29 at week 26 (VNR for week 52) | 1.8 | –0.1 | –2% TC | ||||
| –2% LDL-Ca | ||||||||
| 5% HDL-C | ||||||||
| –4% TG | ||||||||
| Blonde et al. (2015 )[83] (AWARD-4) | 52 | 884 | Insulin±OADs→(insulin lispro+Met)+insulin glargine | 0.75 mg/day | +0.18 at week 26 (VNR for week 52) | 2.27 | 1.04 | 0.94% TC |
| –1.02% LDL-C | ||||||||
| 3.54% HDL-Ca | ||||||||
| 5.73% TG | ||||||||
| 1.5 mg/day | –0.87 at week 26 (VNR for week 52) | 2.38a | –0.26 | 0.00% TC | ||||
| –1.95% LDL-C | ||||||||
| 2.44% HDL-Ca | ||||||||
| 2.96% TG | ||||||||
| Nauck et al. (2014) [84] (AWARD-5) | 52 | 1,098 | (None, 1, or combination of OAD)+Sit or placebo (placebo→Sit at week 26) | 0.75 mg/day | –2.60a | 2.1a | –0.5 | –0.03 TC |
| 0.02 LDL-C | ||||||||
| 0.07 HDL-C | ||||||||
| –0.15 TG | ||||||||
| 1.5 mg/day | –3.03a | 2.4a | –0.8 | –0.03 TC | ||||
| –0.06 LDL-Ca | ||||||||
| 0.05 HDL-C | ||||||||
| –0.16 TG | ||||||||
| Weinstock et al. (2015) [85] (AWARD-5 extension) | 2 years | 1,098 | (None, 1, or combination of OAD)+Sit or placebo (placebo→Sit at week 26) | 0.75 mg/day | –2.39 | 2.8a | 1.3 | No significant changes in lipids (VNR) |
| 1.5 mg/day | –2.88a | 2.3a | –0.1 | |||||
| Dungan et al. (2014) [86] (AWARD-6) | 26 | 599 | Met+liraglutide | 1.5 mg/day | –2.90 | 2.37 | –3.36 | b |
GLP-1 RA, glucagon-like peptide-1 receptor agonist; BW, body weight; HR, heart rate; BP, blood pressure; CVD, cardiovascular disease; BID, twice a day; DURATION, Diabetes therapy Utilization: Researching changes in A1c, weight and other factors Through Intervention with exenatide Once weekly; Met, metformin; SU, sulfonylurea; TZD, thiazolidinediones; QW, weekly; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride; hs-CRP, high-sensitivity C-reactive protein; LEAD, liraglutide effects and action in diabetes; FFA, free fatty acids; ApoB, apolipoprotein B; OAD, oral antidiabetic drug; Sit, sitagliptin; VNR, value not reported; BNP, B-type natriuretic peptide; QD, daily; AE, adverse event; AWARD, Assessment of Weekly Administration of LY2189265 in Diabetes-1.
aStatistically significant from comparator; bNot determined or not checked; cPerformed significantly more poorly than comparator.
