Articles
- Page Path
- HOME > Endocrinol Metab > Volume 28(1); 2013 > Article
-
Original ArticleTumor Necrosis Factor-α as a Predictor for the Development of Nonalcoholic Fatty Liver Disease: A 4-Year Follow-Up Study
- Yun Yong Seo1*, Yong Kyun Cho1*, Ji-Cheol Bae2, Mi Hae Seo3, Se Eun Park3, Eun-Jung Rhee3, Cheol-Young Park3, Ki-Won Oh3, Sung-Woo Park3, Won-Young Lee3
-
Endocrinology and Metabolism 2013;28(1):41-45.
DOI: https://doi.org/10.3803/EnM.2013.28.1.41
Published online: March 25, 2013
1Department of Gastroenterology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
3Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding author: Won-Young Lee. Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 110-746, Korea. Tel: +82-2-2001-2579, Fax: +82-2-2001-1588, drlwy@hanmail.net
- *These authors contributed equally to this work.
Copyright © 2013 Korean Endocrine Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,445 Views
- 34 Download
- 60 Crossref
ABSTRACT
-
Background
- Tumor necrosis factor (TNF)-α is associated with insulin resistance and systemic inflammatory responses. The aim of this study was to investigate the relationship between TNF-α and the development of nonalcoholic fatty liver disease (NAFLD) in a longitudinal study.
-
Methods
- Three hundred and sixty-three apparently healthy subjects (mean age, 40.5±6.1 years; male, 57.6%) without NAFLD were enrolled in 2003. Anthropometric and laboratory measurements were performed. The participants were grouped into tertiles according to their serum TNF-α levels from samples taken in 2003. At a 4-year follow-up, we compared the odds ratios (ORs) of the development of NAFLD according to the tertiles of TNF-α levels measured in 2003.
-
Results
- At the 4-year follow-up, the cumulative incidence of NAFLD was 29.2% (106/363). The group that developed NAFLD had higher levels of TNF-α than those in the group without NAFLD (3.65±1.79 pg/mL vs. 3.15±1.78 pg/mL; P=0.016). When the 2003 serum TNF-α levels were categorized into tertiles: incidence of NAFLD observed in 2007 was significantly higher with increasing tertiles (22.6%, 35.8%, and 41.5%, respectively; P<0.05). The risk of developing NAFLD was significantly greater in the highest tertile of TNF-α than in the lowest tertile after adjusting for age, smoking, and BMI (OR, 2.20; 95% confidence interval, 1.12 to 4.01; P<0.05).
-
Conclusion
- Higher serum TNF-α levels in subjects without NAFLD were associated with the development of NAFLD. The results of study might suggest a pathologic role of inflammation in NAFLD.
- Nonalcoholic fatty liver disease (NAFLD) is characterized by excessive hepatic accumulation of triglycerides and free fatty acids in the liver [1]. The pathologic spectrum of NAFLD is broad, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), potentially progressing to fibrosis and cirrhosis [2]. After fat infiltrates the liver, progression to hepatocellular inflammation and fibrosis may occur [3,4]. Additional factors, such as oxidative stress, mitochondrial abnormalities, and cytokines such as tumor necrosis factor (TNF)-α, are important in mediating this process. Serum levels of TNF-α are significantly increased in fatty liver disease, and are well correlated with the severity of liver disease [4].
- TNF-α appears to play a central role in the development of hepatic steatosis. TNF-α is a proinflammatory cytokine that mediates hepatic inflammation, oxidative stress, and apoptosis or necrosis of liver cells [5,6]. The cytokine TNF-α is an important cytokine, exerting biological effects in different tissues and species at multiple levels [7].
- Several clinical studies have investigated the role of TNF-α as a marker for NAFLD in cross-sectional analyses, but the results have been contradictory [8-10]. There have been no studies that have demonstrated a relationship between TNF-α and NAFLD in a longitudinal analysis.
- Therefore, in this study, we investigated the relationship between TNF-α levels at baseline and the incidence of NAFLD development in a 4-year follow-up of a cohort of 363 apparently healthy Korean subjects.
INTRODUCTION
- Participants
- This study included 363 subjects in an observational cohort. Participants underwent medical screening at an industrial medical check-up in 2003 and at a follow-up in 2007. The results were previously reported as the Kangbuk Samsung Medical Center-Adipokine Study (KBSMC-Adipokine Study) [11]. Subjects with viral hepatitis B, hepatitis C, other liver diseases, acute or chronic inflammation, malignancy, or excessive alcohol consumption (>20 g/day) were excluded. Questionnaires were used to determine alcohol consumption (g/day). In addition, participants taking medications such as peroxisome proliferator-activated receptor-γ agonists, metformin, or antioxidants (vitamin E or C) were excluded. Alcohol intake, smoking habits, medication, and medical history were assessed by chart review and standardized questionnaire. The study protocol was approved by the Institutional Review Board and the Ethics Committee of Kangbuk Samsung Hospital.
- Biochemical assays
- Blood samples were obtained after 12 hours of overnight fasting and used to measure fasting plasma glucose, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, fasting insulin, creatinine, direct bilirubin, and the following liver function parameters: aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), and alkaline phosphatase (ALP). Samples for measuring TNF-α were separated and stored at -80℃ prior to measurement of serum levels by enzyme-linked immunosorbent assay (ELISA, Bio Vendor Laboratory Medicine, Modrice, the Czech Republic). As a marker of insulin resistance (IR), homeostatic model assessment (HOMA) of IR was calculated as follows [12]: HOMA-IR=[fasting insulin (µIU/mL)×fasting glycaemia (mmol/L)]/22.5.
- Ultrasonography
- Abdominal ultrasonography (Logic Q700 MR, GE, Milwaukee, WI, USA) was performed in all subjects. Fatty liver was diagnosed based on standard criteria, including hepatorenal echo contrast, liver brightness, and vascular blurring, using a 3.5 MHz probe [12]. Several experienced radiologists, all of whom were blinded to the subjects' clinical status, performed the ultrasounds. We did not assess interobserver reliability.
- Statistical analysis
- Statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Normality was tested using the Kolmogorov-Smirnov test. The chi-squared test was used to compare categorical variables between groups. For continuous variables, parameters that followed a normal distribution were analyzed with a t test or analysis of variance (ANOVA) and described as the mean±SD. Parameters that did not follow a normal distribution were analyzed with the Mann-Whitney U test or the Kruskal-Wallis test and expressed as the median (interquartile range).
- TNF-α levels were grouped into tertiles, and multiple logistic regression analysis was used to calculate odds ratios (ORs) for NAFLD. Subjects with higher TNF-α tertiles (second and third tertiles) were compared with the lowest tertile. Two-sided values of P<0.05 were considered significant.
METHODS
- The study cohort included 363 subjects (210 males, 153 females) with an average age of 40.5 years (range, 34 to 46). The subjects were divided into two groups: those with NAFLD (n=106) and those without NAFLD (n=257) in 2007. Table 1 shows a comparison of the baseline characteristics between subjects according to the presence or absence of NAFLD in 2007. The mean ages of the NAFLD and non-NAFLD groups were not significantly different. The NAFLD group had a significantly higher proportion of male subjects and a higher body mass index (BMI) (Table 1). Additionally, the baseline serum levels of ALP, GGT, direct bilirubin, and creatinine were significantly higher in the NAFLD group. The activities of the liver aminotransferases AST and ALT and the IR marker HOMA-IR were not significantly different between the two groups. Table 1 also shows the serum levels of TNF-α in 2003 according to the presence or absence of NAFLD in 2007. TNF-α levels were significantly higher in the NAFLD group than in the non-NAFLD group (3.65±1.78 pg/mL vs. 3.15±1.79 pg/mL; P=0.016).
- The range of levels of TNF-α for each tertile was as follows: tertile 1, 0 to 2.4 pg/mL (mean, 1.48); tertile 2, 2.4 to 3.8 pg/mL (mean, 3.14); and tertile 3, 3.8 to 12.0 pg/mL (mean, 5.25). When distributions of serum levels of TNF-α were expressed by tertile, we observed a statistically significant gradient for the development of NAFLD (22.6%, 35.8%, and 41.5%, respectively; P<0.05) (Table 2).
- Table 3 shows the risk of NAFLD after 4 years of follow-up according to TNF-α levels separated by tertiles. The OR of the highest tertile compared with the lowest tertile was 2.20 (95% confidence interval, 1.21 to 4.01; P=0.010), even after adjustment for age, BMI, and smoking.
RESULTS
- In this study, we sought to fill a gap in the existing literature regarding the relationship between TNF-α and NAFLD. Previously, it had been hypothesized that TNF-α plays a role in the pathogenesis of diseases related to IR, including NAFLD [13,14]. Indeed, TNF-α production has been reported to be elevated in peripheral blood cell cultures collected from obese patients with NAFLD [7]. Nevertheless, direct evidence of TNF-α involvement in the early stages of NAFLD has not been previously described.
- Human studies of TNF-α and NAFLD have shown conflicting results, probably due to heterogeneity in study populations or various factors that might affect serum levels of TNF-α. In a cross-sectional study by Hui et al. [8], TNF-α levels were found to be significantly higher in NAFLD patients compared to control patients, but there was no significant difference in TNF-α levels between patients with NAFLD and those with NASH as diagnosed by liver biopsy. In another cross-sectional study of patients with NASH, NAFLD, and control patients, serum TNF-α and soluble TNF receptor 1 were significantly higher in patients with NASH compared to patients with NAFLD and controls [9]. However, in a study by Musso et al. [10], there were no significant differences in TNF-α serum levels among nonobese, nondiabetic NASH patients and matched controls.
- To date, a temporal association between TNF-α and NAFLD has not been demonstrated in a longitudinal analysis. We sought to determine whether or not TNF-α and NAFLD are significantly related over time. We observed a significant relationship between TNF-α and the development of NAFLD after 4 years.
- TNF-α is a central mediator of IR, activating proinflammatory pathways such as c-jun N-terminal kinase and nuclear factor-κB. The proinflammatory cytokine TNF-α has an important role in these pathways, especially in patients with obesity or type 2 diabetes mellitus [15]. Thus, TNF-α may contribute to IR, which then may further promote inflammation by impairing the anti-inflammatory effect of insulin [16]. Furthermore, TNF-α is known to attract inflammatory leukocytes to the liver and to enhance the expression of sterol regulatory element binding protein-1c, which regulates de novo lipogenesis and is more highly expressed in NAFLD [17].
- A study by Satapathy et al. [18] supported a potential role of TNF-α in the pathogenesis of NAFLD. In patients with NASH and elevated ALT, treatment with a TNF-α inhibitor (pentoxifylline) significantly reduced AST, ALT, and serum TNF-α levels and improved the IR index.
- This study had several limitations. First, the diagnosis of NAFLD was not histologically confirmed, but was made by ultrasound, which has a reported sensitivity of 67% to 89% and specificity of 77% to 89% [19,20]. Second, alcohol intake was surveyed by a self-recorded questionnaire; therefore, we cannot rule out the possibility of misreporting or recall bias. In addition, lifestyle risk factors, which can affect future NAFLD, including exercise and dietary habits, were not considered. Thus, the data were subject to potential under- or overestimation. Third, we did not adjust for high sensitivity C-reactive protein (hs-CRP) and HOMA-IR. We did not measure hs-CRP, and there was no significant difference after adjustment for HOMA-IR. Lastly, our cohort was composed of participants who had volunteered for health check-ups, which might also introduce a selection bias.
- In conclusion, this study showed a significant relationship between baseline TNF-α levels and the later development of NAFLD. The role of the cytokine TNF-α in NAFLD needs to be confirmed through further studies.
DISCUSSION
-
Acknowledgements
- This work was supported by MSD Korea research grant # MRI-110907-004 and Daewoong research grant # MRI-110907-005.
ACKNOWLEDGMENTS
- 1. Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis 2001;21:17–26. ArticlePubMedPDF
- 2. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413–1419. ArticlePubMed
- 3. Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107:1103–1109. ArticlePubMed
- 4. McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol 2006;40(Suppl 1):S17–S29. PubMed
- 5. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91. ArticlePubMed
- 6. Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003;112:1785–1788. ArticlePubMedPMC
- 7. Poniachik J, Csendes A, Diaz JC, Rojas J, Burdiles P, Maluenda F, Smok G, Rodrigo R, Videla LA. Increased production of IL-1alpha and TNF-alpha in lipopolysaccharide-stimulated blood from obese patients with non-alcoholic fatty liver disease. Cytokine 2006;33:252–257. ArticlePubMed
- 8. Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004;40:46–54. ArticlePubMed
- 9. Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K, Nakamura M, Komori A, Yano K, Yatsuhashi H, Eguchi K, Ishibashi H. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int 2006;26:39–45. ArticlePubMed
- 10. Musso G, Gambino R, Durazzo M, Biroli G, Carello M, Faga E, Pacini G, De Michieli F, Rabbione L, Premoli A, Cassader M, Pagano G. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology 2005;42:1175–1183. ArticlePubMed
- 11. Kim YC, Cho YK, Lee WY, Kim HJ, Park JH, Park DI, Sohn CI, Jeon WK, Kim BI, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW, Ryu SH. Serum adipocyte-specific fatty acid-binding protein is associated with nonalcoholic fatty liver disease in apparently healthy subjects. J Nutr Biochem 2011;22:289–292. ArticlePubMed
- 12. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. ArticlePubMedPDF
- 13. Bluher M. Clinical relevance of adipokines. Diabetes Metab J 2012;36:317–327. ArticlePubMedPMC
- 14. Peraldi P, Spiegelman BM. Studies of the mechanism of inhibition of insulin signaling by tumor necrosis factor-alpha. J Endocrinol 1997;155:219–220. ArticlePubMed
- 15. Diehl AM. Tumor necrosis factor and its potential role in insulin resistance and nonalcoholic fatty liver disease. Clin Liver Dis 2004;8:619–638. ArticlePubMed
- 16. Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 2005;54:2939–2945. ArticlePubMed
- 17. Endo M, Masaki T, Seike M, Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c). Exp Biol Med (Maywood) 2007;232:614–621. PubMed
- 18. Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2004;99:1946–1952. ArticlePubMed
- 19. Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol 2003;15:539–543. PubMed
- 20. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745–750. ArticlePubMed
References
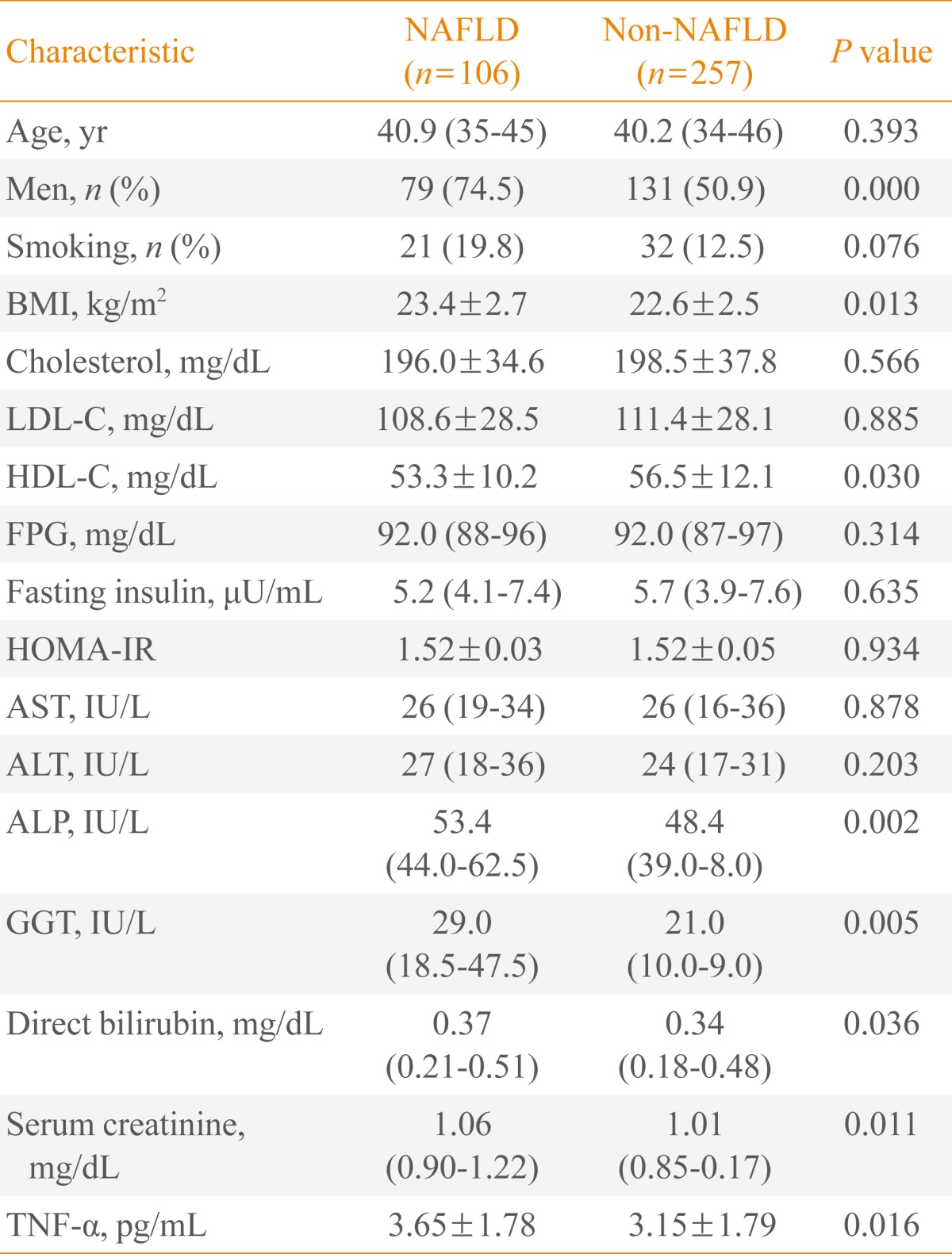
Values are expressed as median (interquartile range) or mean±SD.
NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; FPG, fasting plasma glucose; HOMA-IR, homeostatic model assessment of insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyltransferase; TNF, tumor necrosis factor.
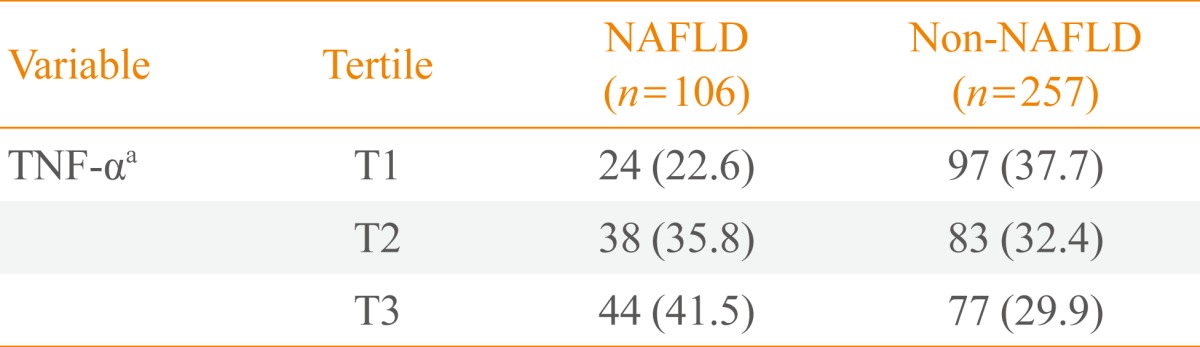
Figure & Data
References
Citations

- Nigella sativa Efficacy in Non-alcoholic Fatty Liver Disease: Mechanisms and Clinical Effects
A.A. Sangouni, A. Jamalzehi, M. Moradpour, H. Mozaffari-Khosravi
Journal of Herbal Medicine.2024; 43: 100833. CrossRef - Berberine prevents NAFLD and HCC by modulating metabolic disorders
Xinyue Lin, Juanhong Zhang, Yajun Chu, Qiuying Nie, Junmin Zhang
Pharmacology & Therapeutics.2024; 254: 108593. CrossRef - Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential
Maria Rosaria Miranda, Vincenzo Vestuto, Giuseppina Amodio, Michele Manfra, Giacomo Pepe, Pietro Campiglia
Life.2024; 14(3): 420. CrossRef - Theabrownin alleviates nonalcoholic fatty liver disease by inhibiting the intestinal farnesoid X receptor–ceramide axis
Jieyi Wang, Dan Zheng, Kun Ge, Fengjie Huang, Yang Li, Xiaojiao Zheng, Wei Jia, Aihua Zhao
Food Frontiers.2024;[Epub] CrossRef - Necroptosis contributes to non-alcoholic fatty liver disease pathoetiology with promising diagnostic and therapeutic functions
Hong-Ju Sun, Bo Jiao, Yan Wang, Yue-Hua Zhang, Ge Chen, Zi-Xuan Wang, Hong Zhao, Qing Xie, Xiao-Hua Song
World Journal of Gastroenterology.2024; 30(14): 1968. CrossRef - RGS7-ATF3-Tip60 Complex Promotes Hepatic Steatosis and Fibrosis by Directly Inducing TNFα
Madhuri Basak, Kiran Das, Tarun Mahata, Abhishek Singh Sengar, Sumit Kumar Verma, Sayan Biswas, Kakali Bhadra, Adele Stewart, Biswanath Maity
Antioxidants & Redox Signaling.2023; 38(1-3): 137. CrossRef - The capsaicinoid nonivamide suppresses the inflammatory response and attenuates the progression of steatosis in a NAFLD‐rat model
Naruemon Wikan, Jiraporn Tocharus, Chio Oka, Sivanan Sivasinprasasn, Waraluck Chaichompoo, Apichart Suksamrarn, Chainarong Tocharus
Journal of Biochemical and Molecular Toxicology.2023;[Epub] CrossRef - Thermoneutral housing shapes hepatic inflammation and damage in mouse models of non-alcoholic fatty liver disease
Jarren R. Oates, Keisuke Sawada, Daniel A. Giles, Pablo C. Alarcon, Michelle S.M.A. Damen, Sara Szabo, Traci E. Stankiewicz, Maria E. Moreno-Fernandez, Senad Divanovic
Frontiers in Immunology.2023;[Epub] CrossRef - 1,25-Dihydroxycholecalciferol down-regulates 3-mercaptopyruvate sulfur transferase and caspase-3 in rat model of non-alcoholic fatty liver disease
Maher N. Ibrahim, Abeer A. Khalifa, Dalia A. Hemead, Amira Ebrahim Alsemeh, Marwa A. Habib
Journal of Molecular Histology.2023; 54(2): 119. CrossRef - Effects of Resveratrol Supplementation on Nonalcoholic Fatty Liver Disease Management
Arefe Nemati, Zeinab Nikniaz, Ali Mota
Topics in Clinical Nutrition.2023; 38(2): 144. CrossRef - The effects of chicory supplementation on liver enzymes and lipid profiles in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of clinical evidence
Elham Maleki, Ali Sadeghpour, Erfan Taherifard, Bahareh Izadi, Mehdi Pasalar, Maryam Akbari
Clinical Nutrition ESPEN.2023; 55: 447. CrossRef - Using bioinformatics and systems biology methods to identify the mechanism of interaction between COVID-19 and nonalcoholic fatty liver disease
Wenbo Dong, Yan Jin, Hongshuo Shi, Xuecheng Zhang, Jinshu Chen, Hongling Jia, Yongchen Zhang
Medicine.2023; 102(23): e33912. CrossRef - The Role of Tumor Necrosis Factor-Alpha in the Pathogenesis and Treatment of Nonalcoholic Fatty Liver Disease
Ilias D. Vachliotis, Stergios A. Polyzos
Current Obesity Reports.2023; 12(3): 191. CrossRef - NAFLD Is Associated With Quiescent Rather Than Active Crohn’s Disease
Scott McHenry, Matthew Glover, Ali Ahmed, Quazim Alayo, Maria Zulfiqar, Daniel R Ludwig, Matthew A Ciorba, Nicholas O Davidson, Parakkal Deepak
Inflammatory Bowel Diseases.2023;[Epub] CrossRef - Gene-regulation modules in nonalcoholic fatty liver disease revealed by single-nucleus ATAC-seq
Fumihiko Takeuchi, Yi-Qiang Liang, Hana Shimizu-Furusawa, Masato Isono, Mia Yang Ang, Kotaro Mori, Taizo Mori, Eiji Kakazu, Sachiyo Yoshio, Norihiro Kato
Life Science Alliance.2023; 6(10): e202301988. CrossRef - Effectiveness of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease Evolution Using In Vivo and In Vitro Studies: A Systematic Review
Cristian Sandoval, Carolina Reyes, Pamela Rosas, Karina Godoy, Vanessa Souza-Mello, Jorge Farías
International Journal of Molecular Sciences.2023; 24(21): 15728. CrossRef - Maternal betaine supplementation ameliorates fatty liver disease in offspring mice by inhibiting hepatic NLRP3 inflammasome activation
Lun Li, Liuqiao Sun, Xiaoping Liang, Qian Ou, Xuying Tan, Fangyuan Li, Zhiwei Lai, Chenghe Ding, Hangjun Chen, Xinxue Yu, Qiongmei Wu, Jun Wei, Feng Wu, Lijun Wang
Nutrition Research and Practice.2023; 17(6): 1084. CrossRef - Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease
Yamei Duan, Xiongfeng Pan, Jiayou Luo, Xiang Xiao, Jingya Li, Prince L. Bestman, Miyang Luo
Frontiers in Immunology.2022;[Epub] CrossRef - A potential link between plasma short‑chain fatty acids, TNF‑α level and disease progression in non‑alcoholic fatty liver disease: A retrospective study
Jing Xiong, Xia Chen, Zhijing Zhao, Ying Liao, Ting Zhou, Qian Xiang
Experimental and Therapeutic Medicine.2022;[Epub] CrossRef - Update on Non-Alcoholic Fatty Liver Disease-Associated Single Nucleotide Polymorphisms and Their Involvement in Liver Steatosis, Inflammation, and Fibrosis: A Narrative Review
Fajar Dwi Astarini, Neneng Ratnasari, Widya Wasityastuti
Iranian Biomedical Journal.2022; 26(4): 252. CrossRef - Clinical significance of epicardial fat assessment in hypertensive patients with non-alcoholic fatty liver disease
M. Е. Statsenko, A. M. Streltsova
"Arterial’naya Gipertenziya" ("Arterial Hypertension").2022; 28(3): 260. CrossRef - The biological clock enhancer nobiletin ameliorates steatosis in genetically obese mice by restoring aberrant hepatic circadian rhythm
Sebastian Larion, Caleb A. Padgett, Joshua T. Butcher, James D. Mintz, David J. Fulton, David W. Stepp
American Journal of Physiology-Gastrointestinal and Liver Physiology.2022; 323(4): G387. CrossRef - Effect of Fiber Supplementation on Systemic Inflammation and Liver Steatosis in Mice Fed with High-Carbohydrate Diets
Cacilda Rocha Hildebrand Budke, Débora Marchetti Chaves Thomaz, Rodrigo Juliano de Oliveira, Rita de Cássia Avellaneda Guimarães, Amariles Diniz Ramires, Doroty Mesquita Dourado, Elisvânia Freitas dos Santos, Adriana Conceicon Guercio Menezes, Andréia Con
Metabolic Syndrome and Related Disorders.2022; 20(10): 558. CrossRef - Association between inflammatory markers and non-alcoholic fatty liver disease in obese children
Yamei Duan, Jiayou Luo, Xiongfeng Pan, Jia Wei, Xiang Xiao, Jingya Li, Miyang Luo
Frontiers in Public Health.2022;[Epub] CrossRef - Efficacy of resveratrol supplementation in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials
Sahar Rafiee, Hamed Mohammadi, Abed Ghavami, Erfan Sadeghi, Zahra Safari, Gholamreza Askari
Complementary Therapies in Clinical Practice.2021; 42: 101281. CrossRef - Association of xenobiotic-metabolizing enzymes (GSTM1 and GSTT 1), and pro-inflammatory cytokines (TNF-α and IL-6) genetic polymorphisms with non-alcoholic fatty liver disease
Narges Damavandi, Sirous Zeinali
Molecular Biology Reports.2021; 48(2): 1225. CrossRef - Potential protective effect of curcumin in high-fat diet-induced nonalcoholic fatty liver disease in rats
AhmedM M. Mahmouda, NohaA.-R A. El-Hagag, HusseinI El-Bitar, Abdel-HalimM Afifi
Journal of Current Medical Research and Practice.2021; 6(1): 92. CrossRef - Current innovations in nutraceuticals and functional foods for intervention of non-alcoholic fatty liver disease
Mengyao Zhao, Shumin Chen, Xiaoguo Ji, Xin Shen, Jiangshan You, Xinyi Liang, Hao Yin, Liming Zhao
Pharmacological Research.2021; 166: 105517. CrossRef - Inflammation in Metabolic Diseases and Insulin Resistance
Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2021; 3(2): 31. CrossRef - Cellular Mechanisms of Liver Fibrosis
Pragyan Acharya, Komal Chouhan, Sabine Weiskirchen, Ralf Weiskirchen
Frontiers in Pharmacology.2021;[Epub] CrossRef - The role of insulin resistance and systemic inflammation in reducing the elasticity of the main arteries in patients with arterial hypertension and non-alcoholic fatty liver disease
M.E. Statsenko, A.M. Streltsova, M.I. Turovets
Profilakticheskaya meditsina.2021; 24(5): 60. CrossRef - Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical therapeutic efficacy evaluation
Victoria L. Slaughter, John W. Rumsey, Rachel Boone, Duaa Malik, Yunqing Cai, Narasimhan Narasimhan Sriram, Christopher J. Long, Christopher W. McAleer, Stephen Lambert, Michael L. Shuler, J. J. Hickman
Scientific Reports.2021;[Epub] CrossRef - L-ergothioneine and metformin alleviates liver injury in experimental type-2 diabetic rats via reduction of oxidative stress, inflammation, and hypertriglyceridemia
Ayobami Dare, Mahendra L. Channa, Anand Nadar
Canadian Journal of Physiology and Pharmacology.2021; 99(11): 1137. CrossRef - Patchouli alcohol ameliorates skeletal muscle insulin resistance and NAFLD via AMPK/SIRT1-mediated suppression of inflammation
Do Hyeon Pyun, Tae Jin Kim, Seung Yeon Park, Hyun Jung Lee, A.M. Abd El-Aty, Ji Hoon Jeong, Tae Woo Jung
Molecular and Cellular Endocrinology.2021; 538: 111464. CrossRef - Low Vitamin D Level was Associated with Non-alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus
Vahid Sheikhi, Zahra Heidari
Shiraz E-Medical Journal.2021;[Epub] CrossRef - Inflammation and Fibrogenesis in MAFLD: Role of the Hepatic Immune System
Pietro Torre, Benedetta Maria Motta, Roberta Sciorio, Mario Masarone, Marcello Persico
Frontiers in Medicine.2021;[Epub] CrossRef Sarcopenia Is an Independent Risk Factor for NAFLD in COPD: A Nationwide Survey (KNHANES 2008–2011)
Kyung Soo Hong, Min Cheol Kim, June Hong Ahn
International Journal of Chronic Obstructive Pulmonary Disease.2020; Volume 15: 1005. CrossRef- A multi-targeting strategy to ameliorate high-fat-diet- and fructose-induced (western diet-induced) non-alcoholic fatty liver disease (NAFLD) with supplementation of a mixture of legume ethanol extracts
Yen-Chun Koh, Yen-Cheng Lin, Pei-Sheng Lee, Ting-Jang Lu, Kai-Yi Lin, Min-Hsiung Pan
Food & Function.2020; 11(9): 7545. CrossRef - The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling
Samukelisiwe C. Shabalala, Phiwayinkosi V. Dludla, Lawrence Mabasa, Abidemi P. Kappo, Albertus K. Basson, Carmen Pheiffer, Rabia Johnson
Biomedicine & Pharmacotherapy.2020; 131: 110785. CrossRef - Redox Regulation by Protein S-Glutathionylation: From Molecular Mechanisms to Implications in Health and Disease
Aysenur Musaogullari, Yuh-Cherng Chai
International Journal of Molecular Sciences.2020; 21(21): 8113. CrossRef - Long-Term Soy Protein Isolate Consumption Reduces Liver Steatosis Through Changes in Global Transcriptomics in Obese Zucker Rats
Melisa Kozaczek, Walter Bottje, Byungwhi Kong, Sami Dridi, Diyana Albataineh, Kentu Lassiter, Reza Hakkak
Frontiers in Nutrition.2020;[Epub] CrossRef - Lycopene Modulates Pathophysiological Processes of Non-Alcoholic Fatty Liver Disease in Obese Rats
Mariane Róvero Costa, Jéssica Leite Garcia, Carol Cristina Vágula de Almeida Silva, Artur Junio Togneri Ferron, Fabiane Valentini Francisqueti-Ferron, Fabiana Kurokawa Hasimoto, Cristina Schmitt Gregolin, Dijon Henrique Salomé de Campos, Cleverton Roberto
Antioxidants.2019; 8(8): 276. CrossRef - A new method to induce nonalcoholic steatohepatitis (NASH) in mice
Feryal Savari, Seyyed Ali Mard, Mohammad Badavi, Anahita Rezaie, Mohammad Kazem Gharib-Naseri
BMC Gastroenterology.2019;[Epub] CrossRef - Zeaxanthin Dipalmitate in the Treatment of Liver Disease
Nisma Lena Bahaji Azami, Mingyu Sun
Evidence-Based Complementary and Alternative Medicine.2019; 2019: 1. CrossRef - A narrative review on effects of vitamin D on main risk factors and severity of Non-Alcoholic Fatty Liver Disease
Abbas Ali Sangouni, Saeid Ghavamzadeh, Atena Jamalzehi
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2019; 13(3): 2260. CrossRef - ABO blood group and risk of newly diagnosed nonalcoholic fatty liver disease: A case-control study in Han Chinese population
Guo-Chao Zhong, Shan Liu, Yi-Lin Wu, Mei Xia, Jin-Xian Zhu, Fa-Bao Hao, Lun Wan, Pavel Strnad
PLOS ONE.2019; 14(12): e0225792. CrossRef - Nonalcoholic Fatty Liver Disease and Diabetes: An Epidemiological Perspective
Eun-Jung Rhee
Endocrinology and Metabolism.2019; 34(3): 226. CrossRef - Telmisartan improves the metabolic, hematological and inflammasome indices in non-alcoholic fatty liver infiltration: A pilot open-label placebo-controlled study
Marwan S.M. Al-Nimer, Vian A.W. Esmail, O. Mohammad
Electronic Journal of General Medicine.2019; 16(3): em142. CrossRef - TRUSS Exacerbates NAFLD Development by Promoting IκBα Degradation in Mice
Chang‐Jiang Yu, Qiu‐Shi Wang, Ming‐Ming Wu, Bin‐Lin Song, Chen Liang, Jie Lou, Liang‐Liang Tang, Xiao‐Di Yu, Na Niu, Xu Yang, Bao‐Long Zhang, Yao Qu, Yang Liu, Zhi‐Chao Dong, Zhi‐Ren Zhang
Hepatology.2018; 68(5): 1769. CrossRef - Association Between Tumor Necrosis Factor-α and the Risk of Hepatic Events: A Median 3 Years Follow-Up Study
Zhi Zhu, Shuofeng Li
Hepatitis Monthly.2018;[Epub] CrossRef - Clinical Characteristics of Non-Alcoholic Fatty Liver Disease Based on Analyses from the Kangbuk Samsung Health Study
Eun-Jung Rhee
The Journal of Korean Diabetes.2017; 18(2): 81. CrossRef - Ezetimibe alleviates non-alcoholic fatty liver disease through the miR-16 inhibiting mTOR/p70S6K1 pathway
Xiang Wang, Yunbing Meng, Junrong Zhang
RSC Advances.2017; 7(60): 37967. CrossRef - Relationship between adipose tissue dysfunction, vitamin D deficiency and the pathogenesis of non-alcoholic fatty liver disease
Flavia A Cimini, Ilaria Barchetta, Simone Carotti, Laura Bertoccini, Marco G Baroni, Umberto Vespasiani-Gentilucci, Maria-Gisella Cavallo, Sergio Morini
World Journal of Gastroenterology.2017; 23(19): 3407. CrossRef - Omega-3 Fatty Acids Protect Fatty and Lean Mouse Livers After Major Hepatectomy
Michael Linecker, Perparim Limani, Patryk Kambakamba, Philipp Kron, Christoph Tschuor, Nicolas Calo, Michelangelo Foti, Jean-François Dufour, Rolf Graf, Bostjan Humar, Pierre-Alain Clavien
Annals of Surgery.2017; 266(2): 324. CrossRef - Exogenous administration of DLK1 ameliorates hepatic steatosis and regulates gluconeogenesis via activation of AMPK
Y-h Lee, M R Yun, H M Kim, B H Jeon, B-C Park, B-W Lee, E S Kang, H C Lee, Y W Park, B-S Cha
International Journal of Obesity.2016; 40(2): 356. CrossRef - Immune Imbalances in Non-Alcoholic Fatty Liver Disease: From General Biomarkers and Neutrophils to Interleukin-17 Axis Activation and New Therapeutic Targets
Feliciano Chanana Paquissi
Frontiers in Immunology.2016;[Epub] CrossRef - Comparison of Serum Adipocytokine Levels according to Metabolic Health and Obesity Status
Tae Hoon Lee, Won Seon Jeon, Ki Joong Han, Shin Yeoung Lee, Nam Hee Kim, Hyun Beom Chae, Choel Min Jang, Kyung Mo Yoo, Hae Jung Park, Min Kyung Lee, Se Eun Park, Hyung Geun Oh, Cheol-Young Park, Won-Young Lee, Ki-Won Oh, Sung-Woo Park, Eun-Jung Rhee
Endocrinology and Metabolism.2015; 30(2): 185. CrossRef - Metabolic Health Is More Important than Obesity in the Development of Nonalcoholic Fatty Liver Disease: A 4-Year Retrospective Study
Min-Kyung Lee, Eun-Jung Rhee, Min Chul Kim, Byung Sub Moon, Jeong In Lee, Young Seok Song, Eun Na Han, Hyo Sun Lee, Yoonjeong Son, Se Eun Park, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
Endocrinology and Metabolism.2015; 30(4): 522. CrossRef - Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease
Sanja Stojsavljević
World Journal of Gastroenterology.2014; 20(48): 18070. CrossRef - Brief Review of Articles in 'Endocrinology and Metabolism' in 2013
Won-Young Lee
Endocrinology and Metabolism.2014; 29(3): 251. CrossRef

 KES
KES
 PubReader
PubReader Cite
Cite

