Articles
- Page Path
- HOME > Endocrinol Metab > Volume 30(3); 2015 > Article
-
Original ArticleClinical Study Efficacy of a Once-Monthly Pill Containing Ibandronate and Cholecalciferol on the Levels of 25-Hydroxyvitamin D and Bone Markers in Postmenopausal Women with Osteoporosis
- In-Jin Cho1, Ho-Yeon Chung1, Sung-Woon Kim1, Jae-Won Lee2, Tae-Won Lee2, Hye-Soon Kim3, Sin-Gon Kim4, Han Seok Choi5, Sung-Hee Choi6, Chan Soo Shin6, Ki-Won Oh7, Yong-Ki Min7, Jung-Min Koh8, Yumie Rhee9, Dong-Won Byun10, Yoon-Sok Chung11, Jeong Hyun Park12, Dong Jin Chung13, Minho Shong14, Eun-Gyoung Hong15, Chang Beom Lee16, Ki Hyun Baek17, Moo-Il Kang17
-
Endocrinology and Metabolism 2014;30(3):272-279.
DOI: https://doi.org/10.3803/EnM.2015.30.3.272
Published online: December 9, 2014
1Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, Korea.
2Dreampharma Corp., Seoul, Korea.
3Department of Endocrinology and Metabolism, Keimyung University School of Medicine, Daegu, Korea.
4Department of Endocrinology and Metabolism, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
5Division of Endocrinology and Metabolism, Department of Internal Medicine, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea.
6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
7Division of Endocrinology and Metabolism, Department of Internal Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea.
8Divison of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
9Department of Internal Medicine, Endocrine Research Institute, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
10Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea.
11Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea.
12Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
13Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
14Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
15Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea.
16Department of Endocrinology and Metabolism, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea.
17Department of Endocrinology and Metabolism, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- Corresponding author: Moo-Il Kang. Department of Endocrinology and Metabolism, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, 222 Banpo-daero, Seocho-gu, Seoul 06591, Korea. Tel: +82-2-2258-6006, Fax: +82-2-599-3589, mikang@catholic.ac.kr
Copyright © 2015 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- The present study evaluated the efficacy of a combination of ibandronate and cholecalciferol on the restoration of the levels of 25-hydroxyvitamin D (25[OH]D) and various bone markers in postmenopausal women with osteoporosis.
-
Methods
- This was a randomized, double-blind, active-controlled, prospective 16-week clinical trial conducted in 20 different hospitals. A total of 201 postmenopausal women with osteoporosis were assigned randomly to one of two groups: the IBN group, which received a once-monthly pill containing 150 mg ibandronate (n=99), or the IBN+ group, which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol (n=102). Serum levels of 25(OH)D, parathyroid hormone (PTH), and various bone markers were assessed at baseline and at the end of a 16-week treatment period.
-
Results
- After 16 weeks of treatment, the mean serum levels of 25(OH)D significantly increased from 21.0 to 25.3 ng/mL in the IBN+ group but significantly decreased from 20.6 to 17.4 ng/mL in the IBN group. Additionally, both groups exhibited significant increases in mean serum levels of PTH but significant decreases in serum levels of bone-specific alkaline phosphatase and C-telopeptide of type 1 collagen (CTX) at 16 weeks; no significant differences were observed between the groups. However, in subjects with a vitamin D deficiency, IBN+ treatment resulted in a significant decrease in serum CTX levels compared with IBN treatment.
-
Conclusion
- The present findings demonstrate that a once-monthly pill containing ibandronate and cholecalciferol may be useful for the amelioration of vitamin D deficiency in patients with postmenopausal osteoporosis. Moreover, this treatment combination effectively decreased serum levels of resorption markers, especially in subjects with a vitamin D deficiency, over the 16-week treatment period.
- Although bisphosphonates are potent antiresorptive drugs and the most widely prescribed pharmacotherapy for the treatment of osteoporosis, the persistence and compliance rates of patients taking bisphosphonates are suboptimal [1]. This is likely because the use of bisphosphonates require the patient to fast overnight, take the pill with a full glass of water, and sit or stand upright for 30 minutes prior to having their first meal or drink of the day. Moreover, there is a high prevalence of osteoporosis in the elderly population, and these patients are more likely to have coexisting cognitive dysfunctions or take multiple drugs to combat other diseases [2]. Therefore, the poor adherence rates for bisphosphonates are likely due to the complexities and side effects of these medications, which include gastrointestinal problems. It is possible that a reduction in the dosing frequency of oral bisphosphonates may improve patient compliance and persistence.
- An adequate intake of vitamin D is crucial for the achievements of optimal calcium metabolism, bone mineralization, and skeletal health in patients with osteoporosis. A vitamin D insufficiency increases serum levels of parathyroid hormone (PTH), activates osteoclasts, and reduces bone mineralization, which in turn lead to aggravation of osteoporosis or the development of osteomalacia [3]. Vitamin D deficiencies are a common affliction among postmenopausal women worldwide but especially among the Korean population, which has the lowest mean serum 25-hydroxyvitamin D (25[OH]D) levels in the world [4]. To improve vitamin D status, a combination of calcium and vitamin D supplements is often prescribed, but many patients do not tolerate this mixture very well [5], and calcium-related adverse effects, such as gastrointestinal irritation, flatulence, and constipation, are often experienced.
- Therefore, two strategies have been developed to overcome the low compliance rates for bisphosphonates and the high rates of vitamin D deficiency: (1) less frequent dosing intervals for bisphosphonates and (2) the administration of bisphosphonate and vitamin D in a combined form. The present study is the first clinical trial of a single, once-monthly pill that contains ibandronate (150 mg) and cholecalciferol (24,000 IU). This study was designed to evaluate the efficacy of this combination pill on the restoration of 25(OH)D and bone marker levels in postmenopausal women with osteoporosis. Additionally, the present study assessed the safety of this medication.
INTRODUCTION
- Study design
- The present study was a multicenter, randomized, double-blind, active-controlled, prospective 16-week clinical trial conducted between December 2011 and September 2012 at 20 different hospitals in Korea. All subjects were postmenopausal females 40 years of age or older. Patients qualified for inclusion if they had a bone mineral density (BMD) T-score of -2.5 or lower for the mean lumbar spine, femoral neck, or total; or a T-score of -1.0 or lower with an osteoporotic fracture of at least one vertebra. At least 3 months prior to screening, all BMD measurements were obtained using dual energy X-ray absorptiometry.
- Menopause was defined based on the presence of at least one of the following criteria: (1) the spontaneous discontinuation of menstruation for at least 12 months; (2) having a serum follicle-stimulating hormone level above 40 IU/L with a history of hysterectomy or with no menstruation for 6 to 12 months; or (3) the absence of menstruation for a duration longer than 6 weeks after a bilateral oophorectomy. Patients with abnormal thyroid function, a history of malignancy, uncontrolled hypercalcemia, or hypocalcemia, chronic diseases known to affect bone metabolism (such as chronic liver disease, chronic alcoholism, and primary hyperparathyroidism), contraindications to oral bisphosphonates (such as esophageal strictures, gastrointestinal malabsorption, and renal insufficiency), and/or 25(OH)D levels less than 9 ng/mL were excluded from the study. Additionally, those patients taking agents known to affect bone or calcium metabolism, with metabolic bone diseases other than osteoporosis, taking more than 200 IU vitamin D per day in the 3 months prior to screening, and who had taken bisphosphonates with an insufficient washout period were also excluded from the study. Inappropriate washout periods were defined as follows: having been administered intravenous bisphosphonates within the 2 years before screening, a period of less than 2 months for patients who had taken oral bisphosphonates for less than 2 weeks, a period less than 6 months for patients who had taken oral bisphosphonates for more than 2 weeks but less than 8 weeks, a period less than 1 year for people who had taken oral bisphosphonates for more than 8 weeks but less than 48 weeks, and a period less than 2 years for people who had taken oral bisphosphonates for more than 48 weeks. Institutional Review Board approval was obtained from all participating hospitals, and all participants provided written informed consent.
- All patients were assigned randomly to one of two groups: the IBN group, which received a once-monthly pill containing 150 mg ibandronate (n=99), or the IBN+ group, which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol (n=102). All patients were instructed to take their assigned pill in the morning after an overnight fast, to stay upright for at least 1 hour after taking the pill, and not to ingest any food, beverages (except water), or other medications for at least 1 hour. All patients were instructed to take one pill every 4 weeks over a 16-week period (four doses at 0, 4, 8, and 12 weeks from the first dose) and received a daily supplement of 500 mg elemental calcium. The patients were not allowed to take any additional vitamin D supplements, and all were provided with a topical sunblock cream to avoid exposure to sunlight. Additionally, the patients were provided with acetaminophen to treat any influenza-like illnesses or pain that may have occurred with the treatment, based on need.
- Evaluations of efficacy and safety
- The primary endpoint in the present study was defined as the change in 25(OH)D levels from baseline to 16 weeks of treatment. The levels of 25(OH)D were measured using a radioimmunoassay (DIAsource ImmunoAssays, Louvain-la-Neuve, Belgium) with an intra-assay coefficient of variation (CV) of 7.3% to 8.8% and an interassay CV of 7.2% to 7.3%. The secondary endpoints were defined as parameters of efficacy, including changes in PTH levels as measured by an electrochemiluminescence immunoassay (Roche Ltd., Mannheim, Germany; intra-assay CV of 1.1% to 2.0% and interassay CV of 2.5% to 3.4%), changes in bone-specific alkaline phosphatase (BSAP) levels as measured by an enzyme immunoassay (QUIDEL Corp., San Diego, CA, USA; intra-assay CV of 3.9% to 5.8% and interassay CV of 5.0% to 7.6%), changes in C-telopeptide of type 1 collagen (CTX) levels as measured by an electrochemiluminescence immunoassay (Roche Ltd.; intra-assay CV of 1.0% to 5.5% and interassay CV of 2.7% to 7.6%), and changes in serum calcium and phosphorus levels at 16 weeks of treatment.
- The IBN and IBN+ groups were each classified into three subgroups according to baseline 25(OH)D levels: (1) vitamin D-sufficient (baseline 25(OH)D ≥20 ng/mL); (2) moderately vitamin D-deficient (baseline 25(OH)D ≥10 and <20 ng/mL); and (3) vitamin D-deficient (baseline 25(OH)D <10 ng/mL). And we also compared changes in 25(OH)D levels among three subgroups. Additionally, the laboratory tests performed for each patient included a complete blood cell count, a liver function test, and electrolyte measurements. The laboratory tests, primary endpoint, included 25(OH)D levels, and secondary endpoints were measured at baseline, 8, and 16 weeks of the treatment period.
- The safety of the treatments was monitored using patient reports of all adverse clinical events and laboratory experiences and the use of any non-study medications during the treatment period. Adverse events could be reported in person at the 8- and 16-week visits or by phone at any time throughout the study.
- Statistical analysis
- All analyses were performed on an intention-to-treat basis. The efficacy analyses were conducted using only patients who took at least one dose of the study medication and for whom at least one efficacy parameter was measured after randomization. To compare differences between the treatment groups, either a t test or the Wilcoxon rank sum test and the chi-square test or the Fisher exact test were used, depending on the distribution of the data. Likewise, either a paired t test or the Wilcoxon signed-rank test was used to compare pretreatment and posttreatment data within a group based on the distribution. Safety analyses were conducted for all patients who took at least one dose of the study medication. A P<0.05 was considered to be statistically significant.
METHODS
- Baseline characteristics of the patients
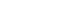
- A total of 201 patients were enrolled in the present study, of whom 102 were assigned to the IBN+ group and 99 to the IBN group. The dispositions of the participants are detailed in Fig. 1. All participants were female, and no significant differences were observed in the baseline characteristics between the two groups (Table 1). At baseline, the percentages of patients with serum 25(OH)D levels <20 ng/mL were 53.1% in the IBN+ group and 60.0% in the IBN group.
- Efficacies of the treatments
- In the IBN+ group, the mean serum level of 25(OH)D exhibited a significant increase after 16 weeks of treatment (baseline, 21.0±10.4 ng/mL; 8 weeks, 24.8±9.00 ng/mL; and 16 weeks, 25.3±9.9 ng/mL; P<0.05), while that in the IBN group significantly decreased (baseline, 20.6±9.3 ng/mL; 8 weeks, 18.3±7.4 ng/mL; and 16 weeks, 17.4±9.2 ng/mL; P<0.001) (Fig. 2). The changes in the distribution of patients according to 25(OH)D levels after 16 weeks of treatment are shown in Fig. 3. The proportion of patients with a 25(OH)D level >20 ng/mL after 16 weeks of treatment increased from 46.9% to 63.5% in the IBN+ group but decreased from 40.0% to 26.3% in the IBN group. Moreover, after 16 weeks of treatment, no patients in the IBN+ group exhibited a 25(OH)D level <10 ng/mL while 4.2% of patients in the IBN group did.
- Compared with baseline, the CTX and BSAP levels exhibited a significant decrease after 16 weeks of treatment in both groups but with no significant differences between the two groups. The level of PTH significantly increased in both groups after 16 weeks of treatment, but no significant differences were observed between the two groups. Additionally, there were significant decreases in the serum calcium and phosphorous levels in both groups after 16 weeks of treatment but with no significant between-group differences (Table 2).
- The IBN+ and IBN groups were each divided into three subgroups based on baseline 25(OH)D levels, and the changes in these levels after 16 weeks of treatment from baseline were assessed for each subgroup. Within the IBN+ group, there were significant increases in 25(OH)D levels in the vitamin D-deficient and moderately vitamin D-deficient subgroups, but no changes were observed in the vitamin D-sufficient subgroup. Within the IBN group, there were no significant changes in 25(OH)D levels in the vitamin D-deficient or moderately vitamin D-deficient subgroups, but there was a significant decrease in the vitamin D-sufficient subgroup (Table 3). Additionally, after 16 weeks of treatment, there was a significant decrease in the CTX levels of the vitamin D-deficient subgroup of IBN+ but not in vitamin D-deficient IBN patients (Table 3).
- Safety
- The overall incidence rates of adverse events (48.7% in the IBN+ group and 51.3% in the IBN group) did not differ significantly between the groups. Generalized pain and headaches were the most frequently reported adverse effects for both groups (19.8% and 16.8% in the IBN+ group vs. 21.4% and 16.3% in the IBN group, respectively) and gastrointestinal discomfort occurred in 14.9% of IBN+ patients and 14.3% of IBN patients. No serious drug-related adverse events or fatal outcomes occurred during the study, and no laboratory-related adverse events, such as hypercalcemia or hyperphosphatemia, were reported.
RESULTS
- In the present study, female patients with postmenopausal osteoporosis who took a single once-monthly pill containing both ibandronate (150 mg) and cholecalciferol (24,000 IU) exhibited a significant increase in serum 25(OH)D levels after 16 weeks of treatment. Moreover, there was a significant increase in the proportion of patients with sufficient vitamin D levels (≥20 ng/mL), and none of the patients exhibited a vitamin D deficiency (<10 ng/mL) after 16 weeks of treatment.
- The nature of optimal vitamin D status remains controversial. The Endocrine Society defines the lower limit of the normal range as a 25(OH)D concentration of 30 ng/mL [6], while the Institute of Medicine considers 20 ng/mL as the lower limit of the normal range [7]. In Korea, widespread vitamin D deficiency exists, even when the more liberal definition of the lower limit of the normal range (20 ng/mL) is applied [48]. The high prevalence of vitamin D deficiency in Korea may be due to behavioral factors, such as an indoor lifestyle, the use of sunscreen, and the low consumption of vitamin D-fortified foods [9]. In fact, in the present study, the IBN group exhibited a decrease in mean serum 25(OH)D levels, which may be due to the study requirements of direct sunlight avoidance and sunscreen use and the restrictions regarding use of other vitamin D supplements. Therefore, supplementation with an adequate amount of vitamin D is essential for the optimal treatment of osteoporosis.
- Several expert societies, including the Korean Society for Bone and Mineral Research, suggest that adults 50 years of age and older should supplement with 800 to 1,000 IU of vitamin D per day [61011]. Based on this recommendation, a majority of studies have investigated the efficacies of regimens using daily combination pills containing 800 to 1,000 IU vitamin D and a bisphosphonate for the treatment of osteoporosis. For example, a once-weekly pill containing risedronate (35 mg) and cholecalciferol (5,600 IU) increased the mean serum 25(OH)D levels of subjects by 12.3 ng/mL after a 16-week treatment course [9]. In the present study, a single once-monthly pill containing ibandronate (150 mg) and cholecalciferol (24,000 IU) increased the mean serum 25(OH)D levels of patients by 4.2 ng/mL after a 16-week treatment period. Taken together, these results demonstrate that a weekly dose of 5,600 IU cholecalciferol caused a greater increase in mean serum 25(OH)D levels than did a monthly dose of 24,000 IU cholecalciferol, even though both drugs provide an equivalent 800 IU dose of vitamin D per day. Shorter dosing intervals are more effective in raising the serum concentrations of 25(OH)D than are longer intervals [12], but this finding is difficult to interpret because this particular study did not directly compare such treatment groups.
- In the present study, the increase of serum 25(OH)D levels was most striking in patients who had low serum vitamin D levels prior to treatment (baseline 25(OH)D, <20 ng/mL). On the other hand, the serum 25(OH)D levels of patients with sufficient levels at baseline (25[OH]D, ≥20 ng/mL) did not increase. This suggests a low risk of vitamin D intoxication when taking a monthly treatment regimen of 24,000 IU cholecalciferol. In fact, vitamin D doses of up to 4,000 IU per day and 50,000 IU per week have been administered to patients without any adverse toxic effects [1314]. Similarly, there were no side effects associated with excessive vitamin D supplementation, such as hypercalcemia or hyperphosphatemia, observed in the present study.
- Bisphosphonate therapy increases the PTH levels of osteoporosis patients via a subclinical decrease in serum calcium levels caused by an inhibition in bone resorption [15]. The present study found that, although there were significant increases in the PTH levels in both treatment groups (but no significant between-group differences), this change was smaller in the IBN+ group than the IBN group. It is possible that treatment with a bisphosphonate in combination with vitamin D increases intestinal calcium absorption, which in turn, results in a slight decrease in PTH levels.
- Although changes in BMD were not measured in the present study, there were significant decreases in the levels of representative bone turnover markers in both treatment groups after 16 weeks of treatment. An earlier decrease in bone turnover markers is related to long-term increases in BMD in patients treated with bisphosphonates [16]. The present study also found a significant decrease in the CTX levels of IBN+ patients with a vitamin D deficiency, but not in vitamin D-deficient IBN patients. Thus, ibandronate and cholecalciferol combination therapy reduced CTX levels in subjects with a vitamin D deficiency more effectively than did ibandronate-only therapy. Corrections in vitamin D status are an important component in the treatment of osteoporosis patients, and vitamin D supplementation is an easily modifiable treatment modality that can drive a favorable bisphosphonate response [17].
- The present study had several limitations. First, 25(OH)D levels in the present study may have been affected by seasonal variations, in that they tend to be low in late winter or spring and to peak in autumn [18], as a result of seasonal variations in the amount of sunlight; the present study was conducted from winter to autumn. However, all patients in the present study were provided with a topical sunblock cream to minimize the effects of sunlight exposure. Additionally, there were no differences in the season when patients were enrolled in the both treatments. Second, the duration of the treatment period (16 weeks) was too short to properly assess changes in BMD and the related risk of fracture. Future studies evaluating the long-term efficacy and safety of potential treatments for osteoporosis are crucial for the identification and characterization of novel approaches. Third, the present study did not address concerns regarding patient compliance with the treatment regimen. There has been a recent increase in concern regarding the cardiovascular risks associated with calcium supplementation regimens [19]. The American Society for Bone and Mineral Research recommend that calcium-containing foods are the best source of calcium intake and that supplementation should occur only in cases of deficient dietary calcium intake [20]. Therefore, if a patient is using food as their primary source of calcium intake while taking a bisphosphonate-vitamin D combination pill, then this convenient option may improve compliance in osteoporosis treatments.
- The present study demonstrated that combination therapy of ibandronate and cholecalciferol was highly efficacious in increasing the 25(OH)D serum levels in patients with osteoporosis and in mediating an early decrease in bone markers in vitamin D-deficient osteoporosis patients. Therefore, a combination pill containing both ibandronate and cholecalciferol may be useful for the treatment of osteoporosis in postmenopausal women with vitamin D deficiency.
- In patients with postmenopausal osteoporosis, a once-monthly pill of ibandronate and cholecalciferol appears to be a promising and efficacious treatment regimen with antiresorptive effects that can aid in the maintenance of optimal vitamin D status without any adverse events.
DISCUSSION
-
CONFLICTS OF INTEREST: This study was supported by Dreampharma Corp., of Seoul, Korea.
Article information
- 1. Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 2007;18:1023–1031. ArticlePubMedPDF
- 2. Srivastava M, Deal C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med 2002;18:529–555. ArticlePubMed
- 3. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc 2011;86:50–60. ArticlePubMedPMC
- 4. Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med 2006;260:245–254. ArticlePubMed
- 5. Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, et al. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int 2006;17:914–921. ArticlePubMedPDF
- 6. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930. ArticlePubMed
- 7. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary reference intakes: calcium, vitamin D; Washington DC: National Academies Press; 2011.
- 8. Choi HS. Vitamin d status in Korea. Endocrinol Metab (Seoul) 2013;28:12–16. ArticlePubMedPMC
- 9. Chung HY, Chin SO, Kang MI, Koh JM, Moon SH, Yoon BK, et al. Efficacy of risedronate with cholecalciferol on 25-hydroxyvitamin D level and bone turnover in Korean patients with osteoporosis. Clin Endocrinol (Oxf) 2011;74:699–704. ArticlePubMed
- 10. National Osteoporosis Foundation. Clinician's guide to prevention and treatment of osteoporosis; Washington DC: National Osteoporosis Foundation; 2014.
- 11. Korean Society for Bone and Mineral Research. Physician's guide for diagnosis and treatment of osteoporosis; Seoul: Korean Society for Bone and Mineral Research; 2013.
- 12. Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int 2008;19:663–671. ArticlePubMedPDF
- 13. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351:805–806. Article
- 14. Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 2001;73:288–294. ArticlePubMedPDF
- 15. Recker R, Lips P, Felsenberg D, Lippuner K, Benhamou L, Hawkins F, et al. Alendronate with and without cholecalciferol for osteoporosis: results of a 15-week randomized controlled trial. Curr Med Res Opin 2006;22:1745–1755. ArticlePubMed
- 16. Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab 1994;79:1693–1700. ArticlePubMed
- 17. Carmel AS, Shieh A, Bang H, Bockman RS. The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/ml. Osteoporos Int 2012;23:2479–2487. ArticlePubMedPMCPDF
- 18. Maxwell JD. Seasonal variation in vitamin D. Proc Nutr Soc 1994;53:533–543. ArticlePubMed
- 19. Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040ArticlePubMedPMC
- 20. The American Society for Bone and Mineral Research. New recommendations for taking calcium and vitamin D: U.S. Preventive services task force releases latest on supplements and bone fracture in adults [Internet]; Washington DC: The American Society for Bone and Mineral Research; c2013. updated 2013 Feb 25. cited 2014 Oct 20. Available from: http://www.asbmr.org/about/pressreleases/detail.aspx?cid=e03036f7-5e78-40ae-a3ad-2749b64a8b50.
References
Randomization scheme and patient disposition. IBN+, the group which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol; IBN, the group which received a once-monthly pill containing 150 mg ibandronate.
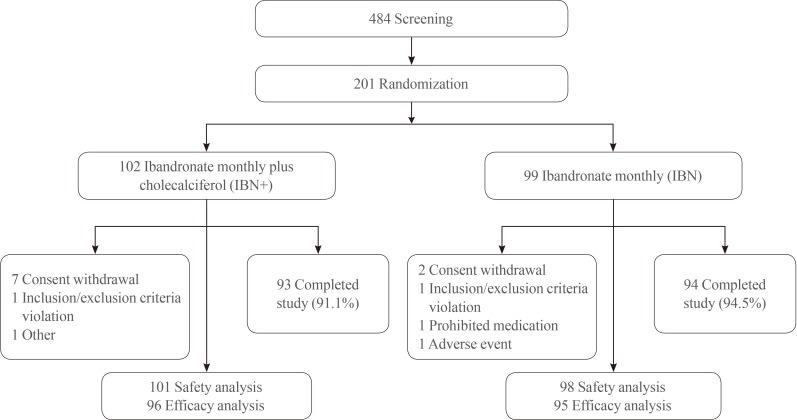
Changes in mean serum 25(OH)D levels over the 16-week treatment period. IBN+, the group which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol; IBN, the group which received a once-monthly pill containing 150 mg ibandronate; 25(OH)D, 25-hydroxyvitamin D. aP<0.001.
Changes in the distribution of patients according to 25-hydroxyvitamin D (25[OH]D) levels after the 16-week treatment period. IBN+, the group which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol; IBN, the group which received a once-monthly pill containing 150 mg ibandronate treatment period, grouped by baseline 25(OH)D levels.

Baseline Characteristics of the Participants
Values are expressed as mean±SD or number (%).
IBN+, the group which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol; IBN, the group which received a once-monthly pill containing 150 mg ibandronate; NS, not significant; BMD, bone mineral density; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; BSAP, bone-specific alkaline phosphatase; CTX, C-telopeptide of type 1 collagen.
Changes in the Serum Levels of 25(OH)D, Bone Turnover Markers, Parathyroid Hormone, Calcium, and Phosphorus after the 16-Week Treatment Course
Values are expressed as mean±SD.
25(OH)D, 25-hydroxyvitamin D; IBN+, the group which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol; IBN, the group which received a once-monthly pill containing 150 mg ibandronate; NS, not significant; CTX, C-telopeptide of type 1 collagen; BSAP, bone-specific alkaline phosphatase; PTH, parathyroid hormone.
aWilcoxon's rank sum test; bWilcoxon's signed rank test; ct test; dPaired t test.
Changes in the Mean Serum Levels of 25(OH)D and CTX after the 16-Week Treatment Course, Grouped by Baseline 25(OH)D Levels
Values are expressed as mean±SD.
25(OH)D, 25-hydroxyvitamin D; CTX, C-telopeptide of type 1 collagen; IBN+, the group which received a once-monthly pill containing 150 mg ibandronate and 24,000 IU cholecalciferol; IBN, the group which received a once-monthly pill containing 150 mg ibandronate; NS, not significant.
at test; bWilcoxon's rank sum test; cPaired t test; dWilcoxon's signed rank test.
Figure & Data
References
Citations

- Effect of vitamin D supplementation or fortification on bone turnover markers in women: a systematic review and meta-analysis
Nasrin Nasimi, Sanaz Jamshidi, Aida Askari, Nazanin Zolfaghari, Erfan Sadeghi, Mehran Nouri, Nick Bellissimo, Shiva Faghih
British Journal of Nutrition.2024; : 1. CrossRef - Quality of life and patient satisfaction with raloxifene/cholecalciferol combination therapy in postmenopausal women
Dong-Yun Lee, Yoon-Sok Chung
Scientific Reports.2022;[Epub] CrossRef - Efficacy of risedronate with cholecalciferol on bone mineral density in Korean patients with osteoporosis
So Young Park, Moo-Il Kang, Hyung Moo Park, Yumie Rhee, Seong Hwan Moon, Hyun Koo Yoon, Jung-Min Koh, Jae Suk Chang, In Joo Kim, Ye Yeon Won, Ye Soo Park, Hoon Choi, Chan Soo Shin, Taek Rim Yoon, Sung-Cheol Yun, Ho-Yeon Chung
Archives of Osteoporosis.2020;[Epub] CrossRef - Efficacy and safety of vitamin D3 B.O.N intramuscular injection in Korean adults with vitamin D deficiency
Han Seok Choi, Yoon-Sok Chung, Yong Jun Choi, Da Hea Seo, Sung-Kil Lim
Osteoporosis and Sarcopenia.2016; 2(4): 228. CrossRef - Pharmacologic treatment of osteoporosis
Yong-Ki Min
Journal of the Korean Medical Association.2016; 59(11): 847. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite