The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma
Article information
Abstract
Background
Tumor-associated macrophages (TAMs) play a tumorigenic role related to advanced staging and poor prognosis in many human cancers including thyroid cancers. Yet, a functional role of TAMs in papillary thyroid carcinoma (PTC) has not been established. The aim of this study was to investigate TAM expression in human PTC with lymph node (LN) metastasis.
Methods
Thirty-six patients who underwent surgery after being diagnosed with PTC with LN metastasis were included. Primary tumor tissues were immunohistochemically stained with an anti-CD68 antibody and clinical characteristics according to TAM density were evaluated.
Results
The TAM densities (CD68+ cells) varied from 5% to 70%, in all tumor areas, while few cells were stained in adjacent normal tissues. TAMs were identified as CD68+ cells with thin, elongated cytoplasmic extensions that formed a canopy structure over tumor cells. Comparing clinicopathologic characteristics between tumors with low (<25%) and high (25% to 70%) TAM densities, primary tumors were larger in the high density group than in the low density group (2.0±0.1 vs. 1.5±0.1; P=0.009).
Conclusion
TAMs were identified in primary PTC tumors with LN metastasis and higher TAM densities were related to larger tumor sizes, suggesting a tumorigenic role of TAMs in human PTCs.
INTRODUCTION
Macrophages are one of the components of bone marrow cells and play roles in innate immunity. Classically, they circulate in the blood and migrate into the inflammatory tissues such as infectious diseases in response to specific, endogenous immune signal [1,2]. Recently their alternative roles in anti-inflammation, cell clearance and tissue regenerations have also been established especially in inflammatory metabolic disorders including obesity and diabetes [1,2]. Therefore, macrophages have been categorized into two subtypes-M1 and M2-depending on their distinctive roles. M1 macrophages play proinflammatory functions in response to TH1 cytokines and M2 macrophages play anti-inflammatory and imunosuppressive activities in response to Th2 cytokines [3]. However, macrophages are not fixed to a single M1 or M2 subtype but suspected to rapidly differentiate and redifferentiate in various microenvironments.
Tumor-associated macrophages (TAMs), macrophages existing tumor microenvironment, were first described in the early 1980s [4,5]. Till now, TAMs have been found to play dual functions, both positively or negatively affect tumor growth through interactions with the microenvironment, and these actions are tissue specific [6,7]. Several clinical studies showed that high density TAMs were present in more advanced stages of cancer with a worse prognosis in breast [8,9], lung [10,11], and bladder cancer [12,13]. Although a high TAM density has been reported in poorly differentiated papillary thyroid carcinoma (PTC) or anaplastic thyroid cancer [14], the distribution and function of TAMs in the most common PTC have not been studied yet. In this study, we have demonstrated the existence of TAMs in PTC tissue with lymph node (LN) metastasis and also used expression distribution and morphological properties to investigate the relationship between TAMs and the frequency of extrathyroidal invasion in PTC.
METHODS
Study subjects
The study was conducted from March to May 2012 at Seoul National University Hospital. The primary PTC lesion and surrounding normal tissues were collected from 36 consecutive patients with a previous thyroidectomy and central region LN dissection followed by a pathological diagnosis of PTC with LN metastasis. This diagnosis was defined as having a metastasized lesion in at least one LN from the histological examination of 1 to 37 LNs obtained from a central region LN dissection. All participating patients signed the informed consent form after receiving a comprehensive explanation of the study. This study was conducted with the approval of the Institutional Review Board of Seoul National University Hospital (IRB approval No. H-0809-0970-258).
Histologic examination
All sample tissues were fixed in 10% neutral formalin solution for 24 hours and washed with distilled water for 20 minutes before histological observation. Tissues were dehydrated with ethyl alcohol, washed with xylene, embedded in paraffin, and sectioned into 3 µm sections. Sections were deparaffinized, using a standard process of anhydrous ethyl alcohol, washed, and stained with hematoxilin and eosin. The size of the primary cancer and presence of extrathyroidal invasion (report of other pathological findings) were measured by two or more pathologists.
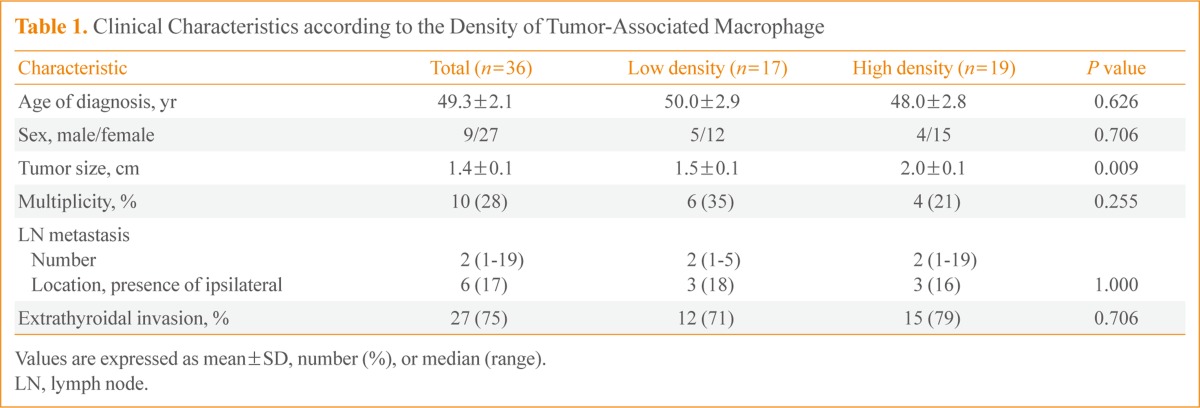
For immunohistochemistry to demonstrate TAM expression, paraffin-embedded tissues were sectioned at 5 µm thick, deparaffinized, placed on a slide, dried at 37℃, and deparaffinized with xylene. They were then incubated with antihuman CD68 antibody PG-M1 (1:50, DakoCytomation, Carpinteria, CA, USA). Color staining was achieved with a DAKO EnVision+ system (DakoCytomation) by using a streptavidin biotin-horseradish peroxidase process and DAB (3,3'-diaminobenzi dine tetrahydrochloride) as a chromogen. Hematoxilin and eosin staining was used as a control. These sections were observed under an optical microscope. The proportion of CD68+ cells in each tumor on each slide was evaluated after staining. Based on a median value of 25%, weak positive (+) and strong positive (++) staining were defined as <25% and ≥25%, respectively (Fig. 1).
Classification of the density of CD68-positive cells in human papillary thyroid carcinoma. Immunohistochemical staining of CD68 was performed and tumor-associated macrophages (TAMs) were scored by the number of CD68+ cells/total tumor cells under × 400 magnification. Patients were divided into two groups according to TAM density; (A) low (<25%) and (B) high (25% to 70%) TAM density.
Statistical analysis
Patients' clinical characteristics are presented as mean±SEM. CD68 immunohistochemistry results were classified according to the pathological analysis along with a correlation analysis of other clinical data. Statistical significance is indicated with the P value, and a significant difference was considered when P values for both were less than 0.05. Statistical analysis was conducted by using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics of study subjects
The mean age of the 36 study subjects was 49.3±2.1 years old, and 27 subjects (75%) were female. The mean tumor size was 1.4±0.1 cm, and extrathyroidal invasion was observed in 27 subjects (75%). Ten subjects (28%) had multiple (two or three) tumors and eight of them had tumors in both lobes. LN metastases (median, 2; range, 1 to 19) were pathologically confirmed. Three metastases were found opposite the tumor, and three patients had bilateral LN metastases (Table 1).
Characteristics of TAM staining in PTC
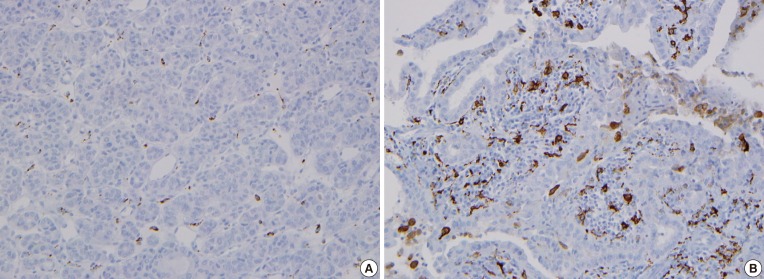
The CD68 immunohistochemistry effectively stained macrophage cytoplasm and allowed us to observe TAMs in PTC. The TAM nuclei in PTC tissue were approximately 1/3 to 1/2 the size of the nuclei in surrounding tumor cells, and the cytoplasm was widely distributed, seeming to wrap around PTC cells. The cytoplasm of TAM and cancer cells appeared to be in close contact (Fig. 2). TAMs with extended nuclei were rare relative to the number of cancer cells due to the multidirectional expansion of TAM cytoplasm. The slide preparation may have affected the visibility of the cytoplasm, but not the layer containing the nucleus.
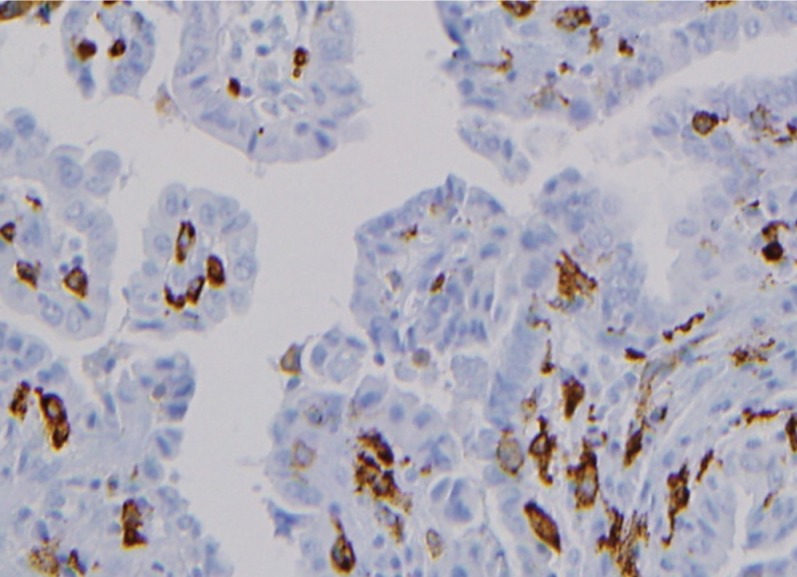
Morphologies of CD68-positive tumor-associated macrophages (TAMs) in papillary thyroid carcinomas (PTCs). CD68+ cells (brownish) with thin elongated cytoplasmic extensions were intercalated into the papillary structures of PTCs. TAMs formed a distinctive canopy-like structure over cuboidal tumor cells (×400).
Clinical characteristics for the distribution of TAM expression
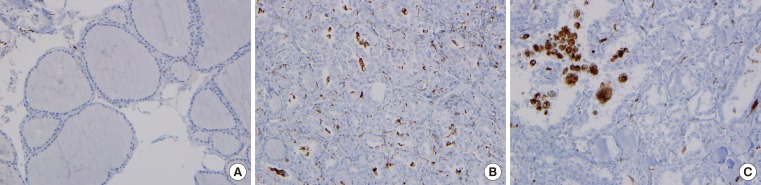
CD68+ cells were rare in normal thyroid tissue (Fig. 3A), but with the presence of TAMs, the CD68+ cells were present in PTC lesions. TAMs accounted 5% to 75% of all cancer cells, and were dispersed throughout the cancer tissues (Fig. 3B) or were grouped together in some lesions (Fig. 3C). This papillary arrangement of the thyroid cancer was maintained regardless of the presence of TAMs. There was no significant difference in clinical characteristics such as age at diagnosis, gender, size or number of primary tumor, or presence of extrathyroidal invasion according to the TAM expression pattern (diffuse or localized).
Clinical characteristics by TAM density
The patients' clinical characteristics were compared in tumors with low (17 patients) and high (19 patients) TAM density. TAM density was the proportion all cancer cells that were CD68+. The mean age at diagnosis and gender were not significantly different between the two groups, but the primary tumors were statistically larger in the group with high TAM density (1.5±0.1 vs. 2.0±0.1; P<0.01) (Table 1). The position and number of LN metastases was not analyzed statistically due to the small number of subject, but two patients with six or more LN metastases had TAM densities of 50% or higher. The number of tumors and presence of extrathyroidal invasion were similar between the two groups (Table 1).
DISCUSSION
This study demonstrated the presence of TAM in a canopy structure over thyroid tumor cells in primary tumor tissues from 36 patients with PTC with LN metastasis. In addition, the primary tumors were significantly larger in the group with high TAM density. More LNs had metastases with a higher TAM density, although the difference was not statistically significant. Based on these findings, TAM density is considered to be related to PTC stage.
Ryder et al. [14] demonstrated a higher TAM density in poorly differentiated PTC and anaplastic thyroid cancer than in well-differentiated PTC, by using CD68 and CD163 immunohistochemistry. Qing et al. [15] also reported a high TAM density in well-differentiated PTC, and the TAM density was significantly higher in tumors with TNM staging over stage III. The results from these studies demonstrated that a high TAM density is present in poorly differentiated PTC and is related to poor prognosis, which indicates TAMs may support PTC growth. However, the role of TAMs in thyroid cancer remains controversial. Fiumara et al. [16], using CD68 immunohistochemistry, found that TAM prevented metastasis in some patients. Of 121 patients with PTC, 15% had TAMs CD68+) that appeared to have a phagocytic function on cancer cells. These patients had significantly less blood vessel invasion and remote metastasis and largely accompanying invasions of lymphocytes and dendritic cells, than patients without an CD68+ cells. These results suggest that these cells may activate the host immune response to cancer cells. However, in our study, 56% of patients had TAMs without evidence of phagocytosis, and their clinical characteristics were similar to those without TAMs.
This study investigated TAM morphologies and evaluated related clinical characteristics in PTC tissue with relatively advanced LN metastasis. Interestingly, CD68+ TAM cells observed in this study appeared to be mostly cytoplasm forming a canopy structure that projected over cancer cells. These canopy structures formed over the interior and exterior papillary projections in PTC cells that normally form papillary structures. These morphological characteristics are a standard of the TAM definition and differ from the rounded amoeba shape of inflammatory response cells, which are generally referred to as M1 macrophages [17]. The morphological characteristics of TAMs support cancer cell growth by forming a structural network between cancer cells and the extracellular matrix and are considered to support endothelial cells in forming blood vessels [18,19]. Caillou et al. [20] described the morphological characteristics of TAMs and their adjacency between cells, and these morphological characteristics suggest that TAMs facilitate cancer cell growth and metastasis. In this study, however, the morphological characteristics of thyroid cancer tissue were maintained regardless of the presence of TAMs, hence we suspect that TAMs do not have a huge effect on cancer development [20].
Interestingly, despite of small number of patients, when we classified patients according to TAM density, the primary tumors were larger in the high TAM density group. Hence, we hypothesize that TAMs inhibit tumor growth in advanced PTC. These results differ from previous findings, where Qing et al. [15] compared the micropapillary cancer smaller than 1 cm and papillary cancer of typical size (1 cm or larger) and found no difference in TAM density. Apart from the etiological interpretation, TAM can be interpreted to be involved in cancer invasiveness after mobilization followed by the cancer growth. Actually, TAM has been related to metastasis in various cancer types [12].
The study has a limitation of not including patients without LN metastasis. This study on TAMs reported that TAMs are rare in PTC, and especially they have only been reported in poorly differentiated cancer or anaplastic thyroid cancer. Therefore, this study only included the subjects with LN metastasis in higher stages of papillary cancer. A further study with more subjects including patients without LN metastasis is warranted.
Studies on cancer treatments targeting TAM have increased since the first report that TAM facilitates cancer growth and metastasis and offers indicates a poor prognosis. Popular treatment strategies that have been investigated involve cytokines such as colony stimulating factor (CSF)-1 [21], which mobilizes TAM to the tumor; interleukin (IL)-10 [21] and IL-4 [22], which facilitate a cancer's affinity for TAM through macrophage differentiation into the M1 and M2 subtypes; and CCR2 [23,24], which facilitates TAM in cancer metastasis. Interestingly, a recent animal study was published that suggests that these strategies might be feasible in treating thyroid cancer [25]. Ryder et al. [25] demonstrated PTC inhibition in a BRAF-mutant, transformed mice model by inhibiting CSF-1 gene expression through genetic modification or use of a CSF-1/CSF-1 receptor signal pathway inhibitor.
Studies on TAM in thyroid cancer are underway, but clinical data and studies on the mechanisms of TAM action are lacking. Thyroid cancer has a relatively favorable prognosis because its growth rate is slow. Therefore, general cancer pathophysiology does not apply to the role of TAMs. Currently the role of TAMs is only known in relatively invasive breast, lung, and pancreatic cancers, but the results of this study indicate that TAMs also exist in PTC, which is generally well differentiated with a favorable prognosis. Their role in cancer development is exciting; especially, the in light of the animal study targeting TAM that found possible inhibition of PTC. Expectations are high for treating patients with thyroid cancer since the BRAF mutation rate is higher among the Korean population than other ethnic groups. Additional study on the relationship between the BRAF mutation and TAM in Korean patients is warranted.
This study was conducted with relatively few patients, but it demonstrated the presence of TAMs in clinically common PTC patients, and the presence of a high TAM density in larger primary cancers. However patients without LN metastasis were not included in the study or the comparative analysis. Another limitation arose from not evaluating the characteristics of TAMs in metastasized LNs. Also, there is a fundamental controversy over whether CD68, the TAM marker, is appropriate for the staining M2 macrophages, so the more specific CD 163 should be stained in future analyses.
As a result of immunohistochemistry by using anti-CD68 antibody in PTC tissue with LN metastasis, TAM was present in all tissues with an extended cytoplasm forming a canopy shape over cancer cells. The density varied from 5% to 70% according to the tissue type. In normal thyroid tissue from the same patients, CD68+ cells were rare. When tumors were classified by TAM density, the primary tumors were statistically significantly larger in the group with a high TAM density. Therefore, TAM is suggested to stimulate cancer growth in PTC tissue with LN metastasis.
ACKNOWLEDGMENTS
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ00954003), Rural Development Administration, Republic of Korea.
Notes
No potential conflict of interest relevant to this article was reported.