Anaplastic Thyroid Carcinoma Following Radioactive Iodine Therapy for Graves' Disease
Article information
Abstract
Radioactive iodine (RAI) therapy has been used as a treatment option for Graves' disease, and it has been widely accepted to be safe. On the other hand, some evidence suggests that RAI therapy is possibly associated with a small increased risk of thyroid cancer. Herein, we report a rare case of anaplastic thyroid carcinoma (ATC) associated with Graves' disease, following RAI treatment. A 42-year-old woman had been diagnosed with Graves' disease and although she was treated with an antithyroid drug, she remained in a hyperthyroid state, which led to two RAI treatments. More than 10 years later, the patient revisited our clinic due to hoarseness, dysphagia, and dyspnea, which had lasted for 2 months. Neck computed tomography suggested thyroid carcinoma and a lymph node biopsy showed metastatic papillary carcinoma. The patient underwent total thyroidectomy and was finally diagnosed as having an ATC. It is not clear if the occurrence of ATC reported here was influenced by the RAI therapy or alternatively, it may only represent the delayed recognition of a rare change in the natural history of Graves' disease. Nevertheless, this report is worthwhile since it presents a very rare case of ATC that occurred eleven years after the RAI therapy for Graves' disease.
INTRODUCTION
The treatment options for Graves' disease consist of antithyroid drugs, radioactive iodine (RAI) therapy, and surgery. RAI therapy has been used for the treatment of Graves' disease for more than seven decades, and in most studies, it has not been associated with an increased risk of cancer [1]. On the other hand, several studies have proposed the potential increase in thyroid malignancies with RAI treatment [2-4].
Here, we describe a very rare case of anaplastic thyroid carcinoma (ATC) associated with Graves' disease, following RAI treatment.
CASE REPORT
A 42-year-old woman was referred to our clinic in 1998, because of palpitations, hand tremor, and laboratory findings of hyperthyroidism (serum thyrotropin [thyroid-stimulating hormone, TSH] 0.05 µIU/mL [reference range, 0.17 to 4.05], free thyroxine 4.01 ng/dL [reference range, 0.79 to 1.86], and positive TSH receptor antibody at 201.7% [percent binding inhibition index, radioreceptor assay, reference range; ±15.0%]), and had been diagnosed as Graves' disease. A technetium-99m thyroid scan showed enlarged and homogeneously increased uptake, and ultrasonography revealed a diffuse goiter without nodules. The patient received 200 mg/day of propylthiouracil and β-adrenergic blocking agents for 1 year. However, due to recurrent hyperthyroidism, she received RAI therapy twice with an administered dose 370 MBq (10 mCi), 296 MBq (8 mCi), each. Two years later, she received thyroid hormone replacement due to subsequent hypothyroidism at a local clinic.
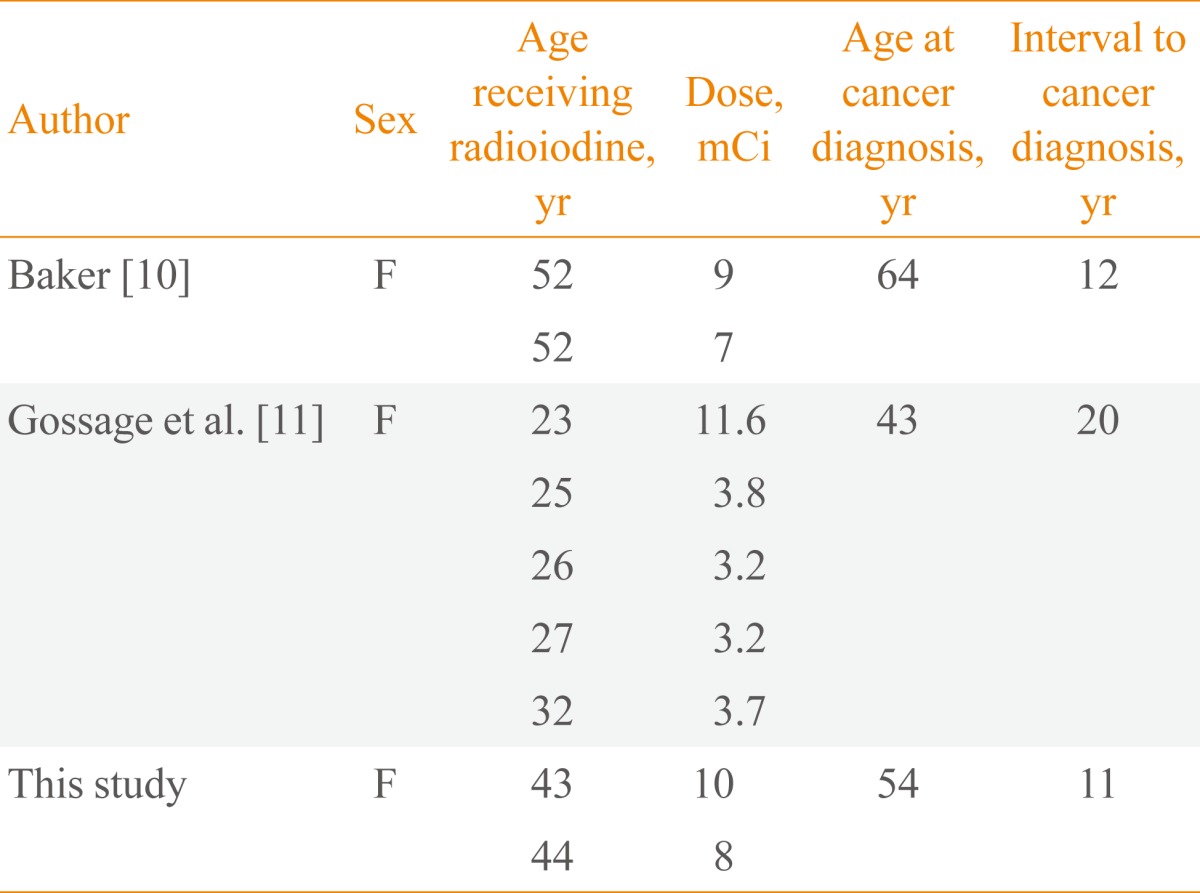
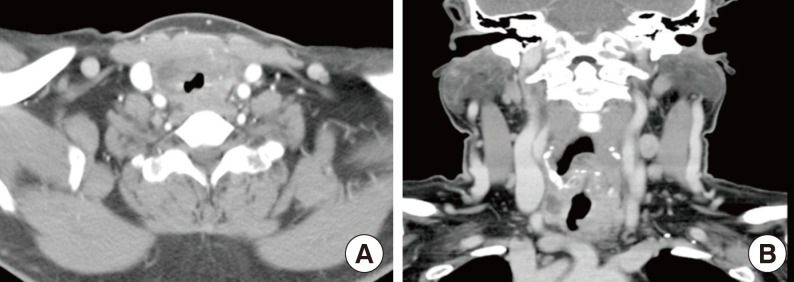
In September 2010, over 10 years later, she revisited us with complaints of hoarseness, dysphagia, and dyspnea, which had been presented for 2 months. An anterior neck mass, which was hard and fixed, and some lymph nodes (LNs) around the mass were palpable. Her chest radiography showed a tracheal narrowing and deviation to the right without active lung lesion. On computed tomography (CT) of the neck, the thyroidal mass invaded the tracheal cartilage and the right tracheoesophageal groove (Fig. 1). In fludeoxyglucose positron emission tomography-CT, an intense hypermetabolic lesion was noted in the thyroid gland and LNs on her left neck without distant metastasis. LN biopsy was performed and microscopically showed metastatic papillary carcinoma (Fig. 2A). In September 2010, a total thyroidectomy with selective neck dissection, tracheal sleeve resection, and end-to-end anastomosis were performed. The surgical specimen from the thyroid was diagnosed as anaplastic carcinoma (Fig. 2B). The final pathologic staging was T4bN1bM0. Subsequently, she had concurrent cisplatin-based chemoradiotherapy for her neck and superior mediastinum (total 6,600 cGy/33 fractions).
Computed tomography (CT) findings of anaplastic thyroid carcinoma. Neck CT shows an exophytic thyroidal mass with extracapsular invasion, tracheal deviation, and narrowing.
The histological findings. (A) The histologic section of lymph node shows clear nuclei and formation of papillary structure (H&E stain, ×200). (B) The surgical specimen shows atypical spindle cells with irregular nuclei; a feature of anaplastic thyroid carcinoma (H&E stain, ×200).
After 4 months of follow-up, despite the aggressive treatment, the disease progressed. She developed shortness of breath, dyspnea, and pain in her left arm. On neck CT, a new focal necrotic mass on the left side of her neck was noted, suggesting local recurrence. The mass extended up to the tracheal lumen and the patient died of sudden uncontrolled bleeding in the airway.
DISCUSSION
The patient in this case received RAI therapy twice for recurrent hyperthyroidism. After more than 10 years, she developed respiratory symptoms and was finally diagnosed with ATC.
This case raises questions about the causal relationship between RAI therapy for Graves' disease and the subsequent occurrence of thyroid cancer. Although it has been widely accepted that RAI is safe, some studies have suggested that RAI therapy is possibly associated with a small increased risk of thyroid cancer [2-4]. DeGroot [5] reported that the RAI treatment can induce an increase of thyroid antibodies, such as TSH receptor antibody, and it was suggested that thyroid stimulating antibodies play an important role in thyroid carcinogenesis [6,7]. These antibodies also can stimulate angiogenesis, which contributes to thyroid tumor cell growth, by activating vascular endothelial growth factor [8]. Sturgis [9] reported cytomorphologic alterations in thyroid follicular epithelium, which is associated with RAI therapy. Before treatment, Graves' disease typically lack significant atypia, while after RAI therapy, nuclear atypia, and progressive cellular metaplasia could be seen microscopically. Furthermore, it was prominent in those who remain in a hyperthyroid state despite RAI treatment.
In addition to our case, two previously reported cases of ATC after RAI therapy for documented Graves' disease are summarized in Table 1. Baker [10] reported a 64-year-old woman with ATC 12 years after RAI therapy. Because thyroid toxicity persisted after one course of RAI therapy, the patient received a second therapy in 4 months. Gossage et al. [11] reported a 46-year-old woman who had her first RAI therapy, when aged only 23 years. Although she underwent partial thyroidectomy prior to the RAI therapy, hyperthyroidism recurred and she received several subsequent doses of radioiodine. Finally, 20 years later, she developed ATC. An analysis of these cases is noteworthy, because since the interval to cancer diagnosis was over 10 years in all cases, it supports the importance of long-term vigilance in patients receiving RAI therapy. In addition, these cases had something in common in terms of the occurrence of ATC after repetitive, small doses of RAI therapy. The Cooperative Thyrotoxicosis Therapy Follow-up Study showed that thyroid neoplasms developed in children treated with lower, rather than higher, doses of RAI therapy [4], which is compatible with our finding. Therefore, it can be hypothesized that multiple courses of RAI therapy, with small doses, may play a role in carcinogenesis, especially in the poorly differentiated type. In particular, Ogawa et al. [12] demonstrated that 131I treatment inactivates the tumor suppressor gene that is frequently found in ATC rather than in the differentiated type. However, to discuss the causal relationship between RAI therapy and the occurrence of cancer, the number of reports is too small, therefore a population-based study is required and this is a limitation of our report.
In addition to the RAI therapy, we cannot exclude that Graves' disease itself might also increase the risk of cancer. Although our patient had no thyroid nodule by ultrasonography, the palpable thyroid nodules found in Graves' patients have a 16.9% malignancy rate, which is higher than that of the general population [13]. Moreover, the finding that thyroid autoantibody stimulates the malignant transformation of the thyroid gland supports this suggestion.
It is not clear that the occurrence of ATC reported here was influenced by RAI therapy or, alternatively, it may only represent the delayed recognition of a rare change in the natural history of Graves' disease. Nevertheless, this report is worthwhile since it presents a very rare case of ATC that occurred 11 years after RAI therapy for Graves' disease.
Notes
No potential conflict of interest relevant to this article was reported.