Figure & Data
References
Citations

- Cardioprotective Actions of a Glucagon‐like Peptide‐1 Receptor Agonist on Hearts Donated After Circulatory Death
Sachiko Kadowaki, M. Ahsan Siraj, Weiden Chen, Jian Wang, Marlee Parker, Anita Nagy, Chun‐Po Steve Fan, Kyle Runeckles, Jing Li, Junko Kobayashi, Christoph Haller, Mansoor Husain, Osami Honjo
Journal of the American Heart Association.2023;[Epub] CrossRef - The role of dipeptidyl peptidase-IV in abdominal aortic aneurysm pathogenesis: A systematic review
Elisha Ngetich, Pierfrancesco Lapolla, Anirudh Chandrashekar, Ashok Handa, Regent Lee
Vascular Medicine.2022; 27(1): 77. CrossRef - Glucagon-like Peptide-1 Receptor Agonists in the Management of Type 2 Diabetes Mellitus and Obesity: The Impact of Pharmacological Properties and Genetic Factors
Jasna Klen, Vita Dolžan
International Journal of Molecular Sciences.2022; 23(7): 3451. CrossRef - Glucagon-like peptide-1 (GLP-1) receptor agonists and cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of double-blind, randomized, placebo-controlled clinical trials
Jing Qin, Li Song
BMC Endocrine Disorders.2022;[Epub] CrossRef - Role of G-protein coupled receptor (GPCRs)/(GPR-120) as an agonists in diabetic wound healing
Jagat Pal Yadav, Dinesh Kumar Patel, Prateek Pathak, Maria Grishina
Obesity Medicine.2022; 36: 100466. CrossRef - Protection against stroke with glucagon-like peptide-1 receptor agonists: a comprehensive review of potential mechanisms
Bruno Vergès, Victor Aboyans, Denis Angoulvant, Pierre Boutouyrie, Bertrand Cariou, Fabien Hyafil, Kamel Mohammedi, Pierre Amarenco
Cardiovascular Diabetology.2022;[Epub] CrossRef - Changing Fields-Diabetes Medications Invading the Cardiovascular Space
Lauren D. Breite, Mackenzie Steck, Brandon Tate Cutshall, Samarth P. Shah, Brandon E. Cave
Current Problems in Cardiology.2021; 46(3): 100736. CrossRef - PEGDA/HA mineralized hydrogel loaded with Exendin4 promotes bone regeneration in rat models with bone defects by inducing osteogenesis
Wei Liu, Xiaowei Jing, Zhiwen Xu, Chong Teng
Journal of Biomaterials Applications.2021; 35(10): 1337. CrossRef - Metabolite G-Protein Coupled Receptors in Cardio-Metabolic Diseases
Derek Strassheim, Timothy Sullivan, David C. Irwin, Evgenia Gerasimovskaya, Tim Lahm, Dwight J. Klemm, Edward C. Dempsey, Kurt R. Stenmark, Vijaya Karoor
Cells.2021; 10(12): 3347. CrossRef - PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice
Marie K. Holt, Daniel R. Cook, Daniel I. Brierley, James E. Richards, Frank Reimann, Alexander V. Gourine, Nephtali Marina, Stefan Trapp
Molecular Metabolism.2020; 39: 101024. CrossRef - A glycosylated Fc‐fused glucagon‐like peptide‐1 receptor agonist exhibits equivalent glucose lowering to but fewer gastrointestinal side effects than dulaglutide
In Bok An, Mi Sun Byun, Sang In Yang, Yuri Choi, Jung Won Woo, Hak Chul Jang, Young Chul Sung
Diabetes, Obesity and Metabolism.2020; 22(8): 1455. CrossRef - Glucagon-Like Peptide-1 Receptor Agonists in Adult Patients With Type 2 Diabetes: Review of Cardiovascular Outcome Trials
Elodie M. Varin, Brent A. McLean, Julie A. Lovshin
Canadian Journal of Diabetes.2020; 44(1): 68. CrossRef - Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors
Alexandros Sachinidis, Dragana Nikolic, Anca Pantea Stoian, Nikolaos Papanas, Omer Tarar, Ali A. Rizvi, Manfredi Rizzo
Metabolism.2020; 111: 154343. CrossRef - GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction
Lili Zhang, Jiali Tian, Sujuan Diao, Guowei Zhang, Mochao Xiao, Dong Chang
Chemico-Biological Interactions.2020; 332: 109252. CrossRef - Predictors of Effectiveness of Glucagon-Like Peptide-1 Receptor Agonist Therapy in Patients with Type 2 Diabetes and Obesity
Alina Yu. Babenko, Daria A. Savitskaya, Yulia A. Kononova, Aleksandra Yu. Trofimova, Anna V. Simanenkova, Elena Yu. Vasilyeva, Evgeny V. Shlyakhto
Journal of Diabetes Research.2019; 2019: 1. CrossRef - Predictors of effectiveness of glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes and obesity
Ekaterina V. Tikhonenko, Alina Y. Babenko, Evgeny V. Shlyakhto
Obesity and metabolism.2019; 15(4): 22. CrossRef - Asian Subpopulations May Exhibit Greater Cardiovascular Benefit from Long-Acting Glucagon-Like Peptide 1 Receptor Agonists: A Meta-Analysis of Cardiovascular Outcome Trials
Yu Mi Kang, Yun Kyung Cho, Jiwoo Lee, Seung Eun Lee, Woo Je Lee, Joong-Yeol Park, Ye-Jee Kim, Chang Hee Jung, Michael A. Nauck
Diabetes & Metabolism Journal.2019; 43(4): 410. CrossRef - Diabetes, Incretin Therapy and Thoracic Aortic Aneurysm – What Does the Evidence Show?
Camilla Krizhanovskii , Anders Franco-Cereceda
Current Vascular Pharmacology.2019; 17(5): 432. CrossRef - Cardiovascular Effects of Different GLP-1 Receptor Agonists in Patients with Type 2 Diabetes
Gül Bahtiyar, Jean Pujals-Kury, Alan Sacerdote
Current Diabetes Reports.2018;[Epub] CrossRef - Efficacy From Strange Sources
Lawrence J. Lesko
Clinical Pharmacology & Therapeutics.2018; 103(2): 253. CrossRef - Exogenous SERP1 attenuates restenosis by restoring GLP-1 receptor activity in diabetic rats following vascular injury
Lishuai Feng, Jianbo Wang, Xu Ma
Biomedicine & Pharmacotherapy.2018; 103: 290. CrossRef - Exenatide exhibits anti‐inflammatory properties and modulates endothelial response to tumor necrosis factor α‐mediated activation
Wojciech Garczorz, Enrique Gallego‐Colon, Agnieszka Kosowska, Agnieszka Kłych‐Ratuszny, Michał Woźniak, Wiesław Marcol, K.J. Niesner, Tomasz Francuz
Cardiovascular Therapeutics.2018;[Epub] CrossRef - Molecular and clinical roles of incretin-based drugs in patients with heart failure
Bassant Orabi, Rasha Kaddoura, Amr S. Omar, Cornelia Carr, Abdulaziz Alkhulaifi
Heart Failure Reviews.2018; 23(3): 363. CrossRef - The effects of Exendin-4 on bone marrow-derived mesenchymal cells
Paola Luciani, Benedetta Fibbi, Benedetta Mazzanti, Cristiana Deledda, Lara Ballerini, Alessandra Aldinucci, Susanna Benvenuti, Riccardo Saccardi, Alessandro Peri
Endocrine.2018; 60(3): 423. CrossRef - Real-world clinical experience of Xultophy in the management of patients with type 2 diabetes in a secondary care clinic
David M. Williams, Natasha Shrikrishnapalasuriyar, Waheeba Syed, Win L. Yin, Richard Chudleigh, Stephen C. Bain, David E. Price, Jeffrey W. Stephens
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2018; 12(6): 1079. CrossRef - Pharmacological Applications of Bile Acids and Their Derivatives in the Treatment of Metabolic Syndrome
Maja Ðanić, Bojan Stanimirov, Nebojša Pavlović, Svetlana Goločorbin-Kon, Hani Al-Salami, Karmen Stankov, Momir Mikov
Frontiers in Pharmacology.2018;[Epub] CrossRef - Cardiovascular Outcome Trials of Diabetes and Obesity Drugs: Implications for Conditional Approval and Early Phase Clinical Development
Andrew J. Krentz, Gerardo Rodriguez-Araujo
Pharmaceutical Medicine.2017; 31(6): 399. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - GLP-1R as a Target for the Treatment of Diabetic Retinopathy: Friend or Foe?
Rafael Simó, Cristina Hernández
Diabetes.2017; 66(6): 1453. CrossRef - GLP-1 receptor agonists and heart failure in diabetes
André J. Scheen
Diabetes & Metabolism.2017; 43: 2S13. CrossRef - Effects of Incretin-Based Therapies on Diabetic Microvascular Complications
Yu Mi Kang, Chang Hee Jung
Endocrinology and Metabolism.2017; 32(3): 316. CrossRef - Historique des études cardiovasculaires : de l’UGDP… aux dernières études
A.-J. Scheen
Médecine des Maladies Métaboliques.2017; 11: 2S15. CrossRef - Cardiovascular safety and benefits of GLP-1 receptor agonists
Niels B. Dalsgaard, Andreas Brønden, Tina Vilsbøll, Filip K. Knop
Expert Opinion on Drug Safety.2017; 16(3): 351. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite