Articles
- Page Path
- HOME > Endocrinol Metab > Volume 29(4); 2014 > Article
-
Original ArticleEndocrine Research Expression of Glucagon-Like Peptide 1 Receptor during Osteogenic Differentiation of Adipose-Derived Stem Cells
- Yun Kyung Jeon1,2*, Min Jung Bae3*, Ju In Kim2, Joo Hyoung Kim3,4, Soo Jong Choi3,4, Su Kyoung Kwon5, Joon Hyop An6, Sang Soo Kim1,2, Bo Hyun Kim1,2, Yong Ki Kim3, In Joo Kim1,2
-
Endocrinology and Metabolism 2014;29(4):567-573.
DOI: https://doi.org/10.3803/EnM.2014.29.4.567
Published online: December 29, 2014
1Department of Internal Medicine, Pusan National University School of Medicine, Korea.
2Biomedical Research Institute, Pusan National University, Korea.
3Kim Yong Ki Internal Medicine Clinic, Korea.
4Department of Plastic and Reconstructive Surgery, Pusan National University Hospital, Korea.
5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kosin University College of Medicine, Korea.
6Department of Internal Medicine, Good Moonhwa Hospital, Busan, Korea.
- Corresponding author: In Joo Kim. Division of Endocrinology and Metabolism, Department of Internal Medicine, Pusan National University School of Medicine, 179 Gudeok-ro, Seo-gu, Busan 602-739, Korea. Tel: +82-51-240-7224, Fax: +82-51-254-3237, injkim@pusan.ac.kr
- *These authors contributed equally to this work.
Copyright © 2014 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Glucagon-like peptide 1 (GLP-1), an incretin hormone well known for its glucose-lowering effect, was recently reported to exert an anabolic effect on bone. Although the exact mechanism is not known, it likely involves the GLP-1 receptor (GLP-1R), which is expressed in some osteoblastic cell lines. Adipose-derived stem cells (ADSCs) have mesenchymal stem cell-specific characteristics, including osteoblastic differentiation potential. We evaluated the expression of GLP-1R during osteogenic differentiation of ADSCs.
-
Methods
- ADSCs were isolated from subcutaneous adipose tissue obtained from three male donors during plastic surgery and were subjected to osteogenic induction. Mineralization was assessed by Alizarin Red staining on day 21. Expression of alkaline phosphatase (ALP), osteocalcin (OC), and GLP-1R was measured by real-time polymerase chain reaction in triplicate for each patient on days 0, 7, 14, and 21. Target mRNA expression levels were normalized to that of β-actin.
-
Results
- ADSCs were fibroblast-like in morphology, adhered to plastic, and had multipotent differentiation potential, as assessed using specific antigen markers. The osteogenic markers ALP and OC were notably upregulated at 21 days. Osteogenic differentiation resulted in a time-dependent increase in the expression of GLP-1R (P=0.013).
-
Conclusion
- We demonstrated upregulation of GLP-1R gene expression during osteogenic differentiation of ADSCs. This finding suggests that GLP-1 may induce osteogenic differentiation in bone tissue.
- Glucagon-like peptide 1 (GLP-1) is a 30-amino-acid incretin hormone produced by intestinal L-cells. Although it is well known to exert beneficial effects by lowering postprandial glucose levels, GLP-1 has been reported to have multiple other functions, including modulation of cell proliferation, differentiation, and apoptosis in various tissues [1]. Many recent studies reported an anabolic effect of GLP-1 on bone, with one study showing that GLP-1 reversed hyperlipidemia-related osteopenia in a rat model [2]. Another study reported an insulin-independent anabolic effect of GLP-1 in an insulin-resistant rat model [3]. However, the exact mechanism of this effect has not been established. Results concerning expression of the GLP-1 receptor (GLP-1R) in osteoblastic cells are inconsistent. GLP-1R expression has been reported in various osteoblastic cell lines, albeit at differing levels [4,5]. Adipose-derived stem cells (ADSCs) are reported to be multipotent and capable of osteogenic differentiation [6,7,8], and studies suggest that they could be an abundant, accessible, and replenishable cell source for bone cell therapy applications [9,10]. In an animal study, critical-size mouse calvarial defects were healed using scaffolds seeded with ADSCs [11].
- To our knowledge, no studies have described the expression of GLP-1R in ADSCs. The aim of this study was to evaluate the expression of GLP-1R during the osteogenic differentiation of ADSCs.
INTRODUCTION
- Design and participants
- ADSCs were isolated from subcutaneous abdominal adipose tissue obtained from three male donors (mean age, 40 years) during plastic surgery. Body mass indices of the donors were 22.6, 26, and 23.6 kg/m2. They had received no medications, including antilipidemic or antidiabetic agents. The donors provided written informed consent, and the study protocol was approved by the Institutional Review Board of Pusan National University Hospital (IRB number 2013-8).
- Isolation and culture of cells
- Knife biopsies of adipose tissue were immediately placed in minimum essential medium-alpha (MEM α, Life Technologies, Carlsbad, CA, USA) supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin (Life Technologies). Samples were transported to the laboratory and processed within 30 minutes of excision. Using a sterile technique, the tissue was finely minced and digested with 0.075% type I collagenase (Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes at 37℃, with vigorous shaking. Then, 25 mL of MEM α containing 10% fetal bovine serum (FBS) were added to neutralize the collagenase, and the suspension was centrifuged at 3,000 rpm for 10 minutes. Next, samples were filtered through a 70µm nylon cell strainer (BD Biosciences, San Diego, CA, USA), washed with phosphate-buffered saline (PBS), and centrifuged at 1,600 rpm for 10 minutes. The isolated ADSCs were maintained and expanded in ADSC culture medium consisting of MEM α containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37℃ in 5% CO2. This initial culture was referred to as passage zero. The medium was replaced twice per week. When the monolayer of adherent cells reached 80% to 90% confluence, cells were trypsinized using 0.25% trypsin-EDTA (Life Technologies) and subcultured to passage three.
- Characterization of cells
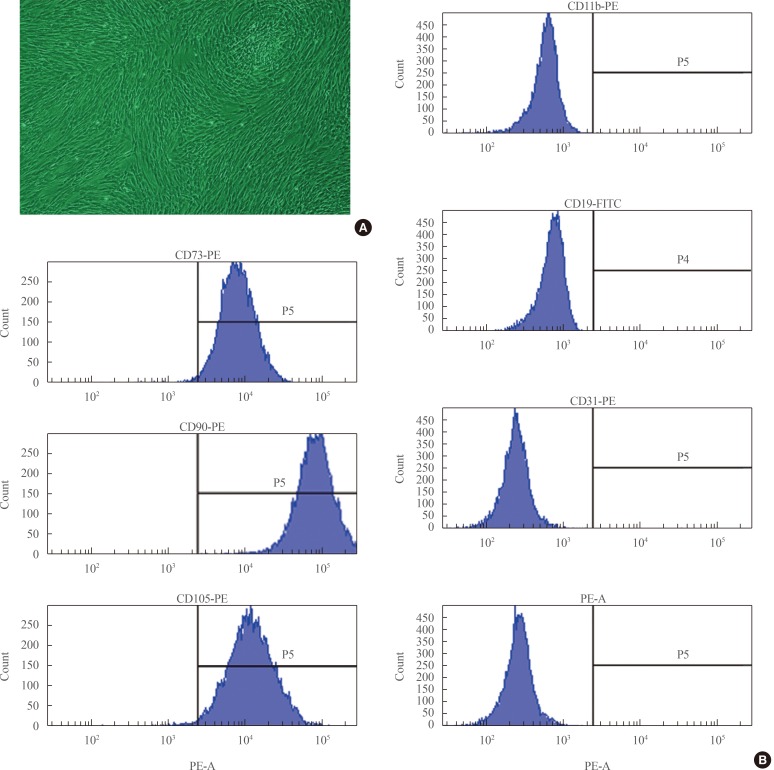
- Flow cytometry was used to characterize the surface marker expression of passage three ADSCs. Briefly, the medium was aspirated and the cell layer was washed with PBS before incubation with 1-mL 0.25% trypsin-EDTA for 3 minutes. Monoclonal antibodies against CD90, CD44, CD73, CD105, CD31, CD19, CD11b, and HLA-DR were used (all from BD Biosciences). Cells were analyzed using a FACSAria flow cytometer (BD Biosciences). Analysis was performed on 10,000 cells per sample and unstained cell samples were used to compensate for background autofluorescence levels.
- Cell differentiation
- For osteogenic induction, ADSCs were seeded on six-well plates at 3×105 cells/well. Next, the medium was replaced with osteogenic medium consisting of MEM α supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, L-glutamine, 0.1 µM dexamethasone, 50 µM ascorbate-2-phosphate, and 10 mM β-glycerophosphate (Sigma-Aldrich). The medium was changed twice weekly.
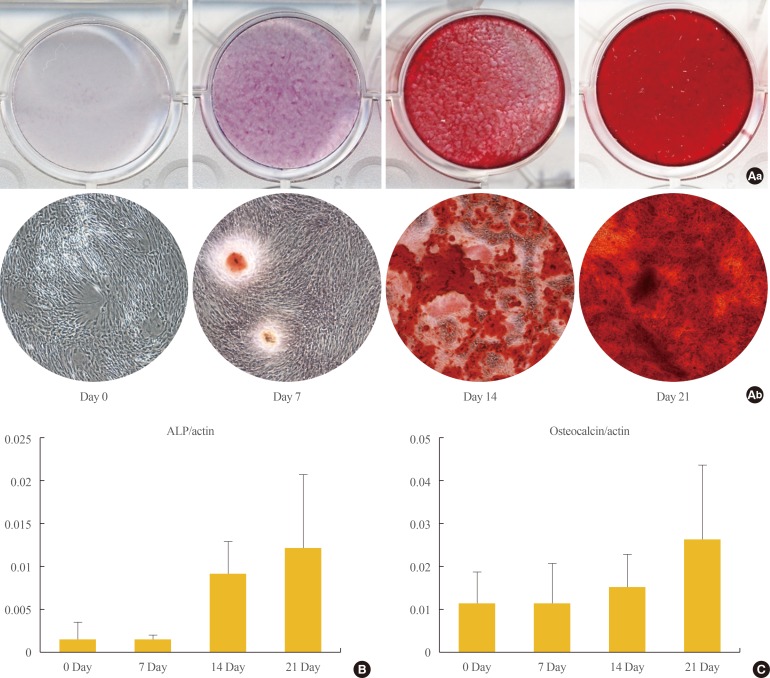
- Alizarin red S staining
- On day 21 of osteogenic induction, the medium was removed and cells were washed with PBS. Cells were fixed with 70% iced ethanol for 15 minutes at 4℃ and washed with distilled water. Alizarin red S staining solution was prepared by dissolving 1 g of Alizarin red S (Sigma-Aldrich) in 100 mL of distilled water, mixing, and adjusting the pH to 4.12 with 0.1% NH4OH. Images of Alizarin red S-stained cells were captured with a DS-U2 digital sight camera (Nikon, Tokyo, Japan).
- Real-time polymerase chain reaction
- Total RNA was extracted using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Each sample, containing 2 µg of RNA, was heated at 65℃ for 15 minutes, before addition of reverse transcriptase. cDNA was prepared through incubation at 50℃ for 60 minutes using the DiaStar RT Kit (SolGent, Seoul, Korea), and real-time polymerase chain reaction (PCR) was performed using a LightCycler instrument (Roche Applied Science, Indianapolis, IN, USA). LightCycler DNA Master SYBR-Green I (Roche Applied Science), cDNA template, primer pairs, and 25 mM MgCl2 were added to microcapillary tubes to give a final volume of 20 µL. PCR was conducted for 40 to 50 cycles, each consisting of predenaturation at 95℃ for 10 seconds, 5 seconds at a specific annealing temperature, and primer extension at 72℃ for 20 seconds. The expression level of the target gene was normalized to the β-actin expression level. Melting curves were visually inspected to confirm the specificity of product detection. The primer sequences were as follows: GLP-1r, TCAAGGTCAACGGCTTATTAG (forward) and TAACGTGTCCCTAGATGAACC (reverse); osteocalcin (OC), AGCAAAGGTGCAGCCTTTGT (forward) and GCGCCTGGGTCTCTTCACT (reverse); and alkaline phosphatase (ALP), CCCCCGTGGCAACTCTATCT (forward) and GATGGCAGTGAAGGGCTTCTT (reverse).
- Western blot analysis
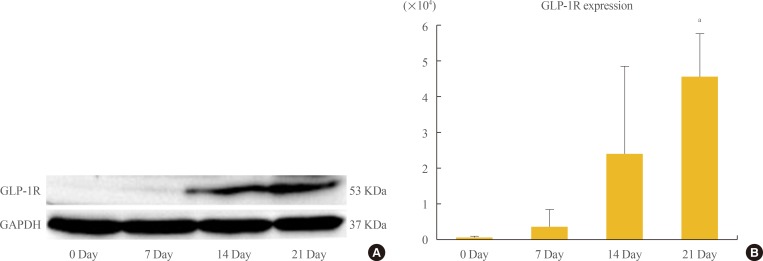
- Cells were isolated using PRO-PREPTM Protein Extraction Solution (iNtRON Biotechnology, Seoul, Korea). Protein concentrations were determined using the BCA protein assay kit (iNtRON). GLP-1R protein was separated by 10% SDS-PAGE and electroblotted onto a Hybond-ECL nitrocellulose membrane (Amersham Biosciences, Little Chalfont, UK). The membrane was blocked with 5% skim milk and incubated with GLP-1R antibody (1:1,000 dilution; Abcam, Cambridge, UK), and subsequently with horseradish peroxidase-conjugated rabbit anti-mouse IgG (Cell Signaling Technology, Danvers, MA, USA). Immunoreactive bands were detected using the West-One Western Blot Detection System (iNtRON).
- Statistical analyses
- All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). For all variables, descriptive statistics, including the mean and standard deviation, were determined for each day. Relative GLP-1R gene expression levels were compared by repeated measures one-way analysis of variance to test for time-dependency. P<0.05 was considered to indicate statistical significance.
METHODS
- Flow cytometric surface marker expression analysis
- ADSCs exhibited fibroblast-like morphology and adhered to plastic (Fig. 1A). To confirm their multipotent differentiation potential, specific surface antigen expression was assessed according to the minimal criteria established by the International Society for Cellular Therapy [12]. The cells showed positive expression (≥95%) of mesenchymal stem cell (MSC)-specific antigen markers, including CD90, CD73, and CD105. Conversely, expression of CD11b (a hematopoietic cell marker prominently expressed on monocytes and macrophages), CD19 (a B cell marker), CD31 (a hematopoietic cell marker expressed on the surfaces of platelets, monocytes, and neutrophils), CD34 (a marker for primitive hematopoietic progenitors and endothelial cells), and HLA-DR was not detected (Fig. 1B).
- Osteoblastic differentiation of ADSCs
- Osteogenic differentiation was confirmed by cytochemical staining and gene expression analysis. After 21 days of osteogenic induction, positive Alizarin red S staining consistent with matrix calcification was observed (Fig. 2A). ALP is a commonly used marker for early osteogenic differentiation. In our study, ALP was notably upregulated at the 21 days (Fig. 2B). Expression of the late osteogenic marker OC is known to be upregulated immediately prior to mineralization, and we observed significant increases in OC mRNA levels by day 21 of differentiation (Fig. 2C).
- Assessment of GLP-1R expression by Western blotting and PCR
- Up-regulation of GLP1-R protein expression during the osteoblastic developmental sequence was confirmed by Western blot data for day 21 (Fig. 3A). Real-time PCR was used to assess the time-course of GLP-1R mRNA expression during osteogenic differentiation. For each time-point (0, 7, 14, and 21 days), the GLP-1R mRNA level of each sample was normalized to that of β-actin. The mean GLP-1R activity of ADSCs increased significantly in a time-dependent manner that was related to the degree of differentiation (P=0.013) (Fig. 3B).
RESULTS
- In this study, we demonstrated that GLP-1R is expressed on ADSC-derived osteogenic cells, and that the level of expression increases in a time-dependent manner during differentiation. GLP-1 has been consistently reported to have a beneficial effect on bone in rodents [2,3]. Also, it is reported to have no adverse effects on bone, despite inducing weight loss in humans [13]. A GLP-1R agonist has been reported to prevent osteopenia by promoting bone formation and by suppressing bone resorption in aged, ovarectomised rats [14]. Furthermore, a GLP-1 agonist increased bone mineral density in type 2 diabetic rats [15]. The mechanism underlying this phenomenon is not clear. One possible explanation is that GLP-1Rs expressed on thyroid C cells promote calcitonin secretion, which inhibits bone resorption; however, GLP-1R was not detected in osteoblasts [16]. A second possibility is the presence of a functional receptor independent of the cAMP-linked GLP-1R. A final possibility is the existence of a GLP-1R.
- The downstream effect of GLP-1 is mediated by a G-protein-coupled receptor that is expressed in various tissues, such as the pancreas, stomach, and vascular system [17]. Expression of GLP-1R by osteoblasts, however, has not been confirmed. GLP-1R has been reported to be expressed on thyroid C cells, which affect bone resorption by promoting calcitonin secretion, but the study did not detect GLP-1R expression in osteoblasts [16]. Conversely, expression of GLP-1R was reported in MC3T3-E1 cells, a well-known mouse osteoblastic cell line [4]. The authors suggested that this result indicates that, like other classic receptors, GLP-1R ha nd temperature-dependent. Another study reported that GLP-1R is expressed by osteoblastic cell lines, but that the expression level is cell line-dependent, perhaps reflecting different stages of osteoblast differentiation [5]. In that study, GLP-1R was expressed by young osteoblasts but not by mature osteoblasts derived from the Saos-2 osteosarcoma cell line. It was suggested that GLP-1R is expressed by osteoblasts, but that the expression decreases with maturation. However, GLP-1R expression was recently reported in osteocytes, which are derived from osteoblasts [15]. In addition, GLP-1R expression may differ among species [18,19].
- We also evaluated the levels of OC and ALP in our ADSC samples. One of the most common methods used to examine mineralization is Alizarin red S staining, which indicates extracellular calcification. Alizarin red staining alone, however, may not be sufficient to confirm osteogenic differentiation [20,21]. ALP is a well-known indicator of the early stage of osteogenic differentiation of MSCs, and expression of ALP increases with osteoblast maturation [22]. In this study, ALP levels increased in a time-dependent manner during osteogenic differentiation of ADSCs. OC is a noncollagenous protein found in bone and dentin and is generally considered to be a relatively late-stage marker for the period of osteoblastic differentiation immediately prior to mineralization [23,24]. In our study, expression of both ALP and OC was elevated on day 21 of differentiation, confirming that osteogenic differentiation of ADSCs had occurred.
- In summary, we demonstrated GLP-1R expression during osteogenic differentiation of ADSCs. Even though it is not yet clear whether GLP-1 has a role in bone metabolism, our results suggest that it may induce osteogenic differentiation in bone tissue. Further studies are needed to explore this possibility.
DISCUSSION
-
Acknowledgements
- This study was supported by a Biomedical Research Institute Grant (2013-10) from Pusan National University Hospital.
ACKNOWLEDGMENTS
- 1. Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol 2003;17:161–171. ArticlePubMedPDF
- 2. Nuche-Berenguer B, Lozano D, Gutierrez-Rojas I, Moreno P, Marinoso ML, Esbrit P, Villanueva-Penacarrillo ML. GLP-1 and exendin-4 can reverse hyperlipidic-related osteopenia. J Endocrinol 2011;209:203–210. ArticlePubMed
- 3. Nuche-Berenguer B, Moreno P, Esbrit P, Dapia S, Caeiro JR, Cancelas J, Haro-Mora JJ, Villanueva-Penacarrillo ML. Effect of GLP-1 treatment on bone turnover in normal, type 2 diabetic, and insulin-resistant states. Calcif Tissue Int 2009;84:453–461. ArticlePubMedPDF
- 4. Nuche-Berenguer B, Portal-Nunez S, Moreno P, Gonzalez N, Acitores A, Lopez-Herradon A, Esbrit P, Valverde I, Villanueva-Penacarrillo ML. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol 2010;225:585–592. ArticlePubMed
- 5. Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol 2011;11:12ArticlePubMedPMC
- 6. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–4295. ArticlePubMedPMC
- 7. Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie 2005;87:125–128. ArticlePubMed
- 8. Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol 2003;58:137–160. ArticlePubMed
- 9. Witkowska-Zimny M, Walenko K. Stem cells from adipose tissue. Cell Mol Biol Lett 2011;16:236–257. ArticlePubMedPMCPDF
- 10. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006;99:1285–1297. ArticlePubMedPMC
- 11. Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 2004;22:560–567. ArticlePubMedPDF
- 12. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. ArticlePubMed
- 13. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006;8:436–447. ArticlePubMed
- 14. Ma X, Meng J, Jia M, Bi L, Zhou Y, Wang Y, Hu J, He G, Luo X. Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J Bone Miner Res 2013;28:1641–1652. ArticlePubMed
- 15. Kim JY, Lee SK, Jo KJ, Song DY, Lim DM, Park KY, Bonewald LF, Kim BJ. Exendin-4 increases bone mineral density in type 2 diabetic OLETF rats potentially through the down-regulation of SOST/sclerostin in osteocytes. Life Sci 2013;92:533–540. ArticlePubMed
- 16. Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology 2008;149:574–579. ArticlePubMedPDF
- 17. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439. ArticlePubMed
- 18. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010;151:1473–1486. ArticlePubMedPDF
- 19. Hirsch PF, Baruch H. Is calcitonin an important physiological substance? Endocrine 2003;21:201–208. ArticlePubMed
- 20. Boyan BD, Schwartz Z, Boskey AL. The importance of mineral in bone and mineral research. Bone 2000;27:341–342. ArticlePubMed
- 21. Declercq HA, Verbeeck RM, De Ridder LI, Schacht EH, Cornelissen MJ. Calcification as an indicator of osteoinductive capacity of biomaterials in osteoblastic cell cultures. Biomaterials 2005;26:4964–4974. ArticlePubMed
- 22. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone 1995;17(2 Suppl):77S–83S. ArticlePubMed
- 23. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol 1990;143:213–221. ArticlePubMed
- 24. Koshihara Y, Kawamura M, Endo S, Tsutsumi C, Kodama H, Oda H, Higaki S. Establishment of human osteoblastic cells derived from periosteum in culture. In Vitro Cell Dev Biol 1989;25:37–43. ArticlePubMedPDF
References

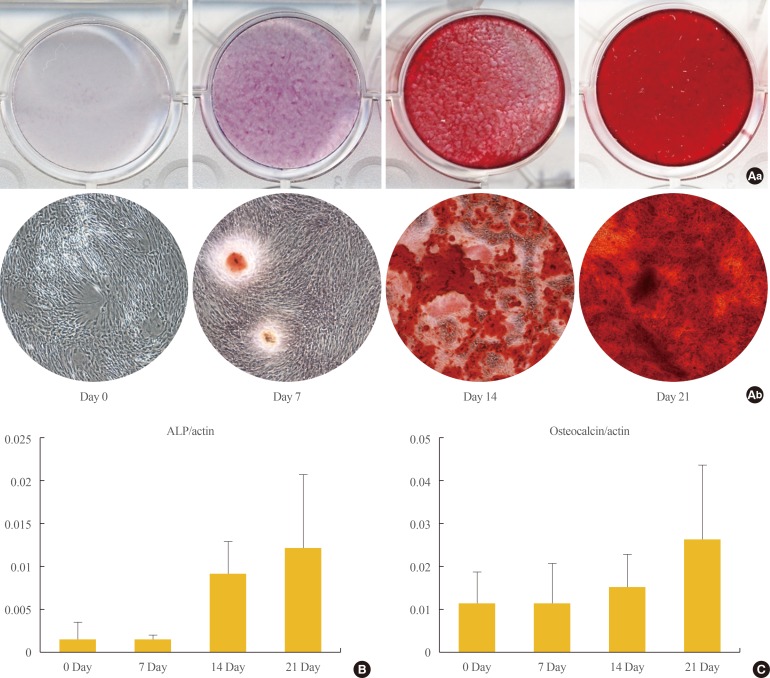
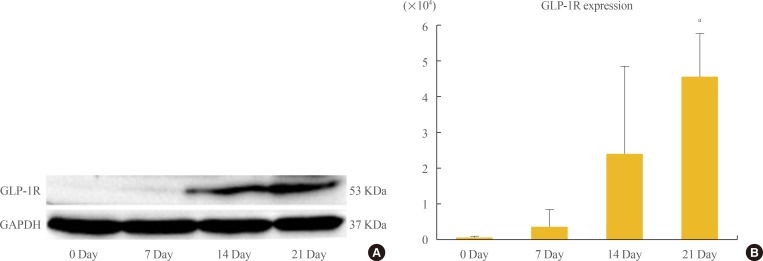
Figure & Data
References
Citations

- Glucagon-like peptide-1 receptor promotes osteoblast differentiation of dental pulp stem cells and bone formation in a zebrafish scale regeneration model
Shafei Zhai, Changkui Liu, Selvaraj Vimalraj, Raghunandhakumar Subramanian, Shahabe Saquib abullais, Suraj Arora, Sekaran Saravanan
Peptides.2023; 163: 170974. CrossRef - The associations of gut microbiota, endocrine system and bone metabolism
Ye Tu, Xinyi Kuang, Ling Zhang, Xin Xu
Frontiers in Microbiology.2023;[Epub] CrossRef - Effect of Liraglutide on Osteoporosis in a Rat Model of Type 2 Diabetes Mellitus: A Histological, Immunohistochemical, and Biochemical Study
Maha Abdelhamid Fathy, Amal Anbaig, Raja Aljafil, Sherein F El-Sayed, Hanim Magdy Abdelnour, Mona Mostafa Ahmed, Eman M A Abdelghany, Sulaiman Mohammed Alnasser, Shaimaa Mohamed Abdelfattah Hassan, Amany Mohamed Shalaby
Microscopy and Microanalysis.2023; 29(6): 2053. CrossRef - Metabolic responses and benefits of glucagon‐like peptide‐1 (GLP‐1) receptor ligands
Neil Tanday, Peter R. Flatt, Nigel Irwin
British Journal of Pharmacology.2022; 179(4): 526. CrossRef - Exendin‐4 enhances osteogenic differentiation of adipose tissue mesenchymal stem cells through the receptor activator of nuclear factor‐kappa B and osteoprotegerin signaling pathway
Sarah A. Habib, Mohamed M. Kamal, Shohda A. El‐Maraghy, Mahmoud A. Senousy
Journal of Cellular Biochemistry.2022; 123(5): 906. CrossRef - Risk of fracture caused by anti-diabetic drugs in individuals with type 2 diabetes: A network meta-analysis
Wen-Hsuan Tsai, Siang-Ke Kong, Chu-Lin Lin, Kai-Hsuan Cheng, Yi-Ting Cheng, Ming-Nan Chien, Chun-Chuan Lee, Ming-Chieh Tsai
Diabetes Research and Clinical Practice.2022; 192: 110082. CrossRef - Comprehensive Analysis of Novel Genes and Pathways Associated with Osteogenic Differentiation of Adipose Stem Cells
Qiuni Gao, Xiaorong Ma, Zuoliang Qi, Jianxin Shi
Disease Markers.2022; 2022: 1. CrossRef - Novel Insights into the Roles and Mechanisms of GLP-1 Receptor Agonists against Aging-Related Diseases
Wei Peng, Rui Zhou, Ze-Fang Sun, Jia-Wei Long, Yong-Qiang Gong
Aging and disease.2022; 13(2): 468. CrossRef - Correlation of Osteoporosis in Patients With Newly Diagnosed Type 2 Diabetes: A Retrospective Study in Chinese Population
Yuhua Wen, Huijuan Li, Xiaoya Zhang, Peipei Liu, Jing Ma, Liya Zhang, Keqin Zhang, Lige Song
Frontiers in Endocrinology.2021;[Epub] CrossRef - Effects of Incretin-Related Diabetes Drugs on Bone Formation and Bone Resorption
Hideki Kitaura, Saika Ogawa, Fumitoshi Ohori, Takahiro Noguchi, Aseel Marahleh, Yasuhiko Nara, Adya Pramusita, Ria Kinjo, Jinghan Ma, Kayoko Kanou, Itaru Mizoguchi
International Journal of Molecular Sciences.2021; 22(12): 6578. CrossRef - Liraglutide regulates bone destruction and exhibits anti-inflammatory effects in periodontitis in vitro and in vivo
Yunxia Zhang, Xuemin Yuan, Yuyan Wu, Minyu Pei, Man Yang, Xuanye Wu, Yunqing Pang, Jing Wang
Journal of Dentistry.2020; 94: 103310. CrossRef - Exendin-4 regulates Wnt and NF-κB signaling in lipopolysaccharide-induced human periodontal ligament stem cells to promote osteogenic differentiation
Honghong Liu, Jiawen Zheng, Taijing Zheng, Ping Wang
International Immunopharmacology.2019; 75: 105801. CrossRef - Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes
Liga Saulite, Kaspars Jekabsons, Maris Klavins, Ruta Muceniece, Una Riekstina
Phytomedicine.2019; 53: 86. CrossRef - Exendin-4 relieves the inhibitory effects of high glucose on the proliferation and osteoblastic differentiation of periodontal ligament stem cells
Zijun Guo, Rui Chen, Fujun Zhang, Ming Ding, Ping Wang
Archives of Oral Biology.2018; 91: 9. CrossRef - Effects of gastric inhibitory polypeptide, glucagon‐like peptide‐1 and glucagon‐like peptide‐1 receptor agonists on Bone Cell Metabolism
Morten S. S. Hansen, Michaela Tencerova, Jacob Frølich, Moustapha Kassem, Morten Frost
Basic & Clinical Pharmacology & Toxicology.2018; 122(1): 25. CrossRef - Liraglutide, a glucagon-like peptide-1 receptor agonist, facilitates osteogenic proliferation and differentiation in MC3T3-E1 cells through phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), extracellular signal-related kinase (ERK)1/2, and cAMP/pro
Xuelun Wu, Shilun Li, Peng Xue, Yukun Li
Experimental Cell Research.2017; 360(2): 281. CrossRef - The Impact of Glucagon-Like Peptide-1 on Bone Metabolism and Its Possible Mechanisms
Chenhe Zhao, Jing Liang, Yinqiu Yang, Mingxiang Yu, Xinhua Qu
Frontiers in Endocrinology.2017;[Epub] CrossRef - Pathophysiology of Bone Fragility in Patients with Diabetes
Andrea Palermo, Luca D’Onofrio, Raffaella Buzzetti, Silvia Manfrini, Nicola Napoli
Calcified Tissue International.2017; 100(2): 122. CrossRef - Activation of GLP-1 Receptor Promotes Bone Marrow Stromal Cell Osteogenic Differentiation through β-Catenin
Jingru Meng, Xue Ma, Ning Wang, Min Jia, Long Bi, Yunying Wang, Mingkai Li, Huinan Zhang, Xiaoyan Xue, Zheng Hou, Ying Zhou, Zhibin Yu, Gonghao He, Xiaoxing Luo
Stem Cell Reports.2016; 6(4): 579. CrossRef - Mechanisms for the cardiovascular effects of glucagon-like peptide-1
H. Poudyal
Acta Physiologica.2016; 216(3): 277. CrossRef - Perspectives in GLP-1 Research: New Targets, New Receptors
Giulia Cantini, Edoardo Mannucci, Michaela Luconi
Trends in Endocrinology & Metabolism.2016; 27(6): 427. CrossRef - Chronic administration of Glucagon-like peptide-1 receptor agonists improves trabecular bone mass and architecture in ovariectomised mice
M. Pereira, J. Jeyabalan, C.S. Jørgensen, M. Hopkinson, A. Al-Jazzar, J.P. Roux, P. Chavassieux, I.R. Orriss, M.E. Cleasby, C. Chenu
Bone.2015; 81: 459. CrossRef - Articles in 'Endocrinology and Metabolism' in 2014
Won-Young Lee
Endocrinology and Metabolism.2015; 30(1): 47. CrossRef - Impact of Anti-hyperglycemic Medications on Bone Health
Naim M. Maalouf
Clinical Reviews in Bone and Mineral Metabolism.2015; 13(1): 43. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